Short summary
The EPIICAL (Early-treated Perinatally HIV-infected Individuals: Improving Children’s Actual Life with Novel Immunotherapeutic Strategies) project arises from the firm belief that perinatally infected children treated with suppressive antiretroviral therapy (ART) from early infancy represent the optimal population model in which to study novel immunotherapeutic strategies aimed at achieving ART-free remission. This is because HIV-infected infants treated within 2–3 months of life have a much reduced viral reservoir size, and rarely show HIV-specific immunity but preserve normal immune development. The goal of EPIICAL is the establishment of an international collaboration to develop a predictive platform using this model to select promising HIV therapeutic vaccine candidates, leading to prioritisation or deprioritisation of novel immunotherapeutic strategies.
To establish this platform, the EPIICAL Consortium aims to: develop predictive models of virological and immunological dynamics associated with response to early ART and to treatment interruption using available data from existing cohorts/studies of early-treated perinatally HIV-infected children; optimise methodologies to better characterise immunological, virological and genomic correlates/profiles associated with viral control; test novel immunotherapeutic strategies using in vivo proof-of-concept (PoC) studies with the aim of inducing virological, immunological and transcriptomic correlates/profiles equivalent to those defined by the predictive model. This approach will strengthen the capacity for discovery, development and initial testing of new therapeutic vaccine strategies through the integrated efforts of leading international scientific groups, with the aim of improving the health of HIV-infected individuals.
Keywords: HIV, children, immunotherapies, therapeutic vaccines, early treated
Introduction
To date, an estimated 3–4 million children are living with HIV and, despite preventive measures, more than 250,000 infants are newly infected every year [1]. With access to antiretroviral treatment (ART), increasing numbers of children are surviving into adolescence and beyond. Advances in treatment in terms of availability of new and increasingly efficacious antiretroviral agents have encouraged the optimistic idea that a ‘cure’ of HIV infection is possible by reducing the HIV reservoir to a level that could maintain HIV ‘remission’ after treatment interruption. However, both the case of the ‘Mississippi baby’ [2] and studies such as the VISCONTI cohort [3] illustrate that only a very small minority of patients achieve prolonged viral control after treatment interruption, with early ART as a single interventional strategy. Moreover, the mechanisms of post-treatment control require further characterisation. Indeed, in all but one case, discontinuation of ART in children without any additional intervention has resulted in rapid viral rebound [4,5]. In line with international guidelines, perinatally HIV-infected infants, should start ART as soon as the diagnosis is confirmed [6], and currently should remain on therapy for their whole life with the risk of accumulating toxicity and viral resistance. The rate of virological failure due to poor adherence increases over time in perinatally HIV-infected children on ART [7]. Similar data have been reported in African settings when access to ART is widely available [8]. This highlights the urgent need to define strategies, such as therapeutic HIV vaccines, to provide long-term viral suppression in the paediatric population, in order to permit safe treatment interruption without viral rebound and its associated complications [9] (Figure 1). In response, an International consortium, named EPIICAL (Early-treated Perinatally HIV-infected Individuals: Improving Children’s Actual Life with Novel Immunotherapeutic Strategies), has been established, gathering together scientists and clinicians in the field of paediatric HIV infection. The EPIICAL project arises from thefirm belief that perinatally infected children treated with suppressive antiretroviral therapy (ART) from early infancy represent the optimal population model in which to study novel immunotherapeutic strategies aimed at achieving ART-free remission
Figure 1.
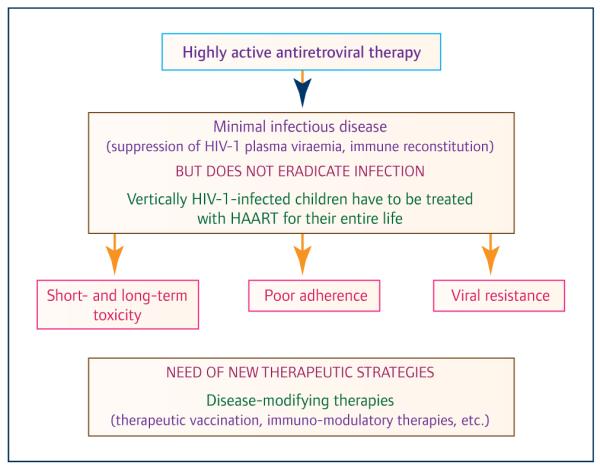
Reasons for the urgent need for new therapeutic strategies for vertically HIV-infected children
Why are early-treated children a unique opportunity to evaluate new immunotherapeutic strategies?
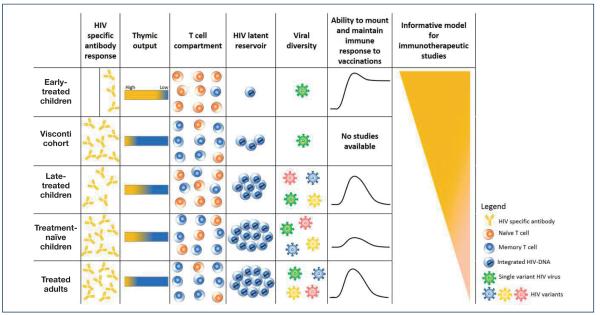
Very early-treated children represent a unique model to evaluate the potential efficacy of different immunotherapeutic strategies [10]. Infants differ immunologically from adults, with an active thymus, very few long-lived CD4+ T memory cells and reduced HIV-specific immune responses (Figure 2). HIV-infected infants treated within 2–3 months of life have a very limited size and diversity of viral reservoir [11,12] compared to early-treated adults. Early virological control in infants prevents ongoing seeding of the HIV reservoir with maintenance of a smaller HIV reservoir over time [12]. Minimising the size of reservoir may optimise conditions for new strategies aimed at achieving ART-free virological remission, including immunotherapy targeting specific viral components in a population with limited viral diversification.
Figure 2.
The unique features of early-treated children as a model. The panel summarises the main differences among early-treated children and other model populations. HIV-infected infants treated within 2–3 months of life rarely exhibit HIV-specific immunity, have a more limited viral reservoir and viral diversity, greater thymic output, lower numbers of central memory T cells and greater ability to develop and maintain protective responses to vaccination, compared with other population models
Infants treated during acute infection rarely exhibit HIV-specific immunity [13–15] but appear to maintain normal immune development [16-18]. Furthermore, compared with adults, children have a more active thymus resulting in a better capacity for immune regeneration, which permits evaluation of the ability of potential immunogenic vaccines to induce new traceable immune responses with novel or available assays [19,20]. Moreover, young infants have high immune tolerance and low immune activation state, a condition not conducive for HIV reservoir seeding, resulting in a better response to vaccination [21,22] (Figure 2). Hence, early-treated children represent the optimal cohort in which to investigate therapeutic vaccines with the ultimate goal of controlling the latent/persistent HIV reservoir without ART [23,24].
The long and winding road towards an effective HIV therapeutic vaccine
Therapeutic vaccines are part of the HIV cure agenda and are a global health priority. Several immunotherapeutic approaches to enhance host immunity and control viral replication have been tested to date. However, the disappointing results of recent clinical trials highlight the urgent need for earlier identification of viable HIV therapeutic vaccine candidates, a step that if left unaddressed will continue to present significant risks of failure at relatively late stages of the development process. Preclinical and early-phase testing of new vaccines requires several years and by the time a vaccine enters the efficacy-testing phase, it is frequently out-dated relative to current scientific research [25]. As a result, vaccine development costs are increasing while financial support is decreasing or at best is unpredictable. Several other factors are affecting the successful development of an HIV therapeutic vaccine. First is the lack of specific correlates of protection that should be induced by an effective therapeutic vaccine. Protective immunity for current routine childhood vaccination is typically associated with antibody responses [26,27]. For HIV vaccines the identification of these biomarkers is more complex and thus far, difficult to achieve with current technologies. Evidence suggests a combination of cellular and humoral immune responses are needed for effective protection; however, studies have failed to produce reliable correlates of protection to be used as end points in proof of concept and subsequently in vaccine efficacy studies [28,29]. Understanding the role of host genetics and the functional attributes of vaccine-induced immunity can improve our knowledge of the immune correlates broadly applicable in a globally efficacious vaccine. Another obstruction in the HIV vaccine development field is the identification of an optimal model population to investigate novel approaches. To date, therapeutic HIV vaccine strategies have been studied almost exclusively in adults. One major limitation of adult cohorts studied up to now is the lack of a uniform population in terms of the timing of diagnosis and commencement of antiretroviral therapy in relation to the date of seroconversion as well as the immunological and virological characteristics of the vaccinees (Figure 2).
The current scenario of immunotherapy in HIV infection
Of the several immunotherapeutic approaches tested in HIV-infected adults, very few have induced even a transitory reduction in viral load in the context of treatment interruption. Dendritic cell-based immunotherapeutic vaccines using autologous inactivated HIV or virus-like particles are being trialled to facilitate recognition and elimination of HIV from the patient’s own latently infected cells. It has been shown that HIV-1-specific immune responses elicited by therapeutic DC vaccines could significantly change plasma viral load set point after treatment interruption in early-treated chronic HIV-1-infected patients [30]. Lévy et al. reported that treatment with dendritic cells generated ex vivo and loaded with HIV lipopeptides in patients on antiretroviral therapy shows an association between vaccine-elicited immune responses and control of viral load [31]. The major limitation of these approaches is the difficulty of implementation on a larger scale, particularly in developing countries. Therefore, further investigation of new vaccine candidates feasible in these settings is a research priority.
DNA vaccination strategies such as HIV-therapeutic vaccines have been explored in several completed trials in adults and, for the first time in children, demonstrating their safety and immunogenicity [32,33]. DNA vaccines can also be combined with other vaccination modalities and molecular adjuvants aimed at increasing the strength and durability of antiviral responses. Heterologous prime-boost approaches with recombinant vaccinia- or adeno-based HIV genes used as vaccine boosting, with or without adjuvants, have induced a broad and strong cellular immunity in healthy adults [34-36].
A peptide-based HIV-1 therapeutic vaccine (Vacc-4x) was shown to significantly modulate the viral set point during treatment interruption in adults, although without apparent clinical benefit [37]. Li et al. reported that a therapeutic trial with a recombinant adenovirus 5 based HIV-1 Gag vaccine (rAd5 HIV-1) was associated with a modest transient effect on residual viraemia [38].
Several other strategies have been proposed as potential adjuncts to ART in the HIV ‘cure’ agenda. These include passive immunisation with highly neutralising antibodies as tested in an SIV model [39] and immunotherapy with pegylated interferon alpha [40]: both appeared to decrease the latent pool of virus. Clinical trials are currently ongoing to evaluate their use in HIV therapy in humans.
Such approaches could potentially achieve durable viral suppression. However, although results reported in adults have been partially positive, such approaches cannot necessarily be extrapolated to children.
The novelty of the EPIICAL project
The goal of EPIICAL is to establish a predictive in vivo platform to select promising HIV therapeutic vaccine candidates and to evaluate a therapeutic vaccine strategy using early-treated perinatally HIV-infected children as the model. The Consortium has access to data from unique cohorts/studies including more than 1,000 vertically HIV-infected children treated with ART at <6 months of life. Most of the children have been virologically suppressed for several years and exhibit heterogeneous serostatus (e.g. HIV seronegative or seropositive). The plan is that children from EPPICC, the Thai cohort, CHERUB-UK, NEVEREST, CHANGES and CHER will be stratified according to the time of first viral suppression: they will be defined as ‘rapid viral suppressors’ if viral control occurred within 12 weeks following ART initiation or ‘slow viral suppressors’ if control occurred after 12 weeks. The CHER and PENTA11 studies include children who underwent planned treatment interruption after early ART and all patients showed a viral rebound. These children will be stratified according to time to viral rebound (defined as viral load over 1000 copies/mL) as ‘rapid rebounders’ or ‘slow rebounders’, with cut-off points for inclusion in the two groups based on statistical analysis and modelling of the data (Figure 3). To validate this predictive platform, we plan to generate new immunological, virological and transcriptomic (by RNA sequencing/Fluidigm-Biomark) [41] correlates/profiles of viral control in early-treated HIV-infected children (Figure 3).
Figure 3.
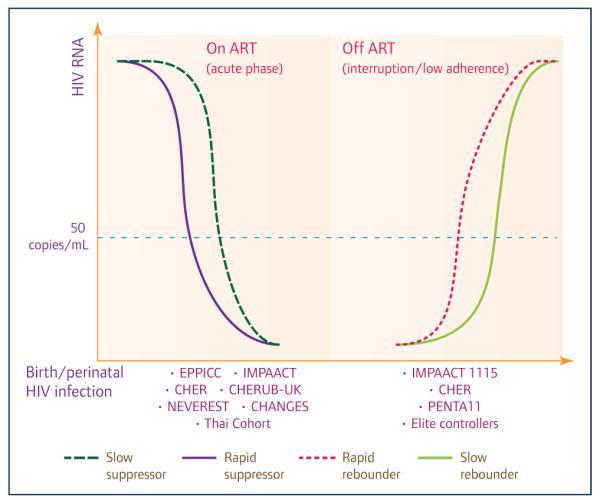
Cohorts/studies of early-treated HIV-infected children around the world (both observational cohorts and clinical trials cohorts) that are available to generate correlates/profiles of viral control after treatment initiation and interruption
The development of mathematical models applied to retrospective data from such cohorts represents a major novel insight for the EPIICAL project. Mathematical modelling will be used to address three issues: (i) predicting the virological response of HIV-infected infants to initiation of ART; (ii) predicting the response of a patient on ART with undetectable viral load (<50 copies/mL) to treatment interruption by defining the endpoint ‘time to viral rebound’; and (iii) to establish transcriptomic profiles that identify patients who are more likely to achieve post-treatment virological control (Figure 3).
Mechanistic mathematical models have proved hugely successful and influential in the HIV field and can provide us with a fundamental understanding of why patients respond to treatment in different ways. For example, how are the risks and kinetics of rebound influenced by the quality and magnitude of immune responses that a patient exhibits pre-interruption? One could hypothesise that a successful vaccine will induce a baseline potential for strong antiviral responses that will limit rebound. One might also propose that a more measured, less aggressive response to treatment interruption might be the optimal targeting of infected cells, while limiting the generation of new susceptible cells through generalised inflammation. Mathematical models of the interactions between viruses, susceptible cells and the immune response are tailor-made for predicting the outcome of competing processes such as these, and predicting how baseline state may tip the balance one way or another. Because these models treat patients’ states as dynamic variables, rather than simply correlating baseline states to long-term outcome, they provide the possibility of identifying early predictors of viral control after ART initiation, or viral rebound after treatment interruption.
To refine these predictive models, we plan to produce new data on the existing cohorts using novel optimised virological and immunological methods. Virological assays can provide not only a static picture of the viral reservoir, but more importantly, provide unique virological data on the evolution of the reservoir following treatment initiation and treatment interruption in large existing international cohorts. A combination of different and complementary techniques quantifying integrated HIV-1 DNA, 2-LTR circles, cell-associated RNA and low level residual viraemia and serology will be used to measure the viral reservoir to evaluate virological outcomes in PoC studies. Furthermore, immunological data together with virological data will be used to feed and refine the predictive model to identify genomic signatures related to virological control (Figure 4a). For this purpose, a novel protocol has been developed and is discussed further in Cotugno et al. (this issue) [41].
Figure 4.
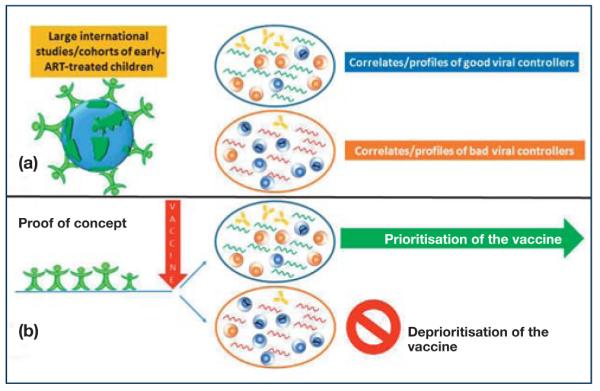
(a) Immunological and virological data as well as the transcriptomic correlates/profiles of infants who rapidly controlled (large retrospective cohorts/studies) and controlled (prospective cohorts/studies) viral replication both in response to therapy initiation and to treatment will provide the correlates/profiles of good or poor viral controllers. (b) The ability of an immunotherapeutic strategy to reproduce such correlates/profiles will be at the basis of the in vitro and in vivo predictive platform.
Selection of promising immunotherapeutic strategies through proof-of-concept studies
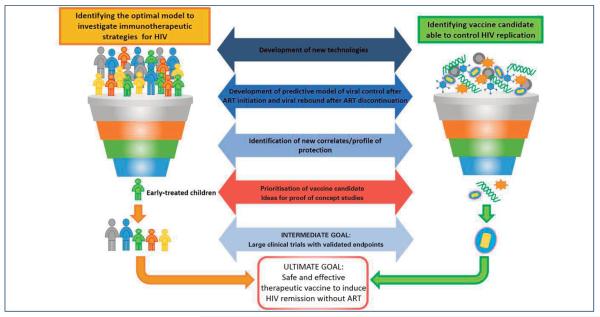
The selection of promising immunotherapeutic strategies through PoC studies in early-treated population models represents a major innovation in the HIV-vaccine field. This approach allows the selection of promising HIV therapeutic vaccine candidates to be tested in humans, thus leading to immediate prioritisation or deprioritisation of the strategy. Immunological and virological data, as well as the transcriptomic correlates/profiles of infants who rapidly control viral replication both in response to therapy initiation and to treatment interruption, will provide the correlates/profiles that we want to observe and to reproduce in the in vivo predictive platform with PoC studies (Figure 4b). This approach stems from the perception, shared by many scientists, that only the combination of in vitro and in vivo testing in humans will provide a relevant impact on the HIV-vaccine field and therapeutic management and lead to immediate prioritisation and deprioritisation of vaccine candidates and strategies [25,42]. Moreover, this approach will strengthen the capacity for discovery, development and initial testing of new therapeutic vaccines for the management of children with HIV infection. The knowledge gained has the potential to transform the lives of HIV-infected children worldwide and has a broader applicability to the treatment of HIV in adults, particularly those who initiate early ART (Figure 5).
Figure 5.
The EPIICAL roadmap to the development of an effective therapeutic HIV vaccine
To perform PoC clinical studies, perinatally infected children and adolescents who started ART early in life (<6 months of age) and who have remained virologically suppressed will be selected. Viral suppression is defined as viral load <50 copies/mL, with blips below 1000 copies/mL allowed. Thus, we will ensure that the enrolled population represents a cohort of children with a very limited viral reservoir [43] and with a normal immune system maintained.
Only children older than 3 years will be considered eligible for PoC studies. The exclusion of children below this age is to minimise, in pilot phase studies, the potential risk to younger HIV-infected children of more rapid disease progression. While there is no uniform definition of early treatment, with different research groups defining early ART as starting anywhere from 72 hours to 6 months of life, in order to capture all possible cases in PoC studies, HIV-infected children treated before 6 months of age will be considered eligible for the study.
HIV-infected children from Europe, South Africa, USA and Thailand fulfilling the clinical and virological characteristics described above will be included. From among the available immunotherapeutic strategies we have initially selected the prime (DNA)–(MVA) boost HIV immunisation schedule, as this currently meets safety criteria allowing proof-of-concept studies in HIV-infected children. Indeed, such prime-boost approaches have been shown to induce broad and long-lasting specific cellular immune responses and functional antibodies in healthy individuals [34,44–46]. Furthermore, safety and immunogenicity data are available in HIV-infected patients, including the paediatric population [32,33]. However, the proposed predictive platform is designed to test any immunotherapeutic candidates that correspond to the safety and regulatory correlates/profiles permitting use in in vivo PoC studies in children.
Treatment interruption: the final goal of HIV therapeutic vaccine. Ethical considerations for the early-treated paediatric population
The final goal of an effective therapeutic vaccine should be viral remission that allows safe periods of treatment interruption. Current paediatric HIV guidelines do not recommend structured treatment interruption, nonetheless, treatment interruptions do occur frequently in clinical practice, with a risk of approximately 30% within 5 years following ART initiation [47]. Most treatment interruptions are unplanned and taken by the family, or the children themselves, due to medication fatigue, poor compliance or adverse events. However, with improvements in survival, treatment interruption during childhood is an important issue that must be addressed for the long-term management of the infection. Few studies have evaluated the risk–benefit ratio of planned treatment interruption in HIV-infected children. The PENTA11 study, a randomised controlled trial of CD4 cell count-guided treatment interruption in children aged 7–15 years, did not report an excess in deaths or disease progression during the treatment interruption period [48,49]. However, a persistent depletion of CD4 memory cells was observed, likely to be a result of a higher residual productive infection in the planned interrupted arm compared to children on continuous therapy [50].
Treatment interruption in early-treated patients appears to be distinct. The CHER trial, a randomised controlled trial of deferred versus early infant therapy with treatment interruption after 40 or 96 weeks, is currently the only published study evaluating the impact of treatment interruption in an early-treated infant population. This study showed no evidences of excess disease during the subsequent period of treatment interruption in the early time-limited ART group. However, a deeper evaluation of the viral reservoir and of the immune function in this population is currently taking place. One implication of the expanded early ART strategy worldwide is the increased number of HIV-infected children treated early who, while on ART test HIV seronegative, which has led to a growing demand by parents, and patients, to question infection status and to request treatment interruption. The question as to whether there is a safe way to achieve treatment interruption has become a focus among the scientific community. While HIV-seronegative children can theoretically achieve a longer period of viral remission before virological relapse, as seen with the Mississippi baby, they may also be more at risk of rapid viral rebound due to the lack of HIV-specific immune responses as reported in the vast majority of cases, emphasising the need for immunotherapy. Owing to the absence of reliable biomarkers predicting safe treatment interruption [51] in perinatally HIV-infected children and unproven efficacy of therapeutic vaccines, such a strategy requires close monitoring and evaluation in clinical trial settings. Many ethical issues surround antiretroviral treatment interruption and undertaking such clinical trials in children. A major concern is the possibility of increasing the viral reservoir in this population. One way to address ethical implications may be the introduction of predictive models of virological and immunological dynamics associated with response to treatment interruption, as proposed by the EPIICAL project. To maintain progress towards sustained HIV remission, further studies in early-treated HIV-infected children are required, with close monitoring and evaluation in the clinical trial setting.
Conclusion
The unique aspects of early-treated HIV-infected children make this the optimal model to investigate promising immunotherapeutic strategies. The EPIICAL project launches a call to action to investigate novel immunotherapeutic strategies in such a model. This call to action invites any institution, scientific group, pharmaceutical company or SME, to explore their promising immunotherapeutic products in the EPIICAL predictive platform, leading to immediate prioritisation or deprioritisation of the candidates.
The combination of the optimal model population, novel available methodologies and the establishment of a research platform may give a new insight into the field of therapeutic HIV vaccine development. This approach will strengthen the capacity for discovery, development and initial testing of new immunotherapeutic strategies through the integrated efforts of leading international scientific groups, with the aim of improving the long-term health of HIV-infected individuals.
Acknowledgements
All authors helped with the conception of this paper. PP, CF, PR and PZ wrote the first draft, and managed all subsequent revisions. KL, SP, EN, ECM, NM, DMG, PR, WB, SB, PZ, VC, AC, BW, CF, MM, ADR, JA, DP, CG, PR provided comments on the draft. All authors critically reviewed the article and approved the final submission.
The work of the authors is supported by PENTA Foundation and by the Children’s Hospital ‘Bambino Gesù’.
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.UNAIDS [accessed June 2015];UNAIDS Report on the Global AIDS Epidemic 2013. 2013 Available at: www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 2.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giacomet V, Vigano A, Erba P, et al. Unexpected vertical transmission of HIV infection. Eur J Pediatr. 2014;173:121–123. doi: 10.1007/s00431-013-2020-9. [DOI] [PubMed] [Google Scholar]
- 5.Butler KM, Gavin P, Coughlan S, et al. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J. 2015;34:e48–51. doi: 10.1097/INF.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 6. [accessed June 2015];Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 7.Pursuing Later Treatment Options, II project team for the Collaboration of Observational HIV Epidemiological Research Europe. Castro H, et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet. 2011;377:1580–1587. doi: 10.1016/S0140-6736(11)60208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernays S, Jarrett P, Kranzer K, Ferrand RA. Children growing up with HIV infection: the responsibility of success. Lancet. 2014;383:1355–1357. doi: 10.1016/S0140-6736(13)62328-4. [DOI] [PubMed] [Google Scholar]
- 10.Payne H, Mkhize N, Otwombe K, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)00087-0. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–1020. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 12.Persaud D, Patel K, Karalius B, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014;168:1138–1146. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol. 2000;74:6984–6991. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanchetta M, Anselmi A, Vendrame D, et al. Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther. 2008;13:47–55. [PubMed] [Google Scholar]
- 15.Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59:1012–1019. doi: 10.1093/cid/ciu432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott ZA, Chadwick EG, Gibson LL, et al. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(+) T cell responses in young HIV-1-infected infants. J Immun. 2001;167:7134–7140. doi: 10.4049/jimmunol.167.12.7134. [DOI] [PubMed] [Google Scholar]
- 17.Aboulker JP, Babiker A, Chaix ML, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18:237–245. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 18.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A. 2009;106:7939–7944. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Rossi A. Primary HIV infection in infants: impact of highly active antiretroviral therapy on the natural course. J Biol Regul Homeostat Agents. 2002;16:53–57. [PubMed] [Google Scholar]
- 20.Sandgaard KS, Lewis J, Adams S, et al. Antiretroviral therapy increases thymic output in children with HIV. AIDS. 2014;28:209–214. doi: 10.1097/QAD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 21.Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS. 2015;10:4–11. doi: 10.1097/COH.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muenchhoff M, Prendergast AJ, Goulder PJ. Immunity to HIV in early life. Front Immunol. 2014;5:391. doi: 10.3389/fimmu.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robb ML, Kim JH. Shot in the HAART: vaccine therapy for HIV. Lancet Infect Dis. 2014;14:259–260. doi: 10.1016/S1473-3099(13)70331-1. [DOI] [PubMed] [Google Scholar]
- 24.Gray GE, Corey L. Reevaluating HIV vaccine clinical trials policy for infants. J Infect Dis. 2015;211:501–503. doi: 10.1093/infdis/jiu445. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro SZ. A proposal to use iterative, small clinical trials to optimize therapeutic HIV vaccine immunogens to launch therapeutic HIV vaccine development. AIDS Res Human Retroviruses. 2015;31:49–55. doi: 10.1089/aid.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56:1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 27.Cagigi A, Cotugno N, Giaquinto C, et al. Immune reconstitution and vaccination outcome in HIV-1 infected children: present knowledge and future directions. Hum Vaccin Immunother. 2012;8:1784–1794. doi: 10.4161/hv.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shasha D, Walker BD. Lessons to be learned from natural control of HIV – future directions, therapeutic, and preventive implications. Front Immunol. 2013;4:162. doi: 10.3389/fimmu.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sui Y, Gordon S, Franchini G, Berzofsky JA. Nonhuman primate models for HIV/AIDS vaccine development. Curr Protocols Immun. 2013;102:14. doi: 10.1002/0471142735.im1214s102. Unit 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia F, Climent N, Guardo AC, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5:166ra162. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 31.Lévy Y, Thiebaut R, Montes M, et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol. 2014;44:2802–2810. doi: 10.1002/eji.201344433. [DOI] [PubMed] [Google Scholar]
- 32.Gudmundsdotter L, Wahren B, Haller BK, et al. Amplified antigen-specific immune responses in HIV-1 infected individuals in a double blind DNA immunization and therapy interruption trial. Vaccine. 2011;29:5558–5566. doi: 10.1016/j.vaccine.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 33.Palma P, Romiti ML, Montesano C, et al. Therapeutic DNA vaccination of vertically HIV-infected children: report of the first pediatric randomised trial (PEDVAC) PloS One. 2013;8:e79957. doi: 10.1371/journal.pone.0079957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakari M, Aboud S, Nilsson C, et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine. 2011;29:8417–8428. doi: 10.1016/j.vaccine.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harari A, Rozot V, Cavassini M, et al. NYVAC immunization induces polyfunctional HIV-specific T-cell responses in chronically-infected, ART-treated HIV patients. Eur J Immun. 2012;42:3038–3048. doi: 10.1002/eji.201242696. [DOI] [PubMed] [Google Scholar]
- 36.Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PloS One. 2011;6:e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard RB, Rockstroh JK, Pantaleo G, et al. Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, Vacc-4x: a phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2014;14:291–300. doi: 10.1016/S1473-3099(13)70343-8. [DOI] [PubMed] [Google Scholar]
- 38.Li JZ, Heisey A, Ahmed H, et al. Relationship of HIV reservoir characteristics with immune status and viral rebound kinetics in an HIV therapeutic vaccine study. AIDS. 2014;28:2649–2657. doi: 10.1097/QAD.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azzoni L, Foulkes AS, Papasavvas E, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cotugno N, De Armas L, Pallikkuth S, et al. Pediatric HIV infection in the ‘omics era. Defining transcriptional signatures of viral control and vaccine responses. J Viral Erad. 2015;1:153–158. [PMC free article] [PubMed] [Google Scholar]
- 42.Barouch DH, Michael NL. Accelerating HIV-1 vaccine efficacy trials. Cell. 2014;159:969–972. doi: 10.1016/j.cell.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210:1529–1538. doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandstrom E, Nilsson C, Hejdeman B, et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198:1482–1490. doi: 10.1086/592507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harari A, Bart PA, Stohr W, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joachim A, Nilsson C, Aboud S, et al. Potent functional antibody responses elicited by HIV-1 DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PloS One. 2015;10:e0118486. doi: 10.1371/journal.pone.0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aupiais C, Faye A, Le Chenadec J, et al. Interruption of cART in clinical practice is associated with an increase in the long-term risk of subsequent immunosuppression in HIV-1-infected children. Pediatr Infect Dis J. 2014;33:1237–1245. doi: 10.1097/INF.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 48.Paediatric European Network for Treatment of A Response to planned treatment interruptions in HIV infection varies across childhood. AIDS. 2010;24:231–241. doi: 10.1097/QAD.0b013e328333d343. [DOI] [PubMed] [Google Scholar]
- 49.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freguja R, De Rossi A, Poulson H, et al. Long-term consequences of planned treatment interruption in HIV-1-infected children. Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. February 2015; Abstract 919. [Google Scholar]
- 51.Li JZ, Smith DM, Mellors JW. The critical roles of treatment interruption studies and biomarker identification in the search for an HIV cure. AIDS. 2015 doi: 10.1097/QAD.0000000000000658. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]