Abstract
Thymic stromal lymphopoietin (TSLP) produced by epithelial cells acts on dendritic cells (DC) to drive differentiation of TH2 cells, and is therefore important in allergic disease pathogenesis. However, DC themselves make significant amounts of TSLP in response to microbial products, but little is known about the key downstream signals that induce and modulate this TSLP secretion from human DC. We show that monocyte-derived DC (mDC) secretion of TSLP in response to C. albicans and β-glucans requires dectin-1, Syk, NFκB and p38 MAPK signalling. In addition TSLP production by mDC is greatly enhanced by IL-1β, but not TNFα, in contrast to epithelial cells. Furthermore, TSLP secretion is significantly increased by signals emanating from the endoplasmic reticulum (ER) stress response, specifically the unfolded protein response sensors, inositol-requiring transmembrane kinase/endonuclease 1 (IRE1α) and protein kinase R-like endoplasmic reticulum kinase (PERK), which are activated by dectin-1 stimulation. Thus, TSLP production by mDC requires the integration of signals from dectin-1, the IL-1 receptor and ER stress signalling pathways. Autocrine TSLP production is likely to play a role in mDC-controlled immune responses at sites removed from epithelial cell production of the cytokine, such as lymphoid tissue.
Keywords: TSLP, dectin-1, ER stress, IL-1β
Introduction
Thymic stromal lymphopoietin (TSLP) is a four-helix bundle cytokine closely related to IL-7, and was initially described as a lymphocyte growth factor [1]. Subsequently it has been shown to promote the development TH2 immunity [2–3], to be important in maintaining tolerance within the gut [4–5], and to be a pathogenic factor in the development of atopic inflammation [6–7] and inflammatory arthritis [8–9]. TSLP signals via a specific receptor (TSLPR) composed of a unique TSLPR chain and the IL-7 receptor α chain (IL-7Rα) [10], leading to JAK-STAT signalling [11–13], and downstream transcription of target genes.
TSLP potently activates dendritic cells (DC), priming DC to drive TH2 cell differentiation [2, 10]. However, in addition to being a target of TSLP, we and others have recently shown that monocyte-derived DC (mDC) themselves produce TSLP in response to pattern recognition receptor (PRR) engagement [14–15]. Furthermore, DC-derived TSLP has been shown to be functionally important in protection against intestinal inflammation by modifying T cell phenotype to promote the development of Foxp3+ T cells [16]. Mechanisms that control autocrine production of TSLP by mDC have not previously been characterised; thus the aim of this work was to determine the signals that induce and modulate human TSLP production by mDC.
Amongst PRRs, dectin-1 stimulation can induce TSLP mRNA expression in mDC [15]. Dectin-1 recognises β-1,3 glucans (β-glucans) on fungi and certain bacteria, initiating downstream signalling events that drive effector responses including phagocytosis [17], inflammasome activation [18–21] and inflammatory cytokine release [22–24], important for anti-fungal immunity. Myeloid cells such as DC mediate these immune responses and are essential for resistance to systemic fungal infections in mice [25]. Therefore continued understanding of signals that control DC functional responses to fungal infection is extremely important.
We have shown that synergistic signalling from PRR and endoplasmic reticulum (ER) stress greatly enhanced TSLP gene transcription in mDC, similar to the effect of ER stress on IL-23p19 expression [14]. Although ER stress has previously been described as regulating cytokine secretion [14, 26–28], and hence influencing inflammatory and autoimmune diseases [26, 29], the effects on IL-23p19 and TSLP were particularly striking [14]. Therefore we wished to determine the key signals that induce TSLP in human mDC, and the pathways of the ER stress response that modulate TSLP production by mDC.
The ER senses dangers to internal homeostasis and initiates the unfolded protein response (UPR) when the protein folding capacity of the cell is exceeded causing disruptions to normal protein folding mechanisms. The UPR is controlled by the three principal ER stress sensing proteins, inositol-requiring transmembrane kinase/endonuclease 1 (IRE1α), protein kinase R-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6) [14, 26–28, 30]. These proteins control discrete yet interconnected signalling pathways which modulate specific genes involved in orchestrating cellular responses to ER stress. The UPR allows the cell to resolve ER stress via various mechanisms including PERK mediated translation shutdown via the phosphorylation of eukaryotic initiation factor 2 α (eIF2α); however, if these mechanisms fail, programmed cell death is initiated [31].
Here we show that dectin-1 signalling stimulates TSLP production from mDC and that this is enhanced by signals from the ER stress sensing proteins, IRE1α and PERK. In addition dectin-1 induced IL-1β is required for optimal TSLP secretion; however, we show that dectin-1 induced TSLP requires independent, mutually exclusive signals emanating from the ER stress response and the IL-1 receptor.
Results
β-glucans induce TSLP in mDC, and require dectin-1 signalling via Syk, NFκB and p38 MAPK
Since previous work showed that the dectin-1/TLR2 agonist zymosan was efficient at inducing TSLP expression in mDC [15], we used three different β-glucan preparations as dectin-1 agonists: curdlan (a large particulate derived from A. faecalis), smaller particulate β-1,3 glucan microparticles (MP) derived from S. cerevisiae, and the hyphal yeast, C. albicans. Dectin-1 signalling stimulated via C. albicans or β-glucan particle engagement induced TSLP secretion in immature mDC (CD83lo, CD86lo, HLA-DR+) (Fig. 1A). The ability to secrete TSLP in response to dectin-1 stimulation was not an artefact of the in vitro differentiation from monocytes, since ex vivo blood-derived CD1c+ DC also demonstrated this property (Fig. 1B). Furthermore, murine bone marrow derived DC (BMDC) can secrete TSLP after β-glucan stimulation (Fig. 1C).
Figure 1.
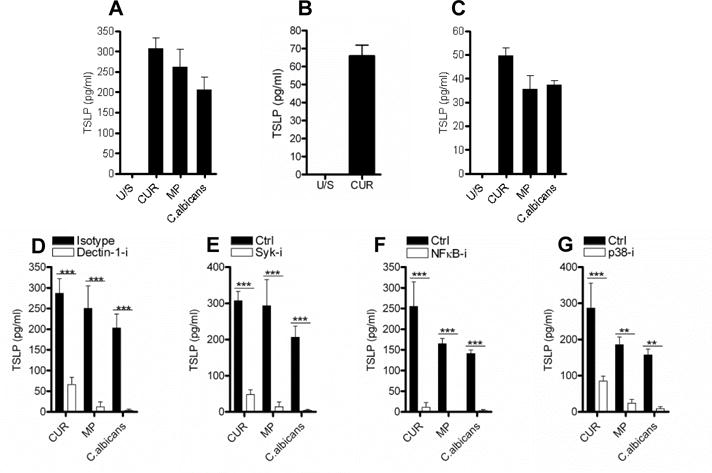
β-glucans induce TSLP in mDC, and require dectin-1 signalling via Syk, NFκB and p38 MAPK. (A) Human monocyte-derived dendritic cells (mDC) were differentiated from CD14+ monocytes for 6 days using GM-CSF (20ng/ml) and IL-4 (4ng/ml) and were stimulated with the dectin-1 agonists curdlan (CUR, 50μg/ml); β-1,3 glucan microparticles (MP, 50μg/ml) or heat-killed C. albicans (MOI 2:1) for 24 hours (n=6). (B) Human ex vivo CD1c+ DC were isolated from PBMC and were stimulated with CUR for 24 hours (n=2). (C) Murine bone marrow-derived DC (BMDC) were differentiated from cells isolated from mouse femurs for 7 days using 5% X63 conditioned media and IL-4 (10ng/ml) and were stimulated with CUR, MP and C. albicans for 24 hours (n=3). (D–G) mDC were pre-incubated for one hour with or without (D) anti-dectin-1 or isotype control (10μg/ml) (n=5), (E) Syk inhibitor (5μM) (n=6), (F) NFκB inhibitor (10μM) (n=6) or (G) p38 MAPK inhibitor (1μM) (n=5) and were then stimulated with CUR, MP or C. albicans for 24 hours. TSLP was measured in 24 hour cell culture supernatants by ELISA. Cumulative data are shown as mean ± SEM. Statistical significance was calculated using (A) one-way or (C–F) two-way ANOVA with Bonferroni post-tests (*** p=0.001, ** p=0.01, * p=0.05).
Blocking dectin-1 with a specific antibody (Fig. 1D), or employing a specific Syk inhibitor (Fig. 1E), potently inhibited TSLP production in response to all three stimuli. β-glucan stimulation induced Syk phosphorylation (Tyr525/526) in mDC with Syk inhibition preventing this phosphorylation event (Supplementary Fig. 1A) and dectin-1 neutralisation had no effect on peptidoglycan induced IL-1β (Supplementary Fig. 1B).
Activation of NFκB [22, 32] and p38 MAPK [32] mediate dectin-1 induced inflammatory cytokine expression, and both NFκB [33–34] and p38 MAPK [35] are required for TSLP induction in epithelial and stromal cells respectively. In agreement with this, we found that β-glucan stimulated mDC induced both NFκB activation, measured by IκBα degradation (Supplementary Fig. 1C) and p38 MAPK phosphorylation (Thr180 and Tyr182) (Supplementary Fig. 1D). Furthermore, C. albicans or β-glucan induced TSLP required activation of both NFκB (Fig. 1F) and p38 MAPK (Fig. 1G) in mDC; however, p38 MAPK inhibition did not affect IL-23 secretion (Supplementary Fig. 1E). This indicated that in human mDC NFκB interaction with the TSLP promoter and signalling by p38 MAPK are requirements for dectin-1 induced TSLP.
IL-1β but not TNFα is required for dectin-1 induced TSLP secretion in mDC
Dectin-1 signalling in DC induces IL-1β [18–19] and TNFα [32, 36] secretion in addition to TSLP. Both of these cytokines alone can induce epithelial cells [33], keratinocytes [37], fibroblasts [8], smooth muscle cells [38] and stromal cells [35] to secrete TSLP. In contrast to these reports, treatment of mDC with IL-1β or TNFα alone, induced negligible quantities of TSLP (Fig. 2A). Although the addition of IL-1β alone failed to induce substantial TSLP secretion in mDC, inhibition of IL-1 signalling using the IL-1 receptor antagonist (IL-1RA) significantly reduced the quantity of TSLP induced by C. albicans or β-glucans (Fig. 2B). Therefore, IL-1β signalling is necessary but not sufficient for the induction of TSLP secretion by dectin-1 agonists. In contrast, TNFα did not exhibit this property, as shown by the inability of a neutralising anti-TNFα antibody (Supplementary Fig 2) to modulate TSLP secretion induced by dectin-1 agonists (Fig. 2B).
Figure 2.
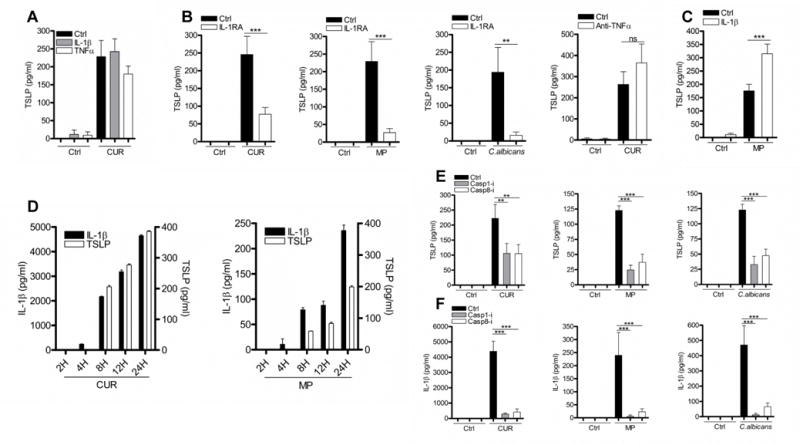
IL-1β but not TNFα is required for dectin-1-induced TSLP secretion in mDC. (A) mDC were stimulated with CUR with or without IL-1β (10ng/ml) or TNFα (10ng/ml) for 24 hours (n=3). (B) mDC were pre-incubated for one hour with or without IL-1RA (1μg/ml) or anti-TNFα (5μg/ml) and were then stimulated with CUR (n=8), MP (n=11) or C. albicans for 24 hours (n=4). (C) mDC were stimulated with MP with or without IL-1β for 24 hours (n=9). (D) mDC were stimulated with CUR or MP for 2, 4, 8, 12 or 24 hours. (E–F) mDC were pre-incubated for one hour with or without caspase-1 (50μM) or caspase-8 (50μM) inhibitors and were then stimulated with CUR, MP or C. albicans for 24 hours (n=5). Cumulative data are displayed as mean ± SEM. TSLP and IL-1β was measured in 24 hour cell culture supernatants by ELISA. Statistical significance was calculated using one-way ANOVA with Bonferroni post-tests (*** p=0.001, ** p=0.01).
In contrast to curdlan, MP are poor inducers of inflammatory cytokines such as IL-6 and IL-23 in DC [23–24]. We also show that compared to large β-glucan particles, MP are poor inducers of IL-1β secretion. Importantly these small quantities of IL-1β were essential for TSLP production, indicated by the profound loss of TSLP expression when IL-1RA was present in mDC culture with MP (Fig. 2B). To determine if the small quantities of IL-1β induced by MP were suboptimal for TSLP secretion, we added additional recombinant IL-1β. This augmented TSLP secretion, but only to an equivalent quantity detected using curdlan stimulation (Fig. 2C). The addition of IL-1β to curdlan stimulated mDC had no effect on TSLP secretion (Fig. 2A), presumably because there were saturating quantities of IL-1β present. The potential for IL-1β to augment TSLP expression is evident by the presence of detectable IL-1β four hours prior to TSLP secretion (Fig. 2D). Although IL-1RA did not inhibit curdlan induced TSLP as potently as TSLP induced by the other dectin-1 agonists (Fig. 2B), this may simply reflect that IL-1RA is an inefficient antagonist and was unable to completely inhibit IL-1β signalling in the presence of high concentrations of IL-1β. These data suggest that even the small quantities of IL-1β provided by MP exceed a critical threshold that enables substantial secretion of TSLP by mDC.
Processing and secretion of IL-1β requires inflammasome activation mediated by caspase recruitment and activation [18–21]. Previous work has described a role for both caspase-1 [18–21] and caspase-8 [19, 21] in fungal induced IL-1β; thus we used specific inhibitors of caspase-1 and caspase-8 to test for a requirement for caspase activation in C. albicans and β-glucan induced TSLP. Activation of both caspase-1 and caspase-8 was required for dectin-1 induced TSLP (Fig. 2E), and IL-1β (Fig. 2F). Furthermore, caspase-1 KO BMDC stimulated with C. albicans or β-glucan showed reduced TSLP and IL-1β (Supplementary Fig. 3A–B). Our findings showed that dectin-1 induced inflammasome/caspase-dependent IL-1β is required for optimal TSLP production from mDC; however, inflammasome-independent signalling events are also required.
Although Syk, NFκB and p38 MAPK signalling is required for IL-1β expression, the addition of exogenous IL-1β could not rescue TSLP expression in the presence of these inhibitors (Supplementary Fig. 4A–F). Therefore the reduction of TSLP expression in the presence of these inhibitors cannot be solely attributed to the loss of IL-1β.
Dectin-1 induced IRE1α and PERK regulate TSLP production
We have previously presented microarray data showing that ER stress can augment PRR induced TSLP and IL-23p19 in mDC [14], though this did not apply to the vast majority of cytokines examined. The importance of crosstalk between signals from PRR and those emanating from the ER stress response in the production of cytokines by DC has been emphasised recently [14, 26–27].
Chemical induction of ER stress using tunicamycin (TM) or thapsigargin (TP) in conjunction with dectin-1 stimulation increased TSLP secretion from mDC (Fig. 3A), and from ex vivo CD1c+ DC (Fig. 3B). Importantly, mDC subjected to ER stress in the absence of dectin-1 stimulation were unable to induce TSLP (Fig. 3A), showing that ER stress modulates but is not sufficient to induce TSLP secretion, and further signifies that multiple independent signalling events are required.
Figure 3.
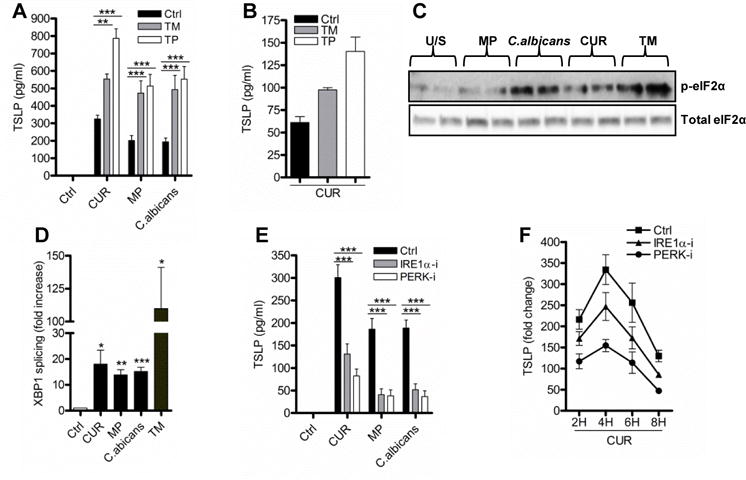
Dectin-1 induces IRE1α and PERK regulates TSLP production. (A–B) mDC (n=7) or ex vivo CD1c+ DC (n=2) were stimulated with CUR, MP or C. albicans with or without the ER stress inducers tunicamycin (TM, 1μg/ml) or thapsigargin (TP, 0.25μM) for 24 hours. mDC were stimulated with CUR, MP, C. albicans or TM for 4 hours and analysed for (C) phospho eIF2α (n=2) and (D) XBP-1 splicing (n=5). (E–F) mDC were pre-incubated for one hour with or without IRE1α (30μM) or PERK (1μM) inhibitors and were then stimulated with (E) CUR, MP or C. albicans for 24 hours for TSLP secretion (n=13) or (F) 4 hours for TSLP mRNA expression (n=3, representative experiment). TSLP and spliced XBP-1 mRNA expression was measured by qRT-PCR, phospho eIF2α was measured by immunoblot and TSLP secretion was measured in 24 hour cell culture supernatants by ELISA. Cumulative data are displayed as mean ± SEM. (D) Statistical significance calculated using t test where p values were calculated as unstimulated vs stimulated mDC, XBP1 splicing p values for (CUR * p=0.0339, (MP ** p=0.0029), (C. albicans *** p=0.0010) and (TM * p=0.0254). (A and E) Statistical significance was calculated using two-way ANOVA with Bonferroni post-tests (*** p=0.001, ** p=0.01).
ER stress results in the activation of three key sentinel proteins, IRE1α, PERK, and ATF6 [14, 26–28, 30]. Activation of IRE1α induces RNAase activity leading to splicing of X-box binding protein 1 (XBP1) mRNA to allow in-frame translation and production of XBP1 protein, whilst activation of the kinase PERK results in eIF2α phosphorylation and activation of the integrated stress response (ISR) [14, 26–28, 30]. The ability of dectin-1 agonists to stimulate both eIF2α phosphorylation (Fig. 3C) and XBP1 splicing (Fig. 3D) suggests stimulation of the dectin-1 pathway can activate components of the ER stress response. Although siRNA approaches were able to knockdown IRE1α and PERK mRNA expression, the degree of knockdown was not sufficient in DC to impact on downstream signalling pathways. However we can conclude that IRE1α and PERK activation with dectin-1 agonists did contribute important signals for TSLP expression, since specific inhibitors reduced dectin-1 agonist-induced TSLP from mDC (Fig. 3E). The efficacy of the inhibitors was shown by loss of β-glucan induced XBP1 splicing (Supplementary Fig. 5A) and eIF2α phosphorylation (Supplementary Fig. 5B).
The ISR can also be activated by other eIF2α kinases including protein kinase R (PKR). Although we show that β-glucan stimulation potently activated PKR (Supplementary Fig. 5C), inhibition of PKR had no effect on β-glucan induced TSLP (Supplementary Fig. 5D). This suggests that activation of PERK is critical for TSLP induction.
The ability of the IRE1α or PERK inhibitors to reduce TSLP secretion suggests that ER stress signals were regulating TSLP expression at the level of gene transcription. Both IRE1α and PERK inhibition reduced TSLP mRNA expression during dectin-1 stimulation without altering its kinetics of expression (Fig. 3F). In contrast, IRE1α inhibitor did not affect osteoprotegerin (OPG) mRNA expression and the PERK inhibitor had no effect on IL-1β mRNA expression (Supplementary Fig. 5E–F). The inability of ER stress inhibitors to modulate surface markers, CD83, CD86 and HLA-DR, indicates that these inhibitors did not affect the maturation of mDC induced by dectin-1 agonists (Supplementary Fig. 5G).
TSLP secretion requires independent activation of the ER stress response and IL-1 receptor dependent signalling
The ability of ER stress to enhance TSLP expression raises the question whether IRE1α and PERK regulated signals exhibit their effect indirectly by modulation of IL-1β expression. Inhibition of IRE1α but not PERK activation reduced IL-1β secretion from mDC (Fig. 4A). The reduction of TSLP expression in the presence of the IRE1α inhibitor cannot be solely attributed to the loss of IL-1β, since addition of exogenous recombinant IL-1β did not rescue TSLP secretion (Fig. 4B). These data established that dectin-1 induced TSLP required independent signals from both the IL-1 receptor and UPR-stimulated sensors.
Figure 4.
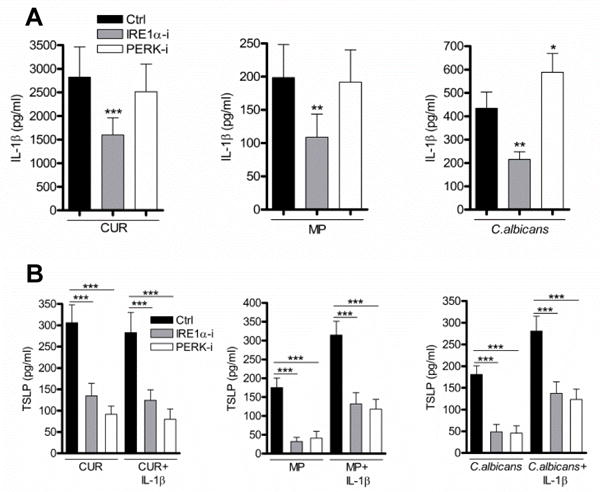
TSLP secretion requires independent activation of ER stress and IL-1 dependent signalling. (A) mDC were pre-incubated for one hour with or without IRE1α or PERK inhibitors and were then stimulated with CUR, MP or C. albicans for 24 hours (n=12). (B) mDC were pre-incubated for one hour with or without IRE1α or PERK inhibitors and were then stimulated with CUR, MP or C. albicans with or without recombinant IL-1β for 24 hours (n=9). TSLP and IL-1β were measured in 24 hour cell culture supernatants by ELISA. Cumulative data are shown as mean ± SEM. Statistical significance was calculated using oneway ANOVA with Bonferroni post-tests (*** p=0.001, ** p=0.01, * p=0.05).
The absence of CHOP does not affect dectin-1 induced TSLP expression
Transcription factors induced during the UPR may be involved in the induction of TSLP gene expression. We have previously shown that ISR-induced C/EBP homologous protein (CHOP) is a critical transcription factor in IL-23p19 expression [14] and hypothesised a role in TSLP expression. We were able reproduce this requirement for CHOP in the expression of IL-23p19 (Supplementary Fig. 6A) induced by β-glucans in BMDC, but found no role for CHOP in dectin-1 activation of TSLP expression (Supplementary Fig. 6B).
Discussion
DC are professional antigen presenting cells and have the ability to sense infection and danger signals and instruct immune cells to differentiate into different cell subsets. TSLP is an important cytokine in immune regulation [2, 4–10] and we have previously demonstrated that TSLP is expressed by human mDC [14]. In this study, we have shown that TSLP secretion from mDC in response to stimulation with C. albicans or β-glucans is regulated by an integration of signals from dectin-1, the ER stress response and the IL-1 receptor.
We noted that mDC generated significant levels of TSLP/cell (~200–300 pg/ml from 0.5×105 cells). It is difficult to directly compare TSLP production by mDC and epithelial cells/fibroblasts; however, stimulation of these cell types typically generates ~50–300 pg/ml from ~2–4×105 cells [8, 39]. Furthermore, bronchoalveolar lavage fluids from severe asthmatic and chronic obstructive pulmonary disease patients have been shown to contain ~50 pg/ml TSLP [40]. Previous work in mice suggests that CD11c+ DC upregulate TSLP mRNA to a higher level compared to epithelial cells when both cell types are challenged with a stimulus to which both can respond [15], implying that mDC may be more potent TSLP producers. Furthermore, contrary to a recent study showing that murine BMDC do not secrete TSLP protein following stimulation with LPS and IL-4 [41], we have shown that BMDC are capable of producing TSLP protein following stimulation with C. albicans and β-glucan, though they may produce lower amounts than their human counterparts. It is likely TSLP secretion is highly stimulation specific. Therefore mDC should not be discounted as potentially important sources of TSLP and we suggest this TSLP could have an important function during an initial fungal challenge.
Previous published work has shown that epithelial and stromal cells can be induced to express TSLP by the addition of exogenous cytokines such as IL-1β and TNFα [8, 33, 35, 37–38]. In contrast, we showed that human mDC had more stringent requirements for TSLP secretion and needed priming signals with a PRR agonist, such as β-glucan, to induce significant TSLP production. These data suggest that dectin-1 stimulation may allow modification of the TSLP promoter in immature mDC that allows subsequent access to cytokine-induced transcription factors.
Initially we showed that dectin-1 induced TSLP from mDC requires the activation of Syk, NFκB and p38 MAPK, suggesting that promoter occupation by NFκB and signalling by p38 MAPK are requirements for TSLP induction. Furthermore, utilising specific inhibitors of IL-1β and TNFα, we identified that IL-1β and not TNFα is critical for the TSLP induction pathway. A recent study using a skin irritation model for TSLP induction also identified IL-1β as an important factor in the induction of TSLP in humans [37]. Given that IL-1β- and TNFα-mediated signalling pathways induce some common downstream pathways including the activation of NFκB, it was surprising that TSLP induction from mDC specifically required IL-1 receptor signalling. These data suggest that there are qualitative differences in the downstream pathways induced by these cytokines. Further investigations into the unique factor/s stimulated by IL-1 receptor signalling required for TSLP induction in human mDC would be a worthwhile avenue of research.
We have previously shown that ER stress can increase TSLP mRNA expression [14] and hypothesised that the induction of the ER stress signalling components IRE1α and PERK via dectin-1 engagement might contribute to TSLP production from mDC. Utilising specific inhibitors of the IRE1α- and PERK-controlled UPR we were able to show that dectin-1-induced TSLP requires independent signals emanating from both IRE1α and PERK and the IL-1 receptor.
These data suggest that dectin-1 induction of ER stress modulates transcriptional regulation of TSLP via an IRE1α-mediated arm. Therefore the transcription factor XBP1, downstream of IRE1α activation, may be an important factor regulating TSLP expression in mDC. We also suggest that PERK-mediated eIF2α phosphorylation is a critical signal for the expression of TSLP. Although eIF2α phosphorylation can attenuate protein translation, it is apparent that activation of this pathway by dectin-1 agonists is not sufficient to prevent the expression of TSLP protein. eIF2α phosphorylation can result in a transcriptional reprogramming of the cell via activation of the ISR [42]. Transcription factors induced by the PERK controlled ISR may be involved in the induction of TSLP gene expression. We have previously shown that ISR-induced CHOP is a critical transcription factor in IL-23p19 expression [14] but we found no role for CHOP in β-glucan activation of TSLP expression in murine BMDC. Although we have found no putative ER stress associated transcription factor binding sites in the human and mouse TSLP promoter, gene expression is clearly ER stress responsive, therefore, a search for potential transcription factors mediating this effect would be worthwhile.
Whether diseases in which ER stress is known to be operating are associated with high levels of TSLP should be investigated, together with examination of any role for TSLP production in the manifestations of these diseases. Where TSLP is implicated in immunopathology, regulating the ER stress response could be useful therapeutically to decrease TSLP production in vivo.
TSLP is an established TH2 differentiation factor, thus it is curious why it would be secreted in such high quantities during dectin-1 stimulation. There is conflicting evidence on whether this signalling pathway induces or actively suppresses TH2 associated cytokines and this appears to be very context dependent. Recent reports show that curdlan can inhibit TH2 cell responses, promoting breast cancer progression [43]; it was also reported to suppress IL-5, IL-13 and surprisingly IL-1β from lymph nodes during epicutaneous sensitisation [44]. Conversely β-glucan treatment can increase TH2 cytokine secretion from co-cultured macrophages and lymphocytes [45] and stimulate production of both pro-allergic (IL-33, CCL17, CCL22) and inflammatory (IL-1β and CXCL1) mediators during lung exposure to Aspergillus fumigatus, in a dectin-1 dependent manner [46]. Furthermore in addition to TSLP we have observed that β-glucan stimulated mDC generate large quantities of CCL22 (data not shown) emphasising that mDC can secrete pro-TH2 factors.
What is the functional relevance of mDC-derived TSLP? We suggest that mDC are likely to be the major source of TSLP at non-epithelial sites, such as draining lymph nodes, and that this TSLP will have the potential to modulate the adaptive immune responses occurring there. This seems feasible, considering that Spadoni et al have clearly established a functional role for DC-derived TSLP, by its actions directly modulating T cell phenotype [16].
We have shown clear differences in the quantities of IL-1β induced by different dectin-1 agonists, whereas TSLP production remained stable. Pioneering experiments that established TSLP as a critical TH2 driver administered large (15 ng/ml) quantities of this cytokine [3]. This raises the possibility that the context of TSLP secretion in vivo may have significant effects on its ability to drive the differentiation of TH2/regulatory T cells or modulate the differentiation of TH17 cells [16, 22–24].
In summary, we have demonstrated that dectin-1 stimulation of mDC by C. albicans or β-glucans produce TSLP, and that this process involves the integration of signals from dectin-1, the ER stress associated proteins IRE1α and PERK, and the IL-1 receptor. These data argue that these signalling pathways may provide novel therapeutic targets to promote or down-regulate TSLP production in relevant clinical settings.
Materials and Methods
Cell Isolation and Generation of Dendritic Cells (DC)
CD14+ monocytes were purified from human PBMC by magnetic bead separation (Miltenyi) and were differentiated into monocyte-derived dendritic cells (mDC) with 20ng/ml GM-CSF (Life Technologies) and 4ng/ml IL-4 (BD Biosciences) by culturing for 6-days in RPMI1640 5% FCS as described previously [14]. Ex vivo CD1c+ DC were isolated from human PBMC by magnetic bead separation (Miltenyi). Murine bone marrow-derived dendritic cells (BMDC) were isolated from the femurs of wild-type (WT) and CHOP KO C57B6 mice and cultured for 7-days in RPMI1640 10% FCS supplemented with 5% X63 conditioned media and 10ng/ml IL-4 (Peprotech).
Cell stimulations
DC were seeded at a density of 2.5×105/ml and were stimulated with 50μg/ml of the β-glucans, curdlan (CUR) derived from Alcaligenes faecalis (A. faecalis) (Wako, Richmond, USA), β-1,3 glucan microparticles (MP) derived from Saccharomyces cerevisiae (S. cerevisiae) (David. L. Williams, East Tennessee State University) or heat-killed hyphal Candida albicans (C. albicans) (MOI 2:1) (gift from John Trowsdale, University of Cambridge) grown in sabouraud dextrose broth for 8 hours at 37°C with shaking to an optical density of 0.2. C. albicans was killed by heating for 1 hour at 70°C.
Reagents
10μg/ml mouse IgG2B dectin-1 blocking antibody (clone-259931 R&D Systems), 10ug/ml mouse IgG2B isotype control (clone-20116 R&D Systems), 5μM Syk inhibitor, R406 (Selleckchem), 10μM NFκB inhibitor, isohelenin (Calbiochem), 1μM p38 MAPK inhibitor, BIRB0796 (Selleckchem), 5μg/ml anti-TNFα, Infliximab (JannsenBiotech), 1μg/ml IL-1 receptor antagonist (IL-1RA) (R&D Systems), 50μM caspase-1 inhibitor (Z-YVAD-FMK) (Calbiochem), 50μM caspase-8 inhibitor (Z-IE(OMe)TD(OMe)-FMK) (Calbiochem), 10ng/ml TNFα (Biolegend), 10ng/ml IL-1β (Miltenyi), 1μg/ml tunicamycin (TM) (Sigma-Aldrich), 0.25μM thapsigargin (TP) (Sigma-Aldrich), 30μM IRE1α inhibitor, 4μ8C (Calbiochem) and 1μM GSK-PERK inhibitor (Toronto Research). Where inhibitors, blocking antibodies and modifiers were used, mDC were pre-treated one hour prior to cell stimulation. Repeated experiments were performed on independent donors unless otherwise stated.
Cytokine production
mDC culture supernatants were harvested after 24 hours of stimulation. The concentrations of TSLP (R&D Systems) and IL-1β (eBioscience) were measured by ELISA as per the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was extracted from cells using RNA Mini Kits (Bioline). Quantitative real-time PCR was carried out using TaqMan Gene Expression Assays (Applied Biosystems) for TSLP, IL-1β and OPG and the SYBR Green method for XBP1 splicing. TSLP (Hs00263639_m1), IL-1β (Hs00174097_m1) and OPG (Hs00171068_m1) TaqMan PCR probe primer sets (Applied Biosystems), and HPRT (MWG-Biotech), probe, 5′-TTCCTCATGGACTAATTATGGACAGG-3′, forward, 5′-TGAGGATTTGGAAAGGGTGTTT-3′ reverse, 5′-ACATCTCGAGCAAGACGTTCAG-3′. SYBR primer sets (MWG-Biotech), XBP1 spliced, forward 5′-TGCTGAGTCCGCAGCAGGTG-3′, reverse 5′-GCTGGCAGGCTCTGGGGAAG-3′, HPRT, forward 5′-GACACTGGCAAAACAATG-3′, reverse 5′-ACAAAGTCTGGCTTATATCC-3′. Gene expression was normalised to HPRT and calculated as fold change from unstimulated control.
Immunoblot
mDC cell were lysed on ice in 250μl of ice cold cytoplasmic lysis buffer (10mM HEPES, 50mM NaCl, 0.5M Sucrose, 0.1mM EDTA, 0.5% v/v Triton X-100, 10mM Tetrasodium pyrophosphate, 17.5mM β-glycerophosphate and one complete mini protease inhibitor cocktail tablet). After lysis the cytoplasmic extract was frozen at −20°C overnight before thawing to aid cell lysis. Lysates were then centrifuged for 15 minutes at 4°C and the supernatant retained. Bradford assay was carried out to determine protein quantification (Thermo) and proteins were separated by SDS-PAGE. Resolved proteins were detected after transfer by immunoblot with indicated primary antibodies, followed by incubation in HRP-conjugated secondary antibodies and ECL (PerkinElmer) and visualised using GBox (Syngene). Monoclonal antibodies for phospho eIF2α (S51) (Abcam, 1090), eIF2α (Cell Signalling, 9722), phospho PKR (Thr451) (Millipore, 07-886), PKR (Santa Cruz, sc-798), IκBα (Santa Cruz, sc-847), phospho Syk (Tyr525/525) (Cell signalling, 2710), Syk (Cell Signalling, 13198), phospho p38 MAPK (Thr180 and 182) (Cell Signalling, 4511), p38 MAPK (Cell Signalling, 8690) and β-actin (Abcam, 8226) were used for detection.
Flow Cytometry
mDC were re-suspended in ice cold PBS supplemented with 2 mM EDTA, 0.1% BSA and 0.01% sodium azide, blocked with 10% mouse serum (Sigma) and stained with anti-CD83 (BD), anti-CD86 (BD) and anti-HLA-DR (Biolegend). Surface expression was evaluated relative to unstimulated stained controls on a BD CANTO II and analysed using FlowJo software (Treestar).
Data Analysis
Data were analysed using GraphPad Prism statistical package. Cumulative data are displayed as mean ± SEM. Statistical analysis using either t test (p values stated in figures legends) or one/two-way ANOVAs with Bonferroni post-tests * = p<0.05 ** = p<0.01, *** = p<0.001.
Supplementary Material
Acknowledgments
We would like to thank Mike Bacon and Tony Ng for their technical assistance during this project. We would also like to thank Professor John Trowsdale for providing us with key reagents. This research was supported by Arthritis Research UK and the Cambridge NIHR BRC Cell Phenotyping Hub. This research was also supported, in part, by NIH GM53522 and GM083016 to DLW.
This work was supported by Arthritis Research UK and by the NIHR Cambridge Biomedical Research Centre.
Abbreviations
- TSLP
Thymic stromal lymphopoietin
- mDC
monocyte derived DC
- BMDC
bone marrow derived dendritic cells
- UPR
unfolded protein response
- IRE1α
inositol-requiring transmembrane kinase/endonuclease 1
- ISR
integrated stress responses
- PERK
protein kinase R-like endoplasmic reticulum kinase
- XBP1
X-box binding protein 1
- eIF2α
eukaryotic initiation factor 2 α
Footnotes
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
- 1.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 2.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 4.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama K, Ozawa T, Hatsushika K, Ando T, Takano S, Wako M, Suenaga F, Ohnuma Y, Ohba T, Katoh R, Sugiyama H, Hamada Y, Ogawa H, Okumura K, Nakao A. A possible role for TSLP in inflammatory arthritis. Biochem Biophys Res Commun. 2007;357:99–104. doi: 10.1016/j.bbrc.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 9.Hartgring SA, Willis CR, Dean CE, Jr, Broere F, van Eden W, Bijlsma JW, Lafeber FP, van Roon JA. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheum. 2011;63:1878–1887. doi: 10.1002/art.30336. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, Wagner KU, Reizis B, Hennighausen L, Ziegler SF. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013;14:364–371. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 17.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 19.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 20.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan S, Rathinam VA, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM, Hise AG, Silverman N, Fitzgerald KA. Caspase-8 Modulates Dectin-1 and Complement Receptor 3-Driven IL-1beta Production in Response to beta-Glucans and the Fungal Pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 23.Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, Brown GD, Taylor PR. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol. 2008;181:3549–3557. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- 24.Hernanz-Falcon P, Joffre O, Williams DL, Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol. 2009;39:507–513. doi: 10.1002/eji.200838687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 31.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, Logan E, Lapaque N, Dore J, Blottiere HM. The NF-kappaB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol. 2013;43:1053–1062. doi: 10.1002/eji.201142340. [DOI] [PubMed] [Google Scholar]
- 35.Urata Y, Osuga Y, Izumi G, Takamura M, Koga K, Nagai M, Harada M, Hirata T, Hirota Y, Yoshino O, Taketani Y. Interleukin-1beta stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrioma stromal cells: possible involvement of TSLP in endometriosis. Hum Reprod. 2012;27:3028–3035. doi: 10.1093/humrep/des291. [DOI] [PubMed] [Google Scholar]
- 36.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumari V, Babina M, Hazzan T, Worm M. TSLP induction by skin irritation is independent of TNF-alpha, but supported by IL-1. Br J Dermatol. 2014 doi: 10.1111/bjd.13465. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–382. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 39.Bhowmick S, Chatterjee D, Chaudhuri K. Human epithelial cells stimulated with Vibrio cholerae produce thymic stromal lymphopoietin and promote dendritic cell-mediated inflammatory Th2 response. Int J Biochem Cell Biol. 2012;44:1779–1790. doi: 10.1016/j.biocel.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 40.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 41.Dewas C, Chen X, Honda T, Junttila I, Linton J, Udey MC, Porcella SF, Sturdevant DE, Feigenbaum L, Koo L, Williams J, Paul WE. TSLP Expression: Analysis with a ZsGreen TSLP Reporter Mouse. J Immunol. 2015;194:1372–1380. doi: 10.4049/jimmunol.1400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 43.Wu TC, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, Anguiano E, Pedroza-Gonzalez A, Snipes GJ, O’Shaughnessy J, Nishimura S, Liu YJ, Pascual V, Banchereau J, Oh S, Palucka K. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. 2014;2:487–500. doi: 10.1158/2326-6066.CIR-13-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin JY, Chen JS, Chen PC, Chung MH, Liu CY, Miaw SC, Wang LF. Concurrent exposure to a dectin-1 agonist suppresses the Th2 response to epicutaneously introduced antigen in mice. J Biomed Sci. 2013;20:1. doi: 10.1186/1423-0127-20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Dong L, Weng D, Liu F, Song L, Li C, Tang W, Chen J. 1,3-beta-glucan affects the balance of Th1/Th2 cytokines by promoting secretion of anti-inflammatory cytokines in vitro. Mol Med Rep. 2013;8:708–712. doi: 10.3892/mmr.2013.1553. [DOI] [PubMed] [Google Scholar]
- 46.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol. 2012;189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


