Abstract
Background
HIV infection and biomarkers of inflammation (measured by interleukin-6 [IL-6]), monocyte activation (soluble CD14 [sCD14]), and coagulation (D-dimer) are associated with morbidity and mortality. We hypothesized that these immunologic processes mediate (explain) some of the excess risk of mortality among HIV infected (HIV+) versus uninfected people independently of co-morbid diseases.
Methods
Among 2350 (1521 HIV+) participants from the Veterans Aging Cohort Study Biomarker Cohort (VACS BC) we investigated whether the association between HIV and mortality was altered by adjustment for IL-6, sCD14 and D-dimer, accounting for confounders. Participants were followed from date of blood draw for biomarker assays (baseline) until death or 7/25/2013. Analyses included ordered logistic regression and Cox Proportional Hazards regression.
Results
During 6·9 years (median), 414 deaths occurred. The proportional odds of being in a higher quartile of IL-6, sCD14 or D-dimer was 2-3 fold higher for viremic HIV+ versus uninfected people. Mortality rates were higher among HIV+ compared to uninfected people (incidence rate ratio (95% CI): 1·31 (1·06-1·62). Mortality risk increased with increasing quartiles of IL-6, sCD14 and D-dimer regardless of HIV status. Adjustment for IL-6, sCD14 and D-dimer partially attenuated mortality risk among HIV+ people with unsuppressed viremia (HIV-1 RNA≥10000 copies/mL) compared to uninfected people – hazard ratio (95% CI) decreased from 2·18 (1·60-2·99) to 2·00 (1·45-2·76).
Conclusions
HIV infection is associated with elevated IL-6, sCD14 and D-dimer, which are in turn associated with mortality. Baseline measures of these biomarkers partially mediate excess mortality risk among HIV+ versus uninfected people.
Keywords: HIV, mortality, inflammation, monocyte activation, coagulation
Introduction
Biomarkers of inflammation (measured by interleukin-6 [IL-6]), monocyte activation (soluble CD14 [sCD14]), and coagulation (D-dimer) are associated with morbidity and increased mortality among HIV infected (HIV+) and uninfected people. 1-5 However, it is unclear whether these immunologic processes explain the excess risk of mortality among HIV+ versus uninfected people 6,7 independently of co-morbid diseases. There are sparse data comparing HIV+ to uninfected people who have similar demographic and behavior characteristics (i.e., prevalence of smoking and alcohol consumption) while also accounting for HIV-specific biomarkers, comorbidities, substance use, biomarkers of inflammation, monocyte activation and coagulation, and complete capture of mortality events.
Our objective, therefore, was to determine whether increased inflammation, monocyte activation and coagulation explain the excess mortality risk among HIV+ compared to uninfected people. Data for these analyses were from the Veterans Aging Cohort Study Biomarker Cohort (VACS BC), an observational, longitudinal cohort of HIV+ and uninfected Veterans in care with detailed phenotypic data, biomarkers of immune function, and thorough capture of mortality outcomes. We assessed whether the association between HIV status and mortality persisted after adjusting for multiple potentially confounding co-morbid conditions alone and when combined with IL-6, sCD14 and D-dimer.
Methods
Cohort
The VACS BC is a subset of VACS 8,8 a prospectively enrolled observational longitudinal study of HIV+ and uninfected veterans matched on age, race-ethnicity, sex and geographic region.8 In 2005-2006, 1,525 HIV+ and 843 uninfected VACS 8 participants consented to provide blood samples forming the VACS BC as previously described.9 These specimens were collected using serum separator and EDTA blood collection tubes and shipped to a central repository at the Massachusetts Veterans Epidemiology Research and Information Center in Boston, Massachusetts. The date of the blood draw was used as the baseline date for each participant in the VACS BC. Those with available measurements of IL-6, sCD14, D-dimer and HIV-1 RNA (for HIV+) were included in analyses. Participants were followed from their baseline date until death or censored on 7/25/2013.
Independent, dependent, and potentially mediating variables
HIV status was the primary independent variable. We collected data on HIV-1 RNA, CD4+ T-cell (CD4) count, and antiretroviral therapy use at baseline. We used HIV-1 RNA measurements obtained as part of clinical care at baseline (±180 days). Death was the primary outcome. It was determined from the VHA vital status file, which uses inputs from the social security administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the VHA Medical Statistical Analysis Systems inpatient datasets. We assessed whether biomarkers of inflammation (IL-6), monocyte activation (sCD14) and altered coagulation (D-dimer) altered the association between HIV and mortality (see description of mediation in statistical analysis below). IL-6, sCD14 and D-dimer were assessed as categorical values (quartiles) or as a composite inflammatory burden score (number of elevated biomarkers i.e. ≥75th percentile threshold among those who died).10 Measurement of these biomarkers has been previously described.9
Covariates
Baseline covariate data, obtained closest to the time of blood draw, have been previously described.9 Briefly, sociodemographic data included age, sex, and race-ethnicity. Cardiovascular disease (CVD) was defined by myocardial infarction,11,12 and diagnostic or procedural codes for congestive heart failure, coronary artery bypass graft, percutaneous coronary intervention, or ischemic stroke. Cancer was determined using VA Central Cancer Registry data.13 Chronic obstructive pulmonary disease (COPD) was defined by ICD-9 code.14 Hypertension was categorized as no hypertension (untreated BP <120/80 mmHg); pre-hypertension (untreated BP 120-139/80-89 mmHg); controlled hypertension (treated BP <140/90 mmHg); or uncontrolled hypertension (BP ≥140/90 mmHg).15 Diabetes was diagnosed using a combination of glucose measurements, use of insulin or oral hypoglycemic agents, and/or ICD-9 codes.16 Smoking was self-reported and obesity was defined as body mass index (BMI) >30 kg/m2. Cholesterol lowering medication use (HMG CoA reductase inhibitor (statins) or gemfibrozil) was assessed using patient pharmacy data. Total cholesterol measurements were obtained from the VA Decision Support System (DSS) and categorized as <200 mg/dL untreated, <200 mg/dL treated, or ≥200 mg/dL.17 Medication data were from the VA Pharmacy Benefits Management database.
Cocaine and alcohol use at baseline were determined by self-report. We categorized alcohol use with data from the Alcohol Use Disorders Identification Test (AUDIT-C) and alcohol abuse and dependence diagnoses using ICD-9 codes based on prior work in VACS as: 1) Low risk current drinking, 2) no current drinking, 3) at-risk or heavy current drinking, and 4) alcohol abuse or dependence diagnosis and current drinking. Current drinking was defined as any drinking reported in the prior 12 months. VACS index was calculated as previously described.18,19 Hepatitis C virus (HCV) infection was defined as a positive HCV antibody test or at least 1 inpatient and/or 2 outpatient ICD-9 codes.20 Liver fibrosis was estimated using FIB-4 scores.21 Hemoglobin was dichotomized at 12g/dL. Renal disease was defined as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73m2.22
Statistical analysis
We compared continuous variables (t-test or median test) and categorical variables (chi-squared test) by HIV status overall and among participants who died. Kaplan-Meier curves were used to describe time to death by HIV status and/or elevations in IL-6, D-dimer, sCD14 and inflammatory burden (number of elevated biomarkers i.e. ≥75th percentile threshold among those who died).
We adapted the method described by Baron and Kearny 23 and MacKinnon et al 24 to assess whether these immunological biomarkers mediate (explain) the relationship between HIV and mortality. This approach requires fulfillment of four conditions: 1) a significant relation between the independent and dependent variables, 2) a significant relation between the independent and mediating variables, 3) a significant relation between the mediating and dependent variables after adjustment for the independent variable, 4) given 1-3 hold, an attenuation (in absolute value) of the association between the independent and dependent variables following adjustment for the mediating variable.
Proportional odds models were used to estimate the association between HIV (stratified by HIV-1 RNA <500, 500-9999, ≥10000 copies/mL) and elevated IL-6, sCD14 and D-dimer. The proportional odds model estimates the proportional odds of being above the Nth quartile of the biomarker distribution versus being in the Nth quartile or lower based on an assumption of proportional odds. To illustrate: the model assumes that coefficients that describe the relationship between the 3rd and 4th quartiles versus 1st and 2nd quartiles of IL-6 are the same as those that describe the relationship between the 2nd, 3rd, and 4th quartiles versus the 1st quartile. We selected this model because it is more parsimonious than a set of logistic regression models for each pair of quartiles while still incorporating all levels of the different outcome variables. This assumption was assessed using the Brant Test (Stata Spost package)25 and found to be valid for all final models except sCD14. Sensitivity analyses using multinomial logistic regression for sCD14 showed consistent results.
Cox proportional hazards models were used to estimate the associations between HIV (stratified by HIV-1 RNA) and mortality adjusting for multiple confounders. All analyses were performed using Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). P-values<0.05 were considered statistically significant.
Results
Of 2389 participants who provided blood specimens, 35 did not have IL-6, sCD14, and D-dimer measured, 4 HIV+ participants had missing HIV-1 RNA and 1 patient subsequently withdrew consent. Of the remainder, 829 were HIV uninfected and 1521 were HIV+. During a median of 6.9 (interquartile range 6·2-7·4) years from baseline (i.e. date of blood draw), 414 deaths occurred (15% of uninfected and 19% of HIV+). Compared to uninfected participants, HIV+ participants were younger and less likely to be female (Table 1). They also had less prevalent cardiovascular disease (14 vs. 25%), diabetes (20 vs. 30%), BMI>30 kg/m2 (16 vs. 46) and alcohol abuse/dependence (28 vs. 24%), and more hepatitis C (47 vs. 31%), FIB-4 greater than 3.25 i.e. suggestive of advanced fibrosis (9 vs. 4%) and hemoglobin <12g/dL (12 vs. 7%) at baseline (Table 1).
Table 1. Characteristics of study population at baseline.
| Data are column percentages (N) unless otherwise specified | Total | Died | |||
|---|---|---|---|---|---|
| HIV- | HIV+ | HIV- | HIV+ | ||
| N | 829 | 1521 | 125 | 289 | |
| Demographics | |||||
| Age | 54·1 (9·4) | 52·3 (8·2) | 59·0 (10·5) | 54·6 (7·6) | |
| Female | 10 (79) | 3 (42) | 2 (2) | 1 (4) | |
| Race | |||||
| White | 21 (174) | 19 (287) | 30 (37) | 16 (47) | |
| Black | 67 (555) | 69 (1050) | 57 (71) | 74 (206) | |
| Hispanic | 8 (66) | 8 (126) | 10 (13) | 6 (18) | |
| Other | 4 (34) | 4 (58) | 3 (4) | 4 (11) | |
| Comorbid diseases | |||||
| Prevalent CVD | 25 (209) | 14 (215) | 44 (55) | 22 (63) | |
| Prevalent cancer | 7 (57) | 6 (97) | 10 (13) | 12 (36) | |
| Controlled hypertension | 57 (473) | 50 (758) | 58 (72) | 50 (144) | |
| Uncontrolled hypertension | 27 (224) | 24 (360) | 37 (46) | 29 (85) | |
| Diabetes | 30 (248) | 20 (302) | 40 (50) | 24 (68) | |
| COPD | 18 (149) | 15 (225) | 28 (36) | 22 (66) | |
| Never smoker | 23 (194) | 24 (366) | 15 (19) | 16 (45) | |
| Current smoker | 47 (391) | 50 (758) | 49 (61) | 61 (176) | |
| Past smoker | 29 (242) | 26 (396) | 36 (45) | 24 (68) | |
| BMI>30 kg/m2 | 46 (385) | 16 (244) | 42 (53) | 16 (45) | |
| Total cholesterol/(mg/dL) | |||||
| <200, no cholesterol-lowering drugs | 37 (303) | 42 (633) | 38 (48) | 40 (117) | |
| <200, on cholesterol-lowering drugs | 36 (300) | 31 (473) | 45 (56) | 37 (108) | |
| >=200 | 26 (213) | 27 (404) | 16 (20) | 21 (62) | |
| Substance use | |||||
| Cocaine use in past year | 38 (312) | 36 (549) | 37 (46) | 46 (132) | |
| Alcohol | |||||
| Not hazardous drinking | 17 (143) | 23 (355) | 17 (21) | 13 (38) | |
| Not current drinking | 40 (330) | 36 (551) | 43 (54) | 43 (123) | |
| At-risk or heavy episodic drinking | 12 (97) | 12 (181) | 6 (8) | 8 (24) | |
| Alcohol abuse/dependence diagnosis | 28 (230) | 24 (372) | 28 (35) | 32 (93) | |
| VACS index components | |||||
| Median (IQR) VACS Index score (includes HIV-1 RNA and CD4 count) | 29 (18, 45) | 46 (33, 62) | |||
| Median (IQR) modified VACS Index score (excludes HIV-1 RNA and CD4 count) | 18 (11, 27) | 21 (11, 33) | 27 (20, 42) | 32 (21, 43) | |
| Hepatitis C | 31 (257) | 47 (714) | 45 (56) | 62 (180) | |
| FIB-4 | |||||
| >3·25 | 4 (33) | 9 (134) | 13 (16) | 20 (59) | |
| 1·45-3·25 | 25 (206) | 36 (549) | 35 (44) | 44 (127) | |
| <1·45 | 69 (575) | 55 (831) | 51 (64) | 35 (102) | |
| Hemoglobin <12 g/dL | 7 (59) | 12 (178) | 11 (14) | 22 (65) | |
| eGFR<60 mL/min/1·73 m2 | 9 (76) | 8 (117) | 22 (27) | 13 (38) | |
| HIV specific factors | |||||
| CD4/ cells/mm3 | Median (IQR) | 392 (232, 583) | 292 (123, 453) | ||
| >=500 | 35 (532) | 22 (65) | |||
| 200-<500 | 46 (704) | 42 (122) | |||
| <200 | 19 (285) | 35 (102) | |||
| HIV-1 RNA/ copies/mL | Median (IQR) | 75 (75, 3339) | 400 (75, 20321) | ||
| <500 | 66 (1006) | 55 (158) | |||
| 500-9999 | 15 (226) | 15 (44) | |||
| ≥10000 | 19 (289) | 30 (87) | |||
| HAART (baseline) | 76 (1156) | 75 (217) | |||
| Inflammatory biomarkers | |||||
| IL-6/ (pg/mL) | Median | 1·8 | 2·1 | 2·6 | 3·1 |
| IQR | (1·2, 3·2) | (1·4, 3·4) | (1·5, 4·6) | (1·8, 5·4) | |
| % elevated (N) | 11 (95) | 12 (177) | 21 (26) | 27 (77) | |
| sCD14/ (ug/mL) | Median | 1731 | 1719 | 1888 | 1879 |
| IQR | (1478, 2043) | (1448, 2085) | (1668, 2339) | (1585, 2333) | |
| % elevated (N) | 15 (124) | 16 (237) | 26 (32) | 25 (71) | |
| D-dimer/ (ug/mL) | Median | 0·30 | 0·26 | 0·47 | 0·37 |
| IQR | (0·21, 0·53) | (0·15, 0·49) | (0·25, 0·88) | (0·22, 0·86) | |
| % elevated (N) | 14 (114) | 13 (192) | 26 (32) | 25 (73) | |
| Inflammatory burden score | |||||
| 0 biomarkers elevated | 67 (556) | 71 (1080) | 42 (53) | 52 (151) | |
| 1 biomarker elevated | 24 (203) | 20 (302) | 42 (52) | 25 (73) | |
| Any 2 biomarkers elevated | 6 (50) | 6 (96) | 14 (17) | 15 (44) | |
| IL-6 & sCD14 elevated | 2 (18) | 3 (48) | 4 (5) | 7 (20) | |
| IL-6 & D-dimer elevated | 2 (19) | 2 (27) | 6 (7) | 5 (14) | |
| D-dimer & sCD14 elevated | 2 (13) | 1 (21) | 4 (5) | 3 (10) | |
| 3 biomarkers elevated | 1 (9) | 2 (37) | 1 (1) | 7 (20) | |
All covariates had complete data during the analysis period except the following: blood pressure was available for 828 HIV uninfected, smoking data were available for 1520 HIV+ and 827 uninfected, BMI was available for 1517 HIV+ and 827 uninfected, cholesterol was available for 1510 HIV+ and 816 uninfected, alcohol use was available for 1459 HIV+ and 800 uninfected, FIB-4 was available for 1512 HIV+ and 804 uninfected, hemoglobin was available for 828 uninfected, eGFR data were available for 823 uninfected, IL-6 was available for 1517 HIV+ and 821 uninfected, and D-dimer was available for 1519 HIV+ and 826 uninfected..
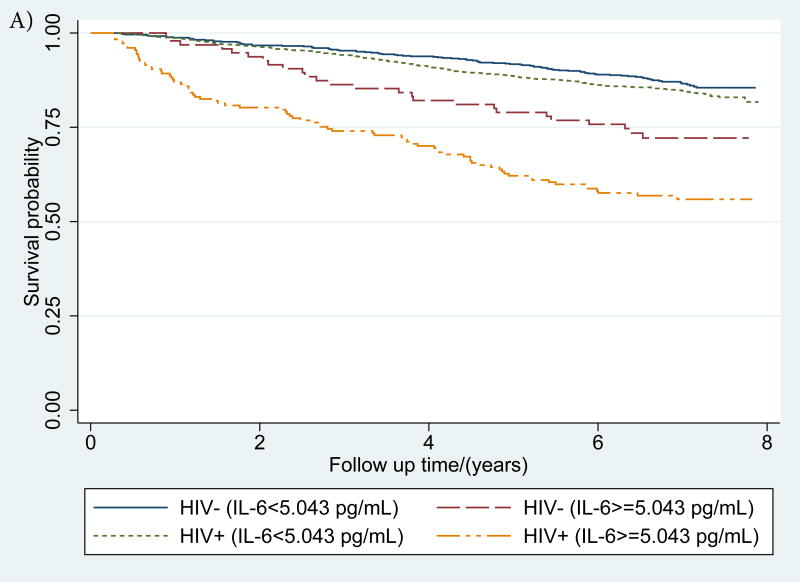
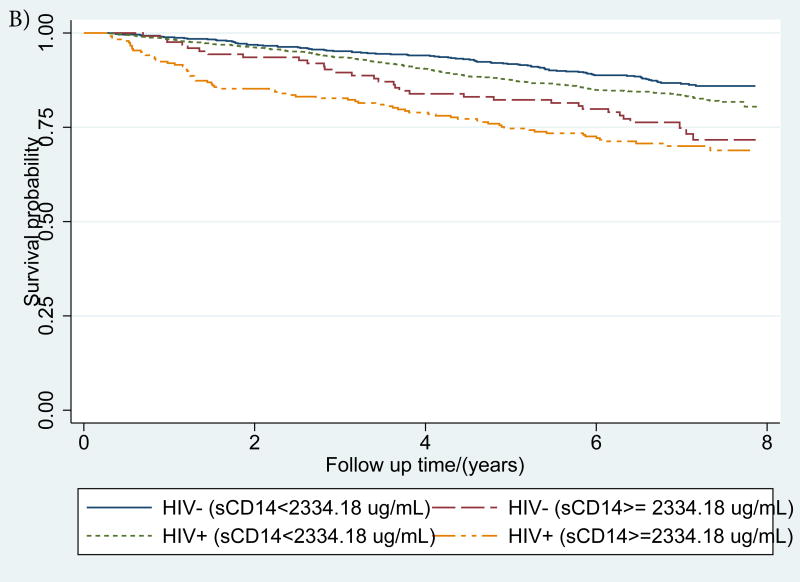
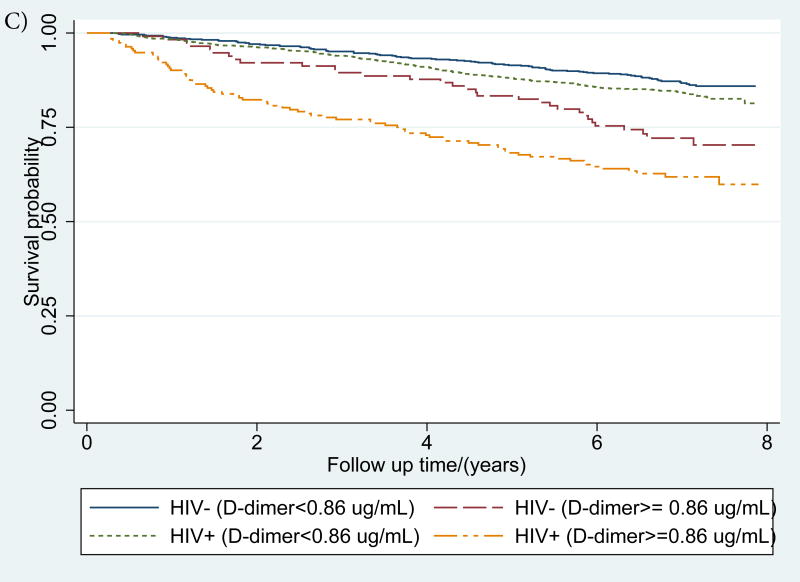
IL-6, sCD14 and D-dimer elevation thresholds were defined as ≥75th percentile among those who died. For, IL-6 this threshold was 5·043 pg/mL, for sCD14 it was 2334·18 ug/mL, and for D-dimer, it was 0·86 ug/mL.
HIV and mortality
Mortality rates per 100 person years were higher among HIV+ versus uninfected people (incidence rate ratio (95% CI): 1·31 (1·06-1·62). Compared to uninfected participants, HIV infection with HIV-1 RNA≥500-9999 and ≥10000 copies/mL was associated with a higher risk of mortality in age and race-ethnicity adjusted models (Hazard ratio (95% CI): 1·55 (1·09-2·19) and 2·94 (2·22-3·91) respectively). This increased risk remained for both HIV groups after further adjusting for comorbid diseases, substance use, and VACS Index components but was only statistically significant among those with HIV-1 RNA≥10000 copies/mL (1·34 (0·93-1·92) and 2·18 (1·60-2·99) respectively). HIV infected people with CD4+ T-cell count below 350 cells/mm3 had increased mortality risk relative to uninfected people (1.67 (1.28-2.18)). The association of HIV status stratified by ART receipt at baseline and mortality did not reach statistical significance (data not shown).
HIV and IL-6, sCD14 and D-dimer
After adjustment for demographics, comorbidities and substance use, HIV+ participants with viremia ≥10000 copies/mL had greater proportional odds of elevated IL-6, sCD14, and D-dimer relative to uninfected participants (Table 2). The proportional odds (95% CI) of being in a higher quartile of IL-6, sCD14 or D-dimer was 2-3 fold higher for HIV+ (HIV-1 RNA≥ 10000 copies/mL) versus uninfected people (Table 2).
Table 2. Association between HIV infection and IL-6, sCD14, and D-dimer adjusted for a) age and race-ethnicity b) all covariates.
| Proportional odds ratio (95% confidence interval)* | |||||
|---|---|---|---|---|---|
| a) Model (age, race-ethnicity adjusted) | Outcomes | HIV uninfected | HIV+ HIV-1 RNA<500 copies/mL | HIV+ HIV-1 RNA 500-9999 copies/mL | HIV+ HIV-1 RNA ≥10000 copies/mL |
| 1 | IL-6 quartiles | 1 (Ref) | 1·14 (0·96-1·35) | 1·32 (1·01-1·73) | 2·99 (2·32-3·84) |
| 2 | sCD14 quartiles | 1 (Ref) | 0·93 (0·78-1·10) | 0·85 (0·65-1·12) | 2·05 (1·61-2·62) |
| 3 | D-dimer quartiles | 1 (Ref) | 0·50 (0·42-0·59) | 0.88 (0·67-1·16) | 1·91 (1·49-2·45) |
| b) Model (Fully adjusted) | Outcomes | HIV uninfected | HIV+ HIV-1 RNA<500 copies/mL | HIV+ HIV-1 RNA 500-9999 copies/mL | HIV+ HIV-1 RNA ≥10000 copies/mL |
| 1 | IL-6 quartiles | 1 (Ref) | 1·35 (1·11-1·64) | 1·46 (1·10-1·95) | 2·78 (2·11-3·65) |
| 2 | sCD14 quartiles | 1 (Ref) | 0·77 (0·64-0·93) | 0·71 (0·54-0·95) | 1·49 (1·14-1·94) |
| 3 | D-dimer quartiles | 1 (Ref) | 0·51 (0·43-0·62) | 0·95 (0·71-1·26) | 1·73 (1·32-2·26) |
The proportional odds model estimates the proportional odds of being above the Nth quartile of the biomarker distribution versus being in the Nth quartile or lower.
Fully adjusted model adjusted for age, race-ethnicity, prevalent cardiovascular disease, cancer, diabetes, chronic obstructive pulmonary disease, hypertension, smoking, hepatitis C, obesity, total cholesterol and cholesterol lowering medication, alcohol use, cocaine use, hemoglobin, FIB-4, estimated glomerular filtration rate. IL-6, sCD14 and D-dimer quartile thresholds were defined using quartile levels (25th, 50th, and 75th percentiles) among those who died. For, IL-6 these thresholds were 1·727, 2·91, and 5·043 pg/mL. For sCD14: 1592·84, 1883·51, and 2334·18 ug/mL. For D-dimer: 0·23, 0·39, and 0·86 ug/mL
IL-6, sCD14, D-dimer and mortality
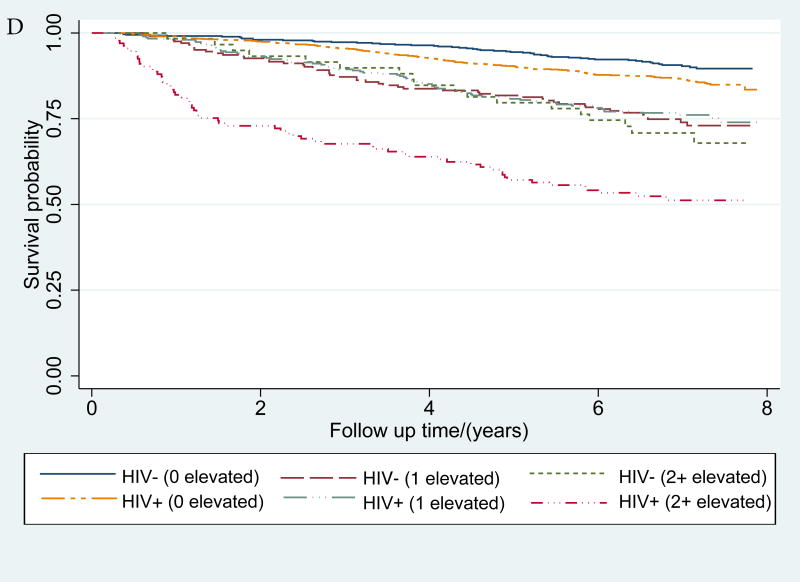
Mortality rates increased with elevations in IL-6, sCD14 and D-dimer (Table 3) and inflammatory burden (Figure 1) among HIV+ and uninfected participants. In Cox proportional hazards models, IL-6, sCD14, and D-dimer elevations (highest quartile) were significantly associated with mortality risk independently of HIV infection or viral suppression (Table 3). These associations were attenuated but persisted after adjustment for comorbid disease, substance use and VACS Index components and the remaining two inflammatory biomarkers (Table 3). The association of elevated sCD14 and mortality persisted after comorbidity adjustment (Supplemental Digital Content Table 1) but was no longer statistically significant after adjustment for IL-6 and D-dimer (Table 3). These results were consistent in analyses excluding HIV infected people with unsuppressed viral replication (HIV-1 RNA≥500 copies/mL; Supplemental Digital Content Table 2).
Table 3. Mortality rates, rate ratios and risks by IL-6, sCD14 and D-dimer quartile mutually adjusted for each other and adjusted for HIV status and co-morbid conditions.
| Number of deaths/ Number of people | Death rate/100py (95% CI) | Mortality IRR for HIV+ vs. uninfected (95% CI) | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| IL-6 quartile | 1 | 103/981 | 1·56 (1·29-1·89) | 1·05 (0·70-1·61) | 1 (ref) | 1 (ref) |
| 2 | 102/651 | 2·44 (2·01-2·96) | 1.00 (0·65-1·58) | 1·25 (0·94-1·65) | 1·11 (0·83-1·47) | |
| 3 | 103/434 | 3·79 (3·12-4·60) | 1·41 (0·89-2·31) | 1·64 (1·23-2·20) | 1·25 (0·92-1·69) | |
| 4 | 103/272 | 6·91 (5·69-8·38) | 1·90 (1·20-3·08) | 2·67 (1·95-3·64) | 1.98 (1·43-2·74) | |
| sCD14 quartile | 1 | 104/896 | 1·73 (1·43-2·10) | 1·46 (0·94-2·34) | 1 (ref) | 1 (ref) |
| 2 | 103/599 | 2·67 (2·20-3·23) | 1·27 (0·83-1·98) | 1·24 (0·94-1·65) | 1·18 (0·89-1·56) | |
| 3 | 104/494 | 3·37 (2·78-4·09) | 1·26 (0·82-1·99) | 1·38 (1·04-1·83) | 1·18 (0·88-1·58) | |
| 4 | 103/361 | 4·87 (4·01-5·90) | 1·27 (0·82-1·99) | 1·57 (1·16-2·12) | 1·27 (0·92-1·73) | |
| D-dimer quartile | 1 | 102/884 | 1·73 (1·43-2·10) | 1·16 (0·73-1·88) | 1 (ref) | 1 (ref) |
| 2 | 113/679 | 2·58 (2·15-3·10) | 2·33 (1·48-3·79) | 1·16 (0·88-1·53) | 1·16 (0·88-1·53) | |
| 3 | 94/476 | 3·09 (2·53-3·79) | 0·93 (0·61-1·44) | 1·17 (0·87-1·58) | 1·09 (0·81-1·48) | |
| 4 | 105/306 | 6.07 (5·02-7·35) | 1·56 (1·01-2·44) | 1·84 (1·36-2·50) | 1·65 (1·20-2·25) | |
All models were adjusted for IL-6, sCD14, D-dimer and HIV status categorized as uninfected, HIV infected (HIV-1 RNA<500 copies/mL), HIV infected (HIV-1 RNA 500-9999 copies/mL), and HIV infected (HIV-1 RNA ≥10000 copies/mL).
Model 1 additionally adjusted for age and race-ethnicity.
Model 2 additionally adjusted for age, race-ethnicity, prevalent cardiovascular disease, diabetes, cancer, chronic obstructive pulmonary disease, hypertension, smoking, hepatitis C, BMI, total cholesterol and cholesterol lowering medication, alcohol use, cocaine use, hemoglobin, FIB-4, and estimated glomerular filtration rate.
IL-6, sCD14 and D-dimer quartile thresholds were defined using quartile levels (25th, 50th, and 75th percentiles) among those who died. For, IL-6 these thresholds were 1·727, 2·91, and 5·043 pg/mL. For sCD14: 1592·84, 1883·51, and 2334·18 ug/mL. For D-dimer: 0·23, 0·39, and 0·86 ug/mL
CI: confidence interval; py: person years; IRR: incidence rate ratio
Association of HIV and mortality adjusting for IL-6, sCD14 and D-dimer
The association between HIV (with HIV-1 RNA ≥10000 copies/mL or CD4+ T-cell count<350 cells/mm3) and mortality was partially attenuated after further adjusting the Cox models for IL-6, sCD14, and D-dimer (Table 4). The degree of attenuation was greatest when all three biomarkers were considered simultaneously as quartiles within a single model (Table 4). The risk of death among those with HIV-1 RNA ≥10000 copies/mL went from 2·18 (1·60-2·99) to 2·00 (1·45-2·76) when IL-6, sCD14 and D-dimer were included in the model (Table 4). Similar attenuation was not observed among those with HIV-1 RNA<10000 copies/mL. Relative to uninfected people, the risk of death for those with CD4+ T-cell counts<350 cells/mm3 went from 1.67 (1.28-2.18) to 1.63 (1.25-2.14) after adjustment for IL-6, sCD14 and D-dimer.
Table 4. Assessing whether addition of inflammatory biomarkers to Cox regression models attenuates the association between HIV infection (stratified by HIV-1 RNA at baseline) and mortality.
| Hazard Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Unadjusted for IL-6, sCD14 or D-dimer | Adjusted for IL-6, sCD14 and D-dimer | Unadjusted for IL-6, sCD14 or D-dimer | Adjusted for IL-6, sCD14 and D-dimer | |
| Model 1 | Model 1 | Model 2 | Model 2 | |
| N (# deaths) | 2324 (411) | 2324 (411) | 2324 (411) | 2324 (411) |
| HIV uninfected | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| HIV+ HIV-1 RNA<500 copies/mL | 1·12 (0·89-1·42) | 1·20 (0·94-1·53) | 1·04 (0·80-1·35) | 1·09 (0·84-1·42) |
| HIV+ HIV-1 RNA 500-9999 copies/mL | 1·55 (1·09-2·19) | 1·58 (1·11-2·24) | 1·34 (0·93-1·92) | 1·42 (0·99-2·05) |
| HIV+ HIV-1 RNA ≥10000 copies/mL | 2·94 (2.22-3·91) | 2·29 (1·72-3·07) | 2·18 (1·60-2·99) | 2·00 (1·45-2·76) |
Model 1 additionally adjusted for age and race-ethnicity.
Model 2 additionally adjusted for age, race-ethnicity, prevalent cardiovascular disease, diabetes, cancer, chronic obstructive pulmonary disease, hypertension, smoking, hepatitis C, BMI, total cholesterol and cholesterol lowering medication, alcohol use, cocaine use, hemoglobin, FIB-4, estimated glomerular filtration rate.
IL-6, sCD14 and D-dimer quartile thresholds were defined using quartile levels (25th, 50th, and 75th percentiles) among those who died. For, IL-6 these thresholds were 1·727, 2·91, and 5·043 pg/mL. For sCD14: 1592·84, 1883·51, and 2334·18 ug/mL. For D-dimer: 0·23, 0·39, and 0·86 ug/mL
We did not find significant interactions between HIV status and biomarker elevations on mortality risk (p≥0.1).
Discussion
We report that HIV infection is associated with biomarkers of inflammation, monocyte activation, and altered coagulation and an increased risk of death compared to those without HIV infection. These biomarkers are also associated with an increased risk of mortality, independently of HIV status or viremia. After adjustment for comorbid diseases and substance use, biomarkers of inflammation, monocyte activation, and altered coagulation partially explain the excess risk of mortality among viremic HIV infected people compared to uninfected people.
Our results are consistent with prior work linking elevated biomarkers of immune function to an increased risk of mortality among HIV infected people.4,5 The lack of uninfected comparators in prior work makes it challenging to assess if these biomarkers contribute to excess mortality among HIV infected people. With our cohort of HIV infected and demographically and behaviorally similar uninfected participants, we have extended these findings. Our results show that some of the excess risk of mortality among viremic HIV infected people is explained by biomarkers of inflammation, monocyte activation, and altered coagulation.
This study brings together a number of important findings within a single, well-phenotyped cohort of HIV infected and uninfected people with thorough capture of mortality outcomes. The fact that 1) mortality decreases with viral suppression (HIV-1 RNA<500 copies/mL in this study), 2) these biomarkers do not attenuate the association between HIV infection and mortality among those with lower HIV-1 RNA, and 3) HIV viremia is associated with higher levels of these biomarkers all support the hypothesis that HIV viremia increases the levels of inflammation, monocyte activation, and altered coagulation, which drive increased mortality. Importantly, our results also demonstrate that the three biomarkers we studied do not explain the majority of the excess risk of mortality associated with HIV infection in our cohort. If immune system activation drives this excess risk, our finding may be explained by the fact that three biomarkers, when measured only at baseline, cannot fully capture the complexity of immune system activation. Additionally, mechanisms beyond inflammation, monocyte activation, and altered coagulation may contribute to the excess risk of mortality associated with viremic HIV infection. Further, these biomarkers can change for reasons unrelated to HIV e.g. comorbid conditions. Lastly, while these specific biomarkers do not explain most of the excess total mortality risk associated with HIV infection, they may explain more cause-specific mortality.
This study has limitations that warrant discussion. First, as the majority of our cohort is men, our results may not be generalizable to women. Second, our analysis did not have multiple longitudinal measures of inflammatory biomarkers to assess the impact of changes in the biomarkers on mortality risk. Third, while all three of our selected biomarkers are associated with HIV infection and increased risk of mortality among those with and without HIV, there are other potentially important biomarkers (e.g., CD163, TNF alpha) and immunologic processes that were not included in our analysis. Finally, like all observational studies, we cannot eliminate the possibility of unmeasured or residual confounding.
In conclusion, increased HIV viral loads are associated with higher levels of biomarkers of inflammation (IL6), monocyte activation (sCD14), and altered coagulation (D-dimer). Elevated levels of these biomarkers are associated with mortality among HIV infected and uninfected people and in combination, these biomarkers partially explain the excess risk of mortality among viremic HIV infected compared to uninfected people.
Supplementary Material
Supplemental digital content Table 1: Mortality risk by a) IL-6, b) sCD14 and c) D-dimer quartile ( IL-6, sCD14 and D-dimer in separate models)
Supplemental digital content Table 2: Mortality risk by IL-6, sCD14 and D-dimer quartile excluding HIV infected people with unsuppressed viremia (IL-6, sCD14 and D-dimer in same model)
Figure 1. (a-d): Kaplan-Meier survival curves describing mortality by HIV status and a) IL-6, b) sCD14 and c) D-dimer and d) inflammatory burden (number of inflammatory biomarkers (0, 1 or 2+) elevated >75th percentile).
IL-6, sCD14 and D-dimer elevation thresholds were defined as ≥75th percentile among those who died. For, IL-6 this threshold was 5·043 pg/mL, for sCD14 it was 2356·12 ug/mL, and for D-dimer, it was 0·88 ug/mL
Key points.
HIV infection is associated with elevated IL-6, sCD14 and D-dimer, which are in turn are associated with mortality. Baseline measures of these biomarkers partially explain the excess risk of mortality among HIV infected compared to uninfected people.
Acknowledgments
We acknowledge the veterans who participate in the Veterans Aging Cohort Study and the study coordinators and staff at each of our sites and at the West Haven Coordinating Center. Without the commitment and care of these individuals, this research would not be possible. We would also like to acknowledge the substantial in-kind support we receive from the Veterans Affairs Healthcare System.
Source of Funding: This work was supported by National Institute on Alcohol Abuse and Alcoholism and National Heart Lung and Blood Institute at the National Institutes of Health [grant number R01HC095136 to M.F; R01AI110259-01A1 and 1I01BX002358-01A1 to MMS]. Veterans Aging Cohort Study funded by: National Institute on Alcohol Abuse and Alcoholism (U10AA13566, U24AA020794, and U01AA020790 to AJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: No conflicts of interest were disclosed except grants to institution from Gilead and AbbVie (Adeel Butt).
Prior presentation: Some of these data were presented at Conference on Retroviruses and Opportunistic Infections 2015, February 23-26, Seattle, WA
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Contributor Information
Kaku A. So-Armah, Boston University School of Medicine, Boston, MA, USA.
Janet P. Tate, VA Connecticut Healthcare System, West Haven, CT; Yale University School of Medicine, New Haven, CT, USA.
Chung-Chou H. Chang, University of Pittsburgh Schools of Medicine and Public Health, Pittsburgh, PA, USA.
Adeel A. Butt, University of Pittsburgh Schools of Medicine and Public Health; VA Pittsburgh Healthcare System, Pittsburgh, PA; Hamad Medical Corporation, Doha, Qatar & USA.
Mariana Gerschenson, John A. Burns School of Medicine, University of Hawaii–Manoa, Honolulu, HI, USA.
Cynthia L. Gibert, VA Medical Center & George Washington University Medical Center, Washington, DC, USA.
David Leaf, VA Greater Los Angeles Healthcare System & David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
David Rimland, VA Medical Center & Emory University School of Medicine, Atlanta, GA.
Maria C. Rodriguez-Barradas, Michael E. DeBakey VA Medical Center & Baylor College of Medicine, Houston, TX, USA.
Matthew J. Budoff, Harbor-UCLA Medical Center and Los Angeles Biomedical Research Institute, Los Angeles, CA, USA.
Jeffrey H. Samet MD, Boston University Schools of Medicine and Public Health, Boston Medical Center, Boston, MA, USA
Lewis H. Kuller, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Steven G. Deeks. MD, Department of Medicine, Positive Health Program, San Francisco General Hospital, San Francisco, CA, USA
Kristina A. Crothers, Division of Pulmonary and Critical Care, Department of Internal Medicine, University of Washington, Seattle, WA, USA.
Russell P. Tracy, Departments of Pathology and Biochemistry, College of Medicine, University of Vermont, Burlington, VT, USA.
Heidi M. Crane, University of Washington School of Medicine, Seattle, WA, USA.
Mohammad M. Sajadi, Baltimore VA Medical Center; Institute of Human Virology at the University of Maryland School of Medicine, Baltimore, MD, USA.
Hilary A. Tindle, Vanderbilt University School of Medicine, Nashville, TN, USA.
Amy C. Justice, VA Connecticut Healthcare System, West Haven, CT; Yale University Schools of Medicine and Public Health, New Haven, CT, USA.
Matthew S. Freiberg, Vanderbilt University School of Medicine, Nashville, TN, USA.
References
- 1.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33(1):158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Castelnuovo A, de Curtis A, Costanzo S, et al. Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica. 2013;98(9):1476–1480. doi: 10.3324/haematol.2012.083410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baune BT, Rothermundt M, Ladwig KH, Meisinger C, Berger K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age. 2011;33(2):209–217. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Napravnik S, Eron JJ, et al. Decreasing excess mortality of HIV-infected patients initiating antiretroviral therapy: comparison with mortality in general population in China, 2003-2009. J Acquir Immune Defic Syndr. 2013;63(5):e150–157. doi: 10.1097/QAI.0b013e3182948d82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armah KA, Quinn EK, Cheng DM, et al. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis. 2013;13(1):399. doi: 10.1186/1471-2334-13-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 12.Every NR, Fihn SD, Sales AE, Keane A, Ritchie JR. Quality Enhancement Research Initiative in ischemic heart disease: a quality initiative from the Department of Veterans Affairs. QUERI IHD Executive Committee. Med Care. 2000;38(6 Suppl 1):I49–59. [PubMed] [Google Scholar]
- 13.Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer Incidence in HIV-Infected Versus Uninfected Veterans: Comparison of Cancer Registry and ICD-9 Code Diagnoses. Journal of AIDS & clinical research. 2014;5(7):1000318. doi: 10.4172/2155-6113.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49(2):225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual review of psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long JS, Freese J. Regression Models for Categorical Dependent Variables, Third Edition. College Station, TX: Stata Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content Table 1: Mortality risk by a) IL-6, b) sCD14 and c) D-dimer quartile ( IL-6, sCD14 and D-dimer in separate models)
Supplemental digital content Table 2: Mortality risk by IL-6, sCD14 and D-dimer quartile excluding HIV infected people with unsuppressed viremia (IL-6, sCD14 and D-dimer in same model)