Abstract
The health of primary sensory afferents supplying muscle has to be a first consideration in assessing deficits in proprioception and related motor functions. Here we discuss the role of a particular proprioceptor, the IA muscle spindle proprioceptor in causing movement disorders in response to either regeneration of a sectioned peripheral nerve or damage from neurotoxic chemotherapy. For each condition, there is a single preferred and widely repeated explanation for disability of movements associated with proprioceptive function. We present a mix of published and preliminary findings from our laboratory, largely from in vivo electrophysiological study of treated rats to demonstrate newly discovered IA afferent defects that seem likely to make important contributions to movement disorders. First, we argue that reconnection of regenerated IA afferents with inappropriate targets, although often repeated as the reason for lost stretch–reflex contraction, is not a complete explanation. We present evidence that despite successful recovery of stretch-evoked sensory signaling, peripherally regenerated IA afferents retract synapses made with motoneurons in the spinal cord. Second, we point to evidence that movement disability suffered by human subjects months after discontinuation of oxaliplatin (OX) chemotherapy for some is not accompanied by peripheral neuropathy, which is the acknowledged primary cause of disability. Our studies of OX-treated rats suggest a novel additional explanation in showing the loss of sustained repetitive firing of IA afferents during static muscle stretch. Newly extended investigation reproduces this effect in normal rats with drugs that block Na+ channels apparently involved in encoding static IA afferent firing. Overall, these findings highlight multiplicity in IA afferent deficits that must be taken into account in understanding proprioceptive disability, and that present new avenues and possible advantages for developing effective treatment. Extending the study of IA afferent deficits yielded the additional benefit of elucidating normal processes in IA afferent mechanosensory function.
Keywords: chemotherapy, mechanotransduction, muscle spindle, sensory encoding, synaptic transmission
Introduction
Primary sensory afferents and their peripheral receptors provide the compulsory first step in detecting and transmitting somatosensory information to the central nervous system (CNS). Just as the elimination of sensory feedback from primary cutaneous afferents impairs one's ability to perceive or manipulate objects through touch (McGlone & Reilly, 2010), we expect the elimination/alteration of information uniquely supplied by large-diameter muscle afferents to impair motor performance. These afferents, which we will refer to as muscle proprioceptors, encode information about static and dynamic features of muscle force and length (Proske & Gandevia, 2012) to contribute to one's sense of body and limb position (proprioceptive), and movement (kinaesthesia; Proske, 2006). Importantly, muscle proprioceptors also coordinate the activities of motor pools and muscles to support and make adjustments as needed for purposeful movement (Proske & Gandevia, 2012). Information transmitted by muscle proprioceptors is indispensable to the stretch reflex, which achieves the fastest possible neural response to limb perturbations (Nichols et al. 1999), as well as to longer latency reflexes responsible for stabilizing body position (e.g. balance during unexpected perturbations) and assisting with smooth trajectory of limbs as they move through biomechanically unstable paths (Shemmell et al. 2010). These functions and associated sensorimotor behaviors are at risk when injury or disease degrades the signals sent by muscle proprioceptors. A long-term goal of our laboratory is to obtain a detailed understanding of the defects in proprioceptor function as needed to develop effective treatment of associated movement disorders.
In this brief account, we report results from our studies of two clinically relevant conditions that damage muscle proprioceptors and that are variously associated with postural instability, clumsiness and/or forms of sensory ataxia. One condition occurs when, despite successful regeneration of severed sensory axons, muscle proprioceptors lose synaptic connections within the spinal cord. In the other condition we describe our preliminary findings relative to the abnormal sensory encoding that we discovered in rats treated with a commonly used chemotherapy agent. For both conditions, we find previously unknown abnormalities in muscle proprioceptors that present themselves as candidate contributors to proprioceptive disorders. We wish to acknowledge that the studies we describe here rest heavily on the seminal contributions made by participants at this meeting. On the occasion of this symposium, we pay special tribute to Dr Banks for the foundations and insights that he contributed to the study of somatosensory primary afferents.
Main body
Sites expressing impaired proprioceptor function – Peripheral and central
From their cell bodies in dorsal root ganglia, pseudo-unipolar proprioceptors project both peripherally to connect with specialized sensory receptors and centrally to form presynaptic connections with specific neurons in the spinal cord. Through these structures, proprioceptors detect, convey and transmit sensory signals from the periphery to the CNS. For descriptive purposes we define central and peripheral processes in relation to our recording site within the dorsal root, where we penetrate axons by micropipette to record from and sometimes label the proprioceptors of anesthetized adult rats in vivo (Fig. 1).
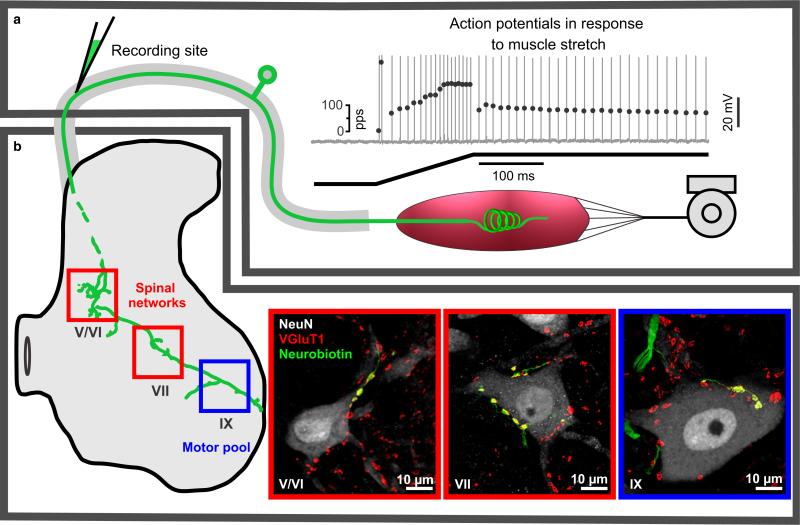
Fig. 1.
Diagram and data representing selected functions and structures of IA afferent in adult rat. (a and b) This IA afferent is divided, respectively, into peripheral and central portions relative to micropipette recording site in dorsal root near its spinal cord entry zone. (a) IA afferent firing that is produced in response to ramp-hold muscle stretch and that represents the culmination of mechanical transduction followed by action potential encoding and orthodromic conduction. Top trace shows action potentials vertical lines with instantaneous firing rates plotted by superimposed black dots; the bottom trace is muscle length during ramp-hold stretch (ramp 20 mm/s to 3 mm and hold for 1 s). (b) Central IA afferent structures, including central axon collaterals (intra-axonal neurobiotin labeled green) that project in the spinal cord to laminae V/VI, VII, IX where they form synapses (VGluT1) with different populations of neurons (labeled with NeuN). Regions (a and b) are divided to assist discussion of where and how IA afferent demonstrate impairment after peripheral nerve injury and chronic chemotherapy.
Peripheral functions: transduction, encoding, conduction
Figure 1a delineates the region of peripheral functions represented by a proprioceptor firing a train of action potentials recorded intra-axonally in response to ramp and hold muscle stretch. This particular proprioceptor was classified as an IA afferent on criteria acknowledged to distinguish this class from other large-diameter proprioceptors, namely type II and IB (Matthews, 1972; Proske & Gregory, 1977; Proske & Morgan, 1999; De-Doncker et al. 2003; Bullinger et al. 2011a). The firing response criteria that we used for proprioceptor classification included the relatively high-frequency ‘initial burst’ firing observed at the onset of muscle stretch, firing entrainment during high-frequency vibration, and firing cessation on the release of stretch (see Figs 3b,c and 4a). Comparatively, spindle afferent firing pauses during muscle twitch, where IB afferent firing increases. Further, IA afferents display high-frequency ‘initial bursts’, and entrainment to high-frequency vibration, where group II spindle afferents fail in both of these categories. In the present report, we restrict all further consideration to IA afferents.
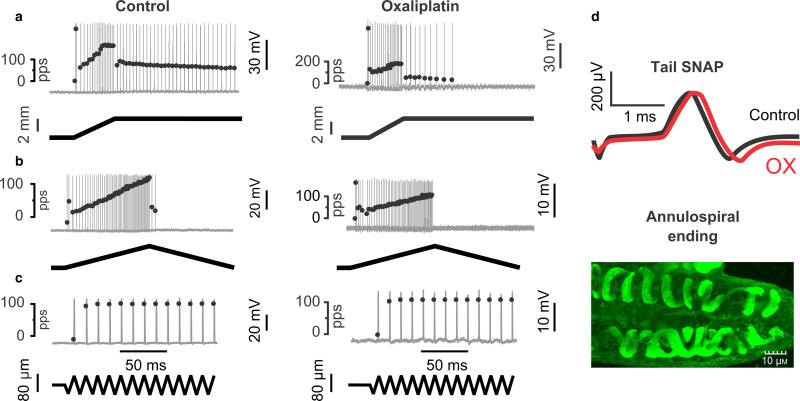
Fig. 3.
Chronic oxaliplatin (OX) treatment abbreviates static phase firing without neuropathy. Electromechanical data arranged vertically for one IA afferent sampled in vivo from a control rat and for one IA afferent taken from a rat 5 weeks after injections of OX (1/week for 4 weeks; cumulative dose 70 mg/kg). Firing of the IA afferents (top traces) is shown in response to three different forms of muscle stretch (bottom traces): (a) ramp-hold; (b) triangular; (c) vibration. Action potentials are shown as vertical lines firing at instantaneous firing rates indicated by superimposed black dots. During the static (hold) phase of muscle stretch (a), IA afferents in normal rats typically sustained firing, but those in OX-treated rats rapidly adapted and ceased firing. By contrast, during dynamic (changing length) phases of muscle stretch (a–c), IA afferents were similarly responsive in control and OX-treated rats. Also, IA afferents in both groups displayed high-frequency initial burst firing at the onset of ramp (a) and triangular (b) stretches, and fired with 1 : 1 entrainment to muscle vibration at high frequency (100 Hz) and low amplitude (0.08 mm) (c). (d) Data demonstrate the absence of neuropathy: top traces show that electrically evoked sensory nerve action potentials (SNAPs) recorded from the tail before and after treatment in the OX rat were indistinguishable; the bottom image represents finding that muscle spindles in OX rats were consistently innervated by normal-looking annulospiral endings (stained with PGP9.5).
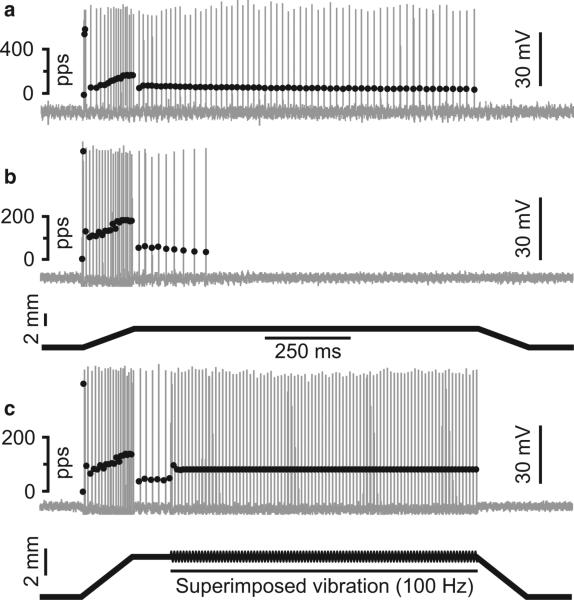
Fig. 4.
IA afferent static phase firing restored by muscle vibration. IA afferent repetitive firing was sustained throughout the static (hold) phase of muscle stretch in normal rat (a), but abbreviated in a rat weeks after oxaliplatin (b). Action potentials shown as vertical lines with instantaneous firing rate are indicated by superimposed black dots; muscle length trace for ramp-hold-release stretch is shown in the bottom trace in (b). (c) Same IA afferent as in (b); 100 Hz muscle vibration superimposed on the hold phase of stretch-elicited sustained firing at 100 Hz.
The arrival of action potentials at the recording site within the dorsal roots relies on progression through three general processes in peripheral signaling: transduction, encoding and conduction. In the initial step, transduction occurs for IA afferents when mechanical perturbation, transmitted through muscle and other non-neural tissues, activates stretch-sensitive channels within annulospiral endings to produce predominately Na+ current (and to a lesser extent Ca2+ current) that results in a depolarizing receptor potential (Hunt et al. 1978). Receptor potential size and shape reflect different dynamic and static components of mechanical perturbations (Hunt & Ottoson, 1975). Recent evidence indicates that epithelial sodium channels may be responsible for the Na+ current of the receptor potential (Simon et al. 2010), but the channel responsible for Ca2+ current has yet to be adequately characterized (Bewick & Banks, 2014). In the next step of peripheral signaling, the receptor potential spreads electronically to the unmyelinated heminode of the IA afferent terminal where its features are encoded in the firing pattern of action potentials generated by voltage-gated Na+ and K+ channels. The final step in signaling proceeds as action potentials propagate from the heminodes orthodromically along the afferent axon as it traverses the peripheral nerve and dorsal root where we record them intra-axonally.
Transduction and encoding in spindle afferents can be modified extrinsically by gamma motoneuron input (Taylor et al. 2006) and intrinsically by Ca2+-activated mechanisms to modulate IA afferent firing (Bewick & Banks, 2014). Our studies of the effects of chemotherapy on spindle firing (see below) attract particular attention to the intrinsic mechanisms, for which there has been exciting recent advances in understanding (Bewick & Banks, 2014). Firing is suppressed by negative feedback mechanisms initiated in the afferent terminal by action potentials that open P/Q-type Ca2+ channels. The resulting Ca2+ influx suppresses afferent firing by increasing K+ current through large-conductance Ca2+-activated K+ channels (BK) within the heminode (Kruse & Poppele, 1991; Simon et al. 2008; Bewick & Banks, 2014), and through small-conductance Ca2+-activated K+ channels (SK) located within the afferent terminal (Bewick & Banks, 2014; Shenton et al. 2014). These mechanisms suppress afferent firing on a short time scale, for example, fractions of a second. A separate intrinsic mechanism increases spindle firing and is essential for maintaining IA afferent responsiveness over a time course of minutes to hours. In this autogenic excitatory mechanism, synaptic-like vesicles in IA afferent annulosprinal endings are stimulated by Ca2+ influx to exocytose glutamate, which binds to a phospholipase D-linked metabotropic glutamate receptor to activate second-messenger signaling that enhances afferent firing (Bewick et al. 2005; Bewick & Banks, 2014). These mechanisms come into consideration below where we discuss the effects of chemotherapy on IA afferent firing.
Central functions: conduction and synaptic transmission
Figure 1b outlines what we identify as the central region of a proprioceptor. In this region, we show a labeled IA afferent projecting central axon branches into a spinal cord segment and making synapses with neurons in specific Rexed laminae (Brown & Fyffe, 1981; Alvarez et al. 2011). Synapses shown in laminae V/VI and VII may have been made with interneurons that are known to be integral to the spinal networks that coordinate the activities of multiple motor pools and the muscles they supply, that is if we can extrapolate from observations made in cat (Jankowska & Edgley, 2010). The IA afferent is also shown in Fig. 1b to synapse with an α-motoneuron, provisionally identified by its morphology (soma diameter > 30 lm) and its location in laminae IX (Swett et al. 1986). Consistent with earlier findings (Alvarez et al. 2011), Fig. 1b shows that all synapses made by this IA afferent expressed the VGLUT 1 isoform of vesicular glutamate transporter.
The operation of these central structures is tested by recording excitatory postsynaptic potentials (EPSPs) intracellularly from postsynaptic neurons. An EPSP initiated by quick stretch of a muscle and recorded intracellularly from a homonymous motoneuron is shown in Fig. 2. The stretch-induced production of an EPSP with monosynaptic latency demonstrated that action potentials recorded in the dorsal roots propagated centrally through IA axonal branches to evoke presynaptic transmitter release onto the motoneuron. It is important to findings presented below that in normal rats (Bullinger et al. 2011a), just as in cats (Henneman & Mendell, 1981), every single IA afferent produces an EPSP in almost every homonymous motoneuron supplying ankle extensor muscles. The sizes and shapes of IA afferent EPSPs vary widely within a pool of motoneurons in relation to a number of pre- and postsynaptic properties (Henneman & Mendell, 1981; Burke et al. 1988).
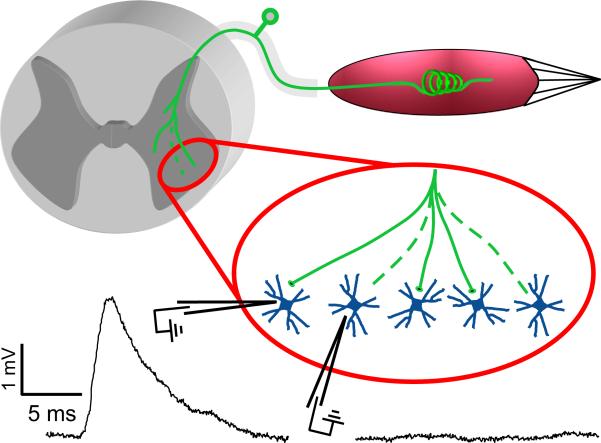
Fig. 2.
Partial disconnection of regenerated IA afferents in the homonymous motor pool. Intracellular records of motoneuron membrane potential during quick stretch (400 m/s; 1 mm) of the homonymous muscle. Representative data collected in terminal study of an anesthetized rat with the muscle re-innervated by its original nerve that was cut and surgically repaired 1 year earlier. EPSPs normally present in all motoneurons were smaller than normal and absent in 2/5 motoneurons.
From these brief descriptions of normal central and peripheral structures and function, we present evidence for abnormality found after two conditions that injure or impair the peripheral axons of IA afferents. For both cases, we suggest that limitation to the recovery of proprioceptive function involves multiple defects in proprioceptors.
Proprioceptor impairment following nerve injury and repair
Peripheral nerves are vulnerable to traumatic injuries that can sever sensory axons and interrupt conduction of sensory signals to the CNS. Although recovery of sensory signaling is made possible by nerve regeneration, difficulty with movement can persist long after nerve repair in many patients (Navarro, 2009; Brushart, 2011); movements that rely on proprioceptors exhibit abnormality, including limb ataxia during walking and permanent loss of stretch reflex contraction (Barker & Young, 1947) (Cope et al. 1994; Abelew et al. 2000; Huyghues-Despointes et al. 2003; Haftel et al. 2005; Maas et al. 2007). The search for explanations for the limited success of nerve regeneration in achieving sensorimotor recovery after nerve injury has motivated extensive experimental examination of peripherally regenerated proprioceptors (Zelena, 1994; Navarro, 2009; Brushart, 2011).
Investigation of regenerated proprioceptors has mostly concentrated on the periphery. It has been shown or inferred that some proprioceptors do not regenerate beyond the injury site and that some may lose sensitivity to muscle stretch (Brown & Butler, 1976; Gregory et al. 1982; Collins et al. 1986; Banks & Barker, 1989). These problems certainly reduce the sensory signal for muscle stretch. A further problem for recovery of muscle stretch signaling by IA afferents is the often emphasized possibility that about half of them mistakenly re-innervate inappropriate targets rather than muscle spindles (Brown & Butler, 1976; Collins et al. 1986; Banks & Barker, 1989). We suggest, however, that the failure of IA afferents to re-innervate muscle spindles may not prevent them from firing in response to muscle stretch. It seems reasonable that IA afferents that erroneously re-innervate tendon organ receptors might generate robust firing in response to muscle stretch as do the IB afferents that normally supply these receptors (Jami, 1992; Bullinger et al. 2011b). We may also expect IA afferents to respond to stretch even when they end as free terminals among extrafusal muscle fibers, just as they do when forced to re-innervate skin (Johnson et al. 1995; Proske et al. 1995). Responsiveness of these misconnected IA afferents to muscle stretch in the absence of gamma motor drive would not be surprising, given that normal IA afferents produce robust stretch-evoked firing when gamma motor drive is eliminated by ventral root section (Matthews, 1972). Thus, stretch-activated firing, even if abnormal from those IA afferents that do not re-innervate spindles (about 60%) seems likely to occur and may be substantial. When added to the signal generated by the remaining 40% of IA afferents that are estimated to succeed in re-innervating spindles (Collins et al. 1986) and that encode stretch similar to normal (Banks & Barker, 1989; Haftel et al. 2005), the stretch signal that the population of regenerated IA afferents delivers to the CNS may be a considerable fraction of normal. Why then is stretch incapable of eliciting reflex contraction or even modulating ongoing contraction of a reinnervated muscle that is otherwise competent to contract? We reasoned that the answer must involve central deficits that we describe next.
We tested the contribution of deficits in central IA afferent processes (Fig. 1b) to lost stretch reflexes by studying adult rats in vivo 1 year after one muscle nerve was severed and immediately repaired by surgical reunion (Haftel et al. 2005; Bullinger et al. 2011a). In terminal experiments we found that the muscle was successfully re-innervated and its motor units were competent to produce reflex contraction evoked by cutaneous stimulation. By contrast, the re-innervated muscle produced only feeble contraction when stretched. The explanation for the inability of the incoming IA afferent stretch signal to evoke a reflex was obtained by recording the synaptic response of motoneurons to muscle stretch. Figure 2 illustrates our finding that monosynaptic excitation produced by quick stretch was present, though weaker than normal in 2/3 motoneurons and completely absent in 1/3 motoneurons. The set of motoneurons that failed to generate EPSPs expressed the full range of postsynaptic electrical properties, for example, input resistance, and were not by these measures restricted to ones that normally produce the smallest EPSPs (Henneman & Mendell, 1981; Burke et al. 1988; Rekling et al. 2000) Recalling that every IA afferent should produce an EPSP in every homonymous motoneuron, these results gave strong evidence for a central IA afferent deficit: if successfully regenerated IA afferents were capable of producing stretch-evoked monosynaptic EPSPs in some motoneurons, then they should have produced EPSPs in all motoneurons, unless they lost functional connections with some motoneurons in the spinal cord. Morphological studies performed in collaboration with the laboratory of Dr Francisco Alvarez show that lost transmission corresponded with permanent physical disconnection and de-clustering of a majority of IA afferent synapses with motoneurons, together with retraction of laminae IX axon projections made by regenerated IA afferents (Alvarez et al. 2011; Rotterman et al. 2014).
These findings lead us to suggest that the nearly complete loss of stretch reflex and other symptoms of proprioceptive dysfunction (ataxia) are caused by deficits in proprioceptors at peripheral (smaller stretch signal) and central (interrupted synaptic transmission) sites illustrated in Fig. 1. We do not rule out the possibility that processes inherent to regenerated motoneurons, for example, postsynaptic amplification, also contributes to weakening IA afferent-motoneuron synaptic effectiveness (Prather et al. 2011; Rotterman et al. 2014). The strong message of our studies is that multiple factors act to limit functional recovery of regenerated IA afferents, including a debilitating contribution from the uninjured central components of IA afferents.
Proprioceptor impairment induced by chemotherapy
Drugs that provide effective chemotherapy for some of the most malignant cancers can be neurotoxic (Dropcho, 2011; Carozzi et al. 2014). Oxaliplatin (OX), for example, has proven highly effective in achieving disease-free survival from colon cancer (Andre et al. 2009); however, its use is associated with distinct acute and chronic neurotoxicities. Acute effects that occur within hours to days following OX infusion are reported by up to 92% of patients who describe symptoms including muscle spasm, paresthesia and dysesthesia including cold hyperalgesia (Argyriou et al. 2008). These symptoms occur with evidence of axonal hyperexcitability (Park et al. 2009). Chronically, OX produces defects that are predominantly sensory owing to its access to and accumulation in dorsal root ganglia, where this platinum-based compound causes DNA damage and cell death or secondary peripheral axonopathy of primary sensory neurons (Boulikas et al. 2007). Chemotherapy-induced peripheral neuropathy (CIPN) with OX has been demonstrated for peripheral axons by clinical electrophysiology [reduction to roughly half normal amplitude of evoked sensory nerve action potentials (SNAPs); Lehky et al. 2004; Park et al. 2011; Bennett et al. 2012] and/or histology (reduced nerve innervation in epidermal biopsies; Koskinen et al. 2011; Sereno et al. 2014). CIPN is the common diagnosis and the dose-limiting side-effect used to explain the movement disability caused by OX, including postural instability, clumsiness, and difficulties with driving and walking, symptoms inferred to reflect deficits in proprioception (Hile et al. 2010; Bennett et al. 2012; Tofthagen et al. 2012). Disability may diminish but persist for months or years after the OX treatment course with little evidence for sensory nerve regeneration (Lehky et al. 2004; Park et al. 2011). All considered, there is strong evidence that degeneration of peripheral sensory axons contributes to the sensory neurotoxicity of OX.
Based on our findings described in the previous section on traumatic nerve damage, we asked whether OX produces additional defects in IA afferents that need to be considered in evaluating proprioceptive dysfunction. This question gains significance from a recent clinical study of OX patients published by neurologists from Johns Hopkins (Burakgazi et al. 2011): “Several of the subjects in this study developed prominent sensory symptoms that interfered with drinking, walking, or dexterity tasks, but they were not associated with axon loss.” This report motivated us to ask, if not by CIPN alone, then in what additional ways does OX impair IA afferents and degrade proprioceptive function? The only other established effect on primary afferent axons is increased axonal excitability, which occurs with acute treatment and progresses chronically in sensory neurons with dose accumulation (Park et al. 2009). In order to determine whether altered axon excitability or other chronic effects of OX interfere with proprioceptor signaling, we studied IA afferents in OX-treated rats.
In our chemotherapy studies, published (Bullinger et al. 2011b) and ongoing, we inject adult female rats with OX once per week for 4 weeks in order to achieve the accumulation of platinum that causes chronic neurotoxicity. During and for weeks after treatment, decreased body weight and blood counts (Bullinger et al. 2011b) verified drug effectiveness, and together with our preliminary behavioral studies (Cope et al. 2013) demonstrated movement difficulties in beam walking without muscle weakness. Five weeks after discontinuing OX in these rats, we obtained electrophysiological measurements in vivo from IA afferents.
Figure 3 illustrates salient findings for chronic OX effects in rats (Bullinger et al. 2011b). For several measures of IA afferent function, we found little or no OX effect. IA afferents fired as they do normally in response to dynamic muscle stretch. This simple observation gives strong evidence that IA afferents are not disconnected from spindle receptors. We verified this conclusion by showing that virtually all spindles that we examined histologically had annulospiral endings with grossly normal appearance (Fig. 3d). Therefore, our current experimental model and dosage does not seem to produce CIPN, the absence of which is further supported by the superimposability of tail SNAPs (Fig. 3d) measured before, during (not shown) and after OX treatment. For these rats, 5 weeks after OX treatment, we observed no evidence of spontaneous or abnormal IA afferent firing before or during muscle stretch (Fig. 3). We have observed, however, that within 1 h of acute OX injection, IA afferents fire erratically consistent with axon hyperexcitability. This effect has the potential to disrupt IA afferent signaling, although the signal for ramp-hold-release stretch remained recognizable. Returning to our chronic study, the most robust and repeatable change was abbreviation of static phase firing when the muscle was stretched and held at fixed length (Fig. 3a). Although we only consider IA afferents here, OX produced a similar abbreviation of static phase repetitive firing in group II and IB afferents (Bullinger et al. 2011b).
The selective effect of OX on IA afferent firing during static as opposed to dynamic stretch provided a clue about the mechanism of impairment. The finding also provided further evidence of differences between the mechanisms underlying dynamic vs. static firing (Kruse & Poppele, 1991). We reasoned that if mechanisms of dynamic firing are independent of those underlying static firing, and if they are unaffected by OX, then dynamic stretch (vibration) should produce IA afferent firing, where it was previously absent. Figure 4 and findings reported by Bullinger et al. (2011b) support this expectation. These results demonstrate that OX does not impair the normal capacity of IA afferents to generate action potentials and conduct them centrally, even following high-frequency repetitive firing evoked during ramp stretch. Thus, we concluded that OX must produce some deficit, peripheral to the recording electrode (Fig. 1), not in action potential conduction, but rather in mechanotransduction or sensory-encoding mechanisms underlying static firing.
Pharmacological study of static repetitive firing mechanisms of IA proprioceptors
We approached identification of candidate mechanisms for OX abbreviation of static phase firing by attempting to reproduce this effect with drugs having known actions. We began by speculating that repetitive firing during sustained muscle stretch might be supported by persistent inward currents (PIC) and, therefore, that drugs that block PIC might reproduce in control rats the observations we made in chronic-OX rats. Based on this reasoning, we tested effects of antiepileptic drugs whose actions include PIC blockade. Here, we report our opening findings on the acute effects of riluzole and phenytoin on IA firing measured in vivo from normal rats.
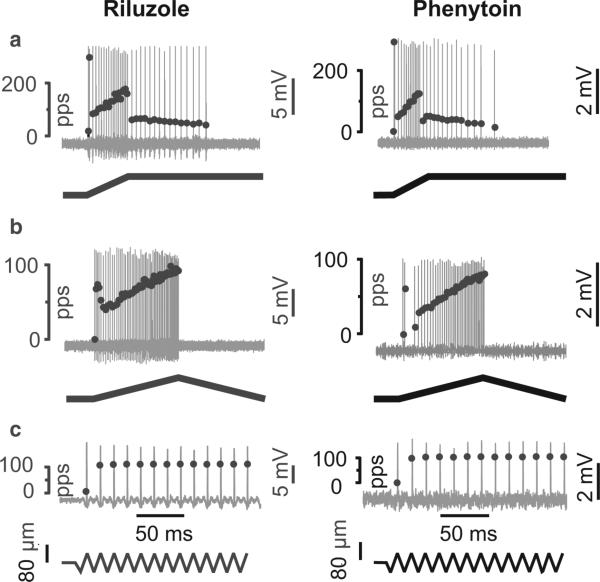
Figure 5 shows cases in which both riluzole and phenytoin qualitatively mimicked the effects of OX on muscle-stretch-evoked firing of IA afferents. Specifically, static phase firing was abbreviated while dynamic firing was unaffected. Just as with OX (Fig. 4c), this effect was not attributable to propagation of action potentials centrally by IA axons that remained competent to support prolonged repetitive firing induced by vibration (data not shown). Note that this effect was not mediated by gamma-motoneurons, as ventral roots were cut in these experiments. These preliminary findings are consistent with our novel notions, first that static phase firing is supported by Na+ PIC and second that Na+ PIC function is impaired by OX. However, alternate or additional mechanisms must be considered because of the non-specificity and multiple actions of these antiepileptic drugs.
Fig. 5.
Antiepileptic drugs abbreviate static phase firing. Similar to oxaliplatin (Fig 3), afferent responses following acute injection of riluzole and phenytoin fail to maintain repetitive firing during the static phase of muscle stretch (a). Otherwise, IA afferents exhibited normal firing during the dynamic phase of ramp (a) and triangular (b) stretch. Also normal was initial burst firing at onset of ramp (a) and triangular (b) stretch and 1 : 1 firing entrainment to 100 Hz muscle vibration (c).
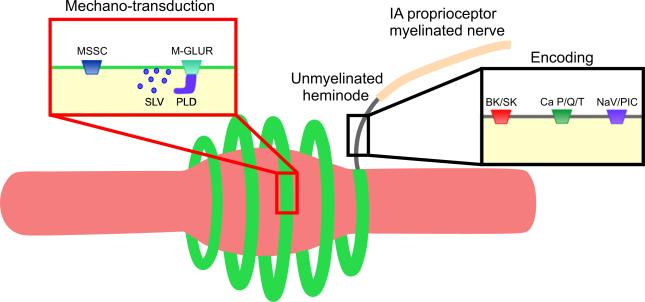
Our examination of mechanisms by which antiepileptics might alter IA afferent firing is fittingly assisted by the model of IA afferent mechanosensory functions recently put forward by Bewick & Banks (2014). Figure 6 is adapted from their model to assist discussion about which of the multiple drug actions of riluzole (Bellingham, 2011) are capable of explaining abbreviated static firing by IA afferents (Fig. 5). We begin by considering riluzole effects on the three mechanisms involved in encoding shown in Fig. 6 and described above in the section entitled, ‘Peripheral functions: transduction, encoding, conduction’. First, in consideration of P/Q-type Ca2+ channels, we discard the possibility that riluzole suppresses static firing by blocking these channels (Huang et al. 1997). It is recently reported that blocking P/Q-type channels with Zn2+ or ω-agatoxin actually increases IA afferent firing (Kruse & Poppele, 1991; Simon et al. 2008), perhaps because with reduced Ca2+ influx, there is less activation of Ca2+-activated outward current through BK/SK channels. Second, activation of BK/SK channels should increase outward current and reduce IA afferent firing (Kruse & Poppele, 1991; Simon et al. 2008). Indeed, through its direct effect on BK/SK channels (Grunnet et al. 2001; Wang et al. 2008), riluzole has the capacity to activate outward current and reduce IA afferent firing. However, this mechanism seems less likely given that it would reduce firing of not only static but also dynamic firing for which we observe no change. Third, we consider the participation of specialized voltage-gated Na+ channels, specifically PIC, which were the focus of our hypothesis for explaining abbreviated IA afferent firing (see above). We selected riluzole for its ability to block Na+ PIC (Urbani & Belluzzi, 2000; Schuster et al. 2012), and its effect in reducing static firing supports our hypothesis. We provide additional support by showing static firing is reduced by phenytoin, which like riluzole blocks Na+ Pic (Zeng et al. 2005), but unlike riluzole does not activate BK/SK (Rogawski & Loscher, 2004).
Fig. 6.
Mechanosensory model of IA proprioceptor adapted from Bewick & Banks (2014). Neural mechanisms in mechano-transduction and sensory encoding for IA afferent fitted to muscle spindle structure including intrafusal muscle fiber (pink) wrapped by annulospiral ending (green) that extends unmyelinated followed by myelinated axon. Mechano-transduction involves mechano-sensitive sodium channels (MSSC) and metabotropic glutamate receptors (M-GLUR) that act through a phospholipase D (PLD) mechanism to modulate glutamate release from synaptic like vesicles (SLV). Sensory-encoding mechanisms include BK and SK potassium channels activated by Ca2+; P/Q- and T-type Ca2+ channels; and voltage-gated Na+ channels (NaV) including Na+ persistent inward current (PIC), which we add to the model based on preliminary findings presented in this report.
IA afferent firing can be modulated not only through encoding processes but also through the mechanotransduction mechanisms that generate receptor potentials. The work of Bewick and Banks gives evidence for a glutamate-dependent autogenic excitatory mechanism (Fig. 6), which increases IA afferent firing (Bewick et al. 2005; Bewick & Banks, 2014). It is reasonable then to speculate that riluzole reduces static firing by impeding glutamate signaling within the IA afferent terminal. Our interest in this possibility is reduced by the knowledge that riluzole modulates glutamate signaling through a phosphokinase C (Noh et al. 2000; Lamanauskas & Nistri, 2008), but does not affect phospholipase D, which is thought to be responsible for glutamate signaling in the muscle spindle (Bewick et al. 2005). Furthermore, blockade of glutamate signaling in IA afferent terminals reduces not only static but also dynamic firing and can abolish firing completely (Bewick et al. 2005; Bewick & Banks, 2014). This result contrasts our findings for selective abbreviation of static firing, suggesting that reduced glutamate signaling is not responsible for our results.
These preliminary findings hint at a novel role for PIC in encoding static muscle length. We tentatively propose that the abbreviation of static firing with riluzole and phenytoin results from their shared action in blocking Na+ PIC. We also propose that OX neurotoxicity targets Na+ PIC in IA afferent nerve terminals. The mechanosensory model put forward by Bewick and Banks is capable of accommodating PIC channels in the category of voltage-gated Na+ channels (Fig. 6).
Concluding remarks
Through our studies of injured proprioceptors, we have gained strong appreciation for the need to identify the complete set of functional deficits. By focusing attention on one deficit, we may fail to develop effective treatments for disability. Absent knowledge of their synaptic disconnection in the spinal cord, it seems unlikely that eventual success in guiding severed proprioceptors back to their original muscle targets will prove sufficient for restoring proprioceptive functions to normal after peripheral nerve injury. By focusing on one deficit, we may overlook problems that are easier to solve and that might provide at least partial recovery. It may prove easier and faster, for example, to restore sensory encoding to those proprioceptors that do not undergo CIPN than it is to promote regrowth of proprioceptors that suffer CIPN. Another value of injury studies is their ability to elucidate as yet unknown normal processes. We were fortunate to find that OX neurotoxicity selectively targets a specific function in sensory encoding, thereby suggesting the existence of a novel mechanism underlying normal operation of proprioceptors.
Acknowledgements
The authors are grateful to Lori Goss for technical assistance. This work was supported by National Institute of Neurological Disorders and Stroke Grant P01-NS-057228.
References
- Abelew TA, Miller MD, Cope TC, et al. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, et al. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol. 2011;106:2450–2470. doi: 10.1152/jn.01095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Polychronopoulos P, Iconomou G, et al. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008;34:368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Banks RW, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol. 1989;408:345–372. doi: 10.1113/jphysiol.1989.sp017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D, Young JZ. Recovery of stretch reflexes after nerve injury. Lancet. 1947;1:704–707. doi: 10.1016/s0140-6736(47)91454-2. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BK, Park SB, Lin CS, et al. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer. 2012;20:2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- Bewick GS, Banks RW. Mechanotransduction in the muscle spindle. Pflugers Arch. 2014;467:175–190. doi: 10.1007/s00424-014-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Reid B, Richardson C, et al. Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol. 2005;562:381–394. doi: 10.1113/jphysiol.2004.074799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T, Pantos A, Bellis E, et al. Designing platinum compounds in cancer: structures and mechanisms. Cancer Ther. 2007;5:537–583. [Google Scholar]
- Brown MC, Butler RG. Regeneration of afferent and efferent fibres to muscle spindles after nerve injury in adult cats. J Physiol. 1976;260:253–266. doi: 10.1113/jphysiol.1976.sp011514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe REW. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. Oxford University Press; New York: 2011. [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, et al. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol. 2011a;106:2471–2485. doi: 10.1152/jn.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Wang Q, et al. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. J Neurophysiol. 2011b;106:704–709. doi: 10.1152/jn.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakgazi AZ, Messersmith W, Vaidya D, et al. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77:980–986. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Fleshman JW, Segev I. Factors that control the efficacy of group Ia synapses in alpha-motoneurons. J Physiol. 1988;83:133–140. [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Chiorazzi A, et al. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2014 doi: 10.1016/j.neulet.2014.10.014. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Collins IWF, Mendell LM, Munson JB. On the specificity of sensory reinnervation of cat skeletal muscle. J Physiol (Lond) 1986;375:587–609. doi: 10.1113/jphysiol.1986.sp016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol. 1994;71:817–820. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Cope TC, Vincent JA, Wieczerzak K, et al. Channelopathy contributes to proprioceptive deficits following chemotherapy. Society for Neuroscience; San Diego: 2013. p. 2013. [Google Scholar]
- De-Doncker L, Picquet F, Petit J, et al. Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J Neurophysiol. 2003;89:442–449. doi: 10.1152/jn.00153.2002. [DOI] [PubMed] [Google Scholar]
- Dropcho EJ. The neurologic side effects of chemotherapeutic agents. Continuum (Minneap Minn) 2011;17:95–112. doi: 10.1212/01.CON.0000394676.67372.87. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Luff AR, Proske U. Muscle receptors in the cross-reinnervated soleus muscle of the cat. J Physiol. 1982;331:367–383. doi: 10.1113/jphysiol.1982.sp014377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, et al. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, et al. Central suppression of regenerated proprioceptive afferents. J Neurosci. 2005;25:4733–4742. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motneuron pool and its inputs. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology . Waverly Press; Bethesda, MD: 1981. pp. 423–508. [Google Scholar]
- Hile ES, Fitzgerald GK, Studenski SA. Persistent mobility disability after neurotoxic chemotherapy. Phys Ther. 2010;90:1649–1657. doi: 10.2522/ptj.20090405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Song JH, Nagata K, et al. Effects of the neuro-protective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;282:1280–1290. [PubMed] [Google Scholar]
- Hunt CC, Ottoson D. Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. J Physiol. 1975;252:259–281. doi: 10.1113/jphysiol.1975.sp011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol. 1978;71:683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of movement history. J Neurophysiol. 2003;90:1547–1555. doi: 10.1152/jn.00719.2002. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992;72:623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci. 2010;32:881–893. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RD, Taylor JS, Mendell LM, et al. Rescue of moto-neuron and muscle afferent function in cats by regeneration into skin I. Properties of afferents. J Neurophysiol. 1995;73:651–661. doi: 10.1152/jn.1995.73.2.651. [DOI] [PubMed] [Google Scholar]
- Koskinen MJ, Kautio AL, Haanpaa ML, et al. Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 2011;31:4413–4416. [PubMed] [Google Scholar]
- Kruse MN, Poppele RE. Components of the dynamic response of mammalian muscle spindles that originate in the sensory terminals. Exp Brain Res. 1991;86:359–366. doi: 10.1007/BF00228959. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via pre-synaptic NMDA receptors on neonatal rat hypoglossal moto-neurons in vitro. Eur J Neurosci. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Lehky TJ, Leonard GD, Wilson RH, et al. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, et al. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res. 2007;181:377–393. doi: 10.1007/s00221-007-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. The Williams and Wilkins Company; Baltimore: 1972. pp. 1–59. [Google Scholar]
- McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34:148–159. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Navarro X. Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. doi: 10.1016/S0074-7742(09)87027-X. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev. 1999;27:255–284. [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, et al. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Park SB, Goldstein D, Lin CS, et al. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. 2009;27:1243–1249. doi: 10.1200/JCO.2008.19.3425. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, et al. Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. Oncologist. 2011;16:708–716. doi: 10.1634/theoncologist.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Nardelli P, Nakanishi ST, et al. Recovery of proprioceptive feedback from nerve crush. J Physiol. 2011;589:4935–4947. doi: 10.1113/jphysiol.2011.210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve. 2006;34:545–558. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Proske U, Gregory JE. The time-course of recovery of the initial burst of primary endings of muscle spindles. Brain Res. 1977;121:358–361. doi: 10.1016/0006-8993(77)90159-7. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle?[see comment]. J Muscle Res Cell Motil. 1999;20:433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- Proske U, Iggo A, Luff AR. Mechanical sensitivity of regenerating myelinated skin and muscle afferents in the cat. Exp Brain Res. 1995;104:89–98. doi: 10.1007/BF00229858. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, et al. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, et al. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci. 2014;34:3475–3492. doi: 10.1523/JNEUROSCI.4768-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster JE, Fu R, Siddique T, et al. Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS. J Neurophysiol. 2012;107:484–492. doi: 10.1152/jn.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno M, Gutierrez-Gutierrez G, Gomez-Raposo C, et al. Oxaliplatin induced-neuropathy in digestive tumors. Crit Rev Oncol Hematol. 2014;89:166–178. doi: 10.1016/j.critrevonc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol. 2010;121:1680–1689. doi: 10.1016/j.clinph.2010.02.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton F, Bewick GS, Banks RW. A study of the expression of small conductance calcium-activated potassium channels (SK1-3) in sensory endings of muscle spindles and lanceolate endings of hair follicles in the rat. PLoS One. 2014;9:e107073. doi: 10.1371/journal.pone.0107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Banks RW, Bewick GS. KCa channels regulate stretch-evoked afferent firing from muscle spindle. Proc Physiol Soc. 2008;15(C):45. [Google Scholar]
- Simon A, Shenton F, Hunter I, et al. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol. 2010;588:171–185. doi: 10.1113/jphysiol.2009.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, Wikholm RP, Blanks RHI, et al. Motoneurons of the rat sciatic nerve. Exp Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, et al. Static and dynamic gamma-motor output to ankle flexor muscles during locomotion in the decerebrate cat. J Physiol. 2006;571:711–723. doi: 10.1113/jphysiol.2005.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Lin MW, Lin AA, et al. Riluzole-induced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life Sci. 2008;82:11–20. doi: 10.1016/j.lfs.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. Chapman and Hall; London: 1994. [Google Scholar]
- Zeng J, Powers RK, Newkirk G, et al. Contribution of persistent sodium currents to spike-frequency adaptation in rat hypoglossal motoneurons. J Neurophysiol. 2005;93:1035–1041. doi: 10.1152/jn.00831.2004. [DOI] [PubMed] [Google Scholar]