Abstract
Objectives
Evidence suggests that non-conventional programming may improve deep brain stimulation (DBS) therapy for movement disorders. The primary objective was to assess feasibility of testing the tolerability of several non-conventional settings in Parkinson’s disease (PD) and essential tremor (ET) subjects in a single office visit. Secondary objectives were to explore for potential efficacy signals and to assess the energy demand on the implantable pulse-generators (IPG).
Materials and Methods
A custom firmware (FW) application was developed and acutely uploaded to the IPGs of 8 PD and 3 ET subjects, allowing delivery of several non-conventional DBS settings, including narrow pulse widths, square biphasic pulses and irregular pulse patterns. Standard clinical rating scales and several objective measures were used to compare motor outcomes with sham, clinically-optimal and non-conventional settings. Blinded and randomized testing was conducted in a traditional office setting.
Results
Overall, the non-conventional settings were well tolerated. Under these conditions it was also possible to detect clinically-relevant differences in DBS responses using clinical rating scales but not objective measures. Compared to the clinically-optimal settings, some non-conventional settings appeared to offer similar benefit (e.g. narrow pulse widths) and others lesser benefit. Moreover, the results suggest that square biphasic pulses may deliver greater benefit. No unexpected IPG efficiency disadvantages were associated with delivering non-conventional settings.
Conclusions
It is feasible to acutely screen non-conventional DBS settings using controlled study designs in traditional office settings. Simple IPG FW upgrades may provide more DBS programming options for optimizing therapy. Potential advantages of narrow and biphasic pulses deserve follow up.
Keywords: Deep brain stimulation, Parkinson’s disease, essential tremor, narrow pulse width, biphasic pulses, irregular patterns
Introduction
Deep brain stimulation (DBS) can be an effective surgical therapy for select patients with medication-refractory symptoms of movement disorders, including Parkinson’s disease (PD) [1], essential tremor (ET) [2], and dystonia [3]. FDA-approved DBS therapies for movement disorders involve implantation of electrodes into the ventral intermedius nucleus (VIM) for medication refractory ET, and either the globus pallidus internus (GPi) or the subthalamic nucleus (STN) for PD, depending on the patient’s disabling features and the results of an interdisciplinary evaluation. The DBS lead is connected to an implantable pulse-generator (IPG), which conventionally delivers charge-balanced, square, cathodic pulses with specific amplitudes and durations at continuous frequencies. The optimization of IPG programming for DBS is most often conducted in the in-office setting at regular intervals.
DBS can be programmed to address specific patient symptoms, including tremor and rigidity. DBS can also be adjusted according to the specific medication side effects such as dyskinesia. DBS therapy for movement disorders is typically delivered at high frequencies (>100 Hz) [4, 5], though DBS at <100Hz may also be beneficial in a subset of PD and dystonia patients [6–10]. Pulse widths for PD and ET therapies typically range from 60–120 μs [2, 4, 11], whereas those for dystonia tend to be longer (120–>200 μs) [12–15]. Therapy customization may also involve changes to the location and shape of the stimulation field along the DBS lead [16, 17]. However, in some cases the therapeutic window of DBS may be unacceptably narrow possibly due to suboptimal lead placement or other unidentified factors[18–21]. In these cases there remains a critical need for additional approaches for optimizing the therapy.
The high energy consumption of neurostimulation therapies such as DBS is also a challenge. Even with clinically-optimal DBS, IPGs with primary cell batteries require replacement approximately every 2–5 years, depending on the stimulation parameters. Not surprisingly, higher DBS pulse amplitudes, longer pulse widths and higher frequencies drain the battery more rapidly. Use of multiple DBS contact cathodes to shape the stimulation field also results in greater battery drain. Although IPGs with rechargeable batteries are currently available, replacement may still be required approximately every 7–9 years. In addition, increased battery consumption carries the distinct disadvantage of shortened recharge intervals with rechargeable IPGs. Therefore, there is also an obvious interest in developing more energy-efficient DBS therapy delivery modalities. There is emerging evidence that some non-conventional approaches to DBS programming may improve both therapy efficacy and efficiency. For example, several studies suggest that more narrow DBS pulse widths than conventionally used may both reduce energy consumption and widen the therapeutic window [4, 5, 11]. In fact, a recent pilot study found that DBS pulse widths even more narrow than commercially available (20–40 μs) may offer further improvements [22]. Findings from several preclinical and clinical studies also suggest that some irregular DBS pulse patterns may improve motor symptoms more effectively and efficiently [23–29]. Furthermore, a number of computer modeling studies exploring alternative DBS pulse shapes suggest similar advantages [30–32].
Although the overall implication is that standard DBS can be optimized with non-conventional programming, more rigorous evaluations in chronically-implanted subjects under real-world conditions are needed. In general, there is a critical need for more well-designed exploratory and feasibility studies of potential central nervous system (CNS) therapeutics, as their underutilization is likely a key contributor to the frequent failure of later-phase and pivotal trials [33]. Designing well-controlled exploratory and feasibility investigations of DBS therapy for movement disorders is especially challenging, but may be accomplished with creative and novel approaches [34, 35]. Therefore, in the current study we assessed whether it was feasible to conduct controlled-testing of several non-conventional DBS settings in chronically-implanted PD and ET patients in a traditional in-office setting.
Materials and Methods
Study Design and Subjects
Others have successfully implemented blinded, randomized study designs for assessing the clinical effects of non-conventional DBS concepts in small to moderate samples (n <10–20) of movement disorder patients[24–26, 29, 35]. The primary objective of this study was to determine if a similar approach could be taken for testing the tolerability several non-conventional DBS settings in PD (n=8) and ET (n=3) patients over 2–3 hours in a single office visit. Tolerability was defined as lack of intolerable side effects that would lead to discontinuation of the non-conventional stimulation-settings. To accommodate the study schedule, approximate 2 minute DBS wash-in and wash-out periods were used; similar periods have been demonstrated to be appropriate in prior studies [24, 26–32, 36] conducted in chronically-implanted patients. All subjects were blinded to the stimulation settings and to whether stimulation was on or off, though they were made aware that the settings had changed. All settings were tested in a pseudorandom order in the same environment as where traditional IPG programming occurs. Motor assessments were videotaped, and independent ratings were collected by three raters: one un-blinded rater, one blinded rater present during testing, and one blinded rater via video recordings (with the exception of PD rigidity, which cannot be assessed via video). Subjects were instructed to report any side effects during the course of the study, including pulling (contractions), tingling, blurry vision, double vision, speech changes, or walking problems. All subjects were assessed in the off-medication state, as all anti-tremor and dopaminergic medications were withheld for at least 12 hours. All procedures were reviewed and approved by the University of Florida Internal Review Board.
Subjects were recruited during routine DBS programming sessions at the University of Florida Health Center for Movement Disorders and Neurorestoration. Since our study was designed to test these non-conventional settings acutely, we included disorders in which acute changes with stimulation alterations are more apparent (e.g. PD and ET vs. dystonia). Inclusion criteria were: 1) PD or ET diagnosis by a fellowship-trained movement disorder neurologist using strict criteria [37], 2) implanted with DBS, and 3) optimized on DBS settings with a minimum of four monthly clinical programming sessions. Exclusion criteria were: 1) diagnosis of another neurodegenerative disorder, 2) multiple DBS surgeries performed due to infection, revision or other complication, 3) DBS settings not optimized, 4) fewer than four DBS outpatient programming, and 5) sub-optimally placed DBS lead as revealed by post-operative imaging. For subjects with bilateral DBS implants, DBS was turn off of the side not being tested for the duration of the study.
Non-Conventional DBS Settings
The non-conventional DBS settings were delivered using a custom temporary downloadable firmware (FW) that was developed to be compatible with Medtronic Activa PC, SC and RC IPGs (Medtronic, Neuromodulation, Minneapolis, MN); 12–13) and controlled by a trained neurologist using a Microsoft Windows-based user interface running on a standard PC laptop that was connected to a telemetry head (Medtronic, Neuromodulation). All stimulation settings included charge-balanced pulses at or below standard clinical amplitudes and within all FDA safety guidelines (30 μC/cm2/phase). The research system allowed stimulation with non-conventional settings to be immediately stopped at the request of the subject in case of discomfort or at any time deemed appropriate by the attending neurologist. The temporary FW was removed from all subjects’ IPGs at the conclusion of the study, and all patients were returned to their clinically-optimal therapeutic settings.
Subjects were tested under 11 total DBS settings (Table 1). To control for fatigue and order effects of the different settings, subjects were evaluated under their clinically-optimal settings (ClinDBS) and with stimulation turned off (DBS-off) both at the beginning and then end of the study (i.e. two assessments for each). Due to technical limitations of the research system, the optimal stimulation frequency sometimes required to be adjusted slightly but remained constant throughout the study (e.g. 185 Hz was changed to 190 Hz; see Results). Whereas standard DBS pulses were charge balanced with a passive recharge (Figure 1A, left), non-conventional charge-balanced biphasic pulses with a square-wave active recharge were tested at the clinically-optimal voltage (BiphClinV; Figure 1A, right). Using the research platform, the IPG was capable of delivering pulse widths as low as 10 μs (Figure 1B), and it was used to deliver pulse widths 50% shorter than clinically-optimal at the clinically-optimal voltage (50%PWClinV). Irregular patterns of stimulation consisting of the same average stimulation frequency as the clinically-optimal settings but with an overall 20% coefficient of variance were tested at the clinically-optimal voltage (20%CVClinV; Figure 1C). Because one objective of the study was to explore the feasibility of assessing the therapeutic efficiency of different DBS settings, the biphasic pulses and the irregular patterns were also tested at an arbitrarily reduced voltage (Biph70%V and 20%CV70%V). To control for the effect of low voltage, the clinically-optimal frequency and pulse width was also tested at the reduced voltage (70%ClinV). On the other hand, the 50% shorter pulse widths were also tested at an increased voltage (50%PW150%V) but with a similar energy consumption profile overall (see Figure 3).
Table 1.
The protocol was for each subject to experience a total of 11 DBS settings. Clinically-optimal settings and off-stimulation were both tested at the beginning and end of the study. All other settings were tested in random order. Subjects were blinded to all settings and were only aware of a change.
| Testing Order | DBS Parameters Tested | Assessments Performed? |
|---|---|---|
| 1 | Clinically-optimal Settings (ClinDBS) | Yes |
| 2 | Stimulation turned off (DBS-off) | Yes |
| Random | Biphasic Pulses (BiphClinV) | Yes |
| – | Stimulation turned off (Washout) | No |
| Random | Biphasic Pulses at 70% Amplitude (Biph70%V) | Yes |
| – | Stimulation turned off (Washout) | No |
| Random | Irregular Patterns (20%CVClinV) | Yes |
| – | Stimulation turned off (Washout) | No |
| Random | Irregular Patterns at 70% Amplitude (20%CV70%V) | Yes |
| – | Stimulation turned off (Washout) | No |
| Random | 50% Shorter Pulse Widths (50%PWClinV) | Yes |
| – | Stimulation turned off (Washout) | No |
| Random | 50% Shorter Pulse Widths at 150% Amplitude (50%PW150%V) | Yes |
| 10 | Stimulation turned off (DBS-off) | Yes |
| Random | Clinically-optimal Settings at 70% Amplitude (70%ClinV) | Yes |
| 11 | Clinically-optimal Settings (ClinDBS) | Yes |
Figure 1.
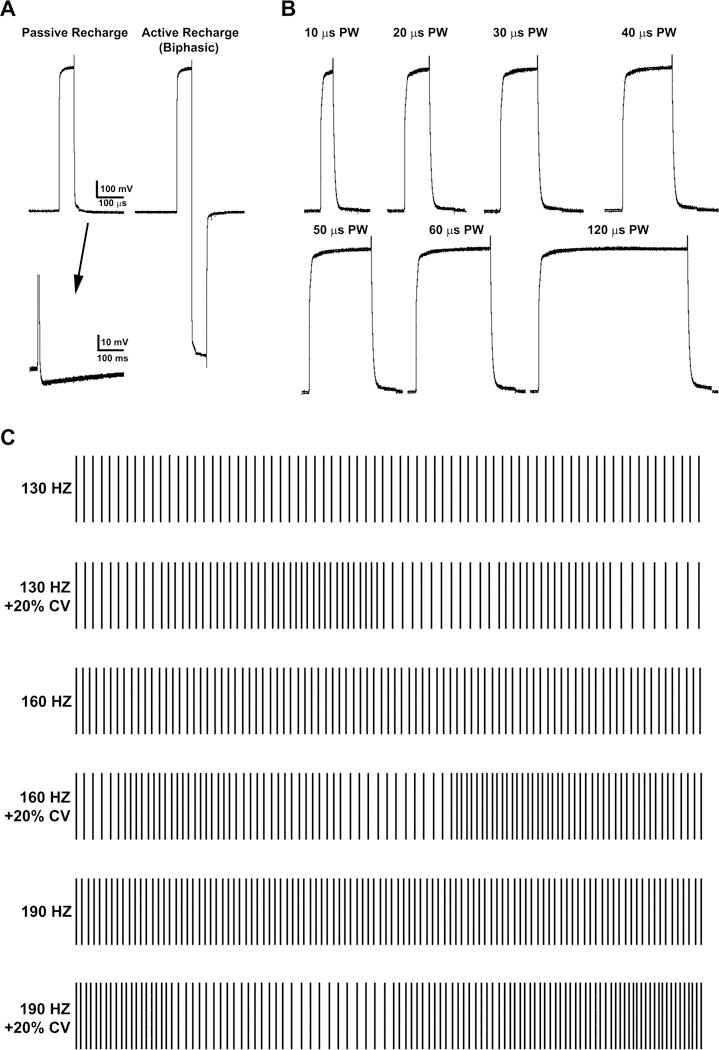
The research programmer system was used to deliver biphasic pulses, which were charge balanced with a square-wave active recharge (A). The research system was capable of delivering pulse widths as low as 10 μs (B), and was used to deliver pulse widths 50% shorter than the clinically-optimal settings. The research system was also used to deliver irregular patterns of stimulation (C), which were the same average stimulation frequency as the clinically-optimal settings but exhibited an overall 20% coefficient of variance (CV).
Figure 3.
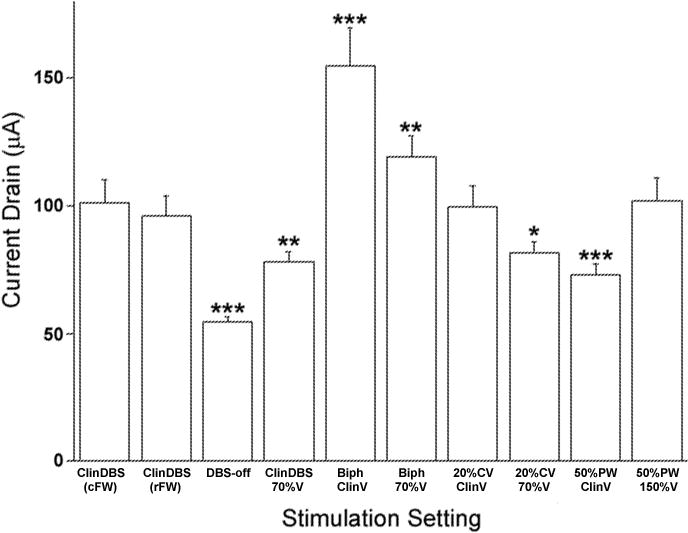
The estimated amount of battery drain associated with each DBS setting. Data are expressed as the average ± SEM current drain across all 11 PD and ET subjects participating in the study for the entire series of settings tested, including the clinically-optimal DBS settings (ClinDBS) delivered using the commercial IPG FW (cFW) and also with the research FW (rFW). Data were analyzed using a paired Students t test, comparing the current drain with clinically-optimal DBS settings delivered using the cFW to the current drain with each non-conventional setting. *p < 0.05, **p < 0.01, ***p < 0.001.
Motor Assessments
A secondary objective of the study was to explore for explore for potential efficacy signals associated with the non-conventional settings. To do so, part III of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) was used for evaluating the PD motor symptoms and the Fahn-Tolosa-Marin Tremor Rating Scale (TRS) was used for evaluating ET motor symptoms contralateral to the site of stimulation. An accelerometer was also used for scoring rest tremor, postural tremor and action tremor during finger tapping on a scale of 0–4 corresponding to tremor severity using algorithms previously validated by the manufacturer (Kinesia, Great Lakes NeuroTechnologies, Cleveland, OH) [38, 39]. Tremor was measured for approximately 10 seconds at rest, for 10 seconds with arms held outstretched in front of the body. Bradykinesia was then measured over 10 finger taps. Gait was assessed using the Timed-up-and-go (TUG) test [40] and the GaitRite walkway and software suite (GaitRite CIR Systems Inc, Havertown, Pennsylvania) [41, 42].
Energy Consumption Comparisons
Another secondary objective of the study was to assess the energy demand on a commercial IPG of delivering the non-conventional DBS settings. To do so IPG battery current drain associated with each DBS setting was estimated in a bench-top setting using either an Activa SC or an Activa PC IPG, depending on the subject (see case series supplement). The IPG circuit board was exposed and connected to a model E3631A power supply (Agilent, Santa Clara, CA) set to 3 V and a model 2001 digital multimeter set to DC mode (Keithley, Cleveland, OH). The IPG header was connected to a DBS lead (3389, Medtronic Neuromodulation) modified at the distal end with a pair of brass pins connected to electrode channels 2 and 3. For testing bipolar electrode configurations, a model TDS460A oscilloscope (Tektronix, Beaverton, OR) was connected to both pins with wiring. For testing monopolar electrode configurations, the oscilloscope was connected to the pin corresponding to electrode 3 and a ground wire that was clipped to the IPG case. In all cases a 500 Ω resistor load was included. The multimeter was configured to display a moving average of current drain, which was recorded for each DBS setting after approximately 1 minute of testing (Figure 3).
Data Analysis
To explore for an efficacy signal, PD motor outcomes associated with the non-conventional DBS settings were compared to those associated with the ClinDBS condition. The data were expressed in box and whisker plots as the median outcome delta relative to the median value with the ClinDBS, whereby the box represents the inter-quartile range, the whiskers represent the spread, the line represents the median and the dots represent outliers (Figure 2). There was no statistically significant difference between the UPDRS III scores when including ratings from all three neurologists and those with the unblinded neurologist removed (not shown). Therefore, median UPDRS-III scores from individual subjects were calculated from the assessments by the three neurologists, except for subject PD1, who was assessed by only the two on-site neurologists due to video equipment malfunction. The UPDRS-III, TUG, PEG and Gait Rite values with ClinDBS and DBS-off expressed were calculated from the median of the assessments taken at the beginning and at end of testing. Because not all of the PD subjects were tested during each DBS setting, the sample sizes expressed range from n = 5–7. The non-parametric Wilcoxon-Mann-Whitney U-test was used to test for pairwise statistical differences within the PD cohort to avoid assumptions of normality and to compensate for the small and unequal sample sizes. Therefore in cases when DBS settings were not tested because of subject dropout, setting intolerability or equipment malfunction, the analyses included data pairs only from subjects who completed the testing. Individual median PD and ET motor outcome values are presented Table 2 (median outcome values for ET subjects were calculated as described above for PD). Given the small number of patients and variable data size, we did not correct for multiple comparisons. The results of this study should be used to guide the design of future statistically-powered studies.
Figure 2.
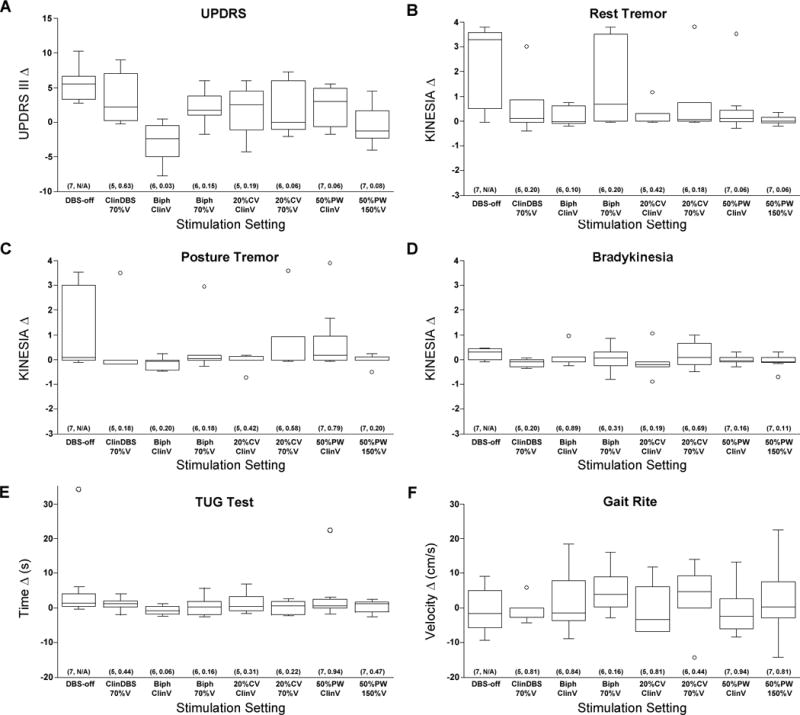
Grouped PD UPDRS-III (A), Kinesia (B–D), TUG (E) and Gait Rite (F) data expressed as the median delta relative to the median value during clinical DBS. The box represents the inter-quartile range, the whiskers represent the spread, the line represents the median and the dots represent outliers. Data were analyzed in pairs using the non-parametric Wilcoxon-Mann-Whitney U-test, comparing the median delta during DBS OFF to the median deltas during the non-conventional DBS settings. Sample sizes ranged from n = 5–7 because not all of the PD subjects were tested during each non-conventional setting. Sample sizes and approximated p values are listed at the bottom of each box (n, p).
Table 2.
Individual subject results. UPDRS-III sub scores, TRS sub scores and Kinesia scores correspond to the limbs contralateral to the DBS brain target. All UPDRS-III and TRS scores are expressed as medians calculated from assessments by the three neurologists, except for subject PD1, which was assessed by only the two on-site neurologists. The median UPDRS scores from clinical DBS and DBS OFF were also calculated from the assessments at the beginning and end of testing. Values are missing from when DBS settings were not tested or from when assessments were not administered because of subject dropout, equipment malfunction or lack of equipment availability.
| Subject | UPDRS-III Scores | Kinesia Scores | PEG Test |
TUG Test |
Gait Rite |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PD1 (L STN, 2− C+, 2.6 V, 110 μs, 190 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0 | 0 | 2.25 | 1.75 | 2.0 | 2.25 | 1.5 | 31.0 | 0.05 | 0 | 2.5 | – | 11.2 | 133.2 |
| DBS OFF | 0.25 | 0 | 0 | 3.0 | 1.75 | 2.0 | 2.75 | 1.5 | 34.25 | 0 | 0 | 2.8 | – | 45.6 | 124.2 |
| Clinical DBS, 70% Amplitude | 0 | 0 | 0 | 3.0 | 2.0 | 2.5 | 3.0 | 1.5 | 38.0 | 0 | 0 | 2.4 | – | 13.3 | 128.9 |
| Biphasic | 0 | 0 | 0 | 2.5 | 1.5 | 2.0 | 2.0 | 1.5 | 30.5 | 0 | 0 | – | – | 10.4 | 141.0 |
| Biphasic, 70 % Amplitude | 0 | 0 | 0 | 2.5 | 1.5 | 2.0 | 2.0 | 1.5 | 32.0 | 0 | 0 | 1.7 | – | 10.0 | 137.0 |
| 20% CV | 0 | 0 | 0 | 3.0 | 1.5 | 2.0 | 2.5 | 2.0 | 35.5 | 0 | 0 | 2.2 | – | 14.7 | 105.6 |
| 20% CV, 70 % Amplitude | 0 | 0 | 0 | 2.5 | 2.0 | 2.5 | 3.0 | 1.5 | 37.0 | 0 | 0 | 2.0 | – | 12.2 | 134.9 |
| 50% PW | 0 | 0 | 0 | 2.5 | 2.5 | 2.5 | 3.0 | 1.5 | 36.5 | 0 | 0 | 2.2 | – | 11.6 | 138.3 |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 2.5 | 1.0 | 2.0 | 2.5 | 1.5 | 33.0 | 0 | 0 | 1.8 | – | 11.3 | 132.9 |
|
PD2 (L STN, 1− C+, 3.0 V, 90 μs, 190 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0 | 0 | 2.5 | 1.5 | 1.5 | 1.75 | 1.0 | 37.09 | 0 | 0 | 0.45 | – | 9.8 | 131.8 |
| DBS OFF | 0 | 0.5 | 0 | 2.75 | 1.75 | 1.5 | 2.0 | 1.0 | 40.5 | 0 | 0 | 0.9 | – | 10.9 | 129.4 |
| Clinical DBS, 70% Amplitude | 0 | 0 | 0 | 3.0 | 2.0 | 1.5 | 2.0 | 1.0 | 46.0 | 0.1 | 0 | 0.1 | – | 10.5 | 129.1 |
| Biphasic | 0 | 0 | 0 | 2.0 | 1.0 | 1.5 | 2.0 | 1.0 | 42.0 | 0 | 0 | 0.2 | – | 10.5 | 128.1 |
| Biphasic, 70 % Amplitude | 0 | 1.0 | 0 | 2.5 | 1.0 | 1.5 | 1.5 | 1.0 | 40.0 | 0 | 0 | 1.3 | – | 10.8 | 135.9 |
| 20% CV | 0 | 0 | 0 | 3.0 | 1.0 | 1.5 | 2.0 | 1.0 | 43.0 | 0 | 0 | 0.5 | – | 11.5 | 125.0 |
| 20% CV, 70 % Amplitude | 0 | 0 | 0 | 2.0 | 2.0 | 1.5 | 2.0 | 1.0 | 39.0 | 0 | 0 | 1.1 | – | 10.3 | 131.8 |
| 50% PW | 0 | 1.0 | 0 | 3.0 | 1.5 | 1.5 | 2.0 | 1.0 | 43.0 | 0.1 | 0 | 0.4 | – | 11.4 | 123.4 |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 2.0 | 2.0 | 1.5 | 1.5 | 1.0 | 43.0 | 0 | 0 | 0.3 | – | 11.1 | 126.4 |
|
PD3 (L STN, 2− C+, 2.5 V, 90 μs, 160 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 1.0 | 1.0 | 0 | 2.0 | 2.0 | 1.0 | 1.5 | 1.0 | 42.5 | 0.1 | 1.0 | 1.5 | – | 8.5 | 137.8 |
| DBS OFF | 3.0 | 2.0 | 1.0 | 3.0 | 2.5 | 1.5 | 2.0 | 1.0 | 48.0 | 3.4 | 4.0 | 1.5 | – | 8.9 | 128.5 |
| Clinical DBS, 70% Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Biphasic | 0 | 1.0 | 0 | 2.0 | 2.0 | 0.5 | 1.5 | 1.0 | 43.0 | 0.7 | 0.6 | 1.6 | – | 8.5 | 128.9 |
| Biphasic, 70 % Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 20% CV | 0 | 2.0 | 0 | 2.0 | 2.0 | 1.0 | 1.5 | 1.0 | 45.0 | 0.4 | 0.3 | 1.6 | – | 8.8 | 134.5 |
| 20% CV, 70 % Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 50% PW | 2.0 | 2.0 | 0 | 2.0 | 2.0 | 1.5 | 2.0 | 1.0 | 48.0 | 0.7 | 2.7 | 1.7 | – | 8.5 | 130.1 |
| 50% PW, 150 % Amplitude | 2.0 | 1.0 | 0 | 2.0 | 2.5 | 1.0 | 2.0 | 1.0 | 47.0 | 0.4 | 0.5 | 1.4 | – | 9.3 | 123.5 |
|
PD4 (L GPi, 1− 2+, 3.2 V, 90 μs, 130 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0 | 0 | 3.0 | 2.0 | 1.5 | 1.5 | 2.0 | 40.0 | – | – | – | – | – | – |
| DBS OFF | 0 | 0 | 0 | 2.0 | 2.5 | 1.5 | 2.0 | 2.0 | 39.0 | – | – | – | – | – | – |
| Clinical DBS, 70% Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Biphasic | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Biphasic, 70 % Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20% CV | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20% CV, 70 % Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 50% PW | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 2.0 | 2.5 | 1.5 | 2.5 | 2.0 | 42.0 | – | – | – | – | – | – |
|
PD5 (L STN, 1− 3+, 2.0 V, 120 μs, 190 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0.5 | 0 | 1.5 | 2.25 | 2.25 | 2.0 | 1.0 | 33.0 | 0.2 | 0.1 | 1.0 | – | 9.8 | 111.4 |
| DBS OFF | 3.5 | 3.0 | 0.5 | 1.5 | 2.5 | 2.75 | 2.25 | 1.0 | 40.0 | 4.0 | 3.65 | 1.45 | – | 10.5 | 120.6 |
| Clinical DBS, 70% Amplitude | 3.0 | 2.0 | 0 | 2.0 | 2.0 | 2.5 | 2.0 | 1.0 | 36.0 | 3.2 | 3.6 | 0.7 | – | 9.9 | 117.3 |
| Biphasic | 0 | 0 | 0 | 1.0 | 1.5 | 2.5 | 1.5 | 1.0 | 28.0 | 0 | 0 | 1.1 | – | 9.2 | 129.9 |
| Biphasic, 70 % Amplitude | 4.0 | 1.0 | 0 | 1.5 | 2.5 | 2.5 | 2.0 | 0 | 39.0 | 4.0 | 0.3 | 1.3 | – | 9.3 | 127.4 |
| 20% CV | 0 | 0 | 0 | 1.5 | 2.0 | 2.5 | 1.5 | 1.0 | 39.0 | 0.2 | 0.3 | 0.8 | – | 9.4 | 123.2 |
| 20% CV, 70 % Amplitude | 4.0 | 3.0 | 1.0 | 1.5 | 2.5 | 2.0 | 2.0 | 1.0 | 32.0 | 4.0 | 3.7 | 1.1 | – | 10.1 | 125.4 |
| 50% PW | 3.0 | 0 | 0 | 1.5 | 2.5 | 2.5 | 2.0 | 1.0 | 36.0 | 3.7 | 4.0 | 0.9 | – | 10.0 | 124.6 |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 1.0 | 1.5 | 2.5 | 1.5 | 1.0 | 29.0 | 0.1 | 0.1 | 1.1 | – | 10.4 | 117.4 |
|
PD6 (R STN, 2− C+, 2.5 V, 150 μs, 130 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0.5 | 0 | 1.5 | 0.75 | 0.5 | 0.75 | 2.0 | 26.75 | 0 | 0.2 | 1.1 | 38.8 | 18.5 | 52.9 |
| DBS OFF | 2.5 | 1.0 | 0 | 2.0 | 1.5 | 0.75 | 1.0 | 2.0 | 37.0 | 3.65 | 0.3 | 1.5 | 57.4 | 21.6 | 56.4 |
| Clinical DBS, 70% Amplitude | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Biphasic | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Biphasic, 70 % Amplitude | 0 | 1.0 | 0 | 1.0 | 1.0 | 0.5 | 1.0 | 2.0 | 28.0 | 0 | 0.3 | 1.3 | 51.2 | 21.5 | 50.1 |
| 20% CV | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20% CV, 70 % Amplitude | 0 | 1.0 | 0 | 3.0 | 1.5 | 0.5 | 1.0 | 2.0 | 34.0 | 0 | 0.2 | 2.1 | 37.7 | 17.4 | 38.6 |
| 50% PW | 0 | 1.0 | 0 | 2.0 | 2.0 | 0.5 | 1.0 | 2.0 | 31.0 | 0 | 0.4 | 1.4 | 48.1 | 29.7 | 50.5 |
| 50% PW, 150 % Amplitude | 0 | 1.0 | 0 | 2.0 | 1.5 | 0.5 | 1.0 | 1.0 | 28.0 | 0 | 0.3 | 1.4 | 44.8 | 17.4 | 75.4 |
|
PD7 (R STN, 2− C+, 1.5 V, 90 μs, 130 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0.5 | 1.0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 24.25 | 0.15 | 0.65 | 1.1 | 38.7 | 9.3 | 134.6 |
| DBS OFF | 0.5 | 1.0 | 0 | 1.5 | 2.0 | 1.0 | 1.0 | 0 | 27.0 | 1.15 | 0.55 | 1.1 | 38.2 | 9.1 | 141.0 |
| Clinical DBS, 70% Amplitude | 1.0 | 0 | 0 | 2.0 | 1.0 | 1.0 | 1.0 | 0 | 24.0 | 1 | 0.5 | 1.1 | 36.1 | 8.4 | 131.9 |
| Biphasic | 0 | 0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 21.0 | 0.9 | 0.9 | 1.0 | 36.3 | 8.2 | 133.9 |
| Biphasic, 70 % Amplitude | 1.0 | 1.0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 28.0 | 1.5 | 0.4 | 1.0 | 40.3 | 8.4 | 143.5 |
| 20% CV | 0 | 0 | 0 | 1.0 | 1.0 | 1.0 | 1.0 | 0 | 20.0 | 1.3 | 0.8 | 1.0 | 45.8 | 8.6 | 140.7 |
| 20% CV, 70 % Amplitude | 0 | 0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 24.0 | 0.9 | 0.6 | 0.9 | 41.5 | 8.4 | 143.9 |
| 50% PW | 0 | 1.0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 23.0 | 0.4 | 0.9 | 1.0 | 37.9 | 8.5 | 130.1 |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 1.0 | 2.0 | 1.0 | 1.0 | 0 | 23.0 | 0.5 | 0.9 | 1.0 | 35.9 | 8.1 | 143.6 |
|
PD8 (R STN, 1− C+, 2.5 V, 90 μs, 190 Hz) |
UE Rest Tremor | UE Action/Postural Tremor | LE Rest Tremor | Finger Tapping Speed | UE Rigidity | LE Rigidity | Neck Rigidity | Gait | Total UPDRS-III Score | UE Rest Tremor | UE Postural Tremor | UE Action Tremor | Time (s) | Time (s) | Speed (cm/s) |
| Clinical DBS | 0 | 0.5 | 2.0 | 1.0 | 1.25 | 0 | 0.5 | 0 | 12.75 | 0.5 | 0.45 | 0.75 | 39.6 | 9.0 | 125.3 |
| DBS OFF | 2.5 | 2.0 | 2.0 | 1.5 | 1.5 | 0 | 0.5 | 0 | 19.0 | 4.0 | 3.5 | 0.65 | 40.2 | 9.2 | 123.6 |
| Clinical DBS, 70% Amplitude | 0 | 1.0 | 2.0 | 1.0 | 1.5 | 0 | 0.5 | 0 | 15.0 | 0.1 | 0.3 | 0.8 | 40.5 | 10.2 | 125.3 |
| Biphasic | 0 | 0 | 1.0 | 0 | 0.5 | 0 | 0.5 | 0 | 5.0 | 0.4 | 0 | 0.7 | 33.4 | 9.3 | 123.1 |
| Biphasic, 70 % Amplitude | 3.0 | 2.0 | 2.0 | 1.0 | 1.0 | 0 | 0.5 | 0 | 15.0 | 4.0 | 3.4 | 0.5 | 41.5 | 9.9 | 125.6 |
| 20% CV | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 20% CV, 70 % Amplitude | 0 | 2.0 | 2.0 | 1.0 | 1.0 | 0 | 0.5 | 0 | 13.0 | 0.6 | 1.4 | 0.7 | 37.0 | 10.5 | 132.8 |
| 50% PW | 0 | 1.0 | 2.0 | 1.0 | 1.5 | 0 | 0.5 | 0 | 11.0 | 0.2 | 0.4 | 0.7 | 39.1 | 10.0 | 125.6 |
| 50% PW, 150 % Amplitude | 0 | 0 | 1.0 | 1.0 | 1.0 | 0 | 0.5 | 0 | 10.0 | 0.3 | 0.6 | 0.8 | 39.1 | 10.0 | 125.6 |
| Subject | TRS Scores | Kinesia Scores |
PEG Test |
TUG Test |
Gait Rite |
||||||||||
|
ET1 (R ViM, 2− C+, 1.0 V, 90 μs, 130 Hz) |
UE Rest Tremor | UE Postural Tremor | UE Action Tremor | LE Rest Tremor | LE Postural Tremor | UE Action Tremor | Drawing A+B+C | Writing | Pouring | Total Score | UE Rest Tremor | UE Postural Tremor | Time (s) | Time (s) | Time (s) |
| Clinical DBS | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | 2.0 | 0.5 | 7.5 | 0 | 0 | – | 11.2 | 115.2 |
| DBS OFF | 0 | 0.5 | 1.0 | 0 | 0 | 0 | 4.0 | 2.0 | 0.5 | 12.0 | 0 | 0.05 | – | 10.8 | 113.3 |
| Clinical DBS, 70% Amplitude | 0 | 0 | 0 | 0 | 0 | 0 | 2.0 | 2.0 | 0 | 6.0 | 0 | 0 | – | 9.6 | 117.0 |
| Biphasic | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | 2.0 | 0 | 4.0 | 0 | 0 | – | 10.6 | 127.4 |
| Biphasic, 70 % Amplitude | 0 | 0 | 1.0 | 0 | 0 | 0 | 1.0 | 2.0 | 0 | 12.0 | 0 | 0 | – | 13.3 | 95.3 |
| 20% CV | 0 | 0 | 1.0 | 0 | 0 | 0 | 2.0 | 2.0 | 0 | 6.0 | 0 | 0 | – | 12.3 | 112.9 |
| 20% CV, 70 % Amplitude | 0 | 0 | 1.0 | 0 | 0 | 0 | 2.0 | 2.0 | 0 | 5.0 | 0.1 | 0 | – | 9.9 | 116.5 |
| 50% PW | 0 | 0 | 1.0 | 0 | 0 | 0 | 0 | 2.0 | 1.0 | 5.0 | 0 | 0 | – | 10.6 | 116.6 |
| 50% PW, 150 % Amplitude | 0 | 0 | 1.0 | 0 | 0 | 0 | 3.0 | 2.0 | 0 | 8.0 | 0.1 | 0 | – | 12.6 | 107.3 |
|
ET2 (R ViM, 1− C+, 1.5 V, 90 μs, 190 Hz) |
UE Rest Tremor | UE Postural Tremor | UE Action Tremor | LE Rest Tremor | LE Postural Tremor | UE Action Tremor | Drawing A+B+C | Writing | Pouring | Total Score | UE Rest Tremor | UE Postural Tremor | Time (s) | Time (s) | Time (s) |
| Clinical DBS | 0 | 0 | 1.5 | 0 | 0 | 0.5 | 3.5 | 0 | 1.0 | 7.5 | 0 | 0 | 20.4 | 95.8 | 88.3 |
| DBS OFF | 0 | 1.0 | 1.5 | 0 | 0.5 | 1.0 | 3.0 | 0 | 1.0 | 10.0 | 0 | 0 | 21.8 | 89.8 | 83.8 |
| Clinical DBS, 70% Amplitude | 0 | 0 | 1.0 | 0 | 0 | 1.0 | 3.0 | 0 | 1.0 | 5.0 | 0 | 0 | 19.3 | 74.1 | 90.1 |
| Biphasic | 0 | 0 | 1.0 | 0 | 0 | 1.0 | 4.0 | 0 | 1.0 | 7.0 | 0 | 0 | 21.9 | 72.5 | 90.6 |
| Biphasic, 70 % Amplitude | 0 | 0 | 1.0 | 0 | 0 | 1.0 | 4.0 | 0 | 1.0 | 6.0 | 0 | 0 | 19.8 | 98.4 | 85.6 |
| 20% CV | 0 | 1.0 | 1.0 | 0 | 0 | 1.0 | 2.0 | 0 | 1.0 | 5.0 | 0 | 0 | 21.2 | 95.1 | 94.2 |
| 20% CV, 70 % Amplitude | 0 | 1.0 | 1.0 | 0 | 0 | 1.0 | 4.0 | 0 | 1.0 | 7.0 | 0.1 | 0 | 21.3 | 69.3 | 83.8 |
| 50% PW | 0 | 1.0 | 2.0 | 0 | 0 | 1.0 | 4.0 | 0 | 1.0 | 9.0 | 0 | 0 | 21.0 | 96.6 | 86.5 |
| 50% PW, 150 % Amplitude | 0 | 1.0 | 1.0 | 0 | 0 | 1.0 | 4.0 | 0 | 1.0 | 7.0 | 0 | 0 | 19.9 | 79.1 | 89.8 |
|
ET3 (L ViM, 2− C+, 2.0 V, 90 μs, 130 Hz) |
UE Rest Tremor | UE Postural Tremor | UE Action Tremor | LE Rest Tremor | LE Postural Tremor | UE Action Tremor | Drawing A+B+C | Writing | Pouring | Total Score | UE Rest Tremor | UE Postural Tremor | Time (s) | Time (s) | Time (s) |
| Clinical DBS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | 0 | 0 | 8.7 | 33.6 | 119.4 |
| DBS OFF | 0 | 0 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 5.5 | 0 | 0 | 10.5 | 38.5 | 116.8 |
| Clinical DBS, 70% Amplitude | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.0 | 0 | 0 | 10.0 | 26.5 | 120.8 |
| Biphasic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.0 | 0 | 0 | 10.2 | 32.9 | – |
| Biphasic, 70 % Amplitude | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.0 | 0 | 0 | 9.8 | 26.7 | 116.0 |
| 20% CV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | 5.0 | 0 | 0 | 11.5 | 35.4 | 98.5 |
| 20% CV, 70 % Amplitude | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.0 | 0 | 0 | 11.1 | 33.1 | 123.2 |
| 50% PW | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.0 | 0 | 0 | 10.7 | 27.1 | 117.3 |
| 50% PW, 150 % Amplitude | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.0 | 0 | 0 | 10.1 | 28.2 | 112.5 |
To compare the relative energy efficiency of the non-conventional DBS settings, IPG current drain data are expressed as mean values ± SEM and analyzed using a paired Students t test, comparing the current drain with clinically-optimal DBS settings delivered using the commercial IPG FW (cFW) to the current drain with each non-conventional setting (Figure 3).
Results
Tolerability of Non-Conventional DBS Settings
Eleven consecutive subjects were enrolled in the study (n = 8 PD and 3 ET). Overall mean age was 62 years (range 47 to 75 years). Mean disease duration for PD subjects was 11 years (range 8–18 years) and for ET subjects was 20 years (range 6–40 years). In all but one of the PD cohort, STN was the surgical target; GPi was the surgical target for subject PD4. VIM was the surgical target for all ET subjects. All implanted electrodes were 3387 model (Medtronic, Neuromodulation, Minneapolis, MN). Only 2 subjects terminated the study prior to completing the full protocol. Subject PD3 withdrew from the study after completing 8 of the 11 DBS settings because of general fatigue. Subject PD4 withdrew from the study after completing only 3 of the settings due to off-medication fatigue. In addition, testing could not be completed in 2 subjects because of intolerable side effects. For subject PD6, intolerable settings included BiphClinV (lip pulling and speaking difficulties), 20%CVClinV (phosphenes), and 70%ClinV (phosphenes). For subject PD8, BiphClinV was the only intolerable setting (dizziness and blurred vision). All other subjects were able to complete the full clinical protocol and accompanying motor assessments. Overall, the proportion of subjects who responded better to the non-conventional settings (i.e. the responder rate) was 1/5 for 70%ClinV, 6/6 for BiphClinV, 1/6 for 70%BiphClinV, 2/5 for 20%CVClinV, 2/6 for 20%CV70%V, and 3/7 for both 50%PWClinV and 50%PW150%V.” In general, motor symptoms appeared discernably better with any of the active settings compared to the DBS-off (sham) condition. Overall, no unexpected adverse events occurred and the non-conventional DBS settings were well tolerated, though in some cases mild side effects were experienced. See Table 2 and the cases series supplement for individual details and results.
PD Motor Assessments
Although the primary objective of this pilot study was to determine the feasibility of acutely testing the tolerability several non-conventional DBS settings, we also explored for any potential efficacy signals in the PD cohort. To do so, first we tested whether a clinically-relevant UPDRS-III difference could even be detected under these conditions in the PD subjects that were able to complete the study from beginning to end (i.e. even if some settings were intolerable; n = 6). As expected, median UPDRS-III scores with DBS-off was significantly worse compared to that with ClinDBS (35.6 vs. 28.9; p = 0.03). In contrast, while median UPDRS-III score with ClinDBS trended upward from the beginning to the end of the study, the difference was not statistically significant (25.5 vs. 31.6; p = 0.44). Similarly, no significant difference was detected in the median UPDRS-III score with DBS-off between the beginning and end of the study (33.8 vs. 35.6; p = 0.41), indicating that fatigue was not likely a major factor in the infrequent intolerability of some non-conventional DBS settings described above or other outcomes described below. Overall, these results demonstrate that the testing conditions were sufficient to detect potential differences in DBS therapeutic efficacy between different DBS settings using standard clinical rating scales in this case on versus off stimulation.
Next we assessed if there was a potential efficacy advantage associated with any of the non-conventional DBS settings. To do so, the UPDRS-III scores were plotted as a delta relative to the scores with ClinDBS (Figure 2A). Consistent with the results above, the median UPDRS-III score delta with DBS-off was ~5 points greater than with ClinDBS. As expected, the UPDRS-III score deltas with 70%ClinV were also generally >0, or suboptimal relative to ClinDBS. On the other hand, the median UPDRS-III score deltas from ClinDBS varied with the non-conventional settings, as some of the non-conventional settings appeared to offer similar benefit and others lesser benefit. Interestingly, the median UPDRS-III score delta with BiphClinV was ~2.5 points less than with ClinDBS, suggesting that this setting delivered greater benefit than ClinDBS. Pairwise statistical tests were then conducted to detect differences between these UPDRS-III score deltas and those with DBS-off, including data only from subjects who completed the testing for the non-conventional setting (n = 5–7 total per condition; see Methods). Whereas the median UPDRS-III score deltas with 20%CV70%V, 50%PWClinV and 50%PW150%V tended to be lower than with DBS-off (p = 0.06–0.08), only the median UPDRS-III delta with BiphClinV was significantly lower than with DBS-off (p = 0.03). Taken together, these results suggest that DBS delivered with the non-conventional biphasic pulse may offer efficacy advantages over standard therapy, though follow up studies specifically designed for testing this concept will be required to confirm.
In the PD subjects that completed the study from beginning to end no significant differences were detected in the median Kinesia, TUG or GaitRite values between ClinDBS between the beginning and end of the study (n = 5–6; p = 0.17–1.0). No significant differences were detected in the median Kinesia, TUG or GaitRite values with DBS-off at the beginning and end of the study (p = 0.20–0.41). Although some of the outcomes of these assessments were positive within individual subjects (Table 2), there were no statistically significant differences detected in the median Kinesia, TUG or GaitRite values between DBS-off and ClinDBS (p = 0.06–0.86), though the Kinesia rest tremor scores trended lower with ClinDBS (p = 0.06). Plots comparing the median deltas of these different assessments relative to ClinDBS provide insight (Figure 2B–F). For example, the median Kinesia rest and postural tremor score deltas were varied considerably with DBS-off (Figure 2B–C), but were approximately 0 with most of the non-conventional settings. On the other hand, the median GaitRite velocity deltas were inconsistent across all settings, whereas median TUG time deltas were relatively static regardless of setting. Moreover, the group-wise outcomes of these objective assessments were likely influenced by the clinical spectrum of the subjects and the applicability of the different assessments under these specific testing conditions. These results can be used as design inputs to follow-up studies incorporating objective motor assessments.
ET Motor Assessments
Three ET subjects were tested (2 females), and in most cases the non-conventional settings appeared to be more effective compared to DBS-off (see Table 2). Specifically, the BiphClinV, 20%CVClinV, and 70%ClinV settings appeared to achieve similar benefits as with ClinDBS settings. Overall, all of the non-conventional settings were well-tolerated. See Table 2 and the cases series supplement for individual details and results.
Energy Consumption of Non-Conventional DBS Settings
The impact on the IPG battery of delivering non-conventional DBS settings may not be straightforward and is an important consideration when proposing alternative programming paradigms. To determine whether delivering any of the non-conventional DBS settings may be associated with unexpected changes in the energy efficiency of the IPG, the amount of current drained from the battery was estimated with each of the 8 DBS settings for all 11 study subjects (Figure 3). Average current drain per minute with each setting delivered using the research FW (rFW) was compared to the average current drain with the ClinDBS settings delivered using the commercial FW (cFW). The average current drain with the ClinDBS was nearly identical with the cFW and cFW (101.34 ± 8.89 μA vs. 96.11 ± 7.69 μA; p = 0.17). As expected, the current drain was significantly less with the IPG turned on but with DBS-off (54.47 ± 1.92 μA; p < 0.0001). Also not surprisingly, reducing stimulation output with a 30% decrease in amplitude or a 50% decrease in the pulse width (70%ClinV and 50%PWClinV) was also associated with significant less current drain (78.24 ± 7.69 μA and 73.09 ± 4.52 μA p < 0.001 and 0.0001). There was no significant difference in current drain associated with delivery of the 20%CVClinV setting (99.83 ± 7.97 μA; p = 0.69). In contrast, delivering BiphClinV or the BiphClin70%V setting was associated with significant increases in current drain (154.75 ± 15.01 μA and 119.35 ± 26.45 μA; p = 0.17p < 0.0001 and p < 0.01). Therefore, there were no disadvantages in IPG efficiency associated with delivery of the non-conventional DBS settings per se, only predictable variations in current drain based on the energy demand of the specific stimulation settings.
Discussion
There is a critical need for novel approaches to optimize the efficacy and efficiency of DBS therapies. While an increasing number of patients benefit from DBS, the improvement for a subset may be limited or offset by side effects. Patients who do benefit from DBS often undergo repeat surgeries to replace IPG batteries, at a rate that depends on the stimulation parameters, electrode configuration and IPG battery type. Thus, improving the efficacy and efficiency of commercially-available IPGs would likely benefit many patients with DBS.
Although several computational and animal model studies have investigated non-conventional DBS parameters, clinical evaluations have been somewhat limited. In this exploratory study, we tested several non-conventional stimulation settings using a custom DBS-device FW application in PD and ET subjects chronically-implanted with DBS using randomization and blinding. Whereas the primary objective was to assess the feasibility of testing tolerability, assessing the feasibility of testing for efficacy and efficiency were secondary objectives. We found that all of the non-conventional stimulation settings were safe in all subjects and generally well-tolerated. Importantly, we also found that a simple FW upgrade to a commercial IPG may provide more DBS programming options without unexpected disadvantages related to the operating efficiency of the device. Moreover, we also provide preliminary evidence that charge-balanced biphasic pulses with a square-wave active recharge may offer therapeutic efficacy advantages. Yet, while standard clinical rating scales were useful for detecting different responses to the different stimulation settings, objective assessments of tremor and gait were not, likely due in part to the phenotypic variability across patients (e.g. tremor-dominant vs. akineticrigid vs. postural instability with gait dysfunction subtype). Nonetheless, the overall results of this study demonstrate critical feasibility of testing these and other non-conventional DBS therapy concepts in a well-controlled manner.
The results of this study can also be used to guide the design of future studies evaluating non-conventional DBS settings. In order to obtain the most generalizable results, we tested subjects in the environment where their DBS adjustments and clinical testing were typically performed. This procedure allowed us to enroll subjects who had already been optimized post-surgically. We chose not to study subjects immediately post-operatively to minimize confounding by the micro-lesion effect, and to provide a valid comparison of non-conventional settings versus clinically optimized settings. The cutoff of 4 monthly programming sessions used to define clinical optimization was a potential limitation of the current study, though in our experience optimization is often achieved within 3 to 4 months after surgery. Although the wash-in plus wash-out period between different stimulation settings may be considered suboptimal, this allowed for screening multiple settings in one session and has been utilized previously [24, 26, 29, 36, 43]. The 2 minute wash-in interval limits our ability to draw firm conclusions about the long-term effects of these non-conventional settings. While acute studies of this nature are ideal for screening multiple non-conventional DBS setting concepts, future statically-powered studies will ideally focus on one specific concept and test in a larger sample over a longer time period.
Stimulation at high frequency (>100 Hz) is typically required to produce motor improvement in PD, and lower frequencies have often been shown to be ineffective [4, 9, 44]. Dorval et al. found evidence suggesting that DBS settings that improve PD bradykinesia during the battery-replacement surgery may do so by entraining basal ganglia activity [26]. Compared to continuous DBS delivered at regular patterns frequencies, irregular DBS patterns with same average frequency (10%, 20% or 30% CV) did not entrain neuronal activity nor alleviate bradykinesia as effectively, suggesting that regular patterns of DBS are superior. On the other hand, Brock et al. [25] tested specific irregular DBS patterns in a similar clinical setting and found that some irregular patterns may in fact deliver greater improvement to PD bradykinesia symptoms. In the current study, we tested irregular DBS patterns with a 20% CV (20%CVClinV) in PD and ET patients in a standard clinical environment. In most subjects, the 20%CVClinV and 20%CV70%V settings appeared to deliver some benefit compared to the DBS-off condition (sham control). In some cases, these irregular DBS pattern settings were even more effective. However, in other cases the irregular DBS patterns not well-tolerated, as in subject PD8 who described a persistent “jolt” sensation. Yet, these results demonstrate that it was feasible to distinguish clinical outcomes associated with different patterns of DBS delivered in chronically-implanted subjects in a standard clinical environment.
There is building evidence that narrower pulse widths than commonly used may be associated with wider therapeutic windows. Moro et al.[4] evaluated the effects of >20 DBS settings by varying pulse width, amplitude and frequency and found that the combination of the highest tolerable voltage with the shortest pulse width was the most effective strategy at treating PD motor symptoms. Results from a more recent open-label study by Reich et al. using a combination of human subject and computational modeling outcomes, suggests that narrower pulse widths may lead to a widening of the therapeutic window through a proportional more efficient activation or neural elements [22]. In the current blinded study, we used a 50% narrower pulse width with a proportional 50% increase in amplitude (50%PW150%V), and found in some cases that this setting was generally both at least as effective and efficient as the clinical settings. The narrower pulse width tested at the clinically-optimal amplitude (50%PWClinV) was not as effective as the clinical settings and was less tolerable, but drained significantly less battery. Regardless, these results also demonstrate the feasibility of studying the potential therapeutic window benefits of shorter pulse widths using a blinded, randomized study design.
The traditional DBS pulse is composed of a rectangular, active phase and an exponential passive recharge phase, designed to produce an appropriate neural response, a coincident behavioral response and balance the cathodic phase to prevent tissue damage [30]. While investigating the effects of modified pulse shapes on neural activity, Hofmann et al. demonstrated that introducing a short gap between the initial cathodic phase and the anodic phase could result in more effective and more efficient neural entrainment [31]. Foutz and McIntyre also evaluated non-traditional pulse shapes, including Gaussian, exponential, triangular and sinusoidal pulse, finding that some novel pulse shapes may deliver the same neural effects with significantly reduce energy requirements [30]. In the current study we tested a non-conventional square-wave biphasic pulse with active cathodic and anodic phases at the subjects’ clinically-optimal voltage (BiphClinV). The BiphClinV setting was well tolerated by nearly all subjects and when delivered at the clinically-optimal voltage appeared even more effective than the clinically-optimal settings. However, the BiphClinV setting was obviously associated with a higher energy demand, requiring approximately 50% greater current drain from the IPG battery than traditional DBS biphasic pulses with a passive recharge. At 70% of the clinically-optimal voltage, the square-wave biphasic pulse (BiphClin70%V) was both less effective and less efficient than the clinically-optimal settings. Nevertheless, the non-conventional square-wave biphasic pulse may have potential clinical utility to provide symptom-specific relief in select patients who do not respond maximally to the commercially-available settings, especially in those implanted with rechargeable IPG batteries. The potential benefits suggested here deserve further exploration.
In summary, there is a clear need to develop more effective and efficient stimulation paradigms. This study demonstrates the feasibility of evaluating non-conventional DBS patterns in a standard clinical environment using controls, blinding and randomization. Whether non-conventional settings can be used to alleviate specific side effects of clinical DBS or address specific unaddressed symptoms was not addressed but also requires further study. Furthermore, the potential for simple FW updates to already implanted DBS devices as a means to improve symptom control is a very innovative and appealing approach for optimizing the therapy. The idea of narrower and biphasic pulses will need to be tested in larger clinical trials.
Supplementary Material
Table 3.
Subjects’ subjective reports of positive and negative perceptions for each of the non-conventional DBS setting tested.
| DBS Setting | Subjective patient comments (positive and negative) |
|---|---|
| ClinDBS | PD1: felt transient current surge |
| DBS-off | PD1: felt tipping over, drunk, shaky, wobbly PD2: felt slower, stiffer, and that they could not speak (mute) PD5: felt transient hand tingling PD6: felt more tremor, stiffness, slowness but that gait was better ET1: felt off-balance and that handwriting was poor |
| BiphClinV | PD1: felt transient current surge PD6:felt lip pulling, experienced difficulty speaking* PD7: felt ipsilateral tremor was better PD8: felt transient strong jolt ET1: felt mild jolt in hand ET2: felt less secure and that movements were more taxing ET3: felt transient hand tingling |
| Biph70%ClinV | PD1: felt transient current surge PD6: felt less tremor, looser, less coordinated hand, but faster gait ET1: felt transient tremor in entire body |
| 20%CVClinV | PD1: felt transient current surge PD5: felt transient arm tingling PD6: felt flashing in the eyes, facial pulling* PD7: felt no tremor at all (bilateral). PD8: felt strong jolt, dizzy, blurry vision* ET1: felt this was a good setting ET2: felt more relaxed. ET3: felt transient tingling but that setting was too strong |
| 20%CV70%V | PD1: felt transient current surge PD2: experienced speech difficulty, felt neck stiffness PD6: felt better coordinated PD7: felt ipsilateral tremor was better ET3: felt that movements were slower |
| 50%PWClinV | PD1: felt transient current surge PD5: felt transient tingling of both hands PD6: felt feel slower and stiffer PD8: felt mild transient jolt |
| 50%PW150%V | PD1: felt transient current surge PD2: felt transient tingling and that speech was much better PD5: felt transient arm tingling PD6: felt gait was much better PD7: felt no tremor at all (bilateral) PD8: felt transient moderate jolt ET1: felt transient jolt in finger tips ET3: felt transient hand tingling |
| 70%ClinV | PD1: felt a little stiff PD2: felt neck was stiffer, speech worse, off-balance PD6: experienced flashes in both eyes* PD8: felt transient mild jolt ET3: felt dexterity was improved |
Intolerable side effects.
Acknowledgments
We thank Medtronic for providing the research FW, equipment and technical support. Medtronic provided no funding.
Footnotes
Authorship Statement: Drs. Akbar, Raike, Hack, Okun, Martinez-Ramirez, Skinner, Hess and DeJesus designed and conducted the study, including patient recruitment, data collection, and data analysis. Dr. Akbar prepared the manuscript draft with important intellectual input from Drs. Raike, Okun, Hess, Hack, DeJesus, Martinez-Ramirez and Skinner. All authors approved the final manuscript. We received no funding for the study. Statistical support in analyzing the data with input from authors Drs. Akbar, Raike, Hack, Okun, Hess and Skinner. Drs. Akbar, Raike and Okun had complete access to the study data. We would like to thank Medtronic Inc. for providing the research firmware, equipment, and technical support.
Disclosures:
Drs. Akbar, Hack, Hess, Martinez-Ramirez, Skinner and DeJesus have no disclosures. Dr. Raike is a paid employee of Medtronic Neuromodulation Global Research.
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, Quantia, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
References
- 1.Deep-Brain Stimulation for Parkinson’s Disease Study, G. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 2.Koller WC, et al. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16(3):464–8. doi: 10.1002/mds.1089. [DOI] [PubMed] [Google Scholar]
- 3.Vercueil L, et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001;248(8):695–700. doi: 10.1007/s004150170116. [DOI] [PubMed] [Google Scholar]
- 4.Moro E, et al. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002;59(5):706–13. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 5.Rizzone M, et al. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: effects of variation in stimulation parameters. J Neurol Neurosurg Psychiatry. 2001;71(2):215–9. doi: 10.1136/jnnp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alterman RL, et al. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69(7):681–8. doi: 10.1212/01.wnl.0000267430.95106.ff. [DOI] [PubMed] [Google Scholar]
- 7.Kim JP, et al. Effects of relative low-frequency bilateral globus pallidus internus stimulation for treatment of cervical dystonia. Stereotact Funct Neurosurg. 2012;90(1):30–6. doi: 10.1159/000333839. [DOI] [PubMed] [Google Scholar]
- 8.Velez-Lago FM, et al. Low-Frequency Deep Brain Stimulation for Dystonia: Lower is Not Always Better. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D85X27PH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merola A, et al. 80 Hz versus 130 Hz subthalamic nucleus deep brain stimulation: effects on involuntary movements. Parkinsonism Relat Disord. 2013;19(4):453–6. doi: 10.1016/j.parkreldis.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Sidiropoulos C, et al. Low-frequency subthalamic nucleus deep brain stimulation for axial symptoms in advanced Parkinson’s disease. J Neurol. 2013;260(9):2306–11. doi: 10.1007/s00415-013-6983-2. [DOI] [PubMed] [Google Scholar]
- 11.Kuncel AM, et al. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Mov Disord. 2006;21(11):1920–8. doi: 10.1002/mds.21087. [DOI] [PubMed] [Google Scholar]
- 12.Blahak C, et al. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol. 2011;18(6):872–5. doi: 10.1111/j.1468-1331.2010.03290.x. [DOI] [PubMed] [Google Scholar]
- 13.Bruggemann N, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. 2015;84(9):895–903. doi: 10.1212/WNL.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vercueil L, et al. Effects of pulse width variations in pallidal stimulation for primary generalized dystonia. J Neurol. 2007;254(11):1533–7. doi: 10.1007/s00415-007-0578-8. [DOI] [PubMed] [Google Scholar]
- 15.Volkmann J, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13(9):875–84. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- 16.Barbe MT, et al. Individualized current-shaping reduces DBS-induced dysarthria in patients with essential tremor. Neurology. 2014;82(7):614–9. doi: 10.1212/WNL.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miocinovic S, et al. Outcomes, management, and potential mechanisms of interleaving deep brain stimulation settings. Parkinsonism Relat Disord. 2014;20(12):1434–7. doi: 10.1016/j.parkreldis.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Hariz MI. Complications of deep brain stimulation surgery. Mov Disord. 2002;17(Suppl 3):S162–6. doi: 10.1002/mds.10159. [DOI] [PubMed] [Google Scholar]
- 19.Pollak P, et al. Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(Suppl 3):S155–61. doi: 10.1002/mds.10158. [DOI] [PubMed] [Google Scholar]
- 20.Tommasi G, et al. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2008;79(7):813–9. doi: 10.1136/jnnp.2007.117507. [DOI] [PubMed] [Google Scholar]
- 21.Tripoliti E, et al. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008;23(16):2377–83. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- 22.Reich MM, et al. Short pulse width widens the therapeutic window of subthalamic neurostimulation. Ann Clin Transl Neurol. 2015;2(4):427–32. doi: 10.1002/acn3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker KB, Zhang J, Vitek JL. Pallidal stimulation: effect of pattern and rate on bradykinesia in the non-human primate model of Parkinson’s disease. Exp Neurol. 2011;231(2):309–13. doi: 10.1016/j.expneurol.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birdno MJ, et al. Tremor varies as a function of the temporal regularity of deep brain stimulation. Neuroreport. 2008;19(5):599–602. doi: 10.1097/WNR.0b013e3282f9e45e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brocker DT, et al. Improved efficacy of temporally non-regular deep brain stimulation in Parkinson’s disease. Exp Neurol. 2013;239:60–7. doi: 10.1016/j.expneurol.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorval AD, et al. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010;104(2):911–21. doi: 10.1152/jn.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamchic I, et al. Coordinated reset neuromodulation for Parkinson’s disease: proof-of-concept study. Mov Disord. 2014;29(13):1679–84. doi: 10.1002/mds.25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tass PA, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol. 2012;72(5):816–20. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]
- 29.Little S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foutz TJ, McIntyre CC. Evaluation of novel stimulus waveforms for deep brain stimulation. J Neural Eng. 2010;7(6):066008. doi: 10.1088/1741-2560/7/6/066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann L, et al. Modified pulse shapes for effective neural stimulation. Front Neuroeng. 2011;4:9. doi: 10.3389/fneng.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wongsarnpigoon A, Grill WM. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J Neural Eng. 2010;7(4):046009. doi: 10.1088/1741-2560/7/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurko O, Ryan JL. Translational research in central nervous system drug discovery. NeuroRx. 2005;2(4):671–82. doi: 10.1602/neurorx.2.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galpern WR, et al. Designing clinical trials for dystonia. Neurotherapeutics. 2014;11(1):117–27. doi: 10.1007/s13311-013-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyam JA, et al. Implementing novel trial methods to evaluate surgery for essential tremor. Br J Neurosurg. 2015;29(3):334–9. doi: 10.3109/02688697.2014.997670. [DOI] [PubMed] [Google Scholar]
- 36.Lopiano L, et al. Temporal changes in movement time during the switch of the stimulators in Parkinson’s disease patients treated by subthalamic nucleus stimulation. Eur Neurol. 2003;50(2):94–9. doi: 10.1159/000072506. [DOI] [PubMed] [Google Scholar]
- 37.Hughes AJ, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuffrida JP, et al. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24(5):723–30. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- 39.Heldman DA, et al. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(6):590–5. doi: 10.1016/j.parkreldis.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81(2):810–8. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 41.Baldewijns G, et al. Validation of the kinect for gait analysis using the GAITRite walkway. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5920–3. doi: 10.1109/EMBC.2014.6944976. [DOI] [PubMed] [Google Scholar]
- 42.Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in individuals with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88–91. doi: 10.1097/MRR.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 43.Temperli P, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60(1):78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 44.Eusebio A, et al. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease. Exp Neurol. 2008;209(1):125–30. doi: 10.1016/j.expneurol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


