Abstract
Clinical mutation screening of the cancer susceptibility genes BRCA1 and BRCA2 generates many Unclassified Variants (UVs). Most of these UVs are either rare missense substitutions or nucleotide substitutions near the splice junctions of the protein coding exons. Previously, we developed a quantitative method for evaluation of BRCA gene UVs – the “integrated evaluation” – that combines a sequence analysis-based prior probability of pathogenicity with patient and/ or tumor observational data to arrive at a posterior probability of pathogenicity. One limitation of the sequence analysis-based prior has been that it evaluates UVs from the perspective of missense substitution severity but not probability to disrupt normal mRNA splicing. Here, we calibrated output from the splice site fitness program MaxEntScan to generate spliceogenicity-based prior probabilities of pathogenicity for BRCA gene variants; these range from 0.97 for variants with high probability to damage a donor or acceptor to 0.02 for exonic variants that do not impact a splice junction and are unlikely to create a de novo donor. We created a database http://priors.hci.utah.edu/PRIORS/ that provides the combined missense substitution severity and spliceogenicity-based probability of pathogenicity for BRCA gene single nucleotide substitutions. We also updated the BRCA gene Ex-UV LOVD, available at http://hci-exlovd.hci.utah.edu, with 77 re-evaluable variants.
Keywords: BRCA1, BRCA2, Cancer Predisposition, Unclassified Variant, Variant of Uncertain Significance, Rare Variant
INTRODUCTION
Clinical resequencing of a high-risk cancer susceptibility gene such as BRCA1 (MIM# 113705) or BRCA2 (MIM# 600185) may reveal that a patient carries a clearly pathogenic sequence variant. Most pathogenic variants in these genes are either nonsense variants, small insertion or deletion variants (indels) that create a frameshift, larger gene rearrangements, variants that create a severe splicing aberration, or severely dysfunctional missense substitutions. However, testing may also reveal that a patient carries an unclassified variant (UV, or VUS for Variant of Uncertain clinical Significance). In BRCA1 or BRCA2, these are usually missense substitutions, in-frame indels, or sequence variants that fall in the splice junction consensus regions but outside of the canonical GT-AG dinucleotides. Even silent substitutions could be pathogenic if they have a severe impact on the regulation of mRNA splicing.
A Bayesian “integrated evaluation” or “multifactorial method” has proven to be a relatively successful approach to classification of BRCA gene UVs (Goldgar et al., 2004; Easton et al., 2007; Goldgar et al., 2008; Spurdle, 2010; Lindor et al., 2012), and there is now a database dedicated to BRCA gene variants that have been classified by this method (Vallée et al., 2012). This approach is also being applied to variants in other high-risk cancer genes, notably the mismatch repair genes (Thompson et al., 2013a). In this approach, UVs are assessed through a Bayesian inference that starts with a prior probability in favor of pathogenicity based on position in the gene and sequence analysis (Easton et al., 2007; Tavtigian et al., 2008). The prior is updated with observational data from segregation analysis, summary personal and family history analysis, co-occurrence between UVs and clearly pathogenic sequence variants, and tumor immunohistochemistry and grade. Each type of observational data is expressed as odds or likelihood ratios in favor of pathogenicity. The resulting posterior probability is then converted to one of 5 qualitative classes, based on defined cutpoints considered to be clinically relevant (Plon et al., 2008).
One limitation to the prior probability is that its sequence analysis component has only been calibrated for missense substitutions and sequence variants that alter the canonical GT-AG splice junction dinucleotides (Easton et al., 2007; Tavtigian et al., 2008). Yet it is well known that sequence variants in the broader proximal splice junction regions can damage function of the wild type splice junctions, sequence variants in either the exons or the introns can create de novo splice junctions, and sequence variants in either the exons or introns can alter splice enhancers or splice silencers; all of these classes of sequence variants have the potential to cause aberrant splicing.
The work presented here focuses on calibrating MaxEntScan (MES) (Yeo and Burge, 2004) based analysis of variants in the proximal splice junction region that may damage the function of wild-type splice junctions, and of exonic variants that may create de novo splice junctions. The analysis covered all exonic bases, plus 20 intronic bases upstream and 6 intronic bases downstream of each exon, and converts the MES score into a probability of pathogenicity so that it can be included in the integrated evaluation of BRCA gene variants.
METHODS
Dataset
The dataset comprised results of full sequence tests carried out at Myriad Genetic Laboratories, as used previously in Easton et al. (2007) and Tavtigian et al. (2008) for modelling of risk associated with BRCA1/2 sequence variation. The analyses described here are based on results of full sequence tests of both genes from 68,000 BRACAnalysis subjects of whom 4,867 were reported to carry a pathogenic BRCA1 variant and 3,561 were reported to carry a pathogenic BRCA2 variant. For a test to have been performed, a test request form must have been completed by the ordering health care provider, and the form must have been signed by an appropriate individual indicating that “informed consent has been signed and is on file”. The mutation screening data are arranged by sequence variant rather than by subject. The dataset includes nucleotide and amino acid nomenclature specifications for all of the exonic single nucleotide substitutions – silent, missense, or nonsense – observed from the 68,000 patient mutations screening set; these are all of the observational data required to calculate the Enrichment Ratio for Single Nucleotide Substitutions (ERS) (Tavtigian et al., 2008).
Analyses of the personal and family history of tested probands to recalculate family history likelihood ratios (FamHx-LRs) derive from a virtually identical series of subjects used previously (Easton et al., 2007). However, this dataset also includes frameshifts, in-frame indels, and sequence variants falling in the intronic portions of the splice junction consensus regions from −20 to +6 of the protein coding exons. We refer to these two overlapping data sets as the B1&2 68K set.
Sequence variant data from the DataBase of Aberrant 3’ and 5’ Splice Sites (DBASS3 and DBASS5) (Buratti et al., 2011) and the Breast Cancer Information Core (BIC) (https://research.nhgri.nih.gov/projects/bic/index.shtml) were obtained in December, 2011; the DBASS data were updated in May, 2015.
Scoring sequence variants with MaxEntScan
MaxEntScan (MES) is a program, based on a maximum entropy model, for scoring the fitness of potential splice donor or splice acceptor sequences (Yeo and Burge, 2004). Rather than scanning an entire sequence to find the best candidates, the program scores fixed k-mers (9-mers with the candidate splice site between the 3 and 4 nucleotide position to evaluate donor splice sites, 23-mers with the candidate splice site between the 20 and 21 nucleotide position for acceptors) and then outputs its maximum entropy-based score for each k-mer. MES was used to obtain scores for the BRCA1 and BRCA2 gene reference sequences. The reference sequences used were: BRCA1 cDNA NM_007294.3, BRCA1 genomic NG_005905.2, BRCA2 cDNA NM_000059.3, and BRCA2 genomic NG_012772.3. We also used MES to score every possible single nucleotide substitution to the coding sequences and proximal splice junction regions plus other individual sequence variants such as in-frame indels present in the B1&2 68K set. For sequence variants that might damage a wild-type splice site, this was done by scoring the wild-type site and the mutated site, with the scoring window set such that the position of the splice junction fell at its normal location.
To detect sequence variants that might create a de novo splice junction, including all possible single nucleotide substitutions to the BRCA gene coding sequences from 20 bp upstream of each coding exon to 6 bp downstream of each coding exon, the following approach was taken. A MaxEntScan scoring window (i.e. 9 bp for scoring a donor, 23 bp for scoring an acceptor) was slid across the substitution-bearing sequence such that k k-mers were scored, with the sequence variant moving from position 1 to position k of the window. Then, the highest score (most fit as a potential splice junction) from each set of k-mers was recorded in a MySQL database (Figure 1). A java implementation of the MES algorithm was developed and incorporated into an application for scoring variants in a vcf file for damage to known splice sites and introduction of novel splice sites. This is released as part of the open source USeq package at (http://useq.sourceforge.net/cmdLnMenus.html#VCFSpliceAnnotator).
Figure 1. Illustration of the MaxEntScan sliding window approach.
A. BRCA1 cDNA reference sequence from 4865 to 4873, highlighting nucleotide C4868 in exon 16. B. Sliding a MaxEntScan donor window across an innocuous possible substitution, c.4868C>T. C. Sliding a MaxEntScan donor window across the de novo donor creating substitution c.4868C>G; note the relatively high MaxEntScan donor score at the 6th frame of the sliding window. D. MaxEntScan evaluation of the intron 16 splice donor; note that this score is actually lower than the score of c.4868C>G.
Enrichment Ratio for Single Nucleotide Substitutions (ERS) calculations
The ERS is similar in spirit to the standard population genetics measure dN/dS (dN is the non-synonymous substitution rate and dS is the synonymous substitution rate per site), where a positive ratio dN/dS is indicative of positive selection (Yang, 1998). For each nucleotide in a canonical DNA sequence, there are three possible single nucleotide substitutions. However, these substitutions are not equally likely to occur because of differences in the underlying substitution rate constants. Using the dinucleotide substitution rate constants given by Lunter and Hein (Lunter and Hein, 2004), averaging sense and antisense orientations, we can estimate a relative substitution rate for every possible single-nucleotide substitution to a DNA sequence, ri. The probability that a new sequence variant (i.e., a new germline sequence variant at the moment that it comes into existence) will fall into a particular algorithmically defined class c† is given by the ratio of the sum of the relative substitution rates of the variants belonging to the class c divided by the sum of all relative substitution rates:
| (1) |
Hence, under the null hypothesis of no selection, we can obtain from the total number of variants observed in a mutation screening study, oT, the number expected in any class, eC=pC × oT, and compare this to the actual number observed, oC. Thus, in general, we define the ERS for any class of substitutions c as the observed / expected ratio for that class normalized by the same ratio for silent (i.e., synonymous) substitutions but excluding the few silent substitutions that are likely to be spliceogenic:
| (2) |
† For this discussion, an “algorithmically defined class” of variants is a class of variants that can be unambigously specified by an algorithm. One example could be, given a specified protein multiple sequence alignment, all substitutions that fall at an invariant position in the alignment and have a Grantham Score ≥65. Another could be all substitutions that fall at the last nucleotide of a protein coding exon.
Summary family history based risk estimates
Sequence variants were stratified by MaxEntScan scores. Then all of the summary personal/family histories of the subjects who carried the variants within a particular stratum (after data cleaning as mentioned below) were used to estimate α, the proportion of variants within that stratum that were pathogenic, using the heterogeneity likelihood ratio defined in Easton et al. (2007). Approximate 95% confidence intervals for the heterogeneity proportion were obtained by finding the values αL and αU for which the overall likelihood differed from that at α by an amount equivalent to a likelihood ratio test significant at the 0.05 level (Easton et al., 2007).
Data Cleaning
One potential confounder in our analyses is that a sequence variant can belong to two algorithmically defined groups at once; for example, a single nucleotide substitution can cause a missense substitution that is likely to damage protein function, and the same nucleotide substitution can be likely to create a de novo splice donor. In general, for ERS calculations, likely spliceogenic nucleotide substitutions that are either silent or create missense substitutions that are predicted to be neutral from a missense loss of function point of view were placed in the likely spliceogenic class and withdrawn from the likely neutral class. For FamHx-LR calculations, likely spliceogenic sequence variants that are also likely to be pathogenic because they create likely damaging missense substitutions were withheld from the analyses. This is because their presence in the calculations could confound the FamHx-LRs in an upwardly biased fashion. On the other hand, likely spliceogenic sequence variants that would probably lead to in-frame indels in regions of BRCA1 or BRCA2 where neither severe missense substitutions nor in frame indels are thought to confer high risk of breast or ovarian cancer (e.g., outside of the BRCA1 RING and BRCT domains and outside of the BRCA2 DNA binding and PALB2 binding domains (Easton et al., 2007; Tavtigian et al., 2008)) were withheld from the analyses because their presence in the calculations could confound the FamHx-LRs in a downwardly biased fashion.
Simple Proportions and 95% confidence intervals were estimated in STATA 11.0 using its exact binomial confidence interval calculator.
Locus-Specific Database URLs
The Breast Cancer Information Core database: https://research.nhgri.nih.gov/projects/bic/index.shtml
The BRCA1 and BRCA2 prior probabilities database: http://priors.hci.utah.edu/PRIORS/
The BRCA1 and BRCA2 classified variants “Ex-UV” LOVD: http://hci-exlovd.hci.utah.edu
[Please note that this Ex-UV database supersedes a similar database located at http://brca.iarc.fr/LOVD.]
RESULTS
To get an approximate idea of MES scores indicative of sequence variants that damage wild-type splice junctions, and of sequence variants that create de novo splice junctions, we mined the DBASS3 and DBASS5 database (Buratti et al., 2011). This database holds records of published variants (single nucleotide variants and indels) that have an impact on splicing of disease susceptibility genes (e.g. congenital hypothyroidism with SLC5A5 gene, hypofibrinogenemia with FGB gene). Our testing dataset was composed of 201 different genes.
On average, sequence variants that damaged function of a wild-type splice junction reduced the MES scores of those junctions by more than two standard deviations of the average MES score of a wild type splice junction (Table 1). Similarly, sequence variants that created a de novo junction raised the MES score of the underlying wild type (non-spliceogenic) k-mer sequence by more than two standard deviations of the average MES score of a wild type splice junction, resulting in MES scores for the de novo junctions that are quite comparable to the MES scores of wild type splice junctions in the data set. While it is understandable that the two DBASS data sets contained fewer examples of sequence variants that create de novo splice sites than that damage wild-type splice sites, we also note that there were fewer examples of de novo acceptors (12) than of de novo donors (65); this holds also for BRCA1 and BRCA2 variants.
Table 1.
MaxEntScan scores for spliceogenic variants from DBASS3 and DBASS5 compared to scores for wildtype splice junctions for disease genes
| Number of Sequence Variants |
MaxEntScan score for wildtype splice junction* |
MaxEntScan score for variant sequence damaging splice junction function** |
MaxEntScan score for wildtype k-mer sequence underlying de novo sites* |
MaxEntScan score for variant k-mer sequence creating de novo sites** |
|
|---|---|---|---|---|---|
| Ave (SD) | Ave (SD) | Ave (SD) | Ave (SD) | ||
| DBASS5 Wild-type donors* | 336 | 8.02 (2.10) | 0.59 (2.88) | n/a | n/a |
| DBASS3 Wild-type acceptors* | 240 | 8.21 (2.74) | 1.53 (3.54) | n/a | n/a |
| DBASS5 De novo donors | 71 | 7.57 (2.42) | n/a | 1.47 (4.94) | 7.29 (3.63) |
| DBASS3 De novo acceptors | 12 | 7.14 (2.26) | n/a | 1.90 (3.62) | 8.33 (2.39) |
|
BRCA1, BRCA2, ATM wild-type donors |
110 | 8.02 (2.31) | n/a | n/a | n/a |
|
BRCA1, BRCA2, ATM wild-type acceptors |
110 | 7.98 (2.44) | n/a | n/a | n/a |
Scores for the relevant wild type splice junction k-mer of exons included in the analysis.
Scores for the k-mer containing spliceogenic variants from DBASS. Excludes scores for BRCA1, BRCA2 and ATM.
Asking whether BRCA gene splice junctions fall in the same MES score range as the junctions recorded in DBASS, we determined MES scores for the reference sequence wild-type splice junctions of the canonical coding exons of BRCA1 and BRCA2. To increase the number of wild type splice junctions from known breast cancer susceptibility genes, we also included ATM (MIM# 607585) (Table 1, last two lines). We note that the average MES scores for the BRCA splice junctions and the wild-type splice junctions reported in DBASS are mutually within one-half standard deviation of each other.
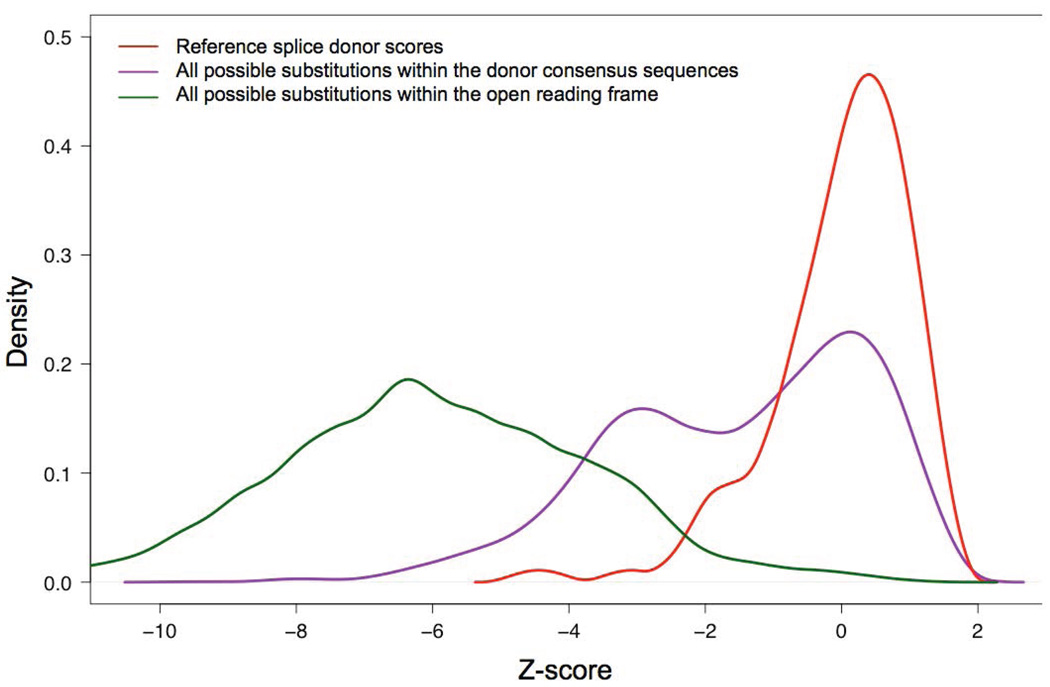
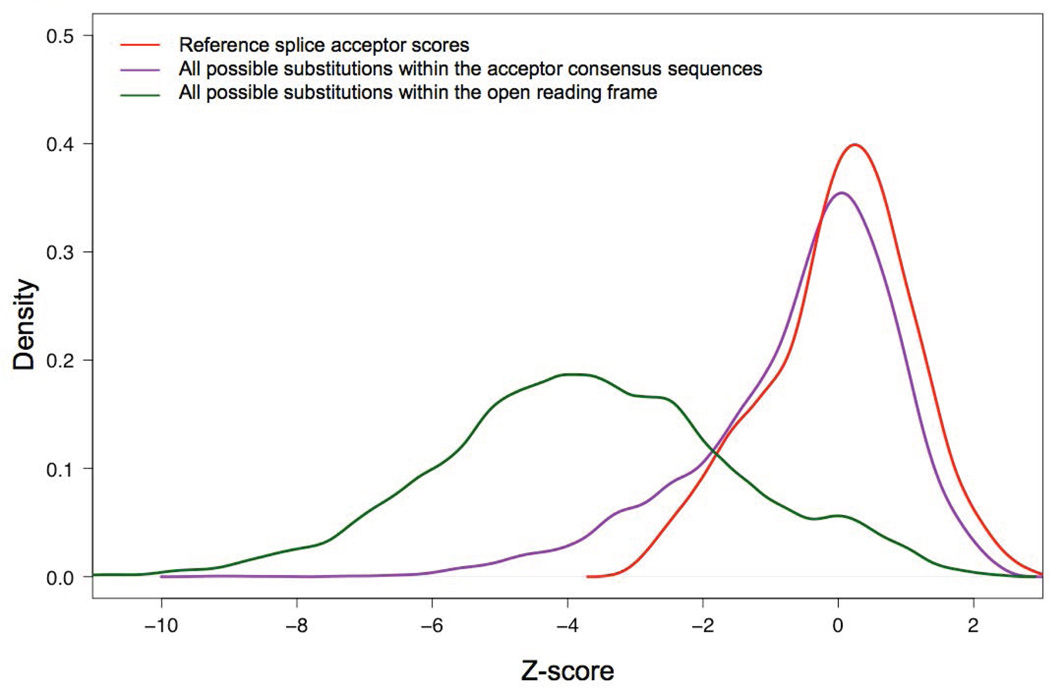
One difficulty in interpreting MES scores is that they are not standard. The program computes maximum entropy of a sequence; the scoring range is not particularly human interpretable and there are no fixed limits to the highest or lowest possible scores. One of the lowest scores that we observed for a possible substitution in the entire BRCA1/2 splice site prediction dataset was ~-46, and the highest score that we observed for a wild-type splice junction was ~+11. The ranges also appeared to be slightly different for donors and acceptors. To standardize the scores, and noting that the scores for wild type splice junctions are approximately normally distributed, we converted the raw MES scores to z-scores based on the average and standard deviations of MES scores for the canonical protein coding exons of ATM, BRCA1, and BRCA2. This was done separately for donors and acceptors (red curves in Figures 2 and 3, respectively).
Figure 2. Distribution of MaxEntScan donor splice site scores.
Red, reference sequence splice donors. Purple, all possible single nucleotide substitutions to the reference splice donors. Green, all possible single nucleotide substitutions to the open reading frames of BRCA1 and BRCA2.
Figure 3. Distribution of MaxEntScan acceptor splice site scores.
Red, reference sequence splice acceptors. Purple, all possible single nucleotide substitutions to the reference splice acceptors. Green, all possible single nucleotide substitutions to the open reading frames of BRCA1 and BRCA2.
Calibration of effects of sequence variation in splice junction consensus sequences
We then examined sequence variation in the splice junction consensus sequences. The distribution of MES scores for all possible single nucleotide substitutions to the splice donors is displayed on Figure 2 (purple curve). The curve is bimodal, with about half of the variants causing a notable drop in MES score. In contrast, when we examined the distribution of MES scores for all possible single nucleotide substitutions to the splice acceptors, we found that most single nucleotide substitutions altering wildtype acceptor sequences have little effect on the MES score (Figure 3, purple curve).
In Table 2, we summarize the number of sequence variants that damage wild-type splice sites (from DBASS, excluding BRCA1/2 variants) and single nucleotide substitutions that damage wild type BRCA1 or BRCA2 splice sites (from the Breast cancer Information Core (BIC)) as a function of MES z-score. We also summarize the total number of all possible single nucleotide substitutions to the BRCA1 and BRCA2 splice consensus regions (9 mer for donor site and 23 mer for acceptor site), and the fraction of these reported in the BIC, as a function of MES z-score. Clear trends in the data are that the number of reported spliceogenic variants increases in both DBASS and BIC as the MES z-score decreases, and the fraction of possible BRCA1/2 variants that are actually reported in BIC follows the same pattern. For variants that damage the splice donors, the increase appears to begin as the MES z-score drops below 0.0, whereas for acceptors the increase appears to begin as the MES z-score drops below +0.5.
Table 2.
MaxEntScan z-score based assessment of sequence variants that damage wild-type splice junctions, recorded in DBASS5, DBASS3, or the BIC
| Potential splice donor damage | |||||
|---|---|---|---|---|---|
| MES Z-score interval |
DBASS5* | % in DBASS5 | BIC donors** | # Possible BRCA1 or BRCA2 single nucleotide substitutions |
% Possible BRCA1 or BRCA2 single nucleotide substitutions reported in BIC |
| (1.5, +inf) | 0 | 0.00 | 0 | 6 | 0.00 |
| (1, 1.5] | 0 | 0.00 | 0 | 77 | 0.00 |
| (0.5, 1] | 2 | 0.74 | 0 | 125 | 0.00 |
| (0, 0.5] | 1 | 0.37 | 0 | 161 | 0.00 |
| (−0.5, 0] | 2 | 0.74 | 1 | 133 | 0.75 |
| (−1, −0.5] | 2 | 0.74 | 1 | 99 | 1.01 |
| (−1.5, −1] | 11 | 4.06 | 3 | 104 | 2.88 |
| (−2, −1.5] | 15 | 5.54 | 3 | 58 | 5.17 |
| (−2.5, −2] | 36 | 13.28 | 8 | 126 | 6.35 |
| (-inf, −2.5] | 202 | 74.54 | 49 | 353 | 13.88 |
| Potential splice acceptor damage | |||||
|---|---|---|---|---|---|
| MES Z-score interval |
DBASS5* | % in DBASS5 | BIC acceptors** | # Possible BRCA1 or BRCA2 single nucleotide substitutions |
% Possible BRCA1 or BRCA2 single nucleotide substitutions reported in BIC |
| (1.5, +inf) | 1 | 0.55 | 0 | 202 | 0.00 |
| (1, 1.5] | 1 | 0.55 | 0 | 238 | 0.00 |
| (0.5, 1] | 1 | 0.55 | 0 | 438 | 0.00 |
| (0, 0.5] | 5 | 2.75 | 0 | 530 | 0.00 |
| (−0.5 , 0] | 5 | 2.75 | 0 | 489 | 0.00 |
| (−1 , −0.5] | 7 | 3.85 | 0 | 339 | 0.00 |
| (−1.5 , −1] | 8 | 4.40 | 3 | 314 | 0.96 |
| (−2 , −1.5] | 27 | 14.84 | 6 | 243 | 2.47 |
| (−2.5 , −2] | 36 | 19.78 | 9 | 151 | 5.96 |
| (-inf , −2.5] | 91 | 50.00 | 39 | 368 | 10.60 |
BRCA1 and BRCA2 sequence variants recorded in DBASS were removed to avoid double counting.
Sequence variants deposited in the BIC (http://research.nhgri.nih.gov/bic/) that are located at the BRCA1 or BRCA2 native donor and acceptor sites. Evidence for spliceogenicity is not recorded in BIC for all these sequence variants.
Next, we divided contiguous intervals of z-score range into an ordered series of four qualitative strata. For donor variants, we started with a stratum 1 of z≥0 and added strata 2–4: −1≤z<0, −2≤z<-1, and z<-2. For acceptor variants, stratum 1 was z≥0.5 and strata 2–4 were −0.5≤z<0.5, −1.5≤z<-0.5, and z<-1.5, respectively. For both donors and acceptors, we added a stratum 0 for variants that improved (increased) the MES score of the splice junction and removed such variants from the z-score category into which they would otherwise fall. Using familial history of cancer as a surrogate for genetic risk to measure risk association with potential damage to a wild-type splice junction as a function of MES score, we then estimated the proportion of variants within the extreme strata that were pathogenic (Easton et al., 2007). For donor variants, the combination of strata 0 and 1 had a proportion pathogenic of 0.00 (95% CI 0.00–0.36); in clear contrast, stratum 4 had an estimated proportion pathogenic of 1.00 (95% CI 0.78–1.00). For acceptors, the combination of strata 0 and 1 had a proportion pathogenic of 0.00 (95% CI 0.00–0.06); in clear contrast, stratum 4 had a proportion pathogenic of 0.93 (95% CI 0.71–1.00). Two important points emerging from this level of analysis were: (1) the 95% confidence intervals of the stratum 0/1 and stratum 4 groupings were clearly non-overlapping, providing unambiguous evidence that MES scores of donor and acceptor sequence variants are predictive of pathogenicity, and (2) as the point estimates for the donor and acceptor stratum 0/1 groupings were mutually within each other’s confidence intervals, and the point estimates for the stratum 4 donor and acceptor categories were within each other’s confidence intervals, it would make sense to combine donor and acceptor z-score strata into qualitative categories.
To add in strata 2 and 3, we first examined three different two-stratum partitions: strata 0–3 vs. stratum 4; strata 0–2 vs. strata 3 and 4; and strata 0 and 1 vs. strata 2–4. Of these, the first grouping had a 4.7-fold better likelihood than the second grouping and a 250-fold better likelihood than the third grouping. We then examined two partitions of the strata into three groups: strata 0–2 vs. stratum 3 vs. stratum 4 and strata 0 and 1 vs. 2 and 3 vs. 4. The latter grouping fit marginally better. We then compared the better 3-group partition vs. the best two-group partition and found that the three-group partition fit the data significantly better (X2=12.67, 1df, p=0.00037). The estimated proportion of pathogenic variants in these three groups are shown in Table 3, as are the calculated splicing priors for pathogenicity per group.
Table 3.
Qualitative z-score ranges and probabilities of pathogenicity for potential damage to reference splice junctions
| Qualitative Category | MES z-score range | Alpha | 95% CI | Number of variants used |
Prior probability |
|---|---|---|---|---|---|
| Improved | Score IMPROVED vs reference sequence |
0.00 | (0.00, 0.08) | 46 | 0.04 |
| Minimal | Donor: z > 0 | 0.00 | (0.00, 0.08) | 24 | 0.04 |
| Acc: z > 0.5 | |||||
| Moderate | Donor: −2 ≤ z ≤ 0 | 0.34 | (0.15, 0.55) | 66 | 0.34 |
| Acc: −1.5 ≤ z ≤ 0.5 | |||||
| High | Donor: z < −2 | 0.97 | (0.82, 1.00) | 94 | 0.97 |
| Acc: z < −1.5 |
In defining these three qualitative categories, we note that there are a few wild-type splice junctions in BRCA1 and BRCA2 that have very low splice fitness scores. For example, the BRCA1 intron 7 splice acceptor has a MES score of just 3.71, resulting in a z-score of −1.75; consequently, any sequence variant in this acceptor that lowers its score at all would fall in the high probability of pathogenicity category and receive a prior probability of 0.97. Out of caution, we add the criterion that for variants falling in acceptors with wild-type scores below z= −1.0 or donors with wild-type scores below z= −1.5, variants must reduce the splice junction z-score by at least 0.5 in order to be placed in the high probability of pathogenicity category. The main consequence of this rule will be to prevent T>C or C>T substitutions in acceptors with low reference sequence MES scores, which are often innocuous, from receiving high prior probabilities of pathogenicity.
Calibration of effects of exonic sequence variation that may create de novo splice junctions
Sequence variants within an exon can create de novo splice donors or de novo splice acceptors, which can in turn disrupt the structure of the mRNA and thus the protein. To assess the bulk potential for sequence variants that would create de novo splice junctions within the open reading frames of BRCA1 and BRCA2, we examined the distribution of the highest possible MES splice donor scores and splice acceptor scores for all possible single nucleotides substitutions (excluding substitutions within three bp of the end of an exon) to the open reading frames of these genes. These are summarized by the green curves in Figures 2 and 3, respectively. The vast majority of possible substitutions have MES scores that are much lower than those for functional (wildtype) splice junctions, but at the high end of the score distribution, there is a tail of scores for possible substitutions that are similar to those of functioning splice junctions.
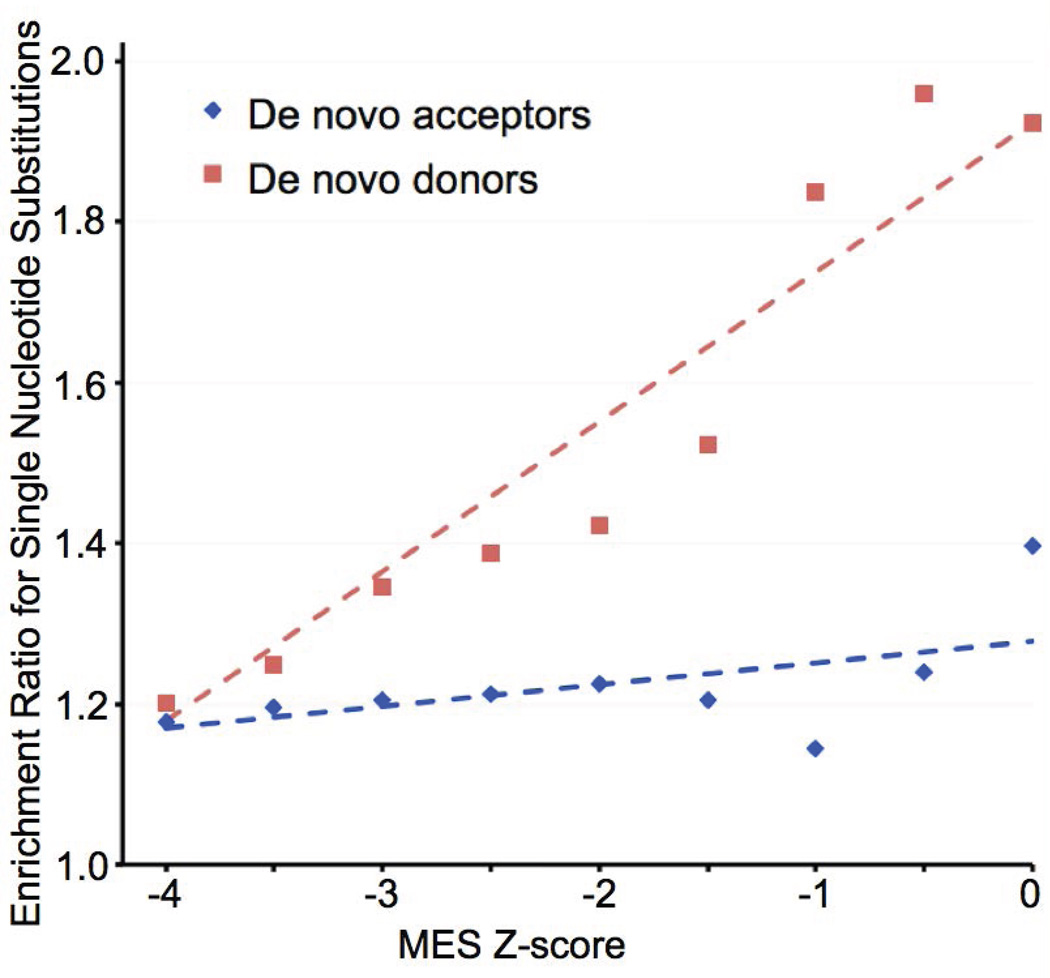
If elevated MES scores for exonic sequence variants are indicative of the creation of de novo splice junctions that are often pathogenic, such variants should be enriched in the Myriad B1&2 68K data set. Excluding variants in the first two or last two nucleotides of the coding exons, 1,698 of the possible 34,840 missense substitutions to BRCA1 and BRCA2 are actually observed in the Myriad B1&2 68K data set. Data from these variants were used to estimate the ERS as a function of MES score separately for potential de novo donors and de novo acceptors (Figure 4); this analysis provides a test of the hypothesis that elevated MES z-scores are indicative of aberrant splicing and thus pathogenicity. Because the data set includes missense substitutions that are pathogenic because of missense dysfunction, which should be randomly distributed with respect to the MES z-score, the baseline ERS is inflated to ~1.2. For the analysis of potential de novo donors, there is a hint of an increased ERS as the MES z-score exceeds −3.0 and greater evidence as the score exceeds −1.0; indeed, a trend test reveals P=2.4×10−5 against the hypothesis that the underlying ERS data are drawn from a trendless series. In contrast, the analysis of potential de novo splice acceptors there is at most a hint of a rise in the ERS when the MES z-score reaches or exceeds 0.0, and a trend test does not reveal significant evidence for an increase in the ERS as a function of MES z-score (P=0.07).
Figure 4. Enrichment for observed exonic substitutions as a function of MaxEntScan de novo donor or de novo acceptor scores.
Red, analysis of de novo donor enrichment as a function of MES donor score. Blue, analysis of de novo acceptor enrichment as a function of MES acceptor score.
Following the same approach described above for variants falling in and around splice donors and acceptors, we then used FamHx-LR analyses to estimate the proportion of pathogenic variants among exonic substitutions with relatively high MES scores. For these analyses, we used the mirror image of the qualitative categories that emerged from our donor and acceptor damage analyses i.e., the most likely deleterious de novo donor category (increased potential to create a de novo donor) that we defined was z≥0, the intermediate category (moderate potential) was −2≤z<0, and the least likely (null/weak/low potential) was z<-2. In setting up this analysis, we noted that there were a few sequence variants in the two lower z-score categories with MES scores that are actually higher than the wild type splice donor for the exon in which they fell. These were promoted from their qualitative category to the next higher category. For example, a variant in the middle of BRCA2 exon 16, c.7709A>C, potentially creates a de novo donor with a z-score of −0.63, thus falling in the “Moderate” category. The wild-type donor of exon 16 has a z-score of −1.44. Therefore this variant is moved to the “Increased” category. The resulting summary family history likelihood ratio point estimates for the three categories were 0.01, 0.30, and 0.64, and the result for the z≥0 category was independently significant (95% CI 0.06–0.98) (Table 4).
Table 4.
Qualitative z-score ranges and probabilities of pathogenicity for creation of exonic de novo donors that would either create a frameshift or alter a key functional domain
| Qualitative Category | MES z-score range | Alpha | 95% CI | Number of variants used |
Prior probability |
|---|---|---|---|---|---|
| Weak/ Null & Low* | z < −2 | 0.01 | (0.00, 0.04) | 977 | 0.02 |
| Moderate* | −2 <= z < 0 | 0.30 | (0.00, 0.88) | 7 | 0.30 |
| Increased | z ≥ 0 | 0.64 | (0.06, 0.98) | 8 | 0.64 |
Potentially spliceogenic sequence variants that have a higher MES score than the wild-type donor for their exon are promoted to the next more severe qualitative category.
As might be predicted from Figures 3 and 4, FamHx-LR analyses of the groups of exonic variants that had some potential to create de novo splice acceptors did not detect any evidence of increased risk (data not shown).
As was the case in our earlier calibration of Align-GVGD for evaluating BRCA1 and BRCA2 key functional domain missense substitutions (Tavtigian et al., 2008), the proportion of potentially spliceogenic variants estimated to be pathogenic based on the FamHx-LR analyses can be used as prior probabilities in favor of pathogenicity for integrated evaluations of BRCA gene sequence variants. Because priors of 0.00 cannot be used in a Bayesian calculation, and in the interest of making the priors for categories with initial point estimates of 0.00 or 0.01 slightly more conservative, we reassign these to the midpoint of their confidence intervals (Table 3 and 4, rightmost column).
Correlation with published assays of spliceogenicity for BRCA1 and BRCA2 sequence variants
To assess how well the splice priors given in Tables 3 and 4 correlate with spliceogenicity assays on BRCA1 and BRCA2 sequence variants, we collated data from 73 papers containing interpretable spliceogenicity assays on variants from these genes. The data included one or more assays on 92 acceptor variants, 116 donor variants, and 239 exonic variants that were not predicted to damage either a donor or an acceptor (Table 5 and Supp. Table S1). Although some publications provided estimates of transcript ratios based on end-point PCR, it should be noted that none of these assays were quantitative in the true sense. This is, aberration severity as defined below is likely overestimated based on the available reported data; transcript proportions were calculated or estimated from endpoint PCR from at best semi-quantitative reactions, and many of the reported aberrations are deletion transcripts that would amplify more efficiently than the longer wildtype transcripts in a competitive PCR reaction.
Table 5.
Summary of published BRCA gene sequence variant spliceogenicity assay results
| No aberration | Aberration | % with aberration (95% CI) |
|
|---|---|---|---|
| Acceptor variants | |||
| High | 0 | 43 | 100.0 (88.0–100.0)† |
| Moderate | 17 | 13 | 43.3 (25.5–62.6) |
| Minimal or Improved | 18 | 1 | 5.3 (0.1–26.0) |
| Donor variants | |||
| High | 2 | 67 | 97.1 (89.9–99.6) |
| Moderate | 5 | 34 | 87.2 (72.6–95.7) |
| Minimal or Improved | 7 | 1 | 12.5 (0.3–52.7) |
|
Combined donor and acceptor variants |
|||
| High | 2 | 110 | 98.2 (93.7–99.8) |
| Moderate | 22 | 47 | 68.1 (55.8–78.8) |
| Minimal or Improved | 25 | 2¥ | 7.4 (0.9–24.3) |
| De novo donor variants | |||
| Increased | 4 | 9 | 69.2 (38.6–90.9) |
| Moderate | 2 | 2 | 50.0 (6.8–93.2) |
| Weak/ Null & Low | 187 | 35 | 15.8 (11.2–21.2) |
Added one discordant observation in order to estimate a 95% confidence interval.
One of the variants in this category, BRCA1 c.591C>T, is IARC Class 1, neutral (Dosil et al., 2010; de la Hoya et al, submitted).
(Friedman et al., 1994; Friedman et al., 1995; Gayther et al., 1995; Petrij-Bosch et al., 1997; Xu et al., 1997; Hoffman et al., 1998; Mazoyer et al., 1998; Fetzer et al., 1999; Ozcelik et al., 1999; Pyne et al., 1999; Santarosa et al., 1999; Scholl et al., 1999; Hartikainen et al., 2000; Pyne et al., 2000; Laskie Ostrow et al., 2001; Vega et al., 2001; Claes et al., 2002; Fackenthal et al., 2002; Howlett et al., 2002; Krajc et al., 2002; Miendl 2002; Agata et al., 2003; Claes et al., 2003; Campos et al., 2003; Hofmann et al., 2003; Keaton et al., 2003; Yang et al., 2003; Brose et al., 2004; Sharp et al., 2004; Tesoriero et al., 2005; Bonatti et al., 2006; Chen et al., 2006; Chenevix-Trench et al., 2006; Beristain et al., 2007; Ang et al., 2007; Anczukow et al., 2008; Bonnet et al., 2008; Farrugia et al., 2008; Goina et al., 2008; Kwong et al., 2008; Machackova et al., 2008; Spearman et al., 2008; Caux-Moncoutier et al., 2009; Gutierrez-Enriquez et al., 2009; Li et al., 2009; Vreeswijk et al., 2009; Willems et al., 2009; Dosil et al., 2010; Gaildrat et al., 2010; Hansen et al., 2010; Rouleau et al., 2010; Sanz et al., 2010; Steffensen et al., 2010; Walker et al., 2010; Whiley et al., 2010; Brandao et al., 2011; Thery et al., 2011; Whiley et al., 2011; Zhang et al., 2011; Acedo et al., 2012; Gaildrat et al., 2012; Houdayer et al., 2012; Joose et al. 2012; Menendez et al., 2012; Thomassen et al., 2012; Wappenshchmidt et al., 2012; Colombo et al., 2013; Di Giacomo et al., 2013; de Garibay et al., 2014; Santos et al., 2014; Whiley et al., 2014a; Acedo et al., 2015; Ahlborn et al., 2015; Parsons et al., 2015).
The category with the proportionately greatest disparity between the probability of pathogenicity and rate of spliceogenicity was de novo donor Weak/ Null & Low. For this category, the probability of pathogenicity was 0.01 (0.00 – 0.04) versus a spliceogenicity rate of 0.158 (0.11 – 0.21). Delving more deeply into the published splice assay results, of 35 Weak/ Null & Low variants with a reported aberration, 32 were published with an RT-PCR or minigene assay from which it was possible to estimate the contribution of the mutant allele to a reference transcript (Supp. Table S2). To evaluate these, we used the proportion of frameshifted transcripts produced from the non-pathogenic BRCA1 allele c.[594-2A>C;641A>G], ~70–80% (de la Hoya et al., submitted), as a guide towards a definition of a severe splicing aberration. To err on the conservative side, we then set >60% aberrant splicing from the variant allele (if the reported result was semi-quantitative) or a visibly strong majority of aberrant splicing from the variant allele (if a qualitative result had to be estimated from a gel) as a standard for a severe splicing aberration. Of the 32 evaluable variants, only seven met this splice defect severity criterion (Supp. Table S2). Taken together, a splice aberration rate of 0.158 multiplied by a severe aberration ratio of 0.219 (7 of 32) results in an overall severe splice aberration rate of 0.035 for this category – which is within the 95% confidence interval for the category’s probability of pathogenicity.
The other category with a notably higher rate of spliceogenicity than probability of pathogenicity was Moderate donor damage. For this category, the probability of pathogenicity was 0.34 (0.15 – 0.55) versus a splice aberration rate of 0.87 (0.73 – 0.96). Examining the published data on Moderate donor variants with a splice aberration, 22 could be evaluated for severity as defined above (Supp. Table S3). For 17 of these (77%), the aberration was reported to be severe. However, two of the variants with a severe splice aberration (BRCA1 c.4484G>T and BRCA2 c.316+5G>C) produce at least one transcript isoform with an in-frame deletion that does not alter any domain already proven to harbor pathogenic missense substitutions. In addition, another two of these variants produced 17–20% of canonical transcript in the semi-quantitative assay cited (BRCA1 c.5072C>T and BRCA2 c.8486A>T) (Houdayer et al., 2012; Santos et al., 2014). If these latter four variants are actually not pathogenic, then the rate of severe pathogenic splice aberrations in the category would fall to 13/22 (59%), just slightly above the 95% confidence interval of our estimated probability of pathogenicity.
Finally, combining across acceptor and donor variants, we note that there were two splice aberrations reported in the Minimal or Improved category. One of these, BRCA1 c.591C>T is considered to be a neutral variant that up-regulates naturally occurring in-frame isoforms (Dosil et al., 2010). The other, BRCA2 c.68-7delT leads only to partial skipping of exon 3, again, a transcript which is also seen in controls (Santarosa et al., 1999).
Expert knowledge added to the Prior Probabilities database
Beyond combining prior probabilities from missense substitution severity and spliceogenicity, we added three expert knowledge elements to the BRCA1/2 prior probabilities database.
First, we have annotated that substitutions of the translation initiation methionines of BRCA1 and BRCA2 have high prior probability of pathogenicity. For BRCA1, this is because the first in-frame methionine codon (p.M18) falls well within the RING domain, and the resulting N-truncated protein would delete several residues that are important for the BARD1 interaction (Starita et al., 2015). For BRCA2, this is because there are several out of frame ATGs located in the mRNA upstream of the first in-frame methionine codon (p.M124); some of these have a high enough translation initiation rate that very little protein synthesis originates from p.M124 (Parsons et al., 2013).
Second, we now know that the prevalence of a BRCA1 delta exon 9–10 transcript, which encodes a functional protein, is high enough that neither spliceogenic nor protein truncating variants in BRCA1 exons 9 or 10 have a high probability to be pathogenic (Colombo et al., 2014, Rosenthal et al., 2015, de la Hoya et al, submitted). To accommodate the observation, we set a ceiling for the probability of pathogenicity at 0.50 for variants in these two exons or their proximal splice junction regions.
Third, we also know that variants that cause skipping of BRCA2 exon 12, which results in an in-frame deletion, do not have a high probability to be pathogenic (Li et al., 2009). As above, we set a ceiling for the probability of pathogenicity at 0.50 for variants in the splice acceptor and splice donor of this exon. However, in contrast to BRCA1 exons 9 and 10, protein truncating variants in BRCA2 exon 12 are still expected to be pathogenic.
Combining prior probabilities of pathogenicity from missense substitution severity and spliceogenicity
Generating an overall prior probability in favor of pathogenicity for sequence variants falling in and around the protein coding exons of BRCA1 and BRCA2 requires considering the priors from analyses of missense substitution severity, potential damage to wild type splice junctions, and potential creation of de novo splice donors. To do this, we look at a sequence variant from each of these perspectives and then assign the variant the highest prior probability in favor of pathogenicity generated by any one of the analyses. The resulting prior probabilities predicted on both missense and splicing analysis are available online for all possible single nucleotide substitutions in and around the protein coding exons of BRCA1 and BRCA2 at http://priors.hci.utah.edu/PRIORS.
Variants added to the Ex-UV database
In our previous effort to populate the BRCA1/2 Ex-UV LOVD with BRCA1/2 missense substitutions that had previously published integrated evaluations (Vallée et al., 2012), we excluded certain missense substitutions from the database because they had (potentially) non-negligible prior probabilities in favor of pathogenicity from a splice effects point of view. Here we have re-assessed those missense substitutions, plus a number of sequence variants falling on the intronic side of the splice junction consensus regions that have published observational data appropriate for use in an integrated evaluation. As a result, a total of 77 re-analyzable variants were added to the BRCA1 and BRCA2 Ex-UV database.
Among these, we find 20 missense substitutions and 19 intronic splice region substitutions previously reported as neutral; 38 of these remain in either Class 1 or Class 2 (not pathogenic or likely not pathogenic). BRCA1 c.5467+5G>C moved from Class 2 (likely not pathogenic) (Whiley et al., 2011) to Class 3 (uncertain) due to the sequence analysis based prior probability in favor of pathogenicity changing from the published value of 0.26 to 0.34 (see Table 6).
Table 6.
BRCA1 and BRCA2 sequence variants reclassified as a consequence of updated prior probabilities of pathogenicity
| Gene | HGVS (nucleotide) | HGVS (amino acid) |
BIC | Motif Location |
Z-score Category |
Published Prior |
Splicing Prior |
Missense Prior |
Selected Prior |
|---|---|---|---|---|---|---|---|---|---|
| BRCA1 | c.4479_4484+2dup † | N/A | IVS14+2ins8 | donor | Z < −2.0 | 0.26 | 0.97 | N/A | 0.97 |
| mRNA analysis: 8bp retention of intron 14 (Whiley et al 2011) | |||||||||
| BRCA1 | c.4868C>G | p.A1623G | A1623G |
de novo donor |
−2 ≤ z < 0 | 0.01 | 0.64* | 0.02 | 0.64 |
| mRNA analysis: 119bp deletion of exon 16, variant also present in wild-type transcripts (Walker et al 2010) | |||||||||
| BRCA1 | c.5278-14C>G | N/A | IVS20-14C>G | acceptor | Z > +0.5 | 0.26 | 0.04 | N/A | 0.04 |
| mRNA analysis: no aberration detected (Spearman et al 2008) | |||||||||
| BRCA1 | c.5467+5G>C | N/A | IVS23+5G>C | donor | −2 ≤ z < 0 | 0.26 | 0.34 | N/A | 0.34 |
| Exon 23 deletion (Whiley et al 2011) | |||||||||
| BRCA2 | c.632-16A>C | N/A | IVS7-16A>C | acceptor | Increased MES score |
0.26 | 0.04 | N/A | 0.04 |
| mRNA analysis: no aberration detected (Thomassen et al 2012) | |||||||||
| Gene | HGVS (nucleotide) | Published source of observational data |
Cosegregation Bayes score |
Pathology LR ¥ |
Co- occurrence LR |
Family History LR |
Published Posterior |
Recalculated Posterior |
IARC Class ¶ |
Class Description |
|---|---|---|---|---|---|---|---|---|---|---|
| BRCA1 | c.4479_4484+2dup † | Whiley et al., 2011 | 0.95 | 2.58 | 1 | 1 | 0.46 | 0.988 | 4 | Likely pathogenic |
| BRCA1 | c.4868C>G | Walker et al., 2010 | 27.72 | 1.23 | 1.09 | 10.47 | 0.80 | 0.999 | 5 | Pathogenic |
| BRCA1 | c.5278-14C>G | Whiley et al., 2011 | 1 | 1 | 1.58 | 0.14 | 0.07 | 0.009 | 2 | Likely not pathogenic |
| BRCA1 | c.5467+5G>C | Whiley et al., 2011 | 0.73 | 0.18 | 1 | 1 | 0.045 | 0.063 | 3 | Uncertain |
| BRCA2 | c.632-16A>C | Thomassen et al., 2012 | 1 | 1 | 1.20 | 0.22 | 0.086 | 0.011 | 2 | Likely not pathogenic |
De novo prior upgraded to the next higher qualitative category because the z score is higher than that of the corresponding wild-type donor.
Pathology likelihood ratios (LRs )based on breast tumour ER, grade or TN status and using revised estimates from Spurdle et al (in press). Cosegregation, co-occurrence and family history LRs are from the cited source of observational data.
Class strata as described in Plon et al (2008).
This variant was originally reported as c.4484+2ins8; insertion is due to duplication of GGAAAGGT
We also find 9 missense substitutions and 19 intronic splice region substitutions previously reported as pathogenic; all of these remain in Class 4 (likely pathogenic) or Class 5 (pathogenic).
Of 8 previously reported as uncertain , 4 remain in Class 3. 2 moved up to Class 4 or Class 5 (likely pathogenic or pathogenic), and 2 moved down to Class 2 (likely not pathogenic); data on which the re-classification of these 4 substitutions rest are summarized in Table 6.
We also added two unusual variants to the Ex-UV database.
The BRCA1 BRCT domain missense substitution p.V1736A. This variant was observed in a woman who also carried the BRCA1 frameshift variant c.2457delC and who was diagnosed at the age of 28 years with stage IV papillary serous ovarian carcinoma. Initially, co-occurence between the BRCA1 frameshift and this missense substitution was taken as strong evidence against pathogenicity of the variant. Subsequent review of medical and photographic records revealed that the patient had abnormalities including microcephaly, macrognathia and developmental delay; moreover, the patient had a severe response to carboplatin treatment that was more reminiscent of the response seen for biallelic BRCA1-mutant mice than heterozygous human patients (Domchek et al., 2013). Following qualitative MMR UV classification rules, potential biallelic mutation carriers with very early onset disease and developmentally abnormal clinical features should not be counted for purposes of calculating a co-occurence likelihood ratio (Thompson et al., 2014). Taking into account that this is an Align-GVGD C65 missense substitution (prior probability =0.81) and the segregation likelihood ratio from ten pedigrees is 234:1 (Domchek et al., 2013), we calculate a posterior probability of pathogenicity of 0.999 (Class 5, clearly pathogenic).
The BRCA2 translation initiator substitution c.3G>A. This variant was treated as a frameshift mutation because (i) it is expected to inactivate the wild-type translation initiator, (ii) the next available AUG in the mRNA is out of frame, and (iii) this out of frame AUG has been shown in transfection experiments to outcompete the next available in-frame AUG (Parsons et al., 2013). For purposes of including the BRCA2 c.3G>A in the Ex-UV database, we use the prior probability of 0.96 suggested by Thomassen et al (2012).
DISCUSSION
The work described here follows a pattern used in Tavtigian et al. (2008) in which we (i) used a sequence analysis algorithm to define what we hypothesized would be an ordered series of increasingly severe grades of sequence variants, (ii) used one kind of data – counts of observed sequence variants and underlying dinucleotide substitution rate constants – available for the sequence variants in those grades to generate evidence that risk actually increased in the expected order, and (iii) used a second quasi-independent kind of data – the FamHx-LRs – to replicate the evidence that risk actually increased in the expected order and to measure the risk for individual grades (or pooled grades) in such a way that the point estimates could become prior probabilities in favor of pathogenicity for clinical analysis of individual sequence variants reported in BRCA1 and BRCA2.
An unusual element of this work, shared with our earlier calibration of missense substitution severity (Tavtigian et al., 2008) is that the prior probability of pathogenicity estimates emerge through direct steps from sequence analysis to estimates of pathogenicity in humans. Thus questions of what fraction of the sequence variants analyzed actually impact mRNA splicing, or the extent to which the resulting splice isoforms encode dysfunctional proteins, were not used to estimate the prior probabilities. Nonetheless, subject to the limitations that (1) sequence variants assessed in an individual paper are often selected through some kind of algorithm and thus do not necessarily represent a random draw from available variants that meet a particular sequence analysis criterion, (2) assays of alternate or aberrant splicing are rarely quantitative and often over-estimate the relative abundance of exon skipping isoforms, and (3) alternate or aberrant splice isoforms sometimes result in in-frame deletions that encode a functional protein, we do observe reasonable agreement between the prior probabilities of pathogenicity for individual sequence analysis defined grades and the rates of severe splice aberrations likely to encode dysfunctional proteins in those same grades.
The resulting calibration of MaxEntScan is almost entirely utilitarian. We have updated our BRCA1/2 prior probability model for missense substitutions, previously based on position in the proteins and a measure of missense substitution severity, to include damage to wild type splice junctions and probability to create exonic de novo splice donors. Thus the model is more complete and covers silent substitutions, missense substitutions, and sequence variants in the proximal splice junction consensus sequences. In principle, the model should apply to other susceptibility genes, and our java implementation of this MES based algorithm can be applied to VCF files and therefore whole exome sequences. As in our previous work, it was reassuring to see that, when we saw significant evidence for increasing risk along an ordered series of sequence variant grades in the first data analysis, we saw the same ordering in the analysis of summary family history likelihood ratios. It was also reassuring that failure to see evidence for risk due to potential de novo exonic splice acceptors by the ERS analysis (Figure 4) was replicated by failure to see evidence for increased risk due to potential exonic splice acceptors by the summary family history likelihood ratio analysis. Of course, why we were unable to usefully predict substitutions that would create de novo splice acceptors remains an interesting question.
A recent study, which examined 272 variants in and around the splice junctions of BRCA1 and BRCA2 variants with the aim of establishing guidelines for transcript analysis in a clinical setting, supports our findings on MaxEntScan’s reliability, as does a larger study of genome-wide splice consensus region variants (Houdayer et al., 2012; Jian et al., 2014). From their dataset of sequence variants’ effects on splicing, Houdayer et al. (2012) were able to perform receiver operating characteristic (ROC) analyses on several splice junction analysis programs; they found that MaxEntScan was the single best performing program, with an area under the ROC curve of 0.956 (an AUC of 1 means 100% specificity, 100% sensitivity). Furthermore, Houdayer et al. affirmed that current programs are ineffective at predicting the effects of sequence variation in candidate exonic splice enhancers and branchpoints. However, the guidelines that they proposed only cover damage to splice sites (their analysis pipeline started with a variant in a consensus site). They did not attempt to analyze sequence variation within exons that could create de novo splice junctions, and they have not used their dataset to determine prior probabilities in favor of pathogenicity – key steps that we take here and then add to the overall integrated evaluation of unclassified BRCA gene sequence variants. Still, the de novo donor analysis presented here requires a cautionary note. Even from the B1&2 68K data set, the number variants with family history data that fell into the Moderate or Increased de novo donor categories was very small, resulting in wide 95% confidence intervals (Table 4). While the ordered result that we obtained in Table 4 and the corresponding categories of Table 5 is reassuring, additional data gathered over the coming years may well refine the current point estimates and prior probabilities.
While we were considering which published BRCA gene sequence variants to move into the Ex-UV database, we ran up against the philosophical question of what combinations of sequence analysis-based prior probability and observation data (expressed as a LR in favor of pathogenicity) actually constitute an integrated evaluation. The general principle is that at least two kinds of data have to be combined. But there are circumstances under which observational data are so uninformative that one could question whether their use actually constitutes a data integration. An extreme example would be a single observation of a BRCA gene missense substitution in an individual who did not carry any pathogenic variant in the same gene, with no other observational data provided. This datum would nominally convert to a co-observation LR of 1.03; if that LR were combined with the sequence analysis-based prior, it would essentially constitute conversion of the prior probability to a posterior probability via only negligible observational data. Referring to the recent calibration of sequence-analysis based prior probabilities for the mismatch repair genes missene substitutions, the minimum and maximum possible priors were truncated at 0.10 and 0.90, respectively (Thompson et al., 2013b). For these two priors, observational data LRs would have to be ≤0.47 or ≥2.2, respectively in order for the variants to reach IARC Class 2 or IARC Class 4. Rounding these values off, we believe that ≤0.5 or ≥2 are reasonable inner boundaries for the magnitude of observational data LR required to perform a valid integrated evaluation. Applying this criterion, 77 re-analyzable variants had sufficient observational data and were added to the Ex-UV database; however, we found 17 published “integrated evaluations” that did not meet this criterion and were consequently excluded (Walker et al., 2010; Whiley et al., 2011; Thomassen et al., 2012; Whiley et al., 2014b).
We close with a bioinformatics challenge. There may remain discrete but small groups of BRCA1/2 sequence variants to which we wrongly assign low prior probabilities in favor of pathogenicity. Start from the list of all possible single nucleotide substitutions to the open reading frames of BRCA1 and BRCA2. Exclude BRCA1/2 nonsense substitutions and missense substitutions that are likely to be pathogenic because of missense dysfunction to BRCA1s RING or BRCT domains, or BRCA2s PALB2 binding domain, DNA binding domains, or exon 27 RAD51 binding site. Exclude substitutions that are likely to damage a BRCA1/2 wild type splice junction. Exclude substitutions that are likely to create a de novo splice donor within an exon of BRCA1 or BRCA2. Now, from the remaining list of exonic BRCA1/2 gene single nucleotide substitutions, identify an algorithmically defined subset of substitutions that confers a statistically significantly increased risk of breast or ovarian cancer. We will be pleased to help calibrate prior probabilities in favor of pathogenicity for sequence variants grouped by that algorithm and then add the new priors into an ever-evolving prior probability structure.
Supplementary Material
Acknowledgments
Grant Sponsors
This work was supported by the United States National Institutes of Health (NIH) National Cancer Institute (NCI) grants R01CA164138 and R01CA164944. The work was also supported by grants from the Australian National Health and Medical Research Council (NHMRC ID1010719, ID1061779), and The Cancer Council Queensland (ID1086286)
Footnotes
Competing Interests
The authors of this manuscript have no conflicts of interest to report.
REFERENCES
- Acedo A, Sanz DJ, Duran M, Infante M, Perez-Cabornero L, Miner C, Velasco EA. Comprehensive splicing functional analysis of DNA variants of the BRCA2 gene by hybrid minigenes. Breast Cancer Res. 2012;14:R87. doi: 10.1186/bcr3202. PMID: 22632462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acedo A, Hernandez-Moro C, Curiel-Garcia A, Diez-Gomez B, Velasco EA. Functional classification of BRCA2 DNA variants by splicing assays in a large minigene with 9 exons. Hum Mutat. 2015;36:210–221. doi: 10.1002/humu.22725. PMID: 25382762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata S, De Nicolo A, Chieco-Bianchi L, D’Andrea E, Menin C, Montagna M. The BRCA2 sequence variant IVS19+1G-->A leads to an aberrant transcript lacking exon 19. Cancer Genet Cytogenet. 2003;141:175–176. doi: 10.1016/s0165-4608(02)00763-x. PMID: 12606139. [DOI] [PubMed] [Google Scholar]
- Ahlborn LB, Dandanell M, Steffensen AY, Jonson L, Nielsen FC, Hansen TV. Splicing analysis of 14 BRCA1 missense variants classifies nine variants as pathogenic. Breast Cancer Res Treat. 2015;150:289–298. doi: 10.1007/s10549-015-3313-7. PMID: 25724305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anczukow O, Buisson M, Salles MJ, Triboulet S, Longy M, Lidereau R, Sinilnikova OM, Mazoyer S. Unclassified variants identified in BRCA1 exon 11: Consequences on splicing. Genes Chromosomes Cancer. 2008;47:418–426. doi: 10.1002/gcc.20546. PMID: 18273839. [DOI] [PubMed] [Google Scholar]
- Ang P, Lim IH, Lee TC, Luo JT, Ong DC, Tan PH, Lee AS. BRCA1 and BRCA2 mutations in an Asian clinic-based population detected using a comprehensive strategy. Cancer Epidemiol Biomarkers Prev. 2007;16:2276–2284. doi: 10.1158/1055-9965.EPI-07-0403. PMID: 18006916. [DOI] [PubMed] [Google Scholar]
- Beristain E, Martinez-Bouzas C, Guerra I, Viguera N, Moreno J, Ibanez E, Diez J, Rodriguez F, Mallabiabarrena G, Lujan S, Gorostiaga J, De Pablo JL, et al. Differences in the frequency and distribution of BRCA1 and BRCA2 mutations in breast/ovarian cancer cases from the Basque country with respect to the Spanish population: implications for genetic counselling. Breast Cancer Res Treat. 2007;106:255–262. doi: 10.1007/s10549-006-9489-0. PMID: 17262179. [DOI] [PubMed] [Google Scholar]
- Bonatti F, Pepe C, Tancredi M, Lombardi G, Aretini P, Sensi E, Falaschi E, Cipollini G, Bevilacqua G, Caligo MA. RNA-based analysis of BRCA1 and BRCA2 gene alterations. Cancer Genet Cytogenet. 2006;170:93–101. doi: 10.1016/j.cancergencyto.2006.05.005. PMID: 17011978. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Krieger S, Vezain M, Rousselin A, Tournier I, Martins A, Berthet P, Chevrier A, Dugast C, Layet V, Rossi A, Lidereau R, et al. Screening BRCA1 and BRCA2 unclassified variants for splicing mutations using reverse transcription PCR on patient RNA and an ex vivo assay based on a splicing reporter minigene. J Med Genet. 2008;45:438–446. doi: 10.1136/jmg.2007.056895. PMID: 18424508. [DOI] [PubMed] [Google Scholar]
- Brandao RD, van Roozendaal K, Tserpelis D, Gomez Garcia E, Blok MJ. Characterisation of unclassified variants in the BRCA1/2 genes with a putative effect on splicing. Breast Cancer Res Treat. 2011;129:971–982. doi: 10.1007/s10549-011-1599-7. PMID: 21638052. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Paul K, Stopfer JE, Colligon TA, Calzone KA, Weber BL. Characterization of two novel BRCA1 germ-line mutations involving splice donor sites. Genet Test. 2004;8:133–138. doi: 10.1089/gte.2004.8.133. PMID: 15345110. [DOI] [PubMed] [Google Scholar]
- Buratti E, Chivers M, Hwang G, Vorechovsky I. DBASS3 and DBASS5: databases of aberrant 3’- and 5’-splice sites. Nucleic Acids Res. 2011;39:D86–D91. doi: 10.1093/nar/gkq887. PMID: 20929868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos B, Diez O, Domenech M, Baena M, Balmana J, Sanz J, Ramirez A, Alonso C, Baiget M. RNA analysis of eight BRCA1 and BRCA2 unclassified variants identified in breast/ovarian cancer families from Spain. Hum Mutat. 2003;22:337. doi: 10.1002/humu.9176. PMID: 12955719. [DOI] [PubMed] [Google Scholar]
- Caux-Moncoutier V, Pages-Berhouet S, Michaux D, Asselain B, Castera L, De Pauw A, Buecher B, Gauthier-Villars M, Stoppa-Lyonnet D, Houdayer C. Impact of BRCA1 and BRCA2 variants on splicing: clues from an allelic imbalance study. Eur J Hum Genet. 2009;17:1471–1480. doi: 10.1038/ejhg.2009.89. PMID: 19471317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Truong TT, Weaver J, Bove BA, Cattie K, Armstrong BA, Daly MB, Godwin AK. Intronic alterations in BRCA1 and BRCA2: effect on mRNA splicing fidelity and expression. Hum Mutat. 2006;27:427–435. doi: 10.1002/humu.20319. PMID: 16619214. [DOI] [PubMed] [Google Scholar]
- Chenevix-Trench G, Healey S, Lakhani S, Waring P, Cummings M, Brinkworth R, Deffenbaugh AM, Burbidge LA, Pruss D, Judkins T, Scholl T, Bekessy A, et al. Genetic and histopathologic evaluation of BRCA1 and BRCA2 DNA sequence variants of unknown clinical significance. Cancer Res. 2006;66:2019–2027. doi: 10.1158/0008-5472.CAN-05-3546. PMID: 16489001. [DOI] [PubMed] [Google Scholar]
- Claes K, Vandesompele J, Poppe B, Dahan K, Coene I, De Paepe A, Messiaen L. Pathological splice mutations outside the invariant AG/GT splice sites of BRCA1 exon 5 increase alternative transcript levels in the 5’ end of the BRCA1 gene. Oncogene. 2002;21:4171–4175. doi: 10.1038/sj.onc.1205520. PMID: 12037674. [DOI] [PubMed] [Google Scholar]
- Claes K, Poppe B, Machackova E, Coene I, Foretova L, De Paepe A, Messiaen L. Differentiating pathogenic mutations from polymorphic alterations in the splice sites of BRCA1 and BRCA2. Genes Chromosomes Cancer. 2003;37:314–320. doi: 10.1002/gcc.10221. PMID: 12759930. [DOI] [PubMed] [Google Scholar]
- Colombo M, De Vecchi G, Caleca L, Foglia C, Ripamonti CB, Ficarazzi F, Barile M, Varesco L, Peissel B, Manoukian S, Radice P. Comparative in vitro and in silico analyses of variants in splicing regions of BRCA1 and BRCA2 genes and characterization of novel pathogenic mutations. PLoS One. 2013;8:e57173. doi: 10.1371/journal.pone.0057173. PMID: 23451180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Blok MJ, Whiley P, Santamarina M, Gutierrez-Enriquez S, Romero A, Garre P, Becker A, Smith LD, De Vecchi G, Brandao RD, Tserpelis D, et al. Comprehensive annotation of splice junctions supports pervasive alternative splicing at the BRCA1 locus: a report from the ENIGMA consortium. Hum Mol Genet. 2014;23:3666–3680. doi: 10.1093/hmg/ddu075. PMID: 24569164. [DOI] [PubMed] [Google Scholar]
- de Garibay GR, Acedo A, Garcia-Casado Z, Gutierrez-Enriquez S, Tosar A, Romero A, Garre P, Llort G, Thomassen M, Diez O, Perez-Segura P, Diaz-Rubio E, et al. Capillary electrophoresis analysis of conventional splicing assays: IARC analytical and clinical classification of 31 BRCA2 genetic variants. Hum Mutat. 2014;35:53–57. doi: 10.1002/humu.22456. PMID: 24123850. [DOI] [PubMed] [Google Scholar]
- Di Giacomo D, Gaildrat P, Abuli A, Abdat J, Frebourg T, Tosi M, Martins A. Functional analysis of a large set of BRCA2 exon 7 variants highlights the predictive value of hexamer scores in detecting alterations of exonic splicing regulatory elements. Hum Mutat. 2013;34:1547–1557. doi: 10.1002/humu.22428. PMID: 23983145. [DOI] [PubMed] [Google Scholar]
- Domchek SM, Tang J, Stopfer J, Lilli DR, Hamel N, Tischkowitz M, Monteiro AN, Messick TE, Powers J, Yonker A, Couch FJ, Goldgar DE, Davidson HR, Nathanson KL, Foulkes WD, Greenberg RA. Biallelic deleterious BRCA1 mutations in a woman with early-onset ovarian cancer. Cancer Discov. 2013;3:399–405. doi: 10.1158/2159-8290.CD-12-0421. PMID: 23269703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosil V, Tosar A, Canadas C, Perez-Segura P, Diaz-Rubio E, Caldes T, de la Hoya M. Alternative splicing and molecular characterization of splice site variants: BRCA1 c.591C>T as a case study. Clin Chem. 2010;56:53–61. doi: 10.1373/clinchem.2009.132274. PMID: 19892845. [DOI] [PubMed] [Google Scholar]
- Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet. 2007;81:873–883. doi: 10.1086/521032. PMID: 17924331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackenthal JD, Cartegni L, Krainer AR, Olopade OI. BRCA2 T2722R is a deleterious allele that causes exon skipping. Am J Hum Genet. 2002;71:625–631. doi: 10.1086/342192. PMID: 12145750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia DJ, Agarwal MK, Pankratz VS, Deffenbaugh AM, Pruss D, Frye C, Wadum L, Johnson K, Mentlick J, Tavtigian SV, Goldgar DE, Couch FJ. Functional assays for classification of BRCA2 variants of uncertain significance. Cancer Res. 2008;68:3523–3531. doi: 10.1158/0008-5472.CAN-07-1587. PMID: 18451181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzer S, Tworek HA, Piver MS, DiCioccio RA. Classification of IVS1-10T-->C as a polymorphism of BRCA1. Cancer Genet Cytogenet. 1999;113:58–64. doi: 10.1016/s0165-4608(99)00005-9. PMID: 10459348. [DOI] [PubMed] [Google Scholar]
- Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, King MC. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. PMID: 7894493. [DOI] [PubMed] [Google Scholar]
- Friedman LS, Szabo CI, Ostermeyer EA, Dowd P, Butler L, Park T, Lee MK, Goode EL, Rowell SE, King MC. Novel inherited mutations and variable expressivity of BRCA1 alleles, including the founder mutation 185delAG in Ashkenazi Jewish families. Am J Hum Genet. 1995;57:1284–1297. PMID: 8533757. [PMC free article] [PubMed] [Google Scholar]
- Gaildrat P, Krieger S, Thery JC, Killian A, Rousselin A, Berthet P, Frebourg T, Hardouin A, Martins A, Tosi M. The BRCA1 c.5434C->G (p.Pro1812Ala) variant induces a deleterious exon 23 skipping by affecting exonic splicing regulatory elements. J Med Genet. 2010;47:398–403. doi: 10.1136/jmg.2009.074047. PMID: 20522429. [DOI] [PubMed] [Google Scholar]
- Gaildrat P, Krieger S, Di Giacomo D, Abdat J, Revillion F, Caputo S, Vaur D, Jamard E, Bohers E, Ledemeney D, Peyrat JP, Houdayer C, et al. Multiple sequence variants of BRCA2 exon 7 alter splicing regulation. J Med Genet. 2012;49:609–617. doi: 10.1136/jmedgenet-2012-100965. PMID: 22962691. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, van Rensburg EJ, Dunning AM, Love R, Evans G, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. PMID: 7493024. [DOI] [PubMed] [Google Scholar]
- Goina E, Skoko N, Pagani F. Binding of DAZAP1 and hnRNPA1/A2 to an exonic splicing silencer in a natural BRCA1 exon 18 mutant. Mol Cell Biol. 2008;28:3850–3860. doi: 10.1128/MCB.02253-07. PMID: 18391021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Deffenbaugh AM, Monteiro AN, Tavtigian SV, Couch FJ. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am J Hum Genet. 2004;75:535–544. doi: 10.1086/424388. PMID: 15290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Byrnes GB, Spurdle AB, Iversen ES, Greenblatt MS. Genetic evidence and integration of various data sources for classifying uncertain variants into a single model. Hum Mutat. 2008;29:1265–1272. doi: 10.1002/humu.20897. PMID: 18951437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Enriquez S, Coderch V, Masas M, Balmana J, Diez O. The variants BRCA1 IVS6-1G>A and BRCA2 IVS15+1G>A lead to aberrant splicing of the transcripts. Breast Cancer Res Treat. 2009;117:461–465. doi: 10.1007/s10549-008-0154-7. PMID: 18712473. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Steffensen AY, Jonson L, Andersen MK, Ejlertsen B, Nielsen FC. The silent mutation nucleotide 744 G --> A, Lys172Lys, in exon 6 of BRCA2 results in exon skipping. Breast Cancer Res Treat. 2010;119:547–550. doi: 10.1007/s10549-009-0359-4. PMID: 19267246. [DOI] [PubMed] [Google Scholar]
- Hartikainen JM, Pirskanen MM, Arffman AH, Ristonmaa UK, Mannermaa AJ. A Finnish BRCA1 exon 12 4216-2nt A to G splice acceptor site mutation causes aberrant splicing and frameshift, leading to protein truncation. Hum Mutat. 2000;15:120. doi: 10.1002/(SICI)1098-1004(200001)15:1<120::AID-HUMU31>3.0.CO;2-E. PMID: 10612843. [DOI] [PubMed] [Google Scholar]
- Hoffman JD, Hallam SE, Venne VL, Lyon E, Ward K. Implications of a novel cryptic splice site in the BRCA1 gene. Am J Med Genet. 1998;80:140–144. PMID: 9805131. [PubMed] [Google Scholar]
- Hofmann W, Horn D, Huttner C, Classen E, Scherneck S. The BRCA2 variant 8204G>A is a splicing mutation and results in an in frame deletion of the gene. J Med Genet. 2003;40:e23. doi: 10.1136/jmg.40.3.e23. PMID: 12624152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer C, Caux-Moncoutier V, Krieger S, Barrois M, Bonnet F, Bourdon V, Bronner M, Buisson M, Coulet F, Gaildrat P, Lefol C, Leone M, et al. Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat. 2012;33:1228–1238. doi: 10.1002/humu.22101. PMID: 22505045. [DOI] [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. PMID: 12065746. [DOI] [PubMed] [Google Scholar]
- Jian X, Boerwinkle E, Liu X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res. 2014;42:13534–13544. doi: 10.1093/nar/gku1206. PMID: 25416802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse SA, Brandwijk KI, Devilee P, Wesseling J, Hogervorst FB, Verhoef S, Nederlof PM. Prediction of BRCA2-association in hereditary breast carcinomas using array-CGH. Breast Cancer Res Treat. 2012;132:379–389. doi: 10.1007/s10549-010-1016-7. PMID: 20614180. [DOI] [PubMed] [Google Scholar]
- Keaton JC, Nielsen DR, Hendrickson BC, Pyne MT, Scheuer L, Ward BE, Brothman AR, Scholl T. A biochemical analysis demonstrates that the BRCA1 intronic variant IVS10-2A--> C is a mutation. J Hum Genet. 2003;48:399–403. doi: 10.1007/s10038-003-0044-0. PMID: 14513821. [DOI] [PubMed] [Google Scholar]
- Krajc M, De Greve J, Goelen G, Teugels E. BRCA2 founder mutation in Slovenian breast cancer families. Eur J Hum Genet. 2002;10:879–882. doi: 10.1038/sj.ejhg.5200886. PMID: 12461697. [DOI] [PubMed] [Google Scholar]
- Kwong A, Wong LP, Chan KY, Ma ES, Khoo US, Ford JM. Characterization of the pathogenic mechanism of a novel BRCA2 variant in a Chinese family. Fam Cancer. 2008;7:125–133. doi: 10.1007/s10689-007-9155-7. PMID: 17657584. [DOI] [PubMed] [Google Scholar]
- Laskie Ostrow K, DiCioccio RA, McGuire V, Whittemore AS. A BRCA1 variant, IVS23+1G-->A, causes abnormal RNA splicing by deleting exon 23. Cancer Genet Cytogenet. 2001;127:188–190. doi: 10.1016/s0165-4608(01)00433-2. PMID: 11428389. [DOI] [PubMed] [Google Scholar]
- Li L, Biswas K, Habib LA, Kuznetsov SG, Hamel N, Kirchhoff T, Wong N, Armel S, Chong G, Narod SA, Claes K, Offit K, et al. Functional redundancy of exon 12 of BRCA2 revealed by a comprehensive analysis of the c.6853A>G (p.I2285V) variant. Hum Mutat. 2009;30:1543–1550. doi: 10.1002/humu.21101. PMID: 19795481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, Guidugli L, Wang X, Vallee MP, Monteiro AN, Tavtigian S, Goldgar DE, Couch FJ. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS) Hum Mutat. 2012;33:8–21. doi: 10.1002/humu.21627. PMID: 21990134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunter G, Hein J. A nucleotide substitution model with nearest-neighbour interactions. Bioinformatics. 2004;20(Suppl 1):i216–i223. doi: 10.1093/bioinformatics/bth901. PMID: 15262802. [DOI] [PubMed] [Google Scholar]
- Machackova E, Foretova L, Lukesova M, Vasickova P, Navratilova M, Coene I, Pavlu H, Kosinova V, Kuklova J, Claes K. Spectrum and characterisation of BRCA1 and BRCA2 deleterious mutations in high-risk Czech patients with breast and/or ovarian cancer. BMC Cancer. 2008;8:140. doi: 10.1186/1471-2407-8-140. PMID: 18489799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer S, Puget N, Perrin-Vidoz L, Lynch HT, Serova-Sinilnikova OM, Lenoir GM. A BRCA1 nonsense mutation causes exon skipping. Am J Hum Genet. 1998;62:713–715. doi: 10.1086/301768. PMID: 9497265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez M, Castellsague J, Mirete M, Pros E, Feliubadalo L, Osorio A, Calaf M, Tornero E, del Valle J, Fernandez-Rodriguez J, Quiles F, Salinas M, Velasco A, Teule A, Brunet J, Blanco I, Capella G, Lazaro C. Assessing the RNA effect of 26 DNA variants in the BRCA1 and BRCA2 genes. Breast Cancer Res Treat. 2012;132:979–992. doi: 10.1007/s10549-011-1661-5. PMID: 21735045. [DOI] [PubMed] [Google Scholar]
- Meindl A. Comprehensive analysis of 989 patients with breast or ovarian cancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. Int J Cancer. 2002;97:472–480. doi: 10.1002/ijc.1626. PMID: 11802209. [DOI] [PubMed] [Google Scholar]
- Ozcelik H, Nedelcu R, Chan VW, Shi XH, Murphy J, Rosen B, Andrulis IL. Mutation in the coding region of the BRCA1 gene leads to aberrant splicing of the transcript. Hum Mutat. 1999;14:540–541. doi: 10.1002/(SICI)1098-1004(199912)14:6<540::AID-HUMU13>3.0.CO;2-C. PMID: 10571952. [DOI] [PubMed] [Google Scholar]
- Parsons MT, Whiley PJ, Beesley J, Drost M, de Wind N, Thompson BA, Marquart L, Hopper JL, Jenkins MA, Brown MA, Tucker K, Warwick L, et al. Consequences of germline variation disrupting the constitutional translational initiation codon start sites of MLH1 and BRCA2: Use of potential alternative start sites and implications for predicting variant pathogenicity. Mol Carcinog. 2015;54:513–522. doi: 10.1002/mc.22116. PMID: 24302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MT, Whiley PJ, Beesley J, Drost M, de Wind N, Thompson BA, Marquart L, Hopper JL, Jenkins MA, Brown MA, Tucker K, Warwick L, et al. Consequences of germline variation disrupting the constitutional translational initiation codon start sites of MLH1 and BRCA2: Use of potential alternative start sites and implications for predicting variant pathogenicity. Mol Carcinog. 2015;54:513–522. doi: 10.1002/mc.22116. PMID: 24302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrij-Bosch A, Peelen T, van Vliet M, van Eijk R, Olmer R, Drusedau M, Hogervorst FB, Hageman S, Arts PJ, Ligtenberg MJ, Meijers-Heijboer H, Klijn JG, et al. BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet. 1997;17:341–345. doi: 10.1038/ng1197-341. PMID: 9354803. [DOI] [PubMed] [Google Scholar]
- Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. PMID: 18951446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne MT, Pruss D, Ward BE, Scholl T. A characterization of genetic variants in BRCA1 intron 8 identifies a mutation and a polymorphism. Mutat Res. 1999;406:101–107. doi: 10.1016/s1383-5726(99)00006-0. PMID: 10479726. [DOI] [PubMed] [Google Scholar]
- Pyne MT, Brothman AR, Ward B, Pruss D, Hendrickson BC, Scholl T. The BRCA2 genetic variant IVS7 + 2T-->G is a mutation. J Hum Genet. 2000;45:351–357. doi: 10.1007/s100380070007. PMID: 11185744. [DOI] [PubMed] [Google Scholar]
- Rosenthal ET, Bowles KR, Pruss D, van Kan A, Vail PJ, McElroy H, Wenstrup RJ. Exceptions to the rule: Case studies in the prediction of pathogenicity for genetic variants in hereditary cancer genes. Clin Genet. 2015;88:533–541. doi: 10.1111/cge.12560. PMID: 25639900. [DOI] [PubMed] [Google Scholar]
- Rouleau E, Lefol C, Moncoutier V, Castera L, Houdayer C, Caputo S, Bieche I, Buisson M, Mazoyer S, Stoppa-Lyonnet D, Nogues C, Lidereau R. A missense variant within BRCA1 exon 23 causing exon skipping. Cancer Genet Cytogenet. 2010;202(2):144–146. doi: 10.1016/j.cancergencyto.2010.07.122. PMID: 20875879. [DOI] [PubMed] [Google Scholar]
- Santarosa M, Viel A, Boiocchi M. Splice variant lacking the transactivation domain of the BRCA2 gene and mutations in the splice acceptor site of intron 2. Genes Chromosomes Cancer. 1999;26:381–382. doi: 10.1002/(sici)1098-2264(199912)26:4<381::aid-gcc14>3.0.co;2-n. PMID: 10534775. [DOI] [PubMed] [Google Scholar]
- Santos C, Peixoto A, Rocha P, Pinto P, Bizarro S, Pinheiro M, Pinto C, Henrique R, Teixeira MR. Pathogenicity evaluation of BRCA1 and BRCA2 unclassified variants identified in Portuguese breast/ovarian cancer families. J Mol Diagn. 2014;16:324–334. doi: 10.1016/j.jmoldx.2014.01.005. PMID: 24607278. [DOI] [PubMed] [Google Scholar]
- Sanz DJ, Acedo A, Infante M, Duran M, Perez-Cabornero L, Esteban-Cardenosa E, Lastra E, Pagani F, Miner C, Velasco EA. A high proportion of DNA variants of BRCA1 and BRCA2 is associated with aberrant splicing in breast/ovarian cancer patients. Clin Cancer Res. 2010;16:1957–1967. doi: 10.1158/1078-0432.CCR-09-2564. PMID: 20215541. [DOI] [PubMed] [Google Scholar]
- Scholl T, Pyne MT, Russo D, Ward BE. BRCA1 IVS16+6T-->C is a deleterious mutation that creates an aberrant transcript by activating a cryptic splice donor site. Am J Med Genet. 1999;85:113–116. PMID: 10406662. [PubMed] [Google Scholar]
- Sharp A, Pichert G, Lucassen A, Eccles D. RNA analysis reveals splicing mutations and loss of expression defects in MLH1 and BRCA1. Hum Mutat. 2004;24:272. doi: 10.1002/humu.9267. PMID: 15300854. [DOI] [PubMed] [Google Scholar]
- Spearman AD, Sweet K, Zhou XP, McLennan J, Couch FJ, Toland AE. Clinically applicable models to characterize BRCA1 and BRCA2 variants of uncertain significance. J Clin Oncol. 2008;26:5393–5400. doi: 10.1200/JCO.2008.17.8228. PMID: 18824701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurdle AB. Clinical relevance of rare germline sequence variants in cancer genes: evolution and application of classification models. Curr Opin Genet Dev. 2010;20:315–323. doi: 10.1016/j.gde.2010.03.009. PMID: 20456937. [DOI] [PubMed] [Google Scholar]
- Starita LM, Young DL, Islam M, Kitzman JO, Gullingsrud J, Hause RJ, Fowler DM, Parvin JD, Shendure J, Fields S. Massively Parallel Functional Analysis of BRCA1 RING Domain Variants. Genetics. 2015;200:413–422. doi: 10.1534/genetics.115.175802. PMID: 25823446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen AY, Jonson L, Ejlertsen B, Gerdes AM, Nielsen FC, Hansen TV. Identification of a Danish breast/ovarian cancer family double heterozygote for BRCA1 and BRCA2 mutations. Fam Cancer. 2010;9:283–287. doi: 10.1007/s10689-010-9345-6. PMID: 20455026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Byrnes GB, Goldgar DE, Thomas A. Classification of rare missense substitutions, using risk surfaces, with genetic- and molecular-epidemiology applications. Hum Mutat. 2008;29:1342–1354. doi: 10.1002/humu.20896. PMID: 18951461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesoriero AA, Wong EM, Jenkins MA, Hopper JL, Brown MA, Chenevix-Trench G, Spurdle AB, Southey MC. Molecular characterization and cancer risk associated with BRCA1 and BRCA2 splice site variants identified in multiple-case breast cancer families. Hum Mutat. 2005;26:495. doi: 10.1002/humu.9379. PMID: 16211554. [DOI] [PubMed] [Google Scholar]
- Thery JC, Krieger S, Gaildrat P, Revillion F, Buisine MP, Killian A, Duponchel C, Rousselin A, Vaur D, Peyrat JP, Berthet P, Frebourg T, Martins A, Hardouin A, Tosi M. Contribution of bioinformatics predictions and functional splicing assays to the interpretation of unclassified variants of the BRCA genes. Eur J Hum Genet. 2011;19:1052–1058. doi: 10.1038/ejhg.2011.100. PMID: 21673748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M, Blanco A, Montagna M, Hansen TV, Pedersen IS, Gutierrez-Enriquez S, Menendez M, Fachal L, Santamarina M, Steffensen AY, Jonson L, Agata S, Whiley P, Tognazzo S, Tornero E, Jensen UB, Balmana J, Kruse TA, Goldgar DE, Lazaro C, Diez O, Spurdle AB, Vega A. Characterization of BRCA1 and BRCA2 splicing variants: a collaborative report by ENIGMA consortium members. Breast Cancer Res Treat. 2012;132:1009–1023. doi: 10.1007/s10549-011-1674-0. PMID: 21769658. [DOI] [PubMed] [Google Scholar]
- Thompson BA, Goldgar DE, Paterson C, Clendenning M, Walters R, Arnold S, Parsons MT, Michael DW, Gallinger S, Haile RW, Hopper JL, Jenkins MA, et al. A Multifactorial Likelihood Model for MMR Gene Variant Classification Incorporating Probabilities Based on Sequence Bioinformatics and Tumor Characteristics: A Report from the Colon Cancer Family Registry. Hum Mutat. 2013a;34:200–209. doi: 10.1002/humu.22213. PMID: 22949379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BA, Greenblatt MS, Vallee MP, Herkert JC, Tessereau C, Young EL, Adzhubey IA, Li B, Bell R, Feng B, Mooney SD, Radivojac P, Sunyaev SR, Frebourg T, Hofstra RM, Sijmons RH, Boucher K, Thomas A, Goldgar DE, Spurdle AB, Tavtigian SV. Calibration of multiple in silico tools for predicting pathogenicity of mismatch repair gene missense substitutions. Hum Mutat. 2013b;34:255–265. doi: 10.1002/humu.22214. PMID: 22949387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, Bapat B, Bernstein I, Capella G, den Dunnen JT, du Sart D, Fabre A, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2014;46:107–115. doi: 10.1038/ng.2854. PMID: 24362816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, Bapat B, Bernstein I, Capella G, den Dunnen JT, du Sart D, Fabre A, Farrell MP, Farrington SM, Frayling IM, Frebourg T, Goldgar DE, Heinen CD, Holinski-Feder E, Kohonen-Corish M, Robinson KL, Leung SY, Martins A, Moller P, Morak M, Nystrom M, Peltomaki P, Pineda M, Qi M, Ramesar R, Rasmussen LJ, Royer-Pokora B, Scott RJ, Sijmons R, Tavtigian SV, Tops CM, Weber T, Wijnen J, Woods MO, Macrae F, Genuardi M. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2014;46:107–115. doi: 10.1038/ng.2854. PMID: 24362816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee MP, Francy TC, Judkins MK, Babikyan D, Lesueur F, Gammon A, Goldgar DE, Couch FJ, Tavtigian SV. Classification of missense substitutions in the BRCA genes: a database dedicated to Ex-UVs. Hum Mutat. 2012;33:22–28. doi: 10.1002/humu.21629. PMID: 21990165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A, Campos B, Bressac-De-Paillerets B, Bond PM, Janin N, Douglas FS, Domenech M, Baena M, Pericay C, Alonso C, Carracedo A, Baiget M, et al. The R71G BRCA1 is a founder Spanish mutation and leads to aberrant splicing of the transcript. Hum Mutat. 2001;17:520–521. doi: 10.1002/humu.1136. PMID: 11385711. [DOI] [PubMed] [Google Scholar]
- Vreeswijk MP, Kraan JN, van der Klift HM, Vink GR, Cornelisse CJ, Wijnen JT, Bakker E, van Asperen CJ, Devilee P. Intronic variants in BRCA1 and BRCA2 that affect RNA splicing can be reliably selected by splice-site prediction programs. Hum Mutat. 2009;30:107–114. doi: 10.1002/humu.20811. PMID: 18693280. [DOI] [PubMed] [Google Scholar]
- Walker LC, Whiley PJ, Couch FJ, Farrugia DJ, Healey S, Eccles DM, Lin F, Butler SA, Goff SA, Thompson BA, Lakhani SR, Da Silva LM, et al. Detection of splicing aberrations caused by BRCA1 and BRCA2 sequence variants encoding missense substitutions: implications for prediction of pathogenicity. Hum Mutat. 2010;31:E1484–E1505. doi: 10.1002/humu.21267. PMID: 20513136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wappenschmidt B, Becker AA, Hauke J, Weber U, Engert S, Kohler J, Kast K, Arnold N, Rhiem K, Hahnen E, Meindl A, Schmutzler RK. Analysis of 30 putative BRCA1 splicing mutations in hereditary breast and ovarian cancer families identifies exonic splice site mutations that escape in silico prediction. PLoS One. 2012;7:e50800. doi: 10.1371/journal.pone.0050800. PMID: 23239986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley PJ, Pettigrew CA, Brewster BL, Walker LC, Spurdle AB, Brown MA. Effect of BRCA2 sequence variants predicted to disrupt exonic splice enhancers on BRCA2 transcripts. BMC Med Genet. 2010;11:80. doi: 10.1186/1471-2350-11-80. PMID: 20507642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley PJ, Guidugli L, Walker LC, Healey S, Thompson BA, Lakhani SR, Da Silva LM, Tavtigian SV, Goldgar DE, Brown MA, Couch FJ, Spurdle AB. Splicing and multifactorial analysis of intronic BRCA1 and BRCA2 sequence variants identifies clinically significant splicing aberrations up to 12 nucleotides from the intron/exon boundary. Hum Mutat. 2011;32:678–687. doi: 10.1002/humu.21495. PMID: 21394826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley PJ, de la Hoya M, Thomassen M, Becker A, Brandao R, Pedersen IS, Montagna M, Menendez M, Quiles F, Gutierrez-Enriquez S, De Leeneer K, Tenes A, et al. Comparison of mRNA splicing assay protocols across multiple laboratories: recommendations for best practice in standardized clinical testing. Clin Chem. 2014a;60:341–352. doi: 10.1373/clinchem.2013.210658. PMID: 24212087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley PJ, Parsons MT, Leary J, Tucker K, Warwick L, Dopita B, Thorne H, Lakhani SR, Goldgar DE, Brown MA, Spurdle AB. Multifactorial likelihood assessment of BRCA1 and BRCA2 missense variants confirms that BRCA1:c.122A>G(p.His41Arg) is a pathogenic mutation. PLoS One. 2014b;9:e86836. doi: 10.1371/journal.pone.0086836. PMID: 24489791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P, Magri V, Cretnik M, Fasano M, Jakubowska A, Levanat S, Lubinski J, Marras E, Musani V, Thierens H, Vandersickel V, Perletti G, Vral A. Characterization of the c.190T>C missense mutation in BRCA1 codon 64 (Cys64Arg) Int J Oncol. 2009;34:1005–1015. doi: 10.3892/ijo_00000226. PMID: 19287957. [DOI] [PubMed] [Google Scholar]
- Xu CF, Chambers JA, Nicolai H, Brown MA, Hujeirat Y, Mohammed S, Hodgson S, Kelsell DP, Spurr NK, Bishop DT, Solomon E. Mutations and alternative splicing of the BRCA1 gene in UK breast/ovarian cancer families. Genes Chromosomes Cancer. 1997;18:102–110. PMID: 9115959. [PubMed] [Google Scholar]
- Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15:568–573. doi: 10.1093/oxfordjournals.molbev.a025957. PMID: 9580986. [DOI] [PubMed] [Google Scholar]
- Yang Y, Swaminathan S, Martin BK, Sharan SK. Aberrant splicing induced by missense mutations in BRCA1: clues from a humanized mouse model. Hum Mol Genet. 2003;12:2121–2131. doi: 10.1093/hmg/ddg222. PMID: 12915465. [DOI] [PubMed] [Google Scholar]
- Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11:377–394. doi: 10.1089/1066527041410418. PMID: 15285897. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen L, Bacares R, Ruggeri JM, Somar J, Kemel Y, Stadler ZK, Offit K. BRCA1 R71K missense mutation contributes to cancer predisposition by increasing alternative transcript levels. Breast Cancer Res Treat. 2011;130:1051–1056. doi: 10.1007/s10549-011-1732-7. PMID: 21863257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.