Abstract
Background
Myocardial infarction (MI) patients without obstructive coronary artery disease (CAD) are at increased risk for recurrent ischemic events, but angina frequency post-MI has not been described.
Methods and Results
Among MI patients who underwent angiography, we assessed angina at baseline, 1, 6, and 12 months using the Seattle Angina Questionnaire (SAQ). A hierarchical repeated measures modified Poisson model assessed the association between the absence of obstructive CAD (defined as epicardial stenoses >70% or left main >50%) and angina. Among 5539 MI patients from 31 US hospitals (mean age 60, 68% male), 6.9% had no angiographic obstructive CAD. More patients without obstructive CAD (vs. obstructive CAD) were female (57% vs 30%), non-white (51% vs 24%) and had NSTEMI (87% vs 51%). In unadjusted analyses, patients without obstructive CAD had less angina prior to MI but more angina and worse health status post-discharge. After adjustment for socio-demographic and clinical factors, the risk of post-MI angina was similar in patients without vs. with obstructive CAD (IRR=0.89, 95% CI 0.77-1.02). Among patients without obstructive CAD, depression and self-reported avoidance of care due to cost were independently associated with angina (IRR=1.28 per 5 points on PHQ, 95% CI 1.17-1.41; IRR=1.34, 95% 1.02-1.1.74).
Conclusions
Following MI, patients without obstructive CAD experience an angina burden at least as high as those with obstructive CAD, affecting 1 in 4 patients at 12 months. As these patients are not candidates for revascularization, other anti-anginal strategies are needed to improve their health status and quality of life.
Keywords: Angina, Coronary artery disease, Acute myocardial infarction
One in ten patients presenting with an acute myocardial infarction (MI) do not have obstructive coronary artery disease (CAD) on coronary angiography, with the etiology of the MI thought to be a combination of resolved thrombus, coronary spasm, and others.(1) Prior research has shown that these patients generally have lower rates of recurrent MI but similar rates of long-term mortality than those with obstructive CAD.(2) However, quality-of-life outcomes, such as post-MI angina, have not been evaluated in this patient population.
This is particularly relevant as patients without obstructive CAD can experience ischemia-driven angina, presumably via mechanisms such as endothelial dysfunction and abnormal coronary vascular resistance. Residual angina after an MI is a particularly relevant outcome as it is associated with poor quality of life and is a major driver of repeat hospitalizations.(3) In addition, it is a potentially modifiable condition and therefore could be an ideal target to both improve patient symptoms and reduce healthcare costs.(4-6)
To address this knowledge gap, we compared the prevalence of post-MI angina, as well as rehospitalization rates, among MI patients with and without obstructive CAD in 2 large U.S. multicenter MI registries. In addition, we evaluated predictors of residual angina among patients without obstructive CAD, so that efforts of more intense medical management could be directed toward those at highest risk.
METHODS
Study Design and Participants
Details regarding the TRIUMPH (Translational Research Investigating Underlying Disparities in acute Myocardial infarction Patients’ Health Status) and PREMIER (Prospective Registry Evaluating Myocardial Infarction: Events and Recovery) prospective observational registries have been described.(7, 8) Briefly, from 2003-04, 2498 MI patients from 19 U.S. hospitals were enrolled in PREMIER, and between 2005-08, 4340 MI patients from 24 U.S. hospitals were enrolled in TRIUMPH (12 hospitals participated in both registries). Both registries had identical inclusion and exclusion criteria and follow-up protocols. Patients’ 1-year mortality and angina outcomes were also similar between the two registries. To be included, patients were required to have a type 1 myocardial infarction,(9) including biomarker evidence of myocardial necrosis and additional evidence supporting the clinical diagnosis of an MI such as prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes.
Baseline data were obtained through chart abstraction and a structured interview by trained research staff within 24 to 72 hours following admission. Each participating site obtained Institutional Research Board approval, and all patients provided informed consent for baseline and follow-up assessments
Definition of Obstructive CAD and Angina
The reports of all angiograms performed during the MI were obtained and abstracted. In the primary analysis, obstructive CAD was defined as any epicardial coronary stenosis ≥70%, and/or left main stenosis ≥50%. We also performed a sensitivity analysis redefining obstructive CAD as any epicardial stenosis >50%. Patients with prior CABG, in-hospital PCI, or in-hospital CABG were also classified as having obstructive CAD. Patients were excluded from the analysis if they did not have a diagnostic coronary angiogram performed during the MI hospitalization.
Angina and health status were assessed during the MI hospitalization and at 1, 6 and 12 months following MI using the Seattle Angina Questionnaire (SAQ)(10) and the Medical Outcomes Study 12-item Short Form (SF-12).(11) The SAQ is a reliable, responsive, and valid 19-item questionnaire with a 4-week recall that assesses 5 clinically important domains of health in patients with CAD: angina frequency, angina stability, disease perception/quality of life, physical limitations and treatment satisfaction. The scores for each of the SAQ domains range from 0 to 100, with higher scores indicating less angina and better health status. The primary outcome of this study was the SAQ angina frequency domain, which quantifies the frequency and burden of angina and was categorized as absent (score=100) or present (score <100).(12) The angina stability domain was not included in these analyses as it represents a short-term assessment of change and is not appropriate for longitudinal analyses. Generic health status was assessed with the Medical Outcomes Study 12-item Short Form (SF-12),(13) which provides summary scales for overall physical and mental health using norm-based methods that standardize the scores to a mean of 50 and a standard deviation of 10 (higher scores indicate better health status).(11)
Rehospitalization Data
As part of the TRIUMPH study, patients were asked to report interval hospitalizations since their last study contact during the follow-up interviews. If a patient reported being hospitalized, records of that hospitalization were obtained to adjudicate cardiovascular events. Chart abstractions were sent to 2 cardiologists who independently classified the reason for hospitalization. If there was disagreement, the record was adjudicated by a third senior cardiologist and, if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained. For this analysis, we examined both all-cause rehospitalizations and those due to chest pain, which included MI, unstable angina, stable angina, and non-cardiac chest pain. Rehospitalizations were not adjudicated in PREMIER and thus only TRIUMPH patients were included in this sub-analysis.
Statistical Analysis
Baseline characteristics--including socioeconomic status, demographic and clinical factors--and health status scores at baseline and 12 months were compared between patients without vs. with obstructive CAD using chi square test for categorical variables and t-test for continuous variables. The prevalence of angina (i.e., SAQ angina frequency score <100) was compared between groups at each follow-up time point using chi square test. A hierarchical, multivariable repeated measures Poisson model was used to assess the independent association between absence of obstructive CAD and angina over the year following MI. As angina was a common outcome, we incidence rate ratios (IRR) directly by using hierarchical modified Poisson regression models (as opposed to logistic regression) to avoid overestimating the effect size.(14, 15) Covariates included in the multivariable model were selected a priori based on prior literature review and clinical judgment of factors that might confound the association between obstructive CAD and angina: age, sex, race, current smoking, diabetes mellitus, depressive symptoms (assessed with the Patient Health Questionnaire(16)), self-reported avoidance of care due to cost, type of MI (ST- or non ST-elevation), and discharge Global Registry of Acute Coronary Events (GRACE) score(17) (a score calculated at the time of discharge from MI that incorporates several prognostically important factors including age, creatinine, heart failure, and in-hospital revascularization procedures).
In order to explore factors associated with angina among patients without obstructive CAD, we constructed a second repeated measures model among only patients without obstructive CAD. After covariates independently associated with angina were identified, we then examined whether these predictors were unique to patients without obstructive CAD by testing the interaction between these covariates and absence of obstructive CAD in the main model (which included patients both with and without obstructive CAD). In addition, we performed two sensitivity analyses. To ensure that our analytic cohort included only patients with MI, we performed a sensitivity analysis excluding patients without obstructive CAD who had a prior diagnosis of heart failure and a peak troponin <1 ng/mL.(18) We also performed a sensitivity analysis redefining obstructive CAD as any epicardial stenosis >50% to evaluate angina outcomes in patients with a more intermediate burden of coronary disease. Finally, we compared the time to first all-cause rehospitalization and first chest pain rehospitalization over the year following MI between those without vs. with obstructive CAD using Kaplan-Meier curves.
Missing baseline data (mean number of missing items per patients of 0.08) were imputed using IVEware (Imputation and Variance Estimation Software; University of Michigan's Survey Research Center, Institute for Social Research, Ann Arbor, MI). All remaining analyses were conducted using SAS v9.3 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
Study Population
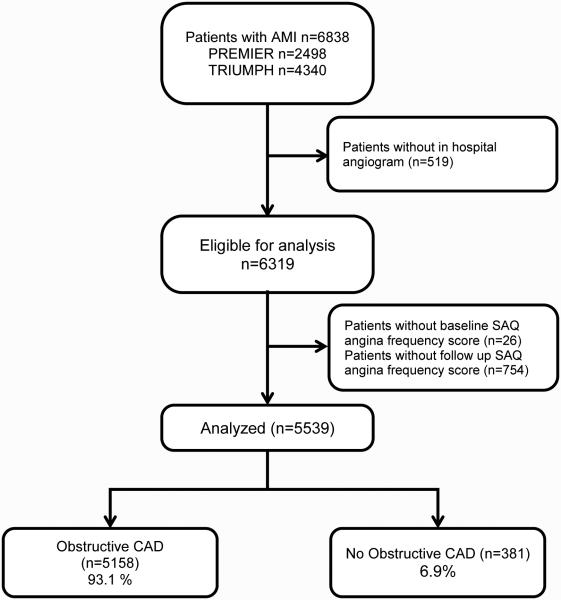
Among 6927 MI patients from 31 U.S. hospitals enrolled in PREMIER and TRIUMPH, 519 (7.5%) patients did not have a coronary angiogram performed during the MI hospitalization, and were thus excluded from the analysis. Twenty-six patients (0.4%) were missing baseline SAQ data and 754 (10.9%) were missing SAQ follow up data (98 of the 754 patients died prior to 1 month and thus had no opportunity to follow up). This left our final analytic sample at 5539 patients (Figure 1). Patients who were missing were more likely to be younger, non-white, and unmarried (Supplemental Table 1). Missing patients were also less likely than analyzed patients to have obstructive CAD (90% vs. 93%, p=0.003), but obstructive CAD was not associated with missingness in a multivariable model adjusted for demographic and clinical factors (p=0.73). The mean age of the cohort was 59.6 years, 68% were men, 74% were white, and 47% presented with an ST-elevation MI (Table 1).
Figure 1.
Flow chart of analytic cohort in the TRIUMPH and PREMIER registries
Table 1.
Baseline characteristics of patients with and without obstructive coronary artery disease in the TRIUMPH and PREMIER registries
| No Obstructive CAD n=381 |
Obstructive CAD n=4941 |
P - Value |
|
|---|---|---|---|
| Mean age (years) | 56.7±12.6 | 59.8±12.1 | <0.001 |
| Female sex | 57.0% | 30.0% | <0.001 |
| Non-white race | 51.1% | 23.6% | <0.001 |
| Married | 46.5% | 59.7% | <0.001 |
| Low Social Support | 19.7% | 15.1% | 0.017 |
| High school education | 76.9% | 80.9% | 0.057 |
| Hypertension | 68.8% | 63.4% | 0.037 |
| Mean total cholesterol | 178.3±51.0 | 173.2±40.0 | 0.074 |
| Mean triglycerides | 157.8±167.4 | 125.7±105.4 | <0.001 |
| Mean HDL cholesterol | 41.9±13.6 | 50.5±18.5 | <0.001 |
| Mean LDL cholesterol | 106.1±41.3 | 98.9±34.4 | 0.002 |
| Prior MI | 16.8% | 20.2% | 0.107 |
| Current Smoker | 33.7% | 37.3% | 0.159 |
| Diabetes mellitus | 20.7% | 28.5% | 0.001 |
| PHQ depression score | 6.2±5.8 | 5.2±5.4 | <0.001 |
| ST-elevation MI | 13.4% | 49.0% | <0.001 |
| Mean GRACE mortality score at discharge | 106.7±28.6 | 100.1±29.4 | <0.001 |
| Troponin peak (ng/dL; Median [IQR]) | 2.4 (0.6, 8.0) | 7.6 (1.9, 37.7) | <0.001 |
| In-hospital PCI | 0.0% | 76.7% | <0.001 |
| In hospital CABG | 0.0% | 12.1% | <0.001 |
| Discharge management | |||
| Cardiac rehab referral | 21.3% | 48.2% | <0.001 |
| Aspirin prescription | 85.6% | 95.1% | <0.001 |
| Clopidogrel prescription | 28.1% | 79.9% | <0.001 |
| Beta-blocker prescription | 76.6% | 91.7% | <0.001 |
| Statin prescription | 70.3% | 88.3% | <0.001 |
| ACE-I or ARB | 69.6% | 75.1% | 0.017 |
| Eplerenone or spironolactone | 6.3% | 3.9% | 0.025 |
CAD, coronary artery disease; MI, myocardial infarction; PHQ, patient health questionnaire; GRACE: Global Registry of Acute Coronary Events; PCI, percutaneous coronary intervention; CABG: coronary artery bypass grafting; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker
Baseline Characteristics
Among the 5539 patients with MI who underwent coronary angiography, 381 (6.9%) did not have obstructive CAD. The baseline demographic and clinical characteristics of patients without vs. with obstructive CAD are shown in Table 1. Greater proportions of patients without obstructive CAD (vs. with obstructive CAD) were female (57% vs. 30%), and non-white (51% vs. 24%). Patients without obstructive CAD were more likely to present with a non-ST-elevation MI (87% vs. 51%) and had lower peak troponin levels (median 2.4 ng/mL vs. 7.6). They were also less likely to have diabetes (21% vs. 29%), but more likely to have hypertension (69% vs. 63%) and chronic lung disease (14% vs. 8%). At discharge, guideline-recommended secondary prevention therapies were less commonly prescribed among patients without obstructive CAD, with lower use of aspirin (86% vs. 95%), clopidogrel (28% vs. 80%), beta-blockers (77% vs. 92%), statins (70% vs. 88%), and referral to cardiac rehabilitation (21% vs. 48%).
Mortality outcomes
Survival rates were similar between patients with and without obstructive CAD at each follow up time point (100% vs 100%, 97.4% vs 98.5%, and 96.1% vs 96.9%, log rank p value =0.08 at 1,6 and 12 months in patients without vs. with obstructive CAD, respectively).
Angina and Health Status
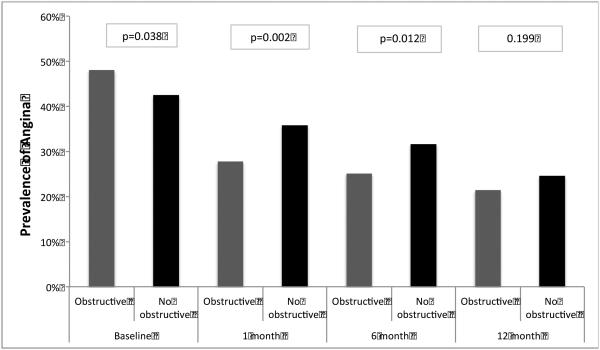
Patients without obstructive CAD had a lower prevalence of angina over the 4-weeks prior to MI (without vs. with obstructive CAD: 42.5% vs. 48.0%, p=0.038), but not at 12 months follow-up (24.6% vs. 21.4%, P=0.199) (Figure 2). In addition, they also reported worse disease-specific and generic health status at 12 months post-MI, including worse quality of life due to angina and lower satisfaction with the treatment of their angina (Table 2). Patterns of antianginal therapies differed between groups; patients without obstructive CAD were treated less frequently with beta-blockers and more frequently with calcium channel blockers during follow-up compared with those who had obstructive CAD (Table 3). In the multivariable repeated measures model, the risk for post-MI angina was similar among patients without vs. with obstructive CAD over the 1-year follow up period (IRR 0.89, 95% CI 0.77-1.02). In the first sensitivity analysis excluding patients without obstructive CAD who had prior heart failure and troponin levels <1 ng/mL, results were unchanged (IRR for post-MI angina in patients without vs. with obstructive CAD 0.90, 95% CI 0.78-1.03). In the second sensitivity analysis redefining obstructive CAD as any epicardial stensosis >50%, results were also similar (IRR 0.96, 95% CI 0.82-1.11).
Figure 2.
Rate of patient reported angina in patients without vs. with obstructive CAD over the year following MI.
Table 2.
Health Status Comparisons at Baseline and 12 months
| No Obstructive CAD n=381 |
Obstructive CAD n=4941 |
P-value | |
|---|---|---|---|
| Baseline | |||
| SAQ Angina Frequency | 85.2±21.0 | 87.6±19.7 | 0.031 |
| SAQ Disease Perception/Quality of Life | 60.5± 24.1 | 63.8± 23.2 | 0.006 |
| SAQ Physical Limitation | 84.4± 24.3 | 86.4± 21.9 | 0.101 |
| SAQ Treatment Satisfaction | 92.3± 11.9 | 94.4± 10.3 | <0.001 |
| SF-12 Mental Component Score | 47.4± 12.6 | 50.2± 11.3 | <0.001 |
| SF-12 Physical Component Score | 42.1± 12.9 | 43.4± 12.1 | 0.065 |
| 12 Months | |||
| SAQ Angina Frequency | 93.3±15.9 | 92.7±16.2 | 0.559 |
| SAQ Disease Perception/Quality of Life | 79.6± 22.7 | 83.2± 19.5 | 0.004 |
| SAQ Physical Limitation | 91.6± 19.3 | 93.9± 16.0 | 0.066 |
| SAQ Treatment Satisfaction | 90.4± 15.4 | 92.9± 13.0 | 0.002 |
| SF-12 Mental Component Score | 50.3± 11.5 | 52.8± 9.8 | <0.001 |
| SF-12 Physical Component Score | 41.3± 12.0 | 44.5± 11.8 | <0.001 |
SAQ, Seattle Angina Questionnaire; SF-12, Medical Outcomes Study 12-item Short Form
*Scores for the SAQ and SF-12 range from 0-100, with higher scores indicating less disease burden
Table 3.
Use of Anti-Anginal Medication At Discharge and 12 Months Following Acute Myocardial Infarction
| No Obstructive CAD n=381 |
Obstructive CAD n=4941 |
P-value | |
|---|---|---|---|
| Discharge | |||
| Beta-Blocker | 76.6% | 91.7% | <0.001 |
| Calcium Channel Blocker | 23.9% | 9.4% | <0.001 |
| Nitrate | 26.2% | 18.0% | <0.001 |
| Mean # Antianginals | 1.3±0.7 | 1.2±0.6 | 0.014 |
| 12 Months | |||
| Beta-Blocker | 62.0% | 78.5% | <0.001 |
| Calcium Channel Blocker | 27.3% | 10.3% | <0.001 |
| Nitrate | 18.8% | 21.6% | 0.303 |
| Mean # Antianginals | 1.1±0.8 | 1.1±0.7 | 0.633 |
Predictors of Post-MI Angina among those without Obstructive CAD
We evaluated the association of demographic and clinical variables with the presence of post-MI angina within the subgroup of patients who did not have obstructive CAD. Depressive symptoms were independently associated with angina (IRR 1.28 per 5 points on PHQ, 95% CI 1.17-1.41) as was self-reported avoidance of care due to cost (IRR 1.34, 95% CI 1.02-1.74) (Supplemental Table 2). In the main model that included all patients, however, the interaction terms between the absence of obstructive CAD and these 2 variables were not significant (p for interaction 0.43 and 0.76, respectively), indicating that neither factor was uniquely associated with a greater risk for post-MI angina among those without (vs. with) obstructive CAD.
Rehospitalizations
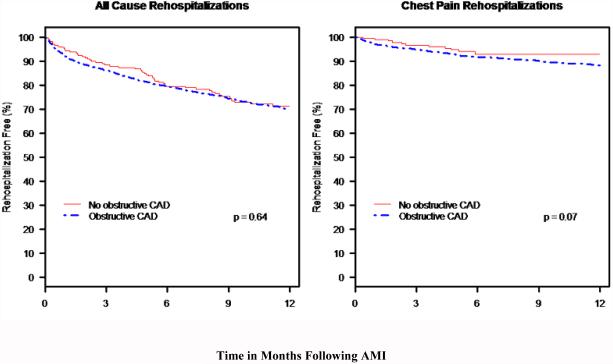
Among the 3440 patients in our analytic cohort from the TRIUMPH registry, patients without vs. with obstructive CAD had similar rates of all-cause rehospitalization over the 12 months after MI (Kaplan-Meier estimated rates 28.8% vs. 30.0%, log-rank-p=0.64; Figure 3). In addition, rates of rehospitalization due specifically to chest pain were also similar between groups (without vs. with obstructive CAD: Kaplan-Meier estimated rates 7.1% vs. 11.9%, log-rank-p=0.07; Figure 3).
Figure 3.
Rates of all-cause rehospitalizations and rehospitalization due to chest pain over the year following MI according to the presence of obstructive CAD.
DISCUSSION
In two large, contemporary multicenter MI registries, we found that patients who presented with an MI and were found to be without obstructive CAD had a high prevalence of angina during follow up, with 1 in 4 patients reporting angina at 1 year after MI. The burden of angina was at least as high in patients without obstructive CAD as in those who had obstructive CAD. Furthermore, patients without obstructive CAD experienced similar rates of rehospitalizations over the year following MI. Collectively, these findings highlight the importance of aggressive medical therapy and follow-up in patients with MI and no obstructive CAD, in order to potentially reduce their burden of angina, improve the quality of life and to prevent rehospitalizations in these patients with limited revascularization options.
Prior Studies
Prior work exploring outcomes of patients without obstructive CAD has suggested that patients who experience MI in the absence of obstructive CAD experience lower rates of reinfarction despite being treated less aggressively with medical management when compared to patients with obstructive CAD.(19, 20) Additionally, Roe et al reported lower adverse ischemic events (death or nonfatal MI by 6-month follow up) in patients without (vs. with) obstructive CAD.(21) However, long-term all cause mortality outcomes for patients without obstructive coronary disease have not been described. Previous studies exploring angina burden in patients with myocardial infarction in the absence of obstructive coronary disease focused on pre-admission angina, and suggested that patients without obstructive CAD had angina less commonly prior to index MI than matched patients with obstructive coronary artery disease, similar to our findings.(22) Our study is unique in that to our knowledge, it is the first to focus on the burden of residual angina post-MI and rehospitalizations in patients without versus with obstructive CAD.
Potential Mechanisms
The reasons as to why patients without angiographically evident obstructive CAD have a high burden of residual angina after an MI are unclear. It has been well-established that patients can have myocardial ischemia and angina in the absence of obstructive epicardial CAD.(23) Potential etiologies include microvascular disease and/or epicardial artery spasm, although the underlying mechanisms for residual angina in these patients require further study. We found that depressive symptoms were associated with an increased risk of angina in patients without obstructive CAD. In patients with CAD, depression has been associated with increased angina, even after adjusting for the degree of myocardial ischemia.(24, 25) Whether or not increased angina occurs due to increased pain reporting or to observable differences in visceral pain processing within the nervous system among these patients is still unclear. Regardless of the mechanism, patients without obstructive CAD had equivalent rates of all-cause and cardiac rehospitalization as those with obstructive CAD, indicating that the downstream effects of the patients’ chest pain, whether truly ischemic or not, were the same in these two groups.
Clinical Implications
Given the high prevalence of residual angina and rehospitalization rates among post-MI patients that do not have obstructive CAD, we believe that these findings are of considerable clinical relevance. Importantly, angina is a potentially modifiable condition, and greater attention to surveillance and aggressive management of angina in post-MI patients without obstructive CAD may improve their symptoms and quality of life, and potentially reduce repeat hospitalizations. However, in concordance with prior studies,(26, 27) we found that patients without obstructive CAD were less aggressively managed with secondary prevention strategies. As these patients are not candidates for coronary revascularization, non-interventional strategies are needed to improve their outcomes; yet, they appear to be prescribed guideline-directed treatments less often. For example, we found that referral to cardiac rehabilitation was far less frequent among those without vs. with obstructive CAD, highlighting one potential opportunity to improve outcomes. Additional potential targets for improvement of angina burden may include psychosocial issues, including mechanisms to decrease medication avoidance due to cost. Admittedly the effectiveness of these secondary prevention strategies, such as clopidogrel and statins, among patients with MI and without obstructive CAD is not well-established as these patients were infrequently included in the pivotal clinical trials.(28, 29) With increasing scrutiny on reducing readmissions post-MI, however, a better understanding is needed of whether strategies such as more frequent follow-up, consideration of aggressive angina management, or referral to cardiac rehabilitation could reduce angina in these patients and also reduce costly rehospitalizations.
Limitations
Our findings should be considered in the context of several potential limitations. First, we relied on adjudication of the coronary angiogram reports to determine the diagnosis of obstructive CAD and did not evaluate the angiograms in a core laboratory. It is possible that some patients without obstructive CAD could have been reclassified as having obstructive CAD if routinely evaluated with intravenous ultrasound or other advanced interventional techniques. However, in the setting of an MI, we suspect that the search for a culprit lesion would likely lead to an over- (rather than under-) estimation of the degree of coronary stenosis of any moderate lesions that would then be treated with PCI. Second, our study was not designed to evaluate the mechanisms underlying the residual angina in these patients. Third, although the entry criteria in our registries were expressly intended to ensure inclusion of patients with Type 1 myocardial infarction (including pre-specified requirements for troponin elevation and presentation within 24 hours of ischemic symptoms onset), it is possible that there could be a few non Type 1 myocardial infarction patients in our study. Last, although the absolute difference in the rate of chest pain-specific rehospitalization was only 4% between groups, it is possible that this difference was found not to be statistically significant due to sample size. Our findings should thus be confirmed in future, larger studies.
Conclusions
Patients who present with an MI and are found to have no obstructive CAD experience a burden of angina that is at least as high as those with obstructive CAD, with 1 in 4 patients reporting angina during one year follow-up. This under recognized group of patients, with substantial angina burden, challenges us to aggressively medically manage their symptoms as they remain at high risk for rehospitalization. As these patients are not candidates for revascularization, non-invasive strategies to reduce angina burden could have a significant impact on their health status and quality of life. Further studies are needed to better determine the etiology of angina as well the effectiveness of antianginal therapies and other treatments, such as cardiac rehabilitation and psychosocial interventions, in improving the symptoms and quality of life of these challenging patients.
Supplementary Material
Acknowledgments
Funding Sources. The PREMIER Registry was supported in part by CV Therapeutics, Palo Alto, CA. Funding support for the TRIUMPH Registry was received from the National Heart, Lung and Blood Institute (P50 HL077113). Dr. Grodzinsky was supported by a T32 training grant from the National Heart Lung and Blood Institute (T32HL110837). The funding agencies had no role in data collection, analysis, interpretation or the decision to submit the results. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Dr. Spertus owns intellectual property rights to the Seattle Angina Questionnaire.
Dr. Spertus reports significant grants from NIH/NHLBI, PCORI, ACCF, Gilead, Lilly, EvaHeart, Amorcyte. Dr. Spertus has consulted for (all modest): United Healthcare, Genentech, Amgen, Janssen, Novartis. He owns the copyright to the Seattle Angina Questionnaire (significant), Kansas City Cardiomyopathy Questionnaire (significant) and Peripheral Artery Questionnaire (modest) and has an equity interest in HealthOutcomesSciences (significant). The other authors have no disclosures or conflicts of interest to report.d Dr. Foody has consulted for (all modest): Novartis, Merck, AstraZeneca, Amgen Inc., Daiichi Sankyo, Sanofi, Janssen, Amarin, Lilly, Aegerion, Pfizer, Boehringer Ingelheim, Regeneron, Genzyme. Dr. Beltrame reports no direct conflicts of interest; potential disclosures include Servier Laboratories (modest). Dr. Maddox is supported with a VA HSR&D career development award. Dr. Mikhail Kosiborod is a consultant for AstraZeneca , Edwards Life Sciences, Gilead Sciences, Roche, Genentech, Regeneron, Eli Lilly, Amgen, Takeda and ZS Pharma. Dr. Kosiborod has received research support from the American Heart Association, Genentech, Gilead Sciences, Glumetrics, Optiscan, Astra Zeneca and Sanofi.
Footnotes
Disclosures. The remaining authors report no disclosures.
REFERENCES
- 1.Nageh T, Sherwood RA, Wainwright RJ, Shah AM, Thomas MR. The clinical relevance of raised cardiac troponin I in the absence of significant angiographic coronary artery disease. International Journal of Cardiology. 2005;100(2):325–30. doi: 10.1016/j.ijcard.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Rossini R, Capodanno D, Lettieri C, Musumeci G, Limbruno U, Molfese M, et al. Long-term outcomes of patients with acute coronary syndrome and nonobstructive coronary artery disease. The American journal of cardiology. 2013;112(2):150–5. doi: 10.1016/j.amjcard.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circulation Cardiovascular quality and outcomes. 2009;2(4):344–53. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 4.Beltrame J, Tavella R, Weekes A, Morgan C. Persistent Angina Symptoms in Stable Angina Patients: The Coronary Artery Disease in gENeral practiCE (CADENCE) Heart, Lung and Circulation. 2008;17(Supplement 3(0)):S100. doi: 10.1016/j.hlc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Jespersen L, Abildstrom SZ, Hvelplund A, Galatius S, Madsen JK, Pedersen F, et al. Symptoms of angina pectoris increase the probability of disability pension and premature exit from the workforce even in the absence of obstructive coronary artery disease. European heart journal. 2013;34(42):3294–303. doi: 10.1093/eurheartj/eht395. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler A, Schrader G, Tucker G, Adams R, Tavella R, Beltrame JF. Prevalence of depression in patients with chest pain and non-obstructive coronary artery disease. The American journal of cardiology. 2013;112(5):656–9. doi: 10.1016/j.amjcard.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circulation Cardiovascular quality and outcomes. 2011;4(4):467–76. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. American heart journal. 2006;151(3):589–97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 11.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110(25):3789–94. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 13.Muller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90(5):523–7. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–5. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 18.TEHRANI DM, SETO AH. Third universal definition of myocardial infarction: Update, caveats, differential diagnoses. Cleveland Clinic Journal of Medicine. 2013;80(12):777–86. doi: 10.3949/ccjm.80a.12158. [DOI] [PubMed] [Google Scholar]
- 19.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the women’s ischemia syndrome evaluation study and the st james women take heart project. Archives of Internal Medicine. 2009;169(9):843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European heart journal. 2012;33(6):734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 21.Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease.The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102(10):1101–6. doi: 10.1161/01.cir.102.10.1101. [DOI] [PubMed] [Google Scholar]
- 22.Larsen AI, Nilsen DW, Yu J, Mehran R, Nikolsky E, Lansky AJ, et al. Long-term prognosis of patients presenting with ST-segment elevation myocardial infarction with no significant coronary artery disease (from the HORIZONS-AMI trial) The American journal of cardiology. 2013;111(5):643–8. doi: 10.1016/j.amjcard.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. Journal of the American College of Cardiology. 1991;17(2):499–506. doi: 10.1016/s0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 24.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: The heart and soul study. JAMA : the journal of the American Medical Association. 2003;290(2):215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SV, Spertus JA, Ciechanowski PS, Soine LA, Jordan-Keith K, Caldwell JH, et al. Psychosocial Modulators of Angina Response to Myocardial Ischemia. Circulation. 2009;120(2):126–33. doi: 10.1161/CIRCULATIONAHA.108.806034. [DOI] [PubMed] [Google Scholar]
- 26.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation. 2000;101(9):948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 27.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of Secondary Prevention Therapies in Patients With Nonobstructive Coronary Artery Disease Identified During Cardiac Catheterization: Insights From the National Cardiovascular Data Registry Cath-PCI Registry. Circulation: Cardiovascular Quality and Outcomes. 2010;3(6):632–41. doi: 10.1161/CIRCOUTCOMES.109.906214. [DOI] [PubMed] [Google Scholar]
- 28.Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. New England Journal of Medicine. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen TR, Faergeman O, Kastelein JP, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The ideal study: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294(19):2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.