Abstract
Socioeconomic disparities in children’s delay of gratification exist, with impoverished children displaying greater difficulties in this developmental domain. The present paper examined the role of vagal tone in predicting the ability to delay gratification across resource rich and resource poor environments. Embedding hypotheses within evolutionary models of children’s conditional adaptation to proximal rearing contexts, Study 1 tested whether elevated vagal tone was associated with lower delay of gratification within impoverished children. Study 2 compared the relative role of vagal tone across two groups of children, one which experienced greater impoverishment and one which was relatively middle-class. Results indicated that within resource rich environments, high vagal tone was associated with greater delay of gratification. In contrast, high vagal tone in children living within resource poor environments was associated with reduced delay of gratification. The results are interpreted within evolutionary-developmental models of children’s stress response system functioning and adaptive behavior across varying contexts of economic risk.
For over 50 years, psychologists have used laboratory tasks in which they ask young children to sit patiently and they’ll receive a larger reward if they can resist the immediate temptation of a smaller reward (Mischel, Shoda, & Rodriguez, 1989). Empirical work has documented that children’s ability to suppress the dominant response and delay gratification is associated with a wide range of positive outcomes including socio-emotional competence, prosocial behavior, and reduced psychopathology (e.g., Eisenberg et al., 2004; Mischel, et al., 1989; Razza & Raymond, 2013). Thus, normative psychological models propose that delay of gratification reflects greater regulation, and this ability to control is the optimal outcome given its association with socioemotional adjustment (e.g., Calkins, 2009). However, research has documented the presence of wide socioeconomic disparities in children’s delay of gratification, with impoverished children displaying greater difficulties compared to more affluent counterparts (e.g., Evans & English, 2002; Raver, et al., 2011). This raises the provocative question, why would children living in heightened economic uncertainty show a greater propensity towards taking the immediate reward if that decision is maladaptive with respect to adjustment?
Perhaps part of the answer lies in the tendency for theories and models in developmental psychology to interpret behavior based upon benchmarks which are largely derived from children living within more secure environments, thus missing the meaning and function of behavior across broad contexts (e.g., Cicchetti & Lynch, 1993; Henrich, Heine, & Norenzaynan, 2010). Evolutionary-developmental models offer an alternative conceptual framework, positing that natural selection has shaped our ability to modify regulatory behaviors towards matching local environmental conditions (e.g., Daly & Wilson, 2005; Ellis, Figueredo, Brumbach, & Schlomer, 2009), suggesting that “'when people encounter stressful environments, this does not so much disturb their development as direct or regulate it toward strategies that are adaptive under stressful conditions, even if those strategies are currently harmful in terms of the long-term welfare of the individual or society as a whole” (Ellis et al., 2011, p. 8). As such, different environments should elicit different strategies based upon the perception of present-future trade-offs (Belsky, Steinberg, & Draper, 1991; Chisholm, 1999). Within environments composed of plentiful resources and support, the ability to control immediate impulses and desires in the service of regulation more than likely results in greater rewards and benefits. In contrast, early rearing contexts consisting of heightened impoverishment, reduced access to resources, and harsher living conditions reduce the likelihood of a future payoff. Under these circumstances, children should lawfully shift preferences toward immediate rewards (Fawcett, McNamara, & Houston, 2012).
However this doesn’t answer the question as to how this occurs, and attention has turned towards understanding the antecedents of children’s ability to delay gratification, particularly within contexts of risk (e.g., Lengua, Zalewski, Fisher, & Moran, 2013). One early biological marker that has been implicated in models of children’s self-regulation is cardiac vagal tone. Tonic vagal tone is typically assessed via resting levels of respiratory sinus arrhythmia (RSA), which captures parasympathetic dominance over cardiac functioning in the autonomic nervous system (e.g., Beauchaine, 2001). In particular, the polyvagal theory (Porges & Furman, 2011) proposes that vagal tone represents a physiological index of an individual’s capacity to respond quickly and flexibly to environmental demands as well as regulate more effectively from heightened arousal. Higher levels of tonic vagal tone are thought to reflect heightened sensitivity to environmental cues and the enactment of behaviors that are tailored to local conditions. In contrast, lower levels of tonic vagal tone are purported to indicate difficulties in attending to and/or reacting to environmental contexts resulting in more ineffective and less malleable behavioral coping strategies (Brosschot & Thayer, 1998). Thus, developmental models of self-regulation consider vagal tone an important mechanism in understanding children’s effortful control.
Empirical work examining associations between RSA and children’s regulation provides support for the suggestion that it may operate as an antecedent of delay control in affective tasks (Beauchaine, 2001; Calkins, 2007). Given its proposed function of supporting appropriate engagement with the environment, research has shown that high basal RSA is associated with sustained attention (Suess, Porges, & Plude, 1994), effortful control (Taylor, Eisenberg, & Spinrad, 2015), and executive function (Marcovitch et al., 2010). However much of the previous work in this area has been conducted in samples of children primarily hailing from middle to higher socioeconomic backgrounds. A small group of studies using primarily low-income and impoverished children have found associations between high vagal tone and poor regulation (e.g., Blair & Peters, 2003; Davies, et al., 2009; Kidwell & Barnett, 2007). If the physiological function of vagal tone is to facilitate the prioritization of behaviors in response to environmental cues in a manner that benefits the individual, these results may not necessarily be contradictory. In conditions characterized by elevated resources and support, high vagal tone may result in delayed gratification with an eye towards a larger, future reward. However, under reduced resource conditions, high vagal tone may support the adoption of quick reward oriented strategies. Thus, both of these strategies may be adaptive within their given contexts.
In summary, the present paper investigated the role of basal vagal tone assessed during the toddler years in associations with children’s decision to delay gratification across resource rich and resource poor environmental contexts. In our first study, we utilized a sample of children living highly impoverished conditions to test the association of basal vagal tone with delay of gratification. Consistent with an evolutionary-developmental framework, we hypothesized that higher vagal tone would predict lower delay of gratification in the context of poverty. Our second study sought to both replicate findings obtained in Study 1 and findings within the larger literature by testing how environmental contexts moderate the role of vagal tone in predicting children’s delay ability. To maximize comparability, procedures were similar across studies and were approved by the Institutional Review Board at the research site.
Study 1
Participants
Participants included 201 two-year-old children (44% female) and their mothers in a moderately-sized metropolitan area in the Northeast who were recruited through agencies who serve disadvantaged children and families, including Women, Infants, and Children, and Temporary Assistance to Needy Families rosters administered through the local Department of Human Services. Participants were recruited to obtain a sample of 200 mothers and recruitment was ended at this achieved goal. Median annual income for the family household was $18,300 (US) per year and a substantial minority of mothers (30%) did not complete high school. Most families were receiving public assistance (95%) and were living below the US Federal Poverty line (99.5%). The majority of the sample of mothers and children were Black (56%), followed by smaller proportions of family members who identified as White (23%), Latino (11%), Multi-Racial (7%), and “Other” (3%). The cumulative retention rate across the two measurement occasions was 87%. To test for selective attrition, we conducted statistical comparisons between the mother-child dyads that participated in each wave and dyads that dropped out during the longitudinal component of the study along the primary, covariate, and demographic variables at the first assessment (e.g., family income, maternal education). No significant differences were identified in the analyses.
Procedures
Assessments were collected when mothers and their toddlers visited our laboratory when children were two and four years old
Heart Monitor Procedure
Basal heart rate data were collected on each child at the first measurement wave using a MiniLoger 2010 Series (Mini Mitter, Inc., Bend, OR). The MiniLogger detects each R-Wave and records every interval between successive R-Waves to the nearest millisecond. Mothers held their children on their laps while a trained experimenter placed two electrodes on the child’s chest, one medially on the right collar bone and one on the child’s left side below the rib cage. Consistent with prior research, children were given low arousal activities during recording to keep them calm and facilitate baseline data collection (Hastings et al., 2008).
Delay of Gratification
At the second wave of data collection, children were placed at a small table with two plates in front of them and a bell. On one plate the experimenter placed two M&Ms and on the other plate placed five M&Ms. Children were instructed on how to ring the bell. Then the experimenter pointed out the difference in the amount of candy on each plate and told the child that if they could wait until the experimenter returned, they would receive the five pieces of candy. If they couldn’t wait, they were to ring the bell to signal the experimenter to return and then they could eat the two pieces of candy. The experimenter then left the room for a 10-minute wait period.
Measures
Respiratory sinus arrhythmia
Interbeat interval (IBI) data were extracted from the EKG assessment using Mini-Log 2000W software (Mini Mitter, Inc., 2001), edited for artifacts, and analyzed using CMet software (Allen, Chambers, & Towers, 2007). Specifically, the CMetX program converted the IBI series into a time series via linear interpolation at a sampling rate of 10 Hz. Then, via a band pass filter, the variance of the heart period within the frequency band of .24– 1.04 Hz (i.e., the band of spontaneous respiration for toddlers; Porges et al., 1996) was extracted. The natural log of this variance was utilized as an estimate of children’s average RSA for the baseline session.
Delay of Gratification
Children’s delay ability was operationalized as the length of time children waited to eat the M&M during the delay of gratification task (Mischel & Ebbesen, 1970). If the child touched the M&Ms or rang the bell, their score was determined from that timepoint. If a child waited the entire time, their score was 10 minutes. In the present sample, only 16% of children waited the entire ten minutes.
Results
For descriptive purposes, Table 1 provides the means and standard deviations among the measures of main constructs.
Table 1.
Means and standard deviations of the main variables in the primary analyses.
| Variable | Mean | SD | Correlations | ||||
|---|---|---|---|---|---|---|---|
| Study 1 | 1 | 2 | 3 | 4 | 5 | ||
| 1. RSA | 6.62 | 1.36 | -- | ||||
| 2. DOG Time | 2:20 | 3:45 | −.27* | -- | |||
| 3. Child Age | 3.79 | 0.41 | .24* | .10 | -- | ||
| 4. Maternal Education | 3.99 | 1.08 | −.21* | .32* | −.08 | -- | |
| 5. Family Income | 20.82 | 12.27 | −.17* | .24* | −.07 | .36* | -- |
| Study 2 | |||||||
| 1. RSA | 5.91 | 2.18 | -- | ||||
| 2. DOG Time | 7:43 | 3:30 | .03 | -- | |||
| 3. Child Age | 5.29 | 0.24 | −.12 | .14 | -- | ||
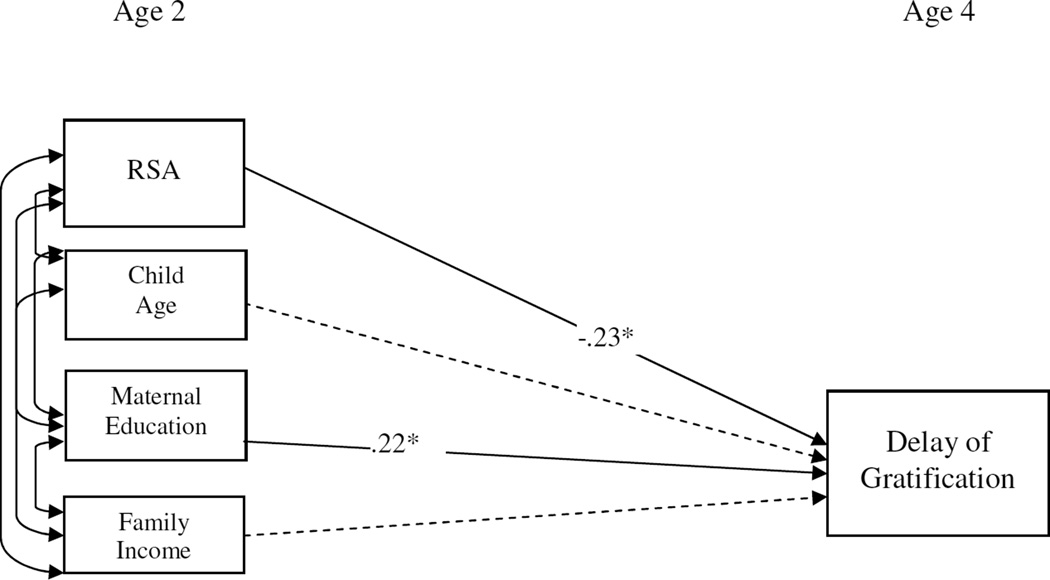
A path model examining the association between children’s RSA at 2 years of age and delay of gratification at 4 years of age was conducted using MPlus software Version 7.0 (Muthén & Muthén, 2012). To maximize our sample size, we utilized the FIML estimation procedure available in MPlus (e.g. Enders, 2001). This method is appropriate when the data are missing completely at random (e.g., no identifiable pattern exists in the missing data) and the amount of missing data is as high as 50% (Schlomer, Bauman, & Card, 2010). Missing data analysis showed that the data were MCAR (χ2 = 0.850 (6), ns). We included two demographic covariates, maternal education and income, to maximize comparability in results with the socio-economic constructs used to create the two groups in Study 2. Child age at the time of delay of gratification was also co-varied. We examined the data for the presence of outliers and found four cases which were greater than 3 standard deviations from the mean on family income. We removed these cases and re-ran the analyses to determine if they were influential and the results were identical to the ones with the cases included in the data. Given the similarity in findings, we elected to retain these cases in our analyses. Finally, delay of gratification evidenced moderate skew. We transformed the variable using a logarithmic function to reduce skew and analyses were run with the transformed variable.
The path model testing associations between basal RSA activity and delay of gratification was fully identified χ2(0). There was a significant effect of basal RSA at age 2 on delay of gratification at age 4 (β = −.23, t= −2.72, p < .01) in the context of other proximal demographic factors entered as simultaneous predictors in the model. Our findings suggest that early occurring elevation in basal RSA activity in the context of poverty was associated with lower delay of gratification at the age of 4.
Study 2
Participants
Data were drawn from a longitudinal study of mother-child relationships. The sample consisted of 140 pairs of mothers and their children (51.6% male) recruited when children were 18 months of age from the metropolitan area of a mid-sized city in the Northeastern United States. Recruitment was conducted so as to obtain two samples of mother-toddler dyads with respect to socioeconomic status (SES). Participants fell into one of two SES based groups: low SES (n = 69) and middle SES (n = 71) groups. For study enrollment, mothers in the middle SES group were required to have completed their Bachelor’s degree at an accredited 4-year college or university, and to have an income not supplemented through government assistance (e.g., food stamps). Mothers in the low SES group were required to be receiving some form of public assistance (verified through Department of Human Services records) and had not completed a degree at an accredited 4-year college or university. Recruitment was ended when two similarly sized samples were obtained. Median family income of participants was $46,093 per year; however, one third of the sample reported earnings below $10,200 annually. Mothers were between 18 and 42 years of age (M = 24). Participants identified themselves as European-American (55% of mothers and 49% of children), African-American/Black (26% of mothers and 25% of children), and Latina (11% of mothers and 12% of children), with smaller percentages of Biracial (5% of mothers and 14% of children), Asian (less than 1 % of mothers, 0 % of children) and Native American/Alaskan (less than 1% of mothers, 0% of children). Retention rate across the waves was 74%.
Procedures
Mothers and their children visited the lab at two time points, when children were 18 months old (Wave 1) and 5 years old (Wave 2). Mothers and children completed laboratory paradigms at the visits and mothers filled out surveys as well.
Heart Monitor Procedure
At Wave 1, Alive Heart Monitors from Alive Technologies Pty. Ltd. (http://www.alivetec.com/index.htm) were utilized to record and store children’s ECG signal during a silent baseline session. These EGC monitors included a precordial, two-pole ECG lead that was placed on children’s chests. Data from these leads were transmitted to a portable unit worn by the child and were stored on an SD card in that unit. The ECG signal was sampled at 300 Hz and had a voltage range of −2.5 to 2.5 V.
Delay of Gratification
At the last measurement occasion, children’s delay of gratification was assessed using the same delay paradigm used in Study 1. However, children were given the choice between 3 and 8 M&Ms.
Measures
Socio-Economic Diversity
At recruitment, participants for the current study were identified as either low SES or middle/high SES. Group membership was used as a measure of socio-economic diversity. Mother-child dyads in the low SES group reported a median family income of $23,518 a year (Range = $20,625 to $26,410) and reported on average 2.5 children in the household (Range = 1 to 11). In contrast, the middle SES group reported an average income of $85,727 (Range = $77,709 to $287,000) and reported on average 1.8 children in the household (Range = 1 to 4). We also utilized mother’s address in order to examine neighborhood statistics. Compared to mothers in the middle SES group, mother-child dyads in the low SES group resided in areas demarcated by high population density and the highest rates of violent crime (US Census Bureau, 2002). Thus, mothers and children in our low SES group were also characterized by experiencing heightened adversity within their immediate residential environment.
Respiratory sinus arrhythmia
Similar to Study 1, RSA was calculated using CMet software (Allen, et al., 2007).
Delay of Gratification
Children’s ability to delay gratification was scored similarly as in Study 1 through using the delay time such that higher scores indicated greater delay.
Results
Table 1 shows the means and standard deviations for the main variables in Study 2. To examine delay ability across the two groups, we utilized a chi-square test which indicated significant differences (χ2 (1) = 3.89, p < .05). Consistent with the larger literature, children in the low SES group were less likely to delay gratification (58%) compared to children in the high SES group (41%). Data were checked for outliers and none were found. As in Study 1, delay of gratification evidenced moderate skew. We transformed the variable using a logarithmic function and used the transformed variable in analyses.
Prior to running analyses, predictor variables were centered to avoid problems with multicollinearity (Aiken & West, 1991). Interaction terms were created by multiplying the centered predictor variables. Path analysis, using the same approach as in Study 1, was used to test our hypothesized model (Figure 2). To evaluate whether data were missing completely at random (MCAR), we examined the patterns of missing data using Little’s MCAR test (Little, 1988). Results showed that the data were MCAR [χ2 = 29.19, ns].
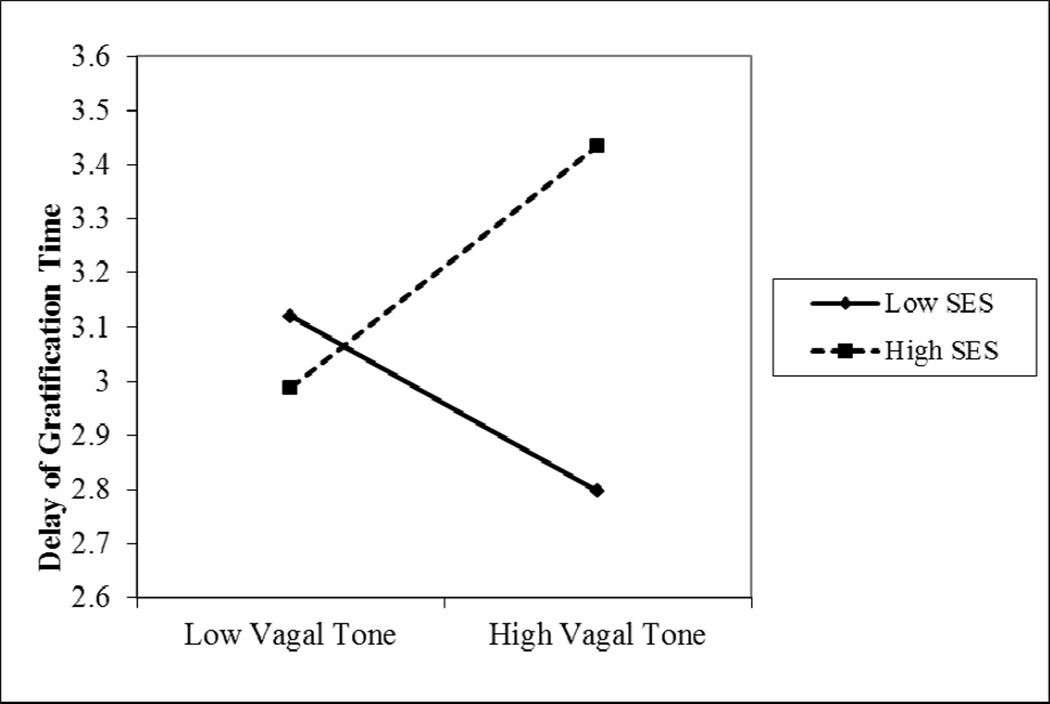
Figure 2.
Plot of the simple slope analysis from Study 2 of the moderating role of socioeconomic group on associations between basal vagal tone at the age of 18 months and children’s delay of gratification at the age of 5. Both simple slopes are significantly different from 0, p < .05. Basal vagal tone values are graphed at −/+ 1 standard deviation.
Model analyses were run in accordance with our study aims. The model was fully saturated with all paths estimated. Replicating previous research, there was a significant effect of SES group on children’s delay of gratification (β = .31, SE = .09, p < .05) such that children in the higher SES group had longer delay time. There was no significant main effect of RSA (β = .07, SE = .02, p = .56), however there was a significant effect of the interaction between RSA and SES on children’s delay of gratification (β = .46, SE = .04, p < .001). The interaction term explained 18% of the variance in children’s delay of gratification. Given the difficulties inherent in detecting moderators in field research (r2 values are typically less than .03; McClelland & Judd, 1993), the magnitude of the effect in the current study is noteworthy.
To probe this effect, simple slope analyses with SES group as the moderator variable were conducted using an online utilities program (Preacher, Curran, & Bauer, 2006; http://www.quantpsy.org/interact/mlr2.htm). Results showed that the simple slope for children in the low SES group was negative and significantly different from zero (B= −.08, t= 2.31, p < .05) whereas the simple slope children in the high SES group was positive and different from zero (B = .10, t=3.02, p < .05). Children’s basal RSA was plotted at +/− 1 SD (See Figure 3). Our findings indicate that higher basal RSA in the context of poverty is associated with lower delay of gratification, while higher basal RSA within more resource rich rearing environments is associated with higher delay of gratification.
Discussion
Psychological models of self-regulation highlight the importance of resisting impulses in order to facilitate health and well-being (Calkins, 2009). Empirical research has identified vagal tone as a potential early physiological precursor of children’s self-regulation however findings have been equivocal to date. As a first foray into testing the predictive value of vagal tone for children’s delay control across environmental conditions, our results across two studies indicate that context matters. Specifically, we found that for children within high resource environments, higher levels of vagal tone were associated with delay of gratification in a predictable fashion according to normative models of regulation. However, the results of Study 1 and Study 2 indicated that higher basal vagal tone within resource poor environments predicted a lower propensity to delay gratification.
Taken together, the present findings do not readily support conceptualizations drawn from normative psychological models proposing that higher vagal tone is a marker of context independent developmentally appropriate regulation. Instead, the current findings suggest that function of high vagal tone may operate in a more curvilinear fashion towards facilitating behavioral fit within specific environmental contexts (Boyce & Ellis, 2005; West-Eberhard, 2003). This dichotomy naturally begs the question, how does context inform our understanding of what is ‘adaptive’ with respect to how vagal tone is associated with the regulation of behavior? Although speculative, we believe that these inconsistencies dovetail nicely with emerging evolutionary-developmental theories which stress placing development within its proximal ecology when interpreting children’s developmental outcomes (Belsky & Pluess, 2013). These frameworks assert that humans adapt their behavior in ways that attempt to increase fitness within a specific local environmental condition. Within a local context where obtaining future rewards is stochastic and uncertain, taking the immediate treat may make perfect sense for children, even though this is viewed as maladaptive by society standards as a whole. In support of this assertion, experimental research showing a positive association between heightened mortality cues and reward-oriented decision-making has attributed this link to greater present-orientation in the context of heightened adversity (Griskevicius, et al., 2001).
By extension, our results also raise the possibility that the way in which the function of vagal tone is interpreted with respect to children’s regulatory behaviors, such as delay of gratification, may depend upon the proximal conditions. Within impoverished contexts where access to resources is more unstable, higher basal vagal tone by virtue of its sensitivity to contextual cues may function to support development through directing behavioral strategies which value immediate reward as opposed to long term gain. In contrast, long-term gains may be more likely in higher resourced and more predictable environments, and thus high vagal tone may support deferring the immediate sweets due to the context-driven (or informed) expectancy that a larger cache is waiting. Thus, across groups higher vagal tone functions to promote the behavioral strategy that is more adaptive to the specific context.
Several limitations must be acknowledged in interpreting our results. First, the current study utilized a single assessment of children’s vagal tone and delay of gratification. Thus, we were unable to model potential transactions across these two domains of regulation as well as developmental change in these constructs over time; and to test this, it would be important for future work to include a broader range of assessments of these constructs. Second, although the focus was to examine tonic or basal vagal tone, our sampling precludes examination of vagal reactivity and regulation in response to stress. It is clear from previous work that both tonic and reactive vagal tone is important to consider in relation to behavioral outcomes. Third, given the homogeneity with respect to SES in Study 1 we were unable to test how different contextual conditions within the context of poverty (e.g., maternal education, varying incomes) may further explain findings presented. Finally, the results of the current study are developmentally specific to the early childhood period and may not generalize to older children and adults.
Against a backdrop of previous empirical research examining the regulatory role of vagal tone in shaping human development, our interpretation of vagal tone as a potential sensitivity factor in determining self-regulation requires confirmatory evidence from future studies. In addition, delineating proximal psychological processes, which operate as critical mediating mechanisms underlying this association, is an important focus of future research. Although we were unable to do so here, it would be interesting to test for potential ‘switch points’ in the gradient of socio-economic status where the association between vagal tone and regulation reverses. This would be particularly informative for interventions targeting chronically marginalized and impoverished children.
These limitations notwithstanding, the present study highlights the potential explanatory utility of integrating evolutionary-developmental models within traditional developmental research (Davies, Sturge-Apple, & Cicchetti, 2011; Sturge-Apple, Davies, Martin, Cicchetti, & Hentges, 2012) and within clinical interventions (Thibodeau, August, Cicchetti, & Symons, 2014). We contend that viewing development within an evolutionary framework naturally shifts the nature of empirical questions from a value laden approach focused primarily on the form of a behavior (inherently ‘good’ vs. inherently ‘bad’) towards understanding the proximate function of children’s behavior when considered within their rearing context. We believe this is particularly true for interpreting behavior within highly stressful and impoverished conditions. Within such contexts, children’s development may actually be elegantly honed to match their environmental constraints and thus necessarily their behavior may not cohere to normative models of child development.
Figure 1.
Path model analysis from Study 1 testing the predictive role of basal vagal tone at age 2 in children’s ability to delay gratification at the age of 4. Standardized coefficients are reported. Dashed line indicates a non-significant effect.
Table 2.
Means and standard deviations of ecological conditions between SES groups in Study 2
| Variable | Full Sample n = 140 |
Low SES n = 71 |
High SES n =69 |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Population Density (100,000 people per square mile) |
1.87 | 1.08 | 2.56 | 0.64 | 1.08 | 0.94 |
| Violent Crime (per 100,000 people) |
6.27 | 3.25 | 8.31 | 1.79 | 3.90 | 2.95 |
Acknowledgments
This research was supported by the National Institute of Nursing Research (Grant R21NR010857) awarded to Fred Rogosch and Melissa Sturge-Apple and the National Institute of Mental Health (R01 MH071256) awarded to Patrick Davies and Dante Cicchetti.
Footnotes
P.T. Davies and D. Cicchetti contributed to the design and data collection of Study 1. M. L. Sturge-Apple, J. H. Suor, M. A. Skibo, and F. A. Rogosch contributed to the design and data collection of Study 2. M. L. Sturge-Apple developed the concept for the manuscript. M. L. Sturge-Apple performed the data analysis and interpretation. M. L. Sturge-Apple drafted the manuscript, and all authors provided critical revisions. All authors approved the final version of the manuscript for submission.
Contributor Information
Melissa L. Sturge-Apple, Department of Clinical and Social Sciences in Psychology, University of Rochester
Jennifer H. Suor, Department of Clinical and Social Sciences in Psychology, University of Rochester
Patrick T. Davies, Department of Clinical and Social Sciences in Psychology, University of Rochester
Dante Cicchetti, Institute of Child Development, University of Minnesota.
Michael A. Skibo, Westchester Community College
Fred A. Rogosch, Mt. Hope Family Center, University of Rochester
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond risk, resilience, and dysregulation: Phenotypic plasticity and human development. Development and Psychopathology. 2013;25:1243–1261. doi: 10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology. 2003;24:479–497. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Regulatory competence and early disruptive behavior problems: The role of physiological regulation. Biopsychosocial regulatory processes in the development of childhood behavioral problems. 2009:86–115. [Google Scholar]
- Chisholm JS. Death, hope and sex: Steps to an evolutionary ecology of mind and morality. Cambridge University Press; 1999. [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children’s development. Psychiatry. 1993;56:96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Daly M, Wilson M. Carpe diem: Adaptation and devaluing the future. The Quarterly Review of Biology. 2005;80(1):55–60. doi: 10.1086/431025. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D. Interparental aggression and children's adrenocortical reactivity: Testing an evolutionary model of allostatic load. Development and Psychopathology. 2011;23:801–814. doi: 10.1017/S0954579411000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Manning LG, Zale E. Children’s patterns of emotional reactivity to conflict as explanatory mechanisms in links between interpartner aggression and child physiological functioning. Journal of Child Psychology and Psychiatry. 2009;50:1384–1391. doi: 10.1111/j.1469-7610.2009.02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Fabes RA, Reiser M, Cumberland A, Shepard SA, Thompson M. The relations of effortful control and impulsivity to children's resiliency and adjustment. Child Development. 2004;75(1):25–46. doi: 10.1111/j.1467-8624.2004.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk. Human Nature. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Enders CK. The impact of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychological Methods. 2001;6:352. [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, McNamara JM, Houston AI. When is it adaptive to be patient? A general framework for evaluating delayed rewards. Behavioural Processes. 2012;89:128–136. doi: 10.1016/j.beproc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Griskevicius V, Tybur JM, Delton AW, Robertson TE. The influence of mortality and socioeconomic status on risk and delayed rewards: a life history theory approach. Journal of Personality and Social Psychology. 2011;100:1015. doi: 10.1037/a0022403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. Most people are not WEIRD. Nature. 2010;466(7302):29–29. doi: 10.1038/466029a. [DOI] [PubMed] [Google Scholar]
- Kidwell SL, Barnett D. Adaptive emotion regulation among low-income African American children. Merrill-Palmer Quarterly. 2007;53:155–183. [Google Scholar]
- Lengua LJ, Zalewski M, Fisher P, Moran L. Does HPA-Axis Dysregulation Account for the Effects of Income on Effortful Control and Adjustment in Preschool Children? Infant and Child Development. 2013;22:439–458. doi: 10.1002/icd.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O'Brien M, Blankson AN. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52:603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological bulletin. 1993;114:376. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB. Attention in delay of gratification. Journal of Personality and Social Psychology. 1970;16:329. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. The comprehensive modelling program for applied researchers: User’s guide. 2012;5 [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and Child Development. 2011;20:106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Raver CC, Jones SM, Li-Grining C, Zhai F, Bub K, Pressler E. CSRP’s impact on low-income preschoolers’ preacademic skills: self-regulation as a mediating mechanism. Child Development. 2011;82:362–378. doi: 10.1111/j.1467-8624.2010.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razza RA, Raymond K. Associations among maternal behavior, delay of gratification, and school readiness across the early childhood years. Social Development. 2013;22:180–196. [Google Scholar]
- Schlomer GL, Bauman S, Card NA. Best practices for missing data management in counseling psychology. Journal of Counseling Psychology. 2010;57:1. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Martin MJ, Cicchetti D, Hentges RF. An examination of the impact of harsh parenting contexts on children's adaptation within an evolutionary framework. Developmental Psychology. 2012;48:791. doi: 10.1037/a0026908. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Taylor ZE, Eisenberg N, Spinrad TL. Respiratory sinus arrhythmia, effortful control, and parenting as predictors of children’s sympathy across early childhood. Developmental Psychology. 2015;51:17. doi: 10.1037/a0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau EL, August GJ, Cicchetti D, Symons FJ. Application of environmental sensitivity theories in personalized prevention for youth substance abuse: a transdisciplinary translational perspective. Translational Behavioral Medicine. :1–9. doi: 10.1007/s13142-015-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; 2003. [Google Scholar]