Abstract
Regulation of load-induced bone formation is considered a local phenomenon controlled by osteocytes, although it has also been hypothesized that functional adaptation may be neuronally regulated. The aim of this study was to examine bone formation in multiple bones, in response to loading of a single bone, and to determine whether adaptation may be neuronally regulated. Load-induced responses in the left and right ulnae and humeri were determined after loading of the right ulna in male Sprague-Dawley rats (69 ± 16 days). After a single period of loading at −760με, −2,000με, or −3,750με initial peak strain, rats were given calcein to label new bone formation. Bone formation and bone neuropeptide concentrations were determined at 10 days. In one group, temporary neuronal blocking was achieved by perineural anesthesia of the brachial plexus with bupivicaine during loading. We found right ulna loading induces adaptive responses in other bones in both thoracic limbs when compared to sham controls, and that neuronal blocking during loading abrogated bone formation in the loaded ulna and other thoracic limb bones. Skeletal adaptation was more evident in distal long bones, when compared with proximal long bones. We also found that the single period of loading modulated bone neuropeptide concentrations persistently for 10 days. We conclude that functional adaptation to loading of a single bone in young rapidly growing rats is neuronally regulated and involves multiple bones. Persistent changes in bone neuropeptide concentrations after a single loading period suggest that plasticity exists in the innervation of bone.
Keywords: Bone innervation, brachial plexus anesthesia, mechanical loading, bone formation, bone neuropeptides
INTRODUCTION
The mechanisms that regulate the process by which bone tissue is added and removed from the skeleton have been a major focus in bone biology since it was recognized that bone is exquisitely sensitive to mechanical loading.(1) However, it is unclear why adaptive failure and fracture of bone is common in older human beings and in human and animal athletes.(2,3) Functional adaptation occurs by modeling and remodeling.(4) During modeling the spatial distribution of bone tissue is altered, whereas remodeling removes and replaces small volumes of existing bone with new bone. (4) Remodeling is a bone repair process that, at least in part, is targeted preferentially to areas of bone microdamage.(5)
Regulation of load-induced bone formation by modeling is currently considered a local phenomenon controlled by the network of osteocytes embedded in the bone matrix, although it has also been hypothesized that functional adaptation may be neuronally regulated.(6,7) The periosteoum is densely innervated with a dense net-like meshwork of nerves, suggesting the existence of a sophisticated and specialized neuronal regulatory mechanism optimized for detection of mechanical distortion of periosteum and bone.(8) Peptidergic nerves are arranged into networks on the surface of bones, and are most numerous in the epiphysis and metaphysis.(9) The periosteum contains both sensory nerves, which release calcitonin gene-related peptide (CGRP) and substance P (SP), as well as sympathetic nerves, which release vasoactive intestinal peptide (VIP) and neuropeptide Y (NPY).(9) The periosteum is the bone envelope with the greatest density of sensory nerve fibres.(10) Neuropeptides are synthesized in the dorsal root or local sympathetic/parasympathetic ganglia, transported within nerve axons to terminals, and released in response to nerve activity. Nerve branches or single neurons enter the bone cortex, often in association with the microvasculature.(9) Unmyelinated sensory peptidergic nerves establish direct connections between individual bone cells and the brain, potentially enabling direct neural regulation of skeletal metabolism.(11) Bone cells express receptors for a wide range of neurotransmitters, including glutamate, catecholamines, and neuropeptides such as CGRP, VIP, and SP.(12) Neuropeptides have pleiotrophic effects on bone cells in vitro and can influence bone formation and the formation and activation of osteoclasts for bone resorption.(13) Functional impairment of sensory or sympathetic nerves can also lead to activation of bone resorption in vivo.(14,15) Collectively, these data suggest that the innervation of bone has a functional role in bone physiology.
If neuronal signaling is an important regulator of load-induced bone formation, it would be expected that this type of signaling might enable cross-talk between appendicular bones in different limbs during functional adaptation. Therefore, the purpose of this study was to determine whether modeling responses to mechanical loading of a single long bone involved multiple bones and whether such responses were neuronally regulated. Using the rat ulna end-loading model, we temporarily blocked neuronal signaling between the right thoracic limb and the spinal cord using perineural anesthesia of the brachial plexus during ulna loading, and found that blockade of neuronal signaling between the limb and the spinal cord during loading significantly reduced skeletal modeling responses in multiple bones. Such responses were also associated with persistent changes in bone neuropeptide concentrations. These phenomena are likely the result of plasticity in the bone innervation - the capacity of neurons to change their function, chemical profile, or structure in response to a stimulus.(16)
MATERIALS AND METHODS
Animals
A homogeneous group of 246 young, male Sprague-Dawley rats (body weight 295 – 320 g; age 69 ± 16 days) were used for the study. Rats were provided with food and water ad libitum. All procedures were performed in accordance with guidelines of the American Veterinary Medical Association and with approval from the Animal Care Committee of the University of Wisconsin-Madison. Humane euthanasia was performed with 390mg pentobarbitone, injected into the peritoneal cavity, at the end of the experimental period.
Experimental design
To determine skeletal adaptive responses to loading of the right ulna, 48 rats were randomly assigned to three treatment groups and treated with a short period of cyclic loading at low, medium, or high initial peak strain (n = 16 rats/group). Twenty rats were also randomly assigned to a sham control group (n = 12), or a baseline control group (n = 8). Rats in the sham control group were given the same treatment regimen as rats in the treatment groups, but were not subjected to any mechanical loading. To determine whether load-induced adaptive bone formation was neuronally regulated, the right ulna of an additional 8 rats was subjected to a single period of cyclic loading at high initial peak strain after neuronal signaling between the limb and the spinal cord was temporarily blocked using perineural anesthesia of the right brachial plexus (see below). All rats were humanely euthanatized at the end of a 10 day experimental period. All rats received two intra-peritoneal injections of 7mg/kg calcein (Sigma, St Louis, MO), the first before recovery from loading or sham loading and the second 7 days after loading, to fluorochrome label new bone formed during the treatment period.(17) The baseline control group was also injected with calcein at times equivalent to the treatment and sham-control groups.
For neuropeptide analysis, 144 rats were randomly assigned to three treatment groups (n = 48 rats/group). Groups were again treated with a period of cyclic loading at low, medium, or high initial peak strain and groups of 12 rats were humanely euthanatized at 1 hour, 1 day, 3 days, or 10 days after loading. An additional 21 rats were assigned to baseline (n = 12) or sham (n = 9) control groups.
Anesthesia of the brachial plexus
Perineural anesthesia of the nerves of the right brachial plexus was performed five minutes before loading using bupivicaine (Marcaine® 0.5%, Hospira Inc., Lake Forest, IL) at a dose of 2mg/kg. Correct positioning of the insulated injection needle (ProBloc™ II, Portex Inc., Smiths Medical, St. Paul, MN) was confirmed using a train-of-four nerve stimulator (Micro Stim, Neuro Technology, Houston, TX). Using a standard regional anesthetic technique, perineural positioning of the injection needle induces an observable twitch in the limb after activation of the nerve stimulator. After perineural injection of bupivicaine, functional blocking of neuronal signaling between the spinal cord and the loaded limb was confirmed by observing paralysis of the limb upon recovery from anesthesia, which resolved within 2 hours of loading. Therefore, lameness associated with blocking was present for <1% of the 10 day adaptive period.
In-vivo ulnar loading
In this bone loading model, the antebrachium of the rat is placed between horizontally orientated loading cups, which are fixed to the loading platen and actuator of a materials testing machine (Model 8800 DynaMight; Instron, Canton, MA) with a 250N load cell (Honeywell Sensotec, Canton, MA). Axial compression accentuates the pre-existing mediolateral curvature of the diaphysis of the ulna and translates most of the axial force into a bending moment, which is maximal near the midshaft. To determine the relationship between peak load and initial peak strain in the rat ulnar end-loading model(17) in our laboratory, we performed an ex-vivo study using 5 rats. A single rosette strain gauge (EA-06-031DE-120, 120Ω, Vishay Micromeasurements, Malvern, PA) was bonded to the diaphysis of the caudal medial surface of the right ulna, approximately 5mm distal to the level of the radial head at 60% of bone length from the proximal end of the ulna. The right antebrachium was then cyclically end-loaded in compression at 4Hz for a small number of cycles (50 to 100 cycles) using a series of cyclic compressive loads (peak loads of −3.3, −10, −16, −18, −20, −22, and −24N) to determine the relationship between initial peak load and initial peak strain (Fig. 1). We selected peak loads that induced low (−3.3N, −760με), medium (−10N, −2,000με), and high initial peak strains (−18N, −3,750με) for the study, based on previous work.(17–20)
FIG. 1.
Load-strain relationship for the Sprague-Dawley rat ulna. Peak cyclic strains were estimated using a series of cyclic peak loads (−3.3, −10, −16, −18, −20, and −22N) for a small number of cycles (50–100 cycles) in an ex-vivo study using 5 male Sprague-Dawley rats. We selected peak loads that induced low (−3.3N, −760με), medium (−10N, −2,000με), and high (−18N, −3,750με) initial peak strains for the present study, based on previous work.(17–20) Error bars represent standard deviation.
In vivo loading of the right ulna was performed under isofluorane-induced general anesthesia. Again, based on previous work,(17–20) the right ulna of each rat was loaded for 1,500 cycles of compression across the antebrachium at 4Hz. The single period of in-vivo loading we used for these studies therefore lasted for 6.25 minutes. For analgesia, butorphanol (0.5mg/kg) was given by subcutaneous injection 15 minutes before induction and again immediately after loading. The right antebrachium was flexed at both the carpus and elbow and placed between an actuator cup and the load cell cup on the materials testing machine. The distance between the actuator cup and the load cell cup was manually adjusted until −0.5N was applied to the ulna to hold it in place. Cyclic compressive load was applied to the ulna using a haversine waveform at 4Hz, with a minimum load of −0.5N and a peak load of either −3.3N, −10.0N, or −18.0N, depending on treatment group. All rats were ambulatory within 20 minutes of recovery from anesthesia.
Bone Histomorphometry
Left and right ulnae and humeri were dissected along with surrounding tissue. Bones were stored at −20°C and then fixed in 70% ethanol. For processing, bones were dehydrated in a graded series of ethanol (70%, 100%) and embedded in methylmethacrylate. Transverse calcified sections, 120μm thick, were made and mounted on standard microscope slides. Ulnae were sectioned at 60% total bone length from the proximal end, where it has been shown maximal adaptation occurs with this model.(21) Humeri were sectioned at the mid-diaphysis (50% of total bone length). Bright-field and confocal microscopy (Bio-Rad MRC-1024 Laser Scanning Confocal Microscope, Bio-Rad, Hercules, CA) were used to examine the bone sections. Sections of the right ulna, which was loaded, were reviewed carefully for bone microdamage. Using confocal fluorescent images of each bone section, periosteal and endosteal labeled bone areas (Ps.L.B.Ar and Es.L.B.Ar, %) were determined using a standard method.(22) In addition, total cortical bone area (Tt.L.B.Ar, %) was determined by thresholding the image and pixel-counting (Image J, NIH, USA). Preliminary work (data not shown) suggested that in young rapidly growing male rats (mean baseline ulna MS/BS and MAR of 73.8 ± 17.9% and 5.4 ± 3.4μm respectively), this morphometric method provided a more sensitive indication of new bone formation, when compared with determination of mineralizing surface, mineral apposition rate, and bone formation rate using classical morphometric methods.(23,24) All measurements were made by a single observer (SS). Data were normalized to the original cortical area to account for minor variations in rat size. Ulnar resorption space density (Rs.N/T.Ar, #/mm2) was also quantified.
Quantification of Bone Neuropeptides
Left and right ulnae were dissected and the surrounding tissue removed. Both the loaded and contralateral bones of each rat were analyzed for CGRP, VIP, and SP neuropeptide concentrations using a previously described method.(25) Each ulna was weighed and crushed into small fragments, which were boiled for ten minutes in 10x volume of 2M acetic acid in 4% EDTA (pH 3.5). After homogenization, the samples were centrifuged at 3,000g for 15 minutes. The supernatants were freeze-dried and then dissolved in ELISA buffer. CGRP, VIP, and SP concentrations were determined using commercial rat-specific ELISA assays (Cayman Chemical, Ann Arbor, MI – CGRP, SP; Phoenix Pharmaceuticals, Belmont, CA – VIP). Data were normalized to the wet weight of the ulna to account for variation in bone size.
Statistical Analysis
The Kolmogorov-Smirnov test was used to confirm that data were normally distributed. A Student’s t test with a hypothesized mean equal to zero was used to determine right-left differences in the loaded and contralateral ulnae. A one-way ANOVA and a post-hoc Dunnett’s test were used for comparison of group effects with the sham control group. A Student’s t test for unpaired data was used to compare the group loaded at high strain and the group loaded at high strain during brachial plexus blocking. The Kruskal Wallis ANOVA test and the Mann-Whitney U test were used to analyze Rs.N/T.Ar data. Results were considered significant at P < 0.05. Data are reported as mean ± standard deviation, or median and range for non-parametric data.
RESULTS
The linear load-strain relationship during end-loading of the rat ulna indicated that the peak loads selected for the low (−3.3N), medium (−10N), and high (−18N) strain groups were associated with average initial peak strains of −760±195με, −2,000±470με, and −3,750±920με, respectively (Fig. 1). As initial peak strain became higher, incrementally larger amounts of labeled new bone formed in response to ulnar loading, with significant increases being detected in the high-strain group (P < 0.05, Table 1). The loading regimens we used did not induce obvious bone fatigue, as displacement amplitude at the end of 1,500 cycles of load did not increase in any of the loading groups. Lameness in the loaded limb was not evident after loading or after reversal of brachial plexus anesthesia in the brachial plexus blocking group.
TABLE 1.
RIGHT-LEFT DIFFERENCES IN ULNAR BONE FORMATION IN RESPONSE TO CYCLIC LOADING OF THE RIGHT ULNA
| Experimental Group | Ps.L.B.Ar, % | P value | Tt.L.B.Ar, % | P Value | Es.L.B.Ar, % | P Value |
|---|---|---|---|---|---|---|
| Baseline Control (n = 8) | −0.32 ± 2.62 | NS | 1.21 ± 5.20 | NS | 0.72 ± 1.13 | P = 0.11 |
| Sham Control (n = 8) | 0.57 ± 2.63 | NS | 1.63 ± 3.88 | NS | 0.50 ± 1.40 | NS |
| Low Initial Peak Strain(−760με, n = 16) | 0.74 ± 2.53 | NS | 0.53 ± 2.75 | NS | 0.20 ± 0.91 | NS |
| Medium Initial Peak Strain (−2,000με, n = 16) | 0.82 ± 1.82 | P = 0.1 | 1.12 ± 3.28 | NS | −0.11 ± 0.88 | NS |
| High Initial Peak Strain (−3,750με, (n = 16) | 2.71 ± 3.90 | P = 0.01 | 3.75 ± 5.72 | P < 0.05 | −0.02 ± 1.68 | NS |
| Brachial Plexus Block and High Initial Peak Strain (−3,750με, n = 8) | 4.24 ± 4.22 | P < 0.05 | 3.81 ± 3.18 | P < 0.05 | 0.51 ± 0.61 | P < 0 .05 |
Note: Data represent mean ± standard deviation. Ps. L.B.Ar – Periosteal labeled bone area; Tt.L.B.Ar – Total labeled bone area; Es.L.B.Ar – Endosteal labeled bone area.
Mechanical loading of the right ulna induced modeling responses in multiple long bones
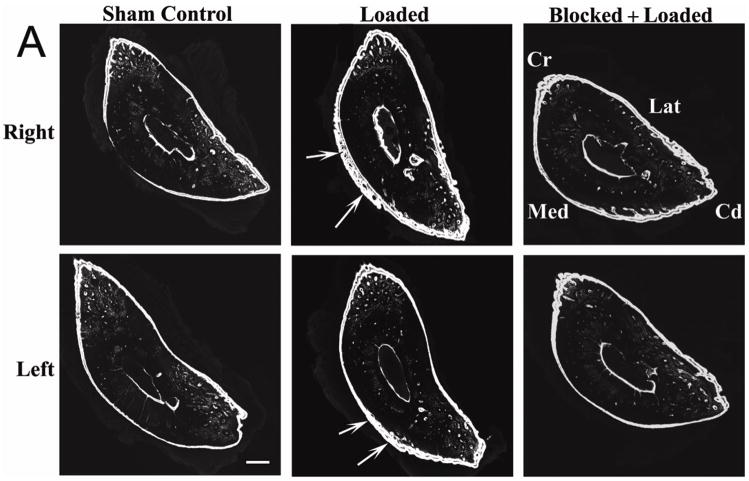
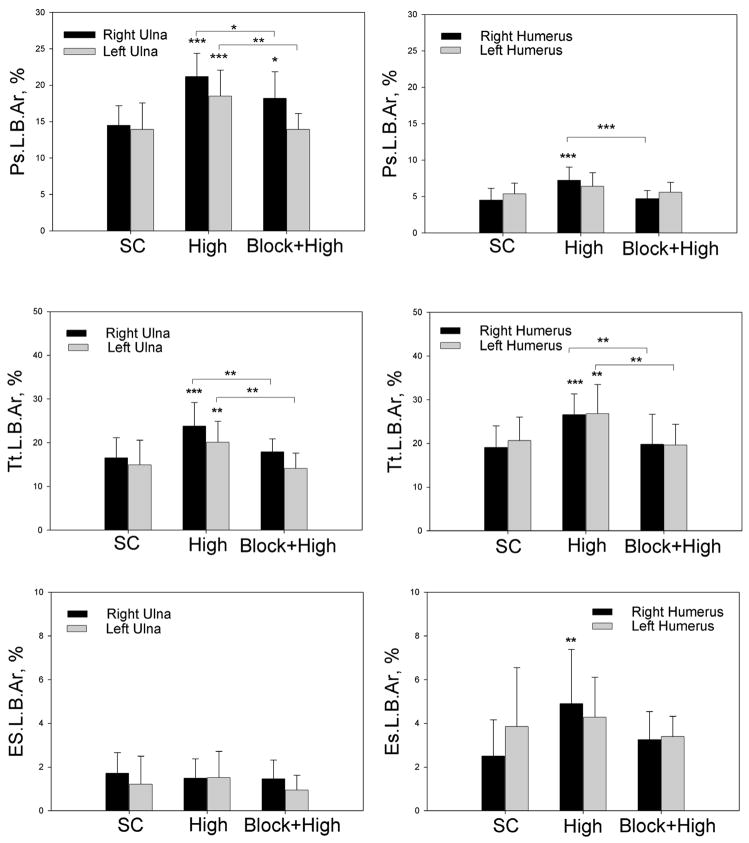
We found that mechanical loading of the right ulna induced increased bone formation in both the loaded bone and in distant bones that were not loaded. In the right ulna, load-induced bone formation was more evident on the periosteal surface of the cortex and within the bone cortex, with increased Ps.L.B.Ar and Tt.L.B.Ar, and less evident in the endosteal surface of the cortex, with no significant changes in Es.L.B.Ar in either the loaded or contralateral ulnae (Figs 2A, 3AB). We also found that loading of the right ulna at high initial peak strain (−3,750με) induced increased Ps.L.B.Ar in the contralateral (left) ulna that was not loaded; Tt.L.B.Ar was also increased when comparisons were limited to the sham control, the high load group and the high load group with brachial plexus blocking (Fig. 3AB). At low and medium strains (−760με, −2,000με, respectively), no significant effects were found in the contralateral ulna (Fig. 3A). Ulnar bone formation in the baseline and sham control groups was not significantly different (Fig. 3A).
FIG. 2.
Cyclic loading of the right ulna induces adaptive bone formation which is neuronally regulated in multiple thoracic limb bones in male Sprague-Dawley rats. (A) Load-induced bone formation was seen in both the loaded (right) ulna and the contralateral (left) ulna that was not loaded (white arrows), and was particularly evident on the periosteal surface. Formation of labeled periosteal new bone was decreased when loading was performed during temporary blocking of neuronal signaling between the right thoracic limb and the spinal cord by anesthesia of the loaded limb’s brachial plexus. Microcracking of the caudomedial region of the right (loaded) ulna, which is typically associated with the development of bone fatigue in this model,(28) was not seen. (B) Bone formation responses in the right and left humeri that were not directly loaded (white arrow heads) were similar to the ulnae, but the extent of the new bone formation was less. Confocal photomicrographs of calcified transverse sections of ulnae at 60% of bone length, from proximal to distal,21 and humerii at 50% of bone length, after 10 days of adaptation to a short period of cyclic loading that was applied to the right ulna (1,500 cycles at 4Hz and an initial peak strain of −3,750με). New bone formation was double-labeled with calcein. Bars = 250μm for Fig. 2A and 500μm for Fig. 2B. Cr – cranial; Cd – caudal; Med – medial; Lat – lateral. Loaded groups n = 16; Sham Control group n = 12; Blocked + Loaded group n = 8.
FIG. 3.
Load-induced bone formation in thoracic limb long bones was most pronounced in the periosteal bone envelope. Neuronal mechanotransduction regulated the majority of adaptive bone formation in distant bones and a large proportion of bone formation in the loaded (right) ulna. (A) Formation of calcein-labeled new bone in thoracic limb bones of Sprague-Dawley rats in response to unilateral cyclic loading of the right ulna. In the high strain group, normalized periosteal labeled bone area (Ps.L.B.Ar) was increased in both the loaded (right) ulna and the contralateral (left) ulna. Similar but reduced responses were also seen in the humerii that were not directly loaded. Changes in total labeled bone area (Tt.L.B.Ar) in response to mechanical loading were less evident. Increased endosteal labeled bone area (Es.L.B.Ar) was only found in the right humerus after high strain loading. Bone formation in baseline and sham control groups of rats was not significantly different. (B) Adaptive bone formation after brachial plexus blocking and loading at high strain was not significantly different from sham control, expect Ps.L.B.Ar in the loaded (right) ulna, which was reduced 45% by neuronal blocking. BC – baseline control; SC – sham control; Low – loading at −3.3N, initial peak strain = −760με Med – loading at −10N, initial peak strain = −2,000με High - loading at −18N, initial peak strain = −3,750με Block+High – loading at high initial peak strain after brachial plexus blocking. * - P < 0.05; ** - P < 0.01; *** - P < 0.001; # - P < 0.15 versus the relevant sham control. Differences between the group loaded at high strain and the blocked + loaded group are also indicated. Error bars represent standard deviation. Loaded groups n = 16; Sham Control group n = 12; Blocked + Loaded group n = 8; Baseline Control group n = 8.
We also found that mechanical loading of the right ulna induced significant changes in bone formation in the right and left humeri that were not directly loaded. Increased bone formation was most evident in the ipsilateral (right) humerus, with increased Ps.L.B.Ar, Tt.L.B.Ar, and Es.L.B.Ar in the high strain group. In addition, increased Tt.L.B.Ar was found in the contralateral left humerus (Fig. 2B, Fig. 3A). Again, at low and medium strains (−760με, −2,000με respectively), few significant effects were found, although Tt.L.B.Ar increased in the right humerus in the low strain group (Fig. 3A). Humeral bone formation in the baseline and sham control groups was not significantly different (Fig. 3A).
Thus, adaptive load-induced bone formation includes responses in multiple bones, including bones that were not directly loaded, suggesting the existence of a cross-talk mechanism between different limbs during functional adaptation. Bright-field and confocal images did not show any evidence of microdamage or increased remodeling in any of the loading groups; ulnar Rs.N/T.Ar was not significantly influenced by mechanical loading (Table 2).
TABLE 2.
RESORPTION SPACE DENSITY IN RAT THORACIC LIMB LONG BONES AFTER CYCLIC LOADING OF THE RIGHT ULNA
| Bone | Baseline Control(n = 8) | Sham Control (n = 12) | Low Initial Peak Strain (−760με) (n = 16) | Medium Initial Peak Strain(−2,000με) (n = 16) | High Initial Peak Strain(−3,750με) (n = 16) | Brachial Plexus Block and High Initial Peak Strain (−3,750με) (n = 8) |
|---|---|---|---|---|---|---|
|
Strain Mode Experimental Groups
| ||||||
| Right (loaded) Ulna | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.77) | 0.00 (0.00, 0.67) | 0.00 (0.00, 0.68) | 0.00 (0.00, 0.63) | |
| Left (contralateral) Ulna | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.66) | 0.00 (0.00, 1.34) | 0.00 (0.00, 0.83) | 0.00 (0.00, 1.29) | |
|
| ||||||
|
Brachial Plexus Blocking Experimental Groups
| ||||||
| Right (loaded) Ulna | 0.00 (0.00, 0.77) | 0.00 (0.00, 0.63) | 0.00 (0.00, 0.82) | |||
| Left (contralateral) Ulna | 0.00 (0.00, 0.66) | 0.00 (0.00, 1.29) | 0.00 (0.00, 0.64) | |||
Note: Data represent median and range. In each experiment, comparisons between groups for the right and left ulnae were not significantly different. Data represent resorption space density - Rs.N/T.Ar, #/mm2.
Brachial plexus anesthesia during cyclic bone loading blocks adaptive modeling responses
Temporary blockade of neuronal signaling between the loaded limb and the spinal cord using brachial plexus anesthesia during cyclic mechanical loading of the right ulna had significant effects on adaptive bone modeling. In the contralateral (left) ulna and in both humeri, neuronal blocking abolished any significant bone modeling effects (Figs. 2, 3B, Table 3). For Tt.L.B.Ar and Es.L.B.Ar in the left ulna and humerus, brachial plexus blocking suppressed bone formation below the sham control (Table 3). Temporary blocking of neuronal signaling between the loaded limb and the spinal cord also reduced Ps.L.B.Ar and Tt.L.B.Ar in the loaded ulna by 45% and 81%, respectively (Figs. 3B and Table 3). Thus, these data support a critical role for neuronal signaling in the bone mechanotransduction mechanism that regulates load-induced bone formation.
TABLE 3.
PROPORTION OF LOAD-INDUCED BONE FORMATION BLOCKED BY BRACHIAL PLEXUS ANESTHESIA IN RAT THORACIC LIMB LONG BONES AFTER CYCLIC LOADING OF THE RIGHT ULNA
| Bone | Sham Contro l(n = 12) | High Initial Peak Strain(−3,750με) (n = 16) | Brachial Plexus Block and High Initial Peak Strain (−3,750με) (n = 8) | Blocking Proportion (%)above Sham Control |
|---|---|---|---|---|
| Ps.L.B.Ar, %
| ||||
| Right (loaded) Ulna | 14.50 ± 2.71 | 21.20 ± 3.17*** | 18.18 ± 3.67* | 45% |
| Left Ulna | 13.93 ± 3.63 | 18.49 ± 3.59*** | 13.94 ± 2.16 | 100% |
| Right Humerus | 4.53 ± 1.59 | 7.25 ± 1.78*** | 4.69 ± 1.14 | 94% |
| Left Humerus | 5.35 ± 1.50 | 6.40 ± 1.87 | 5.58 ± 1.38 | 78% |
|
| ||||
| Tt.L.B.Ar, %
| ||||
| Right (loaded) Ulna | 16.54 ± 4.64 | 23.83 ± 5.38*** | 17.92 ± 2.93 | 81% |
| Left Ulna | 14.91 ± 5.69 | 20.09 ± 4.84** | 14.11 ± 3.52 | >100% |
| Right Humerus | 19.11 ± 4.93 | 26.53 ± 4.76*** | 19.80 ± 6.84 | 91% |
| Left Humerus | 20.63 ± 5.38 | 26.81 ± 6.67** | 19.60 ± 4.73 | >100% |
|
| ||||
| Es.L.B.Ar, %
| ||||
| Right (loaded) Ulna | 1.71 ± 0.95 | 1.50 ± 0.89 | 1.46 ± 0.86 | N/A |
| Left Ulna | 1.21 ± 1.29 | 1.52 ± 1.20 | 0.95 ± 0.68 | >100% |
| Right Humerus | 2.50 ± 1.65 | 4.90 ± 2.47** | 3.25 ± 1.29 | 71% |
| Left Humerus | 3.86 ± 2.70 | 4.28 ± 1.83 | 3.40 ± 0.93 | >100% |
Note: Data represent mean ± standard deviation. Ps. L.B.Ar – Periosteal labeled bone area; Tt.L.B.Ar – Total labeled bone area; Es.L.B.Ar – endosteal labeled bone area. N/A – not applicable as loaded mean was below sham control. >100% indicates brachial plexus blocking suppressed bone formation below sham control.
- P < 0.05;
- P < 0.01;
- P < 0.001 versus the sham control. Blocking proportion (%) = [1-(Block+High group-Sham Control group)/(High Load group-Sham Control group)]*100.
Effect of mechanical loading on ulnar bone neuropeptide concentrations
Loading had significant effects on neuropeptide concentrations in both the loaded ulna and the contralateral ulna that was not loaded (Fig. 4). Compared with the sham control, we found that ulnar CGRP concentrations were persistently decreased bilaterally for at least 10 days after a single short period of mechanical loading (Fig. 4). This phenomenon was evident at all three levels of initial peak strain, but was most pronounced in the group loaded at the high initial peak strain of −3,750με. Such persistent changes in bone neuropeptide concentrations suggest that plasticity exists in the peptidergic innervation of bone. Similar bilateral decreases in ulnar SP concentrations were also found, although this effect was less persistent over the 10 day experimental period; loading at high initial peak strain was associated with increased concentrations of SP in the ulnae bilaterally at 10 days (Fig. 4). Ulnar VIP concentrations were also increased bilaterally at 10 days in response to loading at medium and high strain (Fig. 4); such an effect was not evident at low initial peak strain. The use of general anesthesia for loading also caused significant increases in ulnar SP concentrations, indicated by significant differences in bone neuropeptide concentrations between the sham and baseline control groups (Fig. 4); this effect was not evident with CGRP or VIP.
FIG. 4.
Unilateral cyclic loading of the right ulna induced persistent changes in bone neuropeptide concentrations in both the loaded (right) ulna and the contralateral (left) ulna. Unilateral cyclic loading of the right ulna induced bilateral decreases in ulnar CGRP concentrations that persisted for the 10-day experimental period. Loading with low and medium initial peak strain also induced similar changes in ulnar SP concentrations; these effects were not evident with high strain loading. Ulnar VIP concentrations were also increased bilaterally at 10 days in response to loading at high strain. The Sham Control group was not significantly different from the Baseline Control group for CGRP and VIP, whereas bone concentrations of SP were increased in the Sham Control group. BC – baseline control; SC – sham control; 1H – one hour after loading; 1D – one day after loading; 3D – three days after loading; 10D – ten days after loading; −760με - loading at low initial peak strain using −3.3N; −2,000με - loading at medium initial peak strain using −10N; −3,750με - loading at high initial peak strain using −18N. * - P < 0.05; ** - P < 0.01; *** - P < 0.001; # - P < 0.15 versus the relevant sham control. Error bars represent standard deviation. Loaded groups n = 12; Sham Control group n = 9; Baseline Control group n = 12.
DISCUSSION
This study provides evidence that neuronal signaling acts to regulate load-induced bone formation during skeletal modeling. Using the well-established minimally invasive rat ulna end-loading model,(17) we showed that modeling responses to loading of the right ulna develop in multiple thoracic limb bones. This suggests the existence of a cross-talk mechanism between limbs that facilitates functional adaptation of the skeleton to mechanical loading.
In a novel application in this model, we also used perineural anesthesia of the brachial plexus with bupivicaine to temporarily block neuronal signaling in both sensory and motor nerves between the right thoracic limb and the spinal cord and thus temporarily anesthetize the limb from the elbow distally during ulna loading and block neuronal signaling via the innervation of the ulna.(26) However, brachial plexus anesthesia may not completely block innervation of the humerus. Temporary brachial plexus blocking showed that neuronal signaling regulates a large proportion of bone formation in the loaded bone and the majority of bone formation in distant bones.
Classically, functional adaptation is considered highly site-specific and strain-dependent.(1,6,17,21) Our experiments suggest that bone loading also induces adaptive responses that are not site-specific or strain-dependent, and builds on the observation that loading of one region of a long bone may induce bone formation at a distant site in the same long bone, where bone strain was not altered by loading.(27) In our model, after cyclic loading of the right ulna, modeling responses in the right humerus were more evident than in the left humerus. One possible explanation for this is that right ulna end-loading may have influenced the mechanical environment of the humerus, as joint loading is known to induce diaphyseal bone formation.(27) Could a neuronal mechanism explain these observations? To confirm our hypothesis regarding neuronal regulation of functional adaptation, we temporarily blocked neuronal signaling in the right brachial plexus by perineural anesthesia using bupivicaine and then mechanically loaded the right ulna at high strain (−18N, −3,750με). Our data support a critical role for neuronal signaling in bone mechanotransduction and suggest that a neuronal cross-talk mechanism regulates load-induced bone formation in bones that are directly loaded as well as associated adaptive bone formation in long bones at distant sites in the skeleton that were not loaded. Other mechanotransduction pathways, such as osteocyte signaling,6 likely regulate the remaining bone formation in loaded bones. These neuronal signaling effects were most evident at the periosteal surface, the bone envelope with the greatest density of sensory nerve fibers,10 and were also more evident in the distal long bones than in the proximal long bones. Existence of a neuronal cross-talk mechanism between different bones of the skeleton could also explain how mechanical loading of the ulna might influence blood flow and interstitial fluid flow in the contralateral ulna,(28) and may help to explain the pathology known to be associated with hypertrophic osteoarthropathy(29) and complex regional pain syndrome type 1.(30)
Previous work in this field(14,15,22) has suggested that the innervation of bone has a physiological role in functional adaptation, but data from different models have not always yielded consistent results. This remains a controversial field. One limitation of the use of chemical agents to destroy or block sensory or sympathetic nerves(15,22) is the potential for confounding effects to influence the model during the experimental period. Nerve transection models may also have the confounding effect of inducing disuse in the treated limb.(22) In the present study, the effects of brachial plexus anesthesia were temporary, and yet significant blocking effects were found. This method of nerve blocking may prove advantageous in future studies. Bupivicaine has the potential to influence vasomotor tone,(31) either directly or indirectly, and may have temporarily increased bone blood flow and thus modulated bone formation. However, increased bone blood flow would likely increase bone formation,(32) while we found a blocking effect. In a future work, measurement of ulnar blood flow(28) would determine whether use of bupivicaine influences bone blood flow in this model. A limitation of the rat ulna end-loading model is that the woven bone formation(17) may not completely mirror physiological bone modeling that occurs in the adult skeleton in daily life. Formation of woven bone in both local and distal skeletal sites in this model may represent more of a pathological response or an injury response, rather than physiological skeletal modeling.
Associated with adaptive modeling responses in both loaded (right) and contralateral (left) ulnae were persistent changes in bone neuropeptide concentrations in both of these bones. Our results suggest that plasticity(16) may exist in the peptidergic innervation of bone. It is likely that these changes in bone neuropeptides represent altered release from sensory or sympathetic innervation of bone,(9,33) although autocrine production of neuropeptides by bone cells is another possible explanation. Neuropeptides have pleiotrophic effects on bone cells, which express receptors for a range of neurotransmitters.(13) In vitro, CGRP-α and SP act anabolically on bone cells, where as CGRP-β is not anabolic,(34,35) and in vivo, CGRP+ and SP+ sensory nerves and sympathetic nerves may influence bone mass.(9,14,15,36) However, the sympathetic nervous system does not appear to mediate load-induced cortical bone formation.(22) In contrast, in-vivo load-induced bone formation in the present study was associated with persistent decreases in bone CGRP, suggesting that the regulatory effects of CGRP+ sensory nerves on functional adaptation of bone are complex. Strain-dependent effects on bone concentrations of neuropeptides were most evident with CGRP. As initial peak strain during loading increased, significant depression of bone CGRP concentrations occurred in as little as one hour after loading at high initial peak strain. This suggests that the peptideric innervation of bone is mechanosensitive and capable of a sophisticated response to changes in the loading environment of bone. In contrast, changes in SP and VIP concentrations in bone appeared much less mechanically sensitive. As initial peak strain increased, bone concentrations of these neuropeptides were higher, particularly at 10 days after loading. Such pleiotropic responses to mechanical loading by the innervation of bone again suggest the existence of a sophisticated mechanosensitive physiological pathway. It is unclear whether neuronal signaling acts on bone cells locally within the bone microenvironment via the peripheral innervation of bone, or acts centrally through the hypothalamus and a neural relay.(37)
Although lameness in the right thoracic limb was not observed after loading, a limitation of the study was that lameness was not directly quantified. Therefore, it is possible that subtle lameness in the loaded limb may have caused increased loading of the contralateral limb and influenced functional adapation in the contralateral limb during the study period. However, this is unlikely to be a major factor influencing our results because significant blocking of bone formation was evident with brachial plexus anesthesia. If bone formation in the contralateral thoracic limb was influenced by increased weight-bearing because of right thoracic limb lameness, it might be expected that bone modeling in the contralateral limb bones of the brachial plexus group would be increased because of right thoracic limb paralysis during the period of anesthesia and possible low-grade pain in the right thoracic limb after reversal of the bupivicaine-induced anesthesia. However, we found the opposite effect. In the present work, we only studied male rats of a young age that were rapidly growing (mean baseline ulna MS/BS and MAR of 73.8 ± 17.9% and 5.4 ± 3.4μm respectively). Whether the effects of functional adaptation would be similar in adult or aged rats is unclear. A decline in cross-talk between appendicular long bones with aging or with loss of sex steroids might be expected, since that it is known that bone innervation is preferentially lost in ovariectomized rats.(38) Another limitation was our use of a single fluorochrome color for bone labeling. Use of two fluorochromes of different colors(24) may improve morphometric measurement for new bone formation. However, we believe that the morphometric method of de Souza et al. (2005)(22) is inherently sensitive, particularly in young rapidly growing animals with high baseline bone formation, because it quantifies all labeled bone formation into a measurement of area.
Our findings suggest that skeletal responses to mechanical loading are more complex than previously thought and include adaptive responses in distant bones of the skeleton that were not loaded. Although osteocyte signaling has been previously considered the primary mechanotransduction pathway in bone,6 it is now evident that neuronal signaling also has major effects on bone formation during functional adaptation. Plasticity in the innervation of bone may provide an additional pathway by which sex steroids could influence bone mass.(39,40)
Acknowledgments
This study was supported by grants from the AO Research Fund, Switzerland, and the Graduate School, University of Wisconsin-Madison (to P.M.). Susannah J. Sample received a research training scholarship from the School of Veterinary Medicine Summer Scholars Program and stipend support from the National Institutes of Health. The authors are grateful to Nicholas Keuler for advice with data analysis.
Footnotes
Conflict of Interest
All authors have no conflicts of interest.
References
- 1.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg. 1984;66A:397–402. [PubMed] [Google Scholar]
- 2.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 3.Muir P, McCarthy J, Radtke CL, Markel MD, Santschi EM, Scollay MC, Kalscheur VL. Role of endochondral ossification of articular cartilage and adaptation of the subchondral plate in the development of fatigue microcracking of joints. Bone. 2006;38:342–349. doi: 10.1016/j.bone.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Frost HM. From Wolff’s Law to the Utah Paradigm: Insights about bone physiology and its clinical applications. Anat Rec. 2001;262:398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 5.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 7.Chenu C. Role of innervation in the control of bone remodeling. J Musculoskel Neuron Interact. 2004;4:132–134. [PubMed] [Google Scholar]
- 8.Martin CD, Jiminez-Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: Implications for the generation and maintenance of bone fracture pain. Neurosci Lett. 2007;427:148–152. doi: 10.1016/j.neulet.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 10.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 11.Imai S, Rauvala H, Konttinen YT, Tokunaga T, Maeda T, Hukuda S, Santavirta S. Efferent targets of osseus CGRP-immunoreactive nerve fiber before and after bone destruction in adjuvant arthritic rat: An ultramorphological study on their terminal-target relations. J Bone Miner Res. 1997;12:1018–1027. doi: 10.1359/jbmr.1997.12.7.1018. [DOI] [PubMed] [Google Scholar]
- 12.Spencer GJ, Hitchcock IS, Genever PG. Emerging neuroskeletal signalling pathways: a review. FEBS Lett. 2004;559:6–12. doi: 10.1016/S0014-5793(04)00053-5. [DOI] [PubMed] [Google Scholar]
- 13.Lerner UH. Neuropeptidergic regulation of bone resorption and bone formation. J Musculoskeletal Neuron Interact. 2002;2:440–447. [PubMed] [Google Scholar]
- 14.Sherman BE, Chole RA. Sympathectomy, which induces membranous bone remodeling, has no effect on endochondral long bone remodeling in vivo. J Bone Miner Res. 2000;15:1354–1360. doi: 10.1359/jbmr.2000.15.7.1354. [DOI] [PubMed] [Google Scholar]
- 15.Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsay DP, Jacobs CR, Yao W, Lane NE, Kingery WS. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20:257–267. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 17.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periosteal pressure. Calcif Tissue Int. 1994;54:241–247. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh SF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16:918–924. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 19.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 20.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 21.Kotha SP, Hsieh YF, Strigel RM, Muller R, Silva MJ. Experimental and finite element analysis of the rat ulnar loading model – correlations between strain and bone formation following fatigue loading. J Biomech. 2004;37:541–548. doi: 10.1016/j.jbiomech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical bone formation. J Bone Miner Res. 2005;20:2159–2168. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Comittee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res. 2007;22:984–991. doi: 10.1359/jbmr.070327. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed M, Srinivasan GR, Theodorsson E, Bjurholm A, Kreicbergs A. Extraction and quantitation of neuropeptides in bone by radioimmunoassay. Regul Pept. 1994;51:179–188. doi: 10.1016/0167-0115(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder LE, Horlocker TT, Schroeder DR. The efficacy of axillary block for surgical procedures about the elbow. Anesth Analg. 1996;83:747–751. doi: 10.1097/00000539-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Tanaka SM, Jiang H, Su M, Yokota H. Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol. 2006;100:1452–1459. doi: 10.1152/japplphysiol.00997.2005. [DOI] [PubMed] [Google Scholar]
- 28.Muir P, Sample SJ, Barrett JG, McCarthy J, Vanderby R, Jr, Markel MD, Prokuski LK, Kalscheur VL. Effect of fatigue loading and associated matrix microdamage on bone blood flow and interstitial fluid flow. Bone. 2007;40:948–956. doi: 10.1016/j.bone.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong DJ, McCausland EM, Wright GD. Hypertrophic pulomonary osteoarthropathy (HPOA) (Pierre Marie-Bamberger syndrome): two cases presenting as acute inflammatory arthritis and review of the literautre. Rheumatol Int. 2007;27:399–402. doi: 10.1007/s00296-006-0224-2. [DOI] [PubMed] [Google Scholar]
- 30.Karacan I, Aydin T, Ozaras N. Bone loss in the contralateral asymptomatric hand in patients with complex regional pain syndrome type 1. J Bone Miner Metab. 2004;22:44–47. doi: 10.1007/s00774-003-0447-1. [DOI] [PubMed] [Google Scholar]
- 31.Oda Y, Funao T, Tanaka K, Asada A. Vasodilation increases the threshold for bupivicaine-induced convulsions in rats. Anesth Analg. 2004;98:677–682. doi: 10.1213/01.ane.0000101984.50597.e9. [DOI] [PubMed] [Google Scholar]
- 32.Egrise D, Martin D, Neve P, Vienne A, Verhas M, Schoutens A. Bone blood flow and in vitro proliferation of bone marrow and trabecular bone osteoblast-like cells in ovariectomized rats. Calcif Tissue Int. 1992;50:336–341. doi: 10.1007/BF00301631. [DOI] [PubMed] [Google Scholar]
- 33.Duncan CP, Shim S-S. The autonomic nerve supply of bone. J Bone Joint Surg. 1977;59B:323–330. doi: 10.1302/0301-620X.59B3.19482. [DOI] [PubMed] [Google Scholar]
- 34.Hirt D, Bernard GW. CGRP-beta unlike CGRP-alpha has no osteogenic stimulatory effect in vitro. Peptides. 1997;18:1461–1463. doi: 10.1016/s0196-9781(97)00199-x. [DOI] [PubMed] [Google Scholar]
- 35.Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides. 2007;41:25–31. doi: 10.1016/j.npep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Kingery WS, Offley SC, Guo TZ, Davies MF, Clark JD, Jacobs CR. A substance P receptor (NK1) antagonist enhances the widespread osteoporotic effects of sciatic nerve section. 2003;33:927–936. doi: 10.1016/j.bone.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympatheic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 38.Burt-Pichat B, Lafage-Proust MH, Duboef F, Laroche N, Itzstein C, Vico L, Delmas PD, Chenu C. Dramatic decrease in the innervation density in bone after ovariectomy. Endocrinology. 2005;146:503–510. doi: 10.1210/en.2004-0884. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Bone adaptation requires oestrogen receptor-α. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 40.Hung AJ, Stanbury MG, Shanabrough M, Horvath TL, Garcia-Segura LM, Naftolin F. Estrogen, synaptic plasticity and hypothalamic reproductive aging. Exp Gerontol. 2003;38:53–59. doi: 10.1016/s0531-5565(02)00183-3. [DOI] [PubMed] [Google Scholar]