Abstract
This study was designed to test the hypothesis that the acute toxicity of the nerve agents VX, soman and sarin in guinea pigs is age and sex dependent and cannot be fully accounted for by the irreversible inhibition of acetylcholinesterase (AChE). The subcutaneous doses of nerve agents needed to decrease 24-h survival of guinea pigs by 50% (LD50s) were estimated by probit analysis. In all animal groups, the rank order of LD50s was sarin > soman > VX. The LD50 of soman was not influenced by sex or age of the animals. In contrast, the LD50s of VX and sarin were lower in adult male than in age-matched female or younger guinea pigs. A colorimetric assay was used to determine the concentrations of nerve agents that inhibit in vitro 50% of AChE activity (IC50s) in guinea pig brain extracts, plasma, red blood cell and whole blood. A positive correlation between LD50s and IC50s for AChE inhibition would support the hypothesis that AChE inhibition is a major determinant of the acute toxicity of the nerve agents. However, such a positive correlation was found only between LD50s and IC50s for AChE inhibition in brain extracts from neonatal and prepubertal guinea pigs. These results demonstrate for the first time that the lethal potencies of some nerve agents in guinea pigs are age and sex dependent. They also support the contention that mechanisms other than AChE inhibition contribute to the lethality of nerve agents.
Introduction
The nerve agents VX (S-[2-(diisopropylamino)ethyl]-O-ethyl methylphosphonothioate), soman (O-pinacolyl methylphosphonofluoridate) and sarin (O-isopropyl methylphosphono-fluoridate) are among the most potent organophosphorus (OP) compounds ever developed. They are chemically related to, but far more toxic than OP insecticides used in agriculture and households worldwide. Some of these agents have been used with catastrophic results as chemical weapons of mass destruction in the Second Sino-Japanese War (1937–1945), the 1980’s Iraq-Iran conflict, and the 1994–1995 terrorist attacks in Japan (Romano and King, 2001).
Signs of acute intoxication with nerve agents or OP insecticides have been attributed to their common action as irreversible inhibitors of AChE – the enzyme that hydrolyzes acetylcholine (ACh; Bajgar, 2004). Persistent stimulation of muscarinic receptors by accumulated ACh leads to a syndrome exemplified by miosis, profuse secretions, bradycardia, bronchoconstriction, hypotension, and diarrhea. Overstimulation of nicotinic receptors triggers muscle fasciculation, while their subsequent desensitization contributes to muscle weakness. CNS-related effects include anxiety, restlessness, confusion, ataxia, tremors, seizures, and central cardiorespiratory paralysis (Bajgar, 2004). Nevertheless, there has been considerable debate as to whether AChE inhibition alone accounts for the acute toxicity of OP compounds (Albuquerque et al., 1985; Duysen et al., 2001; Schuh et al., 2002).
Guinea pigs are the best non-primate model to predict the effectiveness of medical countermeasures against OP intoxication in humans (Inns and Leadbeater, 1983). Different levels of OP-metabolizing carboxylesterases in the blood of various animal species contribute to their differential sensitivity to OP agents. Primates, including humans, have almost none, whereas guinea pigs have low levels, and rats and mice have high levels of blood carboxylesterases. Thus, to standardize development of antidotes to OP agents, it is recommended that initial studies be carried out in guinea pigs and results be subsequently confirmed in nonhuman primates (Koplovitz et al., 1992).
An understanding of how the sensitivity to nerve agents depends on age and sex of an animal is essential to guide the development of safe and effective antidotes. Indeed, sex and age dependencies of the toxicity of nerve agents has been observed in victims of the 1995 terrorist attack with sarin (Kawada et al., 2005; Yokoyama et al., 1998) and in laboratory animals (Sterri et al., 1985; Shih et al., 1990). To date, all animal studies reporting sex- and age-related differences in nerve agent toxicity and in the effectiveness of specific countermeasures have been performed using rats. The present study was, therefore, designed to examine sex and age effects on the lethal potencies (LD50s) of soman, sarin, and VX in guinea pigs and to determine whether a positive correlation exists between these LD50s and the potencies of the nerve agents to inhibit AChE in brain extracts and in whole blood, red blood cells (RBC) and plasma of the animals.
Developmental stages of the brain and metabolism of animals are factors known to influence the sensitivity to neurotoxicants. In guinea pigs, like in humans, brain growth spurt – a period of rapid brain development when neurons are especially vulnerable to toxicants, including OP insecticides (Eriksson, 1997) – is mainly a prenatal event (Dobbing and Sands, 1970). However, neurogenesis and synaptogenesis continue to occur throughout life in some brain regions that are exquisitely sensitive to the deleterious effects of OPs, particularly the hippocampus (Eriksson et al., 1998; Wolfer and Lipp, 1995). In the guinea pig hippocampus, recurrent mossy fiber collaterals undergo significant postnatal development, with growth peaking at PND40 (Wolfer and Lipp, 1995) when there is the first surge of sex hormones (Bartesaghi et al., 2003). Female and male guinea pigs reach puberty at PND40-45 and PND45-70, respectively. Finally, hepatic drug-metabolizing enzymes, including the OP-metabolizing carboxylesterases, are not fully developed until about three weeks after birth in guinea pigs (Ecobichon et al., 1978). Thus, three ages were selected to study the sensitivity of guinea pigs to the toxicity of nerve agents: neonatal (PND5-10), prepubertal (PND35-45) and adult (PND120-150).
Results presented herein demonstrate that while the lethal potency of soman is the same regardless of the age and sex of guinea pigs, the lethal potencies of VX and sarin increase with the age of male, but not female guinea pigs. Up to adulthood, the lethal potencies of the nerve agents correlate well with their in vitro potencies for inhibition of AChE in brain extracts. However, in adult male guinea pigs this relationship no longer applies. The lack of positive correlation between LD50s and IC50s for AChE inhibition in blood or brain extracts obtained from adult male guinea pigs indicates that AChE inhibition is not the sole mechanism underlying the acute toxicity of these agents.
Methods
Animals
Male and female Hartley guinea pigs [Crl(HA)Br] at three age groups were purchased from Charles River Laboratories (Wilmington, MA). The guinea pigs were at PND5-10 (neonatal), PND35-45 (prepubertal) and PND120-150 (adult). The animals were housed for two days before experimentation in a temperature- and light-controlled animal-care unit, and they were allowed food and water ad libitum until 2 h prior to experimentation. Handling of the animals was according to the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care, complied with the standards of the Animal Welfare Act, and adhered to the principles of the ‘1996 Guide for the Care and Use of Laboratory Animals.’
Drugs
Soman, sarin and VX were obtained from the U.S. Army Medical Research Institute of Chemical Defense (Aberdeen Proving Ground, MD), and their storage, handling and disposal conformed to the rules set forth by the U.S. Army. On the day of the experiments, a vial containing an aliquot (0.2 to 0.5 ml) of the U.S. Army-issued stock solution of soman (1.88–1.90 mg/ml), sarin (1.88–1.90 mg/ml) or VX (0.89–0.92 mg/ml) was diluted with sterile saline to appropriate concentrations and kept on ice for the duration of the experiment. At the end of the experiments, any remaining diluted OP and materials contaminated with OPs were decontaminated with 5% sodium hypochlorite prior to disposal.
Assessment of lethality of the nerve agents
Approximately 2 h prior to experimentation, the animals were fasted, but allowed free access to water. A given dose of a nerve agent was administered to awake, unrestrained animals, via subcutaneous (sc) injection between the shoulder blades. Challenge of the animals with the nerve agents was performed inside an approved fume hood and with appropriate protection as per the routine for handling OPs set forth by the U.S. Army. A gauze pad soaked with 5% sodium hypochlorite was used to detoxify the site of injection.
To minimize the number of animals required in the experiments, we used the published LD50 of each nerve agent for prepubertal male guinea pigs as the starting dose for animals of both sexes and different ages. That dose was administered to a group of 4 animals. If either no animals or all animals survived at 24 h after that dose, the dose was increased or decreased by 10%, accordingly. If that dose was, however, sufficient to decrease the 24-h survival outcome in the group, the number of animals was increased by four and new groups of animals were tested with doses that were 10% higher and 10% lower than the first dose. Each animal received only one injection of a given nerve agent. Following challenge with the nerve agents, animals were placed in individual cages, allowed to eat and drink, and maintained in the laboratory, which was temperature and light controlled. Animals were examined repeatedly during the first 6-h period after injection and at 24 h following injection. To minimize animal suffering, nerve agent-challenged guinea pigs were euthanized as soon as they presented life-threatening signs as described in the Results section.
Separate dose-mortality relationships were obtained for VX, soman and sarin in male and female guinea pigs at different ages. Probit analysis was used to derive values for the median lethal doses (LD50) and their 95% confidence intervals as well as the slope of the dose-response relationships. The software StatsDirect (StatsDirect Limited, Cheshire, UK) was used for the Probit fitting and the determination of the LD50s and their respective confidence intervals.
Enzyme assay
A colorimetric assay (Ellman et al., 1961) was used to measure the activity of AChE in whole blood, plasma, RBC and brain tissue of neonatal, prepubertal, and adult guinea pigs of both sexes as described herein.
Untreated guinea pigs were deeply anesthetized using CO2, blood was collected by cardiopuncture into heparinized tubes, and the brains were removed, washed in 0.9% saline, pulverized under liquid nitrogen, and pooled according to sex and age. Pulverized brains were mixed with equivalent volume of anti-proteases (Roche Complete Mini, Roche Molecular Biochemicals, Indianapolis, IN)-containing, HEPES-buffered saline, which was composed of 10 mM HEPES (pH 7.5), 5 mM EDTA, 5 mM EGTA, 1 M NaCl and 1% Triton X-100. Samples were then sonicated on ice twice (20 s each time) and centrifuged briefly at low speed (1,877 RCF) to remove insoluble material. The homogenates were transferred to a new tube and diluted with 3 volumes of phosphate buffer (100 mM Na2HPO4, 100 mM KH2PO4, pH 7.4) and stored at −80° C until further processing.
Blood samples were pooled according to sex and age of the guinea pigs and processed as follows. One volume of pooled whole blood was mixed with seven volumes of 100 mM phosphate buffer pH 7.4. Samples were then aliquoted (8.0 ml/tube) and stored at −80°C until needed. To separate plasma and RBCs, pooled whole blood was centrifuged at 4°C at 3000 g for 15 min. The plasma layer was transferred to a new tube and centrifuged for an additional 15 min at 4°C at 3000 g. The resulting plasma was aliquoted (0.5 ml/tube) and stored at −80°C until needed. The pellet, which contained the RBCs, was washed three times with three volumes of phosphate buffer (pH 7.4) and centrifuged at 4°C at 3000 g for 5 minutes. Following the 3rd wash, RBCs were diluted with 15 volumes of phosphate buffer (pH 7.4), aliquoted (8 ml/tube) and stored at −80°C until the assay was conducted.
On the day of the experiments, sample aliquots were thawed at 37°C and incubated at room temperature for 15 min with the butyrylcholinesterase inhibitor tetraisopropylpyro-phosphoramide (iso-OMPA, final concentration, 1 mM). The concentration of iso-OMPA needed to fully inhibit butyrylcholinesterase was empirically estimated using plasma, which had the highest level of enzyme activity, and maintained constant for all other tissues. The nerve agents were diluted in phosphate buffer (100 mM, pH 7.4) to 0.1 nM to 1 μM and added as 1/10 volume of sample. The mixture was incubated at room temperature for an additional 15 min, after which time samples were aliquoted and stored at −80°C.
To measure the AChE activity in the mixtures containing a given concentration of a nerve agent, 0.1 ml DTNB (10 mM in 100 mM HEPES buffer pH 7.0) and 0.1 ml of the sample were added to 2.775 ml of phosphate buffer (100 mM, pH 8.0). Following a 6-min incubation at room temperature, acetylthiocholine iodide in phosphate buffer pH 8.0 (120 mM, 0.025 ml) was added to the reaction mixture and changes in absorbance at 412 nm (for brain and plasma samples) or 436 nm (for whole blood and RBCs) were measured every 15 s for 6 min in a Bio-Rad spectrophotometer (Bio-Rad Laboratories, Hercules, CA). Baseline rates of absorbance changes were measured in samples free of substrate. Protein concentrations were measured in each sample using the bicinchoninic acid assay (Pierce, Rockford, IL). Each experiment was run three times in triplicate. Results are presented as mean and S.E.M. The concentration-response relationships for each nerve agent were fitted using the Hill equation in the SigmaPlot software (version 8, Systat Software, San Jose, CA) to derive the concentrations needed to decrease the enzyme activity by 50% (IC50) and the Hill coefficients (nH). As reported in previous studies, the existence, in crude extracts, of proteins other than AChE or lipids that bind/sequester enzyme inhibitors significantly affects estimates of IC50s (Mortensen et al., 1998). However, the degree of nerve agent-induced AChE inhibition in crude extracts in vitro is more relevant to the overall toxicity of the agents than is the degree to which they inhibit AChE purified from crude extracts (Mortensen et al., 1998). In addition, in clinical settings, levels of OP exposure and the effectiveness of treatments for OP intoxication are generally estimated on the basis of AChE activity measured in whole blood (or RBCs) from OP-exposed patients. Thus, toxicologically and clinically, it is more meaningful to correlate LD50s with IC50s obtained using crude tissue extracts than with IC50s obtained using purified AChE. For meaningful comparison of the IC50 values, in all assays, the amount of tissue among sex and age groups and within all experimental conditions was kept constant and the activity of butyrylcholinesterase was fully inhibited with iso-OMPA.
Results
Acute toxicity of VX, soman and sarin in male and female guinea pigs at different ages
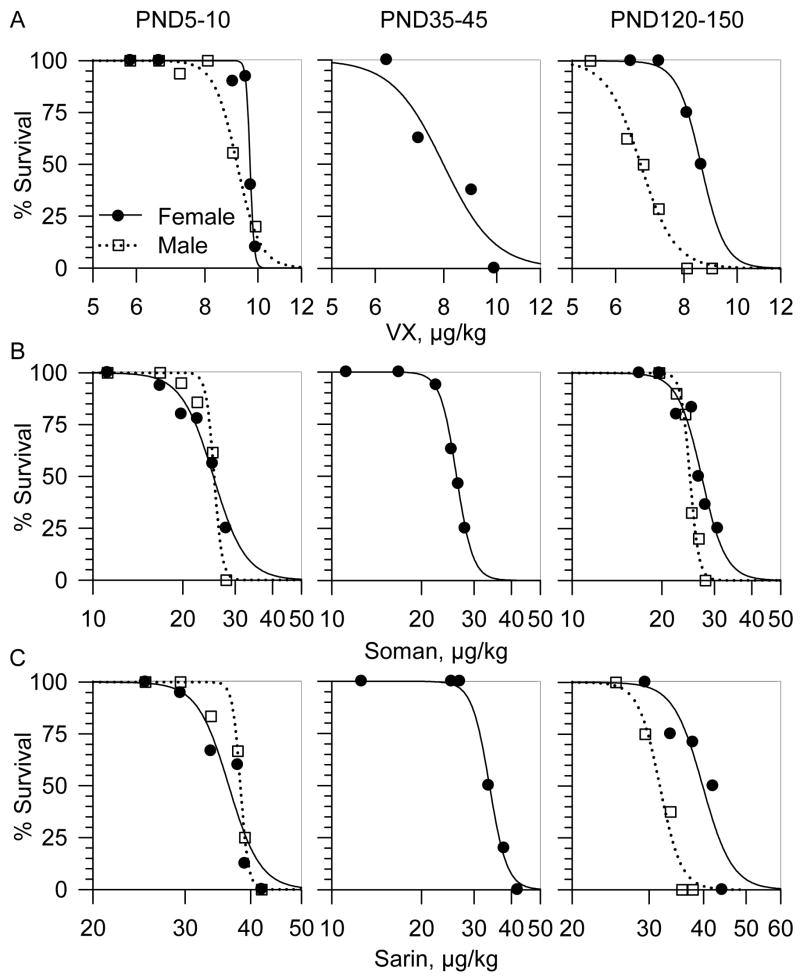
To evaluate the effects of age and sex on the sensitivity of guinea pigs to VX, soman, and sarin, the nerve agents were injected subcutaneously between the shoulder blades of male and female animals at different ages: PND5-10 (neonatal), PND35-45 (prepubertal) ad PND120-150 (adult). As indicated in Methods, the published LD50s of VX, soman and sarin for prepubertal male guinea pigs (see Table 1) were used as the starting test doses for the neonatal and adult guinea pigs. A minimum of six doses of each nerve agent were evaluated in each age group.
Table 1.
Median lethal doses (LD50s) for the nerve agents VX, soman and sarin in neonatal, prepubertal and adult guinea pigs of both sexes.
| LD50s (μg/kg) | ||||
|---|---|---|---|---|
| VX | Soman | Sarin | ||
| PND5-10 | Male | 9.15 (8.61 – 9.90) | 25.1 (24.2 – 26.1) | 37.7 (35.9 – 39.4) |
| Female | 9.94 (9.51 – 9.89) | 25.9 (23.9 – 32.0) | 36.2 (34.2 – 38.2) | |
| PND35-45 | Male | 8† | 28† | 42† |
| Female | 8.05 (7.73 – 9.77) | 26.2 (25.7 – 26.8) | 33.9 (29.3 – 36.5) | |
| PND120-150 | Male | 6.67 (6.13 – 7.17) | 24.9 (24.0 – 26.9) | 31.7 (29.0 – 33.7) |
| Female | 9.37 (8.49 – 10.1) | 27.0 (25.2 – 29.7) | 39.6 (34.6 – 46.5) | |
Agents were injected subcutaneously between the shoulder blades of the animals and 24-h survival in each experimental group was computed. Using the software Stats Direct (StatsDirect Limited, Cheshire, UK), the resulting sigmoid dose-response relationships (see Figures 1, 2, and 3) were linearized by probit transformation to estimate LD50s for each nerve agent in the various animal groups. For each test dose, number of animals ranged from 4 to 12. Numbers in parentheses represent 95% confidence intervals for the estimated LD50s.
LD50 values are from the literature (Shih and McDonough, 2000).
The sc LD50s for VX, soman and sarin were derived from the probit analysis of the 24-h mortality dose-response relationships (Figure 1A–C). The LD50s of soman did not vary significantly with the age or sex of the guinea pigs (Table 1). In contrast, the LD50s of sarin and VX were lower for adult males than for age-matched female guinea pigs as well as male and female guinea pigs of all other ages (Figures 1A–C and Table 1). The probit slopes of the dose-response relationships for each of the three nerve agents for male and female guinea pigs were very steep. For neonatal and adult male guinea pigs, the slopes were, respectively: 19.1 and 21.2 for VX, 11.9 and 13.9 for soman, and 27.1 and 26.6 for sarin. For neonatal, prepubertal and adult female guinea pigs the slopes were, respectively: 21.9, 12.8 and 10.9 for VX, 8.4, 27.9, and 14 for soman, and 19.8, 24.9 and 23.9 for sarin. The steep probit slopes indicate that a small increase in the dose of the nerve agents is sufficient to cause a large reduction in the survival of the guinea pigs.
Figure 1. Mortality dose-response relationships for VX, soman and sarin in male and female guinea pigs at PND5-10, PND35-45 and PND120-150.
Guinea pigs received an sc injection of VX (A), soman (B) or sarin (C) and were then observed for 24 h. Following the IACUC guidelines, animals were euthanized as soon as they developed life-threatening symptoms, including unremitting or recurrent convulsions and/or gasping. The log dose-response relationships were fitted by a sigmoid equation of the form: y = axb/(cb + xb), where “y” is the % of survival after exposure to a given dose of nerve agent, “a” is the maximal % survival, “x” is the dose of the nerve agent, “c” is the median lethal dose, and “b” is the steepness of the curve. Each experimental group showing 100% survival consisted of 4 guinea pigs. Experimental groups in which survival outcome was < 100% consisted of 8 to 12 animals.
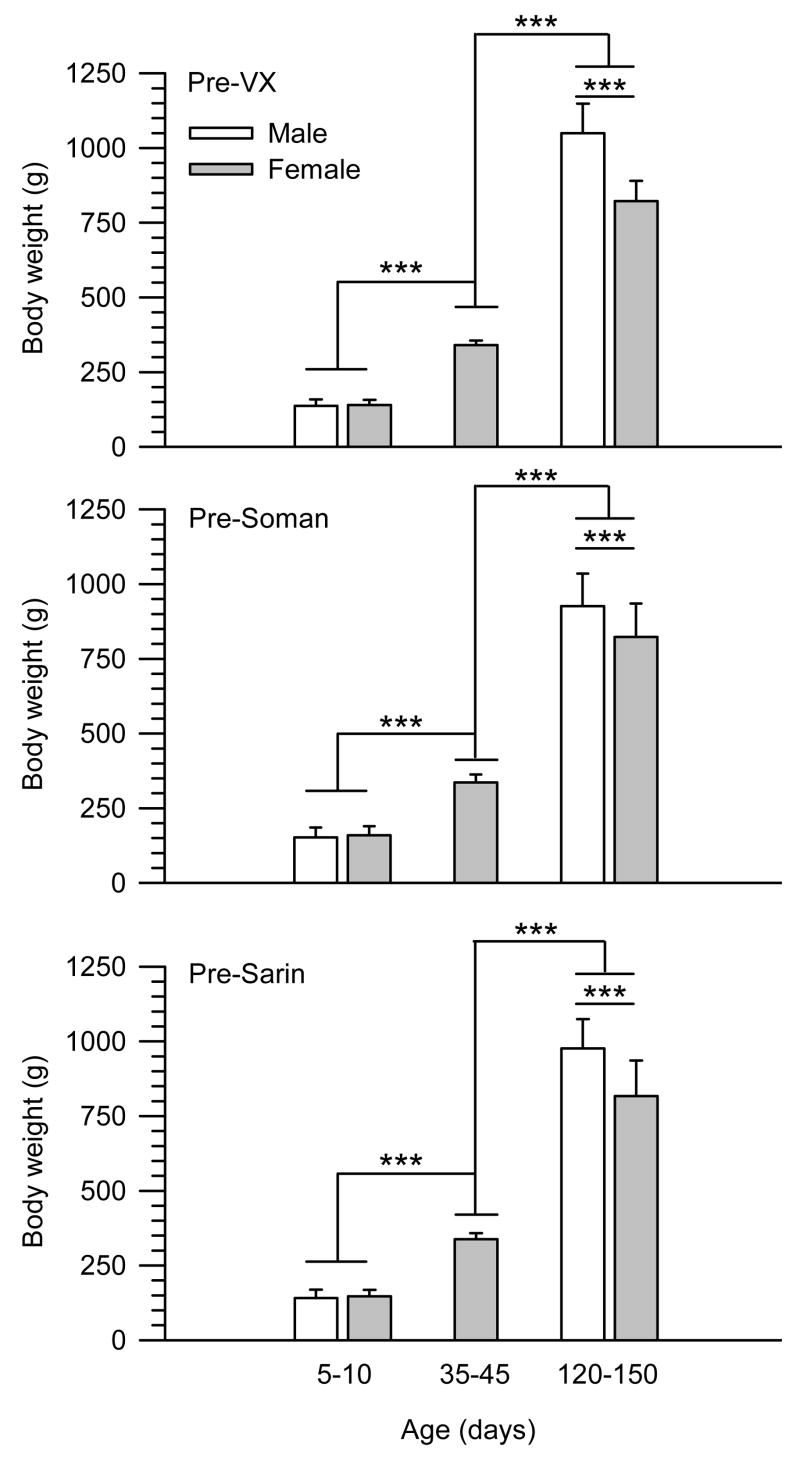
In the present dose-response studies, response variations among animals of differing sizes were taking into account by the use of OPs in doses proportional to the animals’ body weights. The assumption that the amount of OP/unit of animal weight can factor the differences in the subjects’ body weights only holds true if the molecular target for the toxicants increases proportionally to the body weight of the animals. This is not the case for many drugs whose toxicities are significantly influenced by the size of the animals (see Anderson and Weber, 1975 and references therein). As shown here, however, age- and sex-dependent changes in the body weights of the guinea pigs did not parallel the age and sex dependencies of the LD50s of the nerve agents. In each group of animals exposed to a given nerve agent, the mean weights of male and female guinea pigs increased significantly from PND5-10 to PND120-150 (Figure 2). Further, adult male guinea pigs were significantly heavier than adult female guinea pigs (Figure 2). If the total amount of OP/total body weight rather than the amount of OP/kg body weight administered to the animals determined their sensitivity to the OPs, the LD50s of all nerve agents should have decreased gradually from neonatal to prepubertal to adult animals of a given sex. In addition, the LD50s of all nerve agents should have been lower in male than in female guinea pigs. Instead, the LD50 of soman remained unchanged regardless of weight, age or sex of animals. In addition, the LD50s of VX and sarin were within the same range regardless of the weights of neonatal and prepubertal guinea pigs of both sexes and adult female guinea pigs. Thus, higher sensitivity of adult male guinea pigs to VX and sarin cannot be explained by the increase in total body weight of these animals.
Figure 2. Weights of male and female guinea pigs at PND5-10, PND35-45 and PND120-150 measured at 2 h prior to their challenge with VX, soman or sarin.
Guinea pigs were weighed at 2 h prior to the sc injection of any given dose of VX, soman or sarin. In each graph, bar graphs and error bars represent mean and S.D. of weights from all animals that were exposed to the nerve agents. Number of animals in each experimental group ranged from 36 to 80. The weight of the guinea pigs increased with their age, and, in adulthood, the weight of males was significantly higher than that of females (***, p < 0.001 according to one-way ANOVA followed by Tukey post-hoc test for multiple comparisons).
There were qualitative differences in the signs of intoxication presented by guinea pigs challenged with VX, soman or sarin. Of the three nerve agents, VX was the slowest acting, with life-threatening effects occurring only at 3–5 h after the challenge with 1xLD50 (for operational definition of life-threatening effects, see paragraph below). Sarin was the fastest acting nerve agent with life-threatening signs developing within 10–45 min after an exposure to 1xLD50. Life-threatening effects occurred within 30–120 min after the challenge of the guinea pigs with 1xLD50 soman.
In each age and sex group, guinea pigs presented clear signs of acute intoxication when challenged with nerve agent doses ≥ 0.8xLD50. Facial twitches, chewing, slight hyperlocomotion and head tremors were common signs observed in guinea pigs challenged with 0.8xLD50 VX, soman or sarin. With increasing doses of soman or sarin, animals presented progressively with forelimb clonus, increased secretions, muscle fasciculations, rearing, strong grinding, gnashing or clenching of the teeth (bruxism), all limb clonus and convulsions, and severe respiratory distress. Between 10 min and 2 h after the challenge with 1xLD50 sarin or soman, the toxic signs progressed from the peripheral nicotinic and muscarinic effects to the CNS effects. The onset of the CNS effects and the time to lethality were shortened with increasing doses of soman or sarin. At doses > 1xLD50 the signs of acute toxicity progressed from hyperlocomotion and tremors to life-threatening convulsions 10–20 min after the challenge.
Tonic-clonic convulsions triggered by ≥ 1xLD50 soman or sarin became life-threatening when they occurred unremittingly and lasted longer than 10 min or when they recurred for longer than 10 min with the animals exhibiting immobility between the recurrences. Animals that underwent recurrent or continuous convulsions when challenged with ≥ 1xLD50 sarin developed severe respiratory distress within 2–3 minutes of the onset of the convulsions. In contrast, gasping was a condition that developed 10–15 min after the onset of soman-induced convulsions. There is evidence from clinical studies that 10 min of continuous convulsions is sufficient to damage neurons and that unremitting or recurrent seizures lasting longer than 10 min are unlikely to self-terminate (García Peñas et al., 2007). Thus, following the Institutional Animal Care and Use Committee (IACUC) guidelines, the animals were euthanized as soon as life-threatening signs of unremitting or recurrent convulsions developed.
Recurrent or unremitting convulsions were not triggered even by the highest doses of VX tested in this study, i.e., approximately 1.2xLD50. VX-induced convulsions were brief and showed spontaneous termination. In general, peripheral muscarinic and nicotinic signs of intoxication were more prominent with VX than with sarin or soman, and gasping was the most severe sign of VX poisoning. Animals that were challenged with ≥ 1xLD50 VX and developed life-threatening respiratory distress were euthanized. Animals survived the challenge with any given dose of VX, soman or sarin if they showed: (i) no clear signs of intoxication, (ii) only mild peripheral muscarinic and nicotinic signs, or (iii) mild peripheral effects accompanied by short-lasting CNS effects.
Apparent potencies of VX, soman and sarin to inhibit in vitro brain, whole blood, RBC, and plasma AChE from neonatal, prepubertal and adult guinea pigs of both sexes
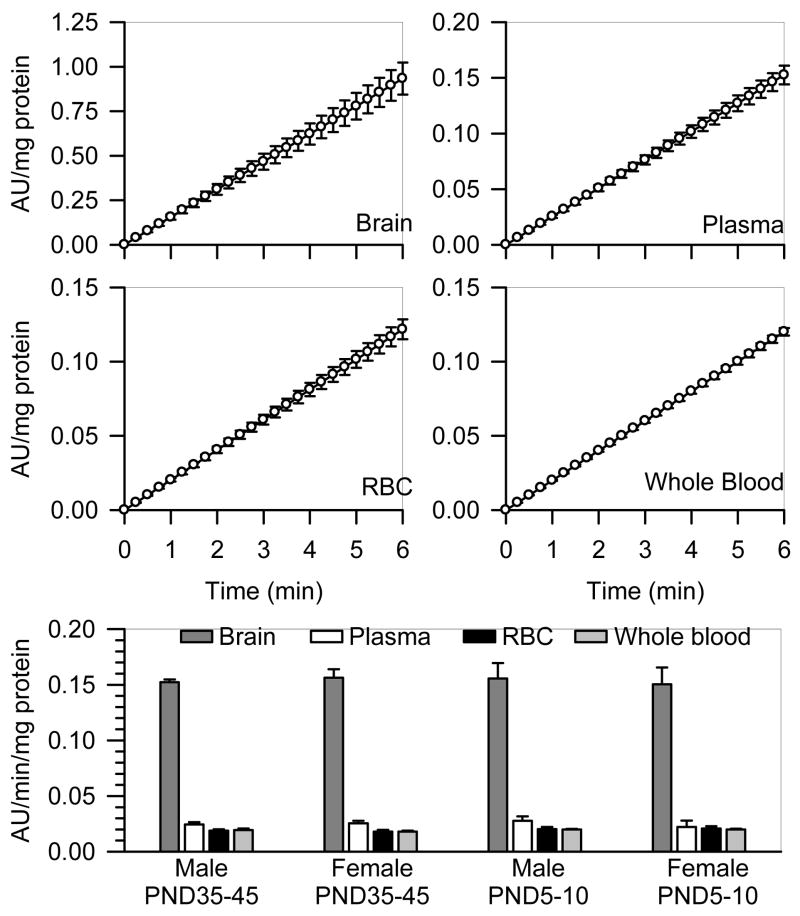
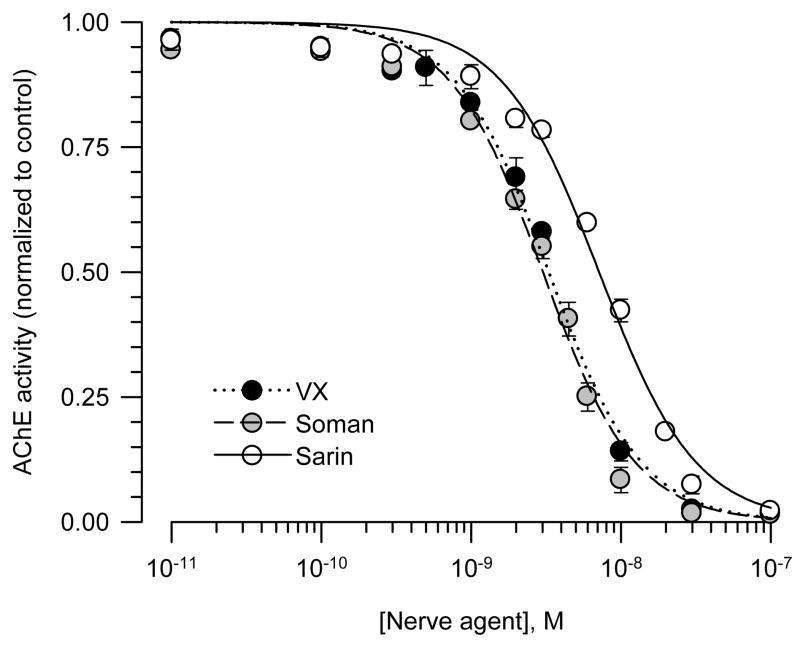
In whole blood, RBC, plasma and brain extracts from prepubertal male guinea pigs, a linear relationship was observed between the change of absorbance units/mg protein and the reaction time (Figure 3). The Pearson’s regression coefficient was 1 in all cases. The same results were obtained using tissue samples from male and female guinea pigs of different ages (data not shown). The AChE activity defined by the rate of hydrolysis of acetylthiocholine normalized to the amount of protein was highest in the brain extracts compared to the plasma, RBC or whole blood in all groups (Figure 3). In addition, as reported in a previous study (Meinecke and Oettel, 1967), levels of AChE activity were not significantly different between guinea pig plasma and RBC (Figure 3). There was no age- or sex-related difference in the AChE activity in any of the tissues analyzed (Figure 3).
Figure 3. Rate of AChE hydrolysis of acetylthiocholine in different tissues from male and female guinea pigs at PND5-10 and PND35-45.
As described in Methods, AChE activity was measured by means of a colorimetric assay (Ellman et al., 1961) in brain extracts, RBC, plasma and whole blood from neonatal and prepubertal guinea pigs of both sexes. A kinetic profile of the enzyme activity (expressed as the changes in absorbance units (AU)/mg protein) was studied spectrophotometrically at 15-s intervals. Scatter graphs show the time changes in absorbance units/mg protein in brain extracts, plasma, RBC, and whole blood pooled from six prepubertal male guinea pigs. Each experiment was carried out in triplicate. Data points and error bars represent the mean and SEM of the actual measurements at any given time. The bar graph shows the rate of acetylthiocholine hydrolysis, estimated as the slope of the plots of AU/mg protein vs. time, in brain extracts, plasma, RBC, and whole blood from neonatal and prepubertal guinea pigs of both sexes. Bar graphs and error bars are mean and SEM of results obtained from triplicate experiments. In all animal groups, AChE activity was significantly higher in the brain extracts than in any blood compartment. However, the total enzyme activity was not sex or age dependent.
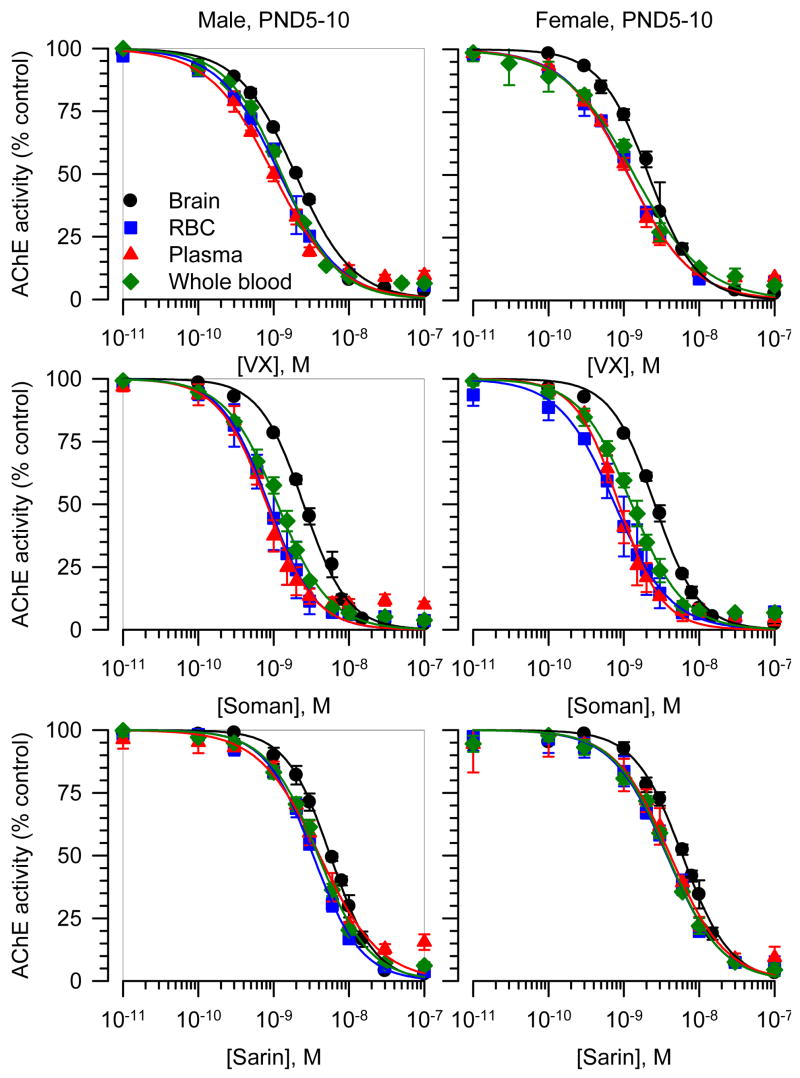
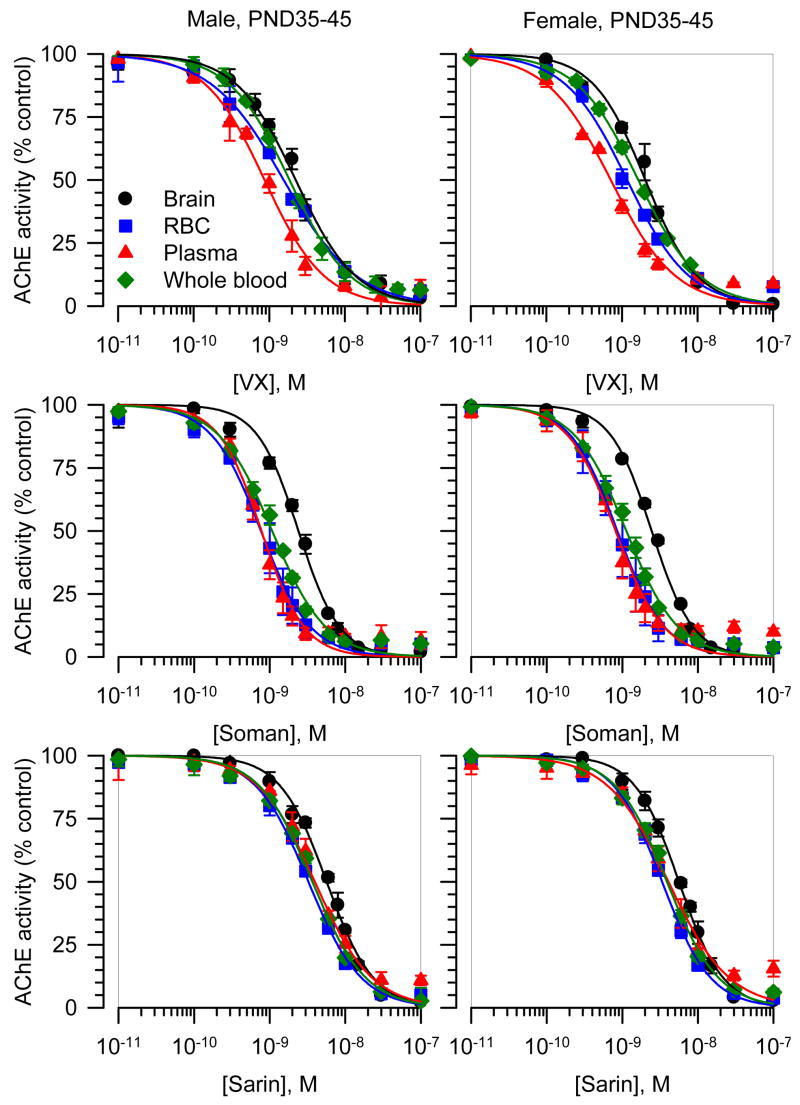
The concentration-response relationships for VX, soman, and sarin to inhibit brain, RBC, plasma and whole blood AChE from the different groups of guinea pigs were fitted by the Hill equation (Figures 4 and 5). The slopes of all curves were very similar and close to unity, indicating no cooperativity in the interactions between a given nerve agent and the enzyme. The IC50s, on the other hand, differed depending on the tissue. The curves for each VX, soman, and sarin to inhibit AChE in brain extracts from neonatal and prepubertal guinea pigs of both sexes were always displaced to the right compared to the other tissues (Figures 4 and 5). Accordingly, the estimated IC50s for VX, soman, and sarin to inhibit AChE in brain extracts were significantly larger than their respective IC50s to inhibit RBC, plasma or whole blood AChE (Table 2). The rank order of potency for the nerve agents to inhibit AChE in RBC and plasma from neonatal and prepubertal guinea pigs of both sexes was: soman > VX > sarin.
Figure 4. Concentration-response relationships for in vitro inhibition of AChE in different tissues from male and female guinea pigs at PND5-10.
The AChE activity (AU/min/mg protein) measured in brain extracts, RBC, plasma, or whole blood each pooled from six male or six female neonatal guinea pigs was taken as 100% and used to normalize the AChE activity measured following in vitro exposure of the tissues to a given nerve agent concentration. The resulting concentration-response relationships were fitted to the Hill equation. Data points and error bars are mean and SEM of results obtained from triplicate experiments.
Figure 5. Concentration-response relationships for in vitro inhibition of AChE in different tissues from male and female guinea pigs at PND35-45.
The AChE activity (AU/min/mg protein) measured in brain extracts, RBC, plasma, or whole blood each pooled from six male or six female prepubertal guinea pigs was taken as 100% and used to normalize the AChE activity measured following in vitro exposure of the tissues to a given nerve agent concentration. The resulting concentration-response relationships were fitted to the Hill equation. Data points and error bars are mean and SEM of results obtained from triplicate experiments.
Table 2.
Apparent potencies (IC50s) for nerve agents to inhibit in vitro AChE activity in brain extracts, RBCs, plasma and whole blood of male and female guinea pigs of different ages.
| Nerve agent | Tissue | IC50s, nM
|
|||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| PND5-10 | PND5-10 | PND35-45 | PND35-45 | ||
| VX | Brain | 1.92 ± 0.07*** | 2.14 ± 0.07*** | 2.07 ± 0.05***† | 2.05 ± 0.24*** |
| RBC | 1.20 ± 0.10 | 1.16 ± 0.06 | 1.55 ± 0.38 | 1.17 ± 0.03 | |
| Plasma | 1.01 ± 0.08 | 1.14 ± 0.02 | 0.88 ± 0.08 | 0.71 ± 0.05 | |
| Whole blood | 1.28 ± 0.06 | 1.35 ± 0.25 | 1.84 ± 0.25 | 1.64 ± 0.22 | |
|
| |||||
| Soman | Brain | 2.52 ± 0.20*** | 2.53 ± 0.13*** | 2.33 ± 0.23*** | 2.47 ± 0.08*** |
| RBC | 0.88 ± 0.21 | 0.79 ± 0.21 | 0.79 ± 0.14 | 0.88 ± 0.16 | |
| Plasma | 0.84 ± 0.12 | 0.87 ± 0.12 | 0.78 ± 0.09 | 0.80 ± 0.13 | |
| Whole blood | 1.21 ± 0.07 | 1.27 ± 0.09 | 1.08 ± 0.04 | 1.12 ± 0.04 | |
|
| |||||
| Sarin | Brain | 5.65 ± 0.55*** | 5.66 ± 0.36*** | 6.15 ± 0.38*** | 6.03 ± 0.12*** |
| RBC | 3.94 ± 0.42 | 3.73 ± 0.48 | 3.27 ± 0.15 | 3.34 ± 0.06 | |
| Plasma | 4.05 ± 0.31 | 4.13 ± 0.65 | 4.29 ± 0.55 | 4.11 ± 0.23 | |
| Whole blood | 3.88 ± 0.04 | 3.80 ± 0.12 | 3.66 ± 0.12 | 3.90 ± 0.34 | |
Data are presented as mean and SD of results obtained from three assays each carried out in triplicate using tissue samples from five animals per age and sex group. IC50s were derived from the fit by the Hill equation of the concentration-response relationships for each nerve agent to inhibit the AChE activity in a given tissue sample.
indicate that the IC50 for a particular nerve agent to inhibit AChE in that tissue is significantly different from the IC50s for that agent to inhibit the enzyme activity in other tissues within a group of animals (one-way ANOVA followed by Tukey, p < 0.001).
indicates that, according to one-way ANOVA followed by Tukey, the IC50 for VX to inhibit AChE in brain extracts differs at p < 0.01 from its IC50s to inhibit RBC and whole blood AChE. No sex or age effect was observed on the IC50s for VX, soman and sarin to inhibit AChE in the various tissues of neonatal and prepubertal guinea pigs of both sexes.
Correlation between LD50s and IC50s for AChE inhibition in neonatal and prepubertal guinea pigs of both sexes
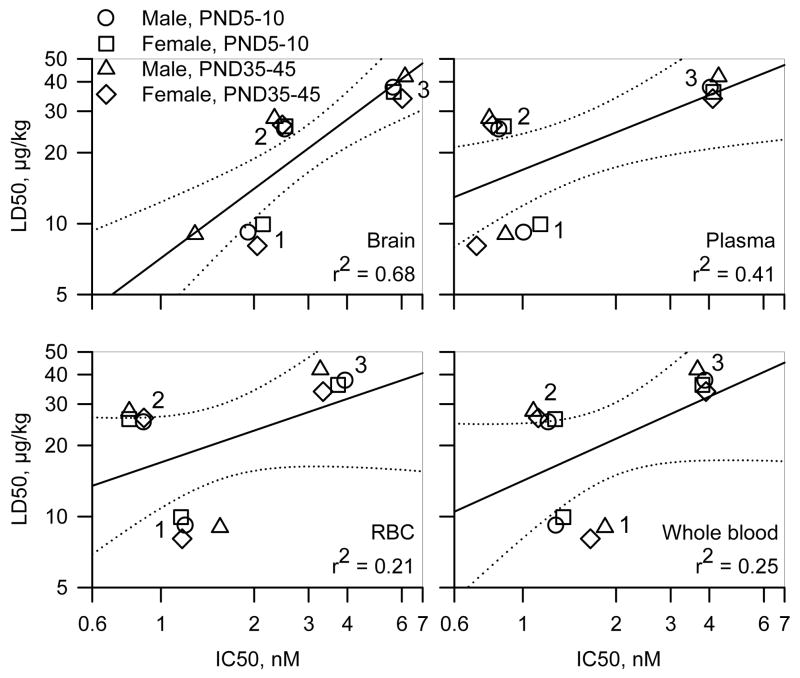
To determine whether the lethal potencies of the nerve agents correlated with their potencies to inhibit AChE in the brain, whole blood, plasma and RBCs from neonatal and prepubertal guinea pigs of both sexes, we analyzed the plots of the LD50s vs. IC50s for all three nerve agents in each tissue from all four animal groups. Given the sex differences identified for VX-induced inhibition of AChE in the various tissues of prepubertal male and female guinea pigs and between this and the younger age groups, the data for VX on prepubertal animals was not included in the plots. The Pearson’s regression coefficient was highest for the correlation between lethal potencies and potencies to inhibit brain AChE (Figure 6). No positive correlations existed between the lethal potencies and the potencies of the nerve agents to inhibit AChE in the plasma, RBC or whole blood.
Figure 6. Correlational analysis of the relationship between LD50s and IC50s for in vitro inhibition of AChE in various tissues from neonatal and prepubertal guinea pigs of both sexes.
Double logarithmic plots of the median lethal doses (LD50s) of each nerve agent for neonatal and prepubertal guinea pigs of both sexes vs. the corresponding in vitro concentrations of the nerve agents needed to inhibit by 50% the AChE activity in brain extracts, plasma, RBC, or whole blood. The symbol clusters labeled 1, 2, and 3 correspond to VX, soman and sarin, respectively. In each graph, the solid lines represent the linear regression of the data points, and the dotted lines illustrate the 95% confidence intervals of the regression. The Pearson coefficients (r2) are shown at the bottom right of each graph. LD50 values for prepubertal male guinea pigs are from the literature (Shih and McDonough, 2000).
AChE inhibition by VX, soman and sarin in brain extracts from adult male guinea pigs
If AChE inhibition was the sole mechanism underlying the toxicity of the nerve agents, the increased lethal potencies of VX and sarin in adult male guinea pigs should be paralleled by an increased apparent potency of each nerve agent to inhibit in vitro AChE activities in brain extracts from those animals. Thus, AChE activity was measured in the absence and in the presence of known concentrations of VX, soman or sarin applied to brain extracts from adult male guinea pigs. The IC50s for each nerve agent to inhibit AChE activity in the brain extracts were estimated by fitting the concentration-response relationships with the Hill equation (Figure 7). The estimated IC50 values were 3.38 ± 0.10 nM, 2.90 ± 0.10 nM, and 7.27 ± 0.12 nM for VX, soman and sarin, respectively. The IC50s for VX and sarin to inhibit AChE activity in brain extracts from adult male brains were significantly higher than those needed to inhibit AChE activity in brain extracts from neonatal and prepubertal male guinea pigs (p < 0.01 according to ANOVA followed by Tukey’s post hock test). The positive correlation observed between the nerve agents’ LD50s and IC50s for inhibition of AChE in brain extracts (see Figure 6) was disrupted by the inclusion of the data obtained from adult male guinea pigs (data not shown). The linear regression of all data points, including data from adult male guinea pigs, had a Pearson’s coefficient (r2) of 0.43.
Figure 7. Concentration-response relationships for in vitro inhibition of AChE in brain extracts from male guinea pigs at PND120-150.
The AChE activity (AU/min/mg protein) measured in brain extracts pooled from four adult male guinea pigs was taken as 100% and used to normalize the AChE activity measured following in vitro exposure of the extracts to a given nerve agent concentration. The resulting concentration-response relationships were fitted to the Hill equation. Data points and error bars are mean and SEM of results obtained from triplicate experiments. Although the lethal potencies of VX and sarin increased with age in male guinea pigs, their potencies to inhibit AChE activity in brain extracts from adult male guinea pigs were lower than those estimated to inhibit the enzyme activity in brain extracts from neonatal and prepubertal guinea pigs.
Discussion
The present study demonstrates that in guinea pigs the lethal potencies of the nerve agents VX and sarin are sex and age dependent, whereas the lethal potency of soman is not. Among neonatal, prepupertal and adult guinea pigs of both sexes, adult males are the most sensitive to VX and sarin. It also provides evidence that the IC50s of the nerve agents to inhibit AChE activity in brain extracts are good predictors of the lethal potencies of the agents in neonatal and prepubertal guinea pigs of both sexes. However, as discussed herein, AChE is not the sole endogenous target that contributes to the lethality of the nerve agents.
Age- and sex-related differences in the lethal potencies of VX, soman, and sarin in guinea pigs: Toxicological relevance
Sex and age effects on the acute toxicity of the nerve agents VX, soman and sarin vary significantly according to the animal species, the nerve agent and its route of administration. A recent study reported no sex differences in the lethal potencies of VX following a whole-body vapor exposure of adult male and female rats to the nerve agent (Benton et al., 2006). Other studies, however, have reported that female rats are more sensitive than male rats to sarin vapor toxicity (Mioduszewski et al., 2002).
Sex-related differences in the lethal potencies have been reported following sc injection of nerve agents in rats. Young adult female rats are more sensitive than age-matched male rats to the lethality of soman and respond better than the male rats to the treatment with the oxime HI-6 and the muscarinic antagonist atropine (Lundy et al., 1989). Female sex hormones increased the effectiveness of the antidotal therapy against nerve agent intoxication in the rats (Lundy et al., 1989). The same study revealed that such sex differences could not be detected in guinea pigs (Lundy et al., 1989). The weight of the guinea pigs in that study suggested that they were prepubertal animals. Therefore, those results are consistent with our current finding that prepubertal female and male guinea pigs are equally sensitive to the lethality of a sc injection of VX, soman and sarin.
As presented in the Results section, for all ages of male and female guinea pigs, the rank order of lethal potencies was VX > soman > sarin. Significant qualitative differences were also observed in the acute toxicity of the nerve agents. First, sarin was the fastest acting, while VX was the slowest acting of the three nerve agents. Second, animals challenged with = 1xLD50 soman or sarin were prone to the development of convulsions that became unremitting and led to life-threatening respiratory distress. In contrast, animals challenged with VX rarely developed convulsions even at the highest doses tested in this study. Gasping was evident in VX-challenged animals without any clear signs of convulsion. The slow onset of the toxic effects of VX and the absence of clear CNS-related effects in VX-challenged animals may be accounted for by the fact that VX has a pKa of 8.5 (see Shih et al., 2005 and references therein). As such, VX is likely to be positively charged at physiological pH and to have more limited access to the brain than the non-charged agents soman and sarin. Brain regional differences in the blood brain barrier permeability have been reported (Banks and Kastin, 1998), with higher degree of permeability being detected in certain regions, including the ventral part of the pons and medulla oblongata, which contains the central “respiratory pacemaker” known to be very sensitive to the actions of AChE inhibitors (Shao and Feldman, 2005).
The present study is the first to evaluate the sensitivity of young adult male and female guinea pigs to the toxicity of the nerve agents. Although the lethal potency of soman remained unchanged in male and female guinea pigs from neonatal ages (PND5-10) to young adult ages (PND120-150), the lethal potencies of VX and sarin were significantly higher in the adult male guinea pigs than in any other age/sex group studied herein. Studies carried out in rats have provided evidence that testosterone increases neuronal vulnerability to chemical hypoxia and enhances the central chemoreflex responsiveness to hypoxia (Ahuja et al., 2007; Nishino et al., 1998). Considering that severe respiratory distress developed quickly after the onset of generalized convulsion in an acute intoxication with sarin and was the leading sign of an acute VX intoxication, it is tempting to speculate that the high levels of testosterone in adult male guinea pigs exacerbate the toxicity of VX and sarin.
Delayed toxic effects resulting from an acute exposure to nerve agents may not follow the same age and sex dependencies as the acute effects. In fact, epidemiological studies have reported that women who were exposed to sarin in the 1995 terrorist attack were more likely to present a delayed effect on the vestibulo-cerebellar type of sway than age-matched men who had been similarly exposed (Yokoyama et al., 1998).
AChE inhibition is not the sole mechanism underlying the toxicity of nerve agents
The apparent potencies of the nerve agents to inhibit AChE in vitro were found to be highly tissue specific. Thus, AChE activity in extracts from brains of neonatal and prepubertal guinea pigs of both sexes was less sensitive than plasma, RBC or whole blood AChE to in vitro inhibition by VX, soman and sarin. Both the G4 and G1 forms of AChE are highly expressed in the mammalian brain (Lintern et al., 1998), whereas the G4 form prevails in the plasma (Massoulié and Bon, 1982). The fact that the G4 is more sensitive than the G1 form of AChE to inhibition by the nerve agents (Bajgar, 1997; Lintern et al., 1998) could explain at least in part the differential sensitivity of AChE in blood and brain extracts to soman, sarin and VX. Another plausible explanation for the tissue-specific IC50s for VX, soman and sarin is the existence of proteins other than AChE that bind the nerve agents with high affinity and, thereby, decrease the agents’ free concentrations to interact with AChE (Mortensen et al., 1998).
The lethal potencies of VX, soman and sarin correlated well with their IC50s to inhibit in vitro AChE activity in brain extracts from neonatal and prepubertal guinea pigs of both sexes. However, although the lethal potencies of VX and sarin increased as the male guinea pigs aged from prepuberty to adulthood, the apparent potencies of these agents to inhibit in vitro AChE activity in brain extracts decreased from prepubertal to adult male guinea pigs. These results are in agreement with the notion that AChE inhibition is not the sole mechanism underlying the toxicity of nerve agents (Duysen et al., 2001). In the brain, muscarinic receptors are among targets that bind OP compounds with high affinity (Jett et al., 1991; Silveira et al., 1990). Therefore, sex- and age-related differences in the expression of such targets could explain the exquisite sensitivity of adult male guinea pigs to the toxicity of sarin and VX.
No significant correlation could be traced between LD50s and IC50s of the nerve agents to inhibit in vitro AChE activity in blood, RBC or whole blood from neonatal and prepubertal guinea pigs of both sexes. These findings support the contention that serum cholinesterase activity has no prognostic value in OP poisoning (Nouira et al., 1994). In the early 1970’s, a grading system for the severity of OP poisoning was developed based on the levels of serum cholinesterase activity (Namba et al., 1971). This score, although never validated, is still used to design the best medical therapy for treatment of OP-poisoned patients (Noiura et al., 1994). More recently, it has been proposed that a field test system should be available for rapid determination of RBC AChE activity to assist physicians in optimizing diagnosis and treatment of acute OP intoxication (reviewed in Thiermann et al., 2007). The goal would be to treat patients with oximes to maintain the reactivation of RBC AChE (see Thiermann et al., 2007 and references therein). However, currently available oximes do not cross the blood brain barrier (reviewed in Lorke et al., 2008) and, as such, do not prevent the irreversible inhibition of brain AChE by the OPs. Thus, while there is no debate regarding the validity of RBC or plasma cholinesterase activity as a biomarker of OP exposure, the levels of enzyme activity in these compartments should not be used as a guide for treatment of OP poisoning.
In conclusion, this study provides the first evidence that adult male guinea pigs are more sensitive to the lethality of VX and sarin than age-matched females or younger guinea pigs of both sexes. It also indicates that the lethal potencies up to adulthood correlate well with the apparent potencies of the nerve agents to inhibit in vitro brain AChE. These results have far reaching implications as they emphasize that development of safe and effective antidotal therapies against OP intoxication has to take into account sex and age and cannot be solely based on the protection of blood AChE against the OP-induced irreversible inhibition.
Acknowledgments
This work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award #UO1NS059344) and by the U.S. Army Research Office Contract W911NF-06-1-0098. Its contents are solely the responsibility of the authors and do not necessarily reflect the official views of the federal government.
The authors would like to thank Ms. Mabel Zelle and Ms. Miriam Akkerman for their technical assistance. The authors are also indebted to Dr. William Randall (University of Maryland School of Medicine) and MAJ Jeremy L. Goodin (US Army Medical Research Institute of Chemical Defense) for insightful discussions and comments on the manuscript.
Abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- IACUC
Institutional Animal Care and Use Committee
- OP
organophosphorus
- PND
postnatal day
- RBC
red blood cell
- sc
subcutaneous
- iso-OMPA
tetraisopropylpyro-phosphoramide
References
- Ahuja D, Mateika JH, Diamond MP, Badr MS. Ventilatory sensitivity to carbon dioxide before and after episodic hypoxia in women treated with testosterone. J Appl Physiol. 2007;102:1832–1838. doi: 10.1152/japplphysiol.01178.2006. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Deshpande SS, Kawabuchi M, Aracava Y, Idriss M, Rickett DL, Boyne AF. Multiple actions of anticholinesterase agents on chemosensitive synapses: molecular basis for prophylaxis and treatment of organophosphate poisoning. Fundam Appl Toxicol. 1985;5:S182–203. doi: 10.1016/0272-0590(85)90129-0. [DOI] [PubMed] [Google Scholar]
- Anderson PD, Weber LJ. Toxic response as a quantitative function of body size. Toxicol Appl Pharmacol. 1975;33:471–483. doi: 10.1016/0041-008x(75)90073-3. [DOI] [PubMed] [Google Scholar]
- Bajgar J. Differential inhibition of the brain acetylcholinesterase molecular forms following sarin, soman and VX intoxication in laboratory rats. Acta Medica (Hradec Kralove) 1997;40:89–94. [PubMed] [Google Scholar]
- Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Guidi S, Severi S, Contestabile A, Ciani E. Sex differences in the hippocampal dentate gyrus of the guinea-pig before puberty. Neuroscience. 2003;121:327–339. doi: 10.1016/s0306-4522(03)00434-2. [DOI] [PubMed] [Google Scholar]
- Benton BJ, McGuire JM, Sommerville DR, Dabisch PA, Jakubowski EM, Matson KL, Mioduszewski RJ, Thomson SA, Crouse CL. Effects of whole-body VX vapor exposure on lethality in rats. Inhal Toxicol. 2006;8:1091–1099. doi: 10.1080/08958370600945598. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: Supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. 2001;299:528–535. [PubMed] [Google Scholar]
- Ecobichon DJ, Dykeman RW, Hansell MM. The development of hepatic drug-metabolizing enzymes in perinatal guinea pigs: a biochemical and morphological study. Can J Biochem. 1978;56:738–745. doi: 10.1139/o78-111. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- García Peñas JJ, Molins A, Salas Puig J. Status epilepticus: Evidence and controversy. Neurologist. 2007;13(6 Suppl 1):S62–73. doi: 10.1097/NRL.0b013e31815bb069. [DOI] [PubMed] [Google Scholar]
- Inns RH, Leadbeater L. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J Pharm Pharmacol. 1983;35:427–433. doi: 10.1111/j.2042-7158.1983.tb04316.x. [DOI] [PubMed] [Google Scholar]
- Jett DA, Abdallah EA, El-Fakahany EE, Eldefrawi ME, Eldefrawi AT. The high affinity activation by paraoxon of a muscarinic receptor subtype in rat brain striatum. Pestic Biochem Physiol. 1991;39:149–157. [Google Scholar]
- Kawada T, Katsumata M, Suzuki H, Li Q, Inagaki H, Nakadai A, Shimizu T, Hirata K, Hirata Y. Insomnia as a sequela of sarin toxicity several years after exposure in Tokyo subway trains. Percept Mot Skills. 2005;100:1121–1126. doi: 10.2466/pms.100.3c.1121-1126. [DOI] [PubMed] [Google Scholar]
- Koplovitz I, Gresham VC, Dochterman LW, Kaminskis A, Stewart JR. Evaluation of the toxicity, pathology, and treatment of cyclohexylmethylphosphonofluoridate (CMPF) poisoning in rhesus monkeys. Arch Toxicol. 1992;66:622–628. doi: 10.1007/BF01981500. [DOI] [PubMed] [Google Scholar]
- Lintern MC, Wetherell JR, Smith ME. Differential recovery of acetylcholinesterase in guinea pig muscle and brain regions after soman treatment. Hum Exp Toxicol. 1998;17:157–162. doi: 10.1177/096032719801700306. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: A review. Curr Med Chem. 2008;15:743–753. doi: 10.2174/092986708783955563. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Goulet JC, Hand BT. Hormone- and dose schedule-dependent protection by HI-6 against soman and tabun poisoning. Fundam Appl Toxicol. 1989;12:595–603. doi: 10.1016/0272-0590(89)90032-8. [DOI] [PubMed] [Google Scholar]
- Massoulié J, Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Meinecke KH, Oettel H. Micromethods for the determination of the acetylcholinesterase activity in erythrocytes and plasma of humans and animals. II. Methods for the blood and the brain of animals. Normal values for rats, rabbits, guinea pigs, dogs, and cattle. Arch Toxikol. 1967;22:244–251. [PubMed] [Google Scholar]
- Mioduszewski R, Manthei J, Way R, Burnett D, Gaviola B, Muse W, Thomson S, Sommerville D, Crosier R. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol Sci. 2002;66:176–184. doi: 10.1093/toxsci/66.2.176. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Brimijoin S, Hooper MJ, Padilla S. Comparison of the in vitro sensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: what do tissue IC50 values represent? Toxicol Appl Pharmacol. 1998;148:46–49. doi: 10.1006/taap.1997.8287. [DOI] [PubMed] [Google Scholar]
- Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am J Med. 1971;50:475–492. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neurosci Res. 1998;30:303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Nouira S, Abroug F, Elatrous S, Boujdaria R, Bouchoucha S. Prognostic value of serum cholinesterase in organophosphate poisoning. Chest. 1994;106:1811–1814. doi: 10.1378/chest.106.6.1811. [DOI] [PubMed] [Google Scholar]
- Romano JA, Jr, King JM. Psychological casualties resulting from chemical and biological weapons. Mil Med. 2001;166:21–22. [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: Altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Pharmacology of nicotinic receptors in preBötzinger complex that mediate modulation of respiratory pattern. J Neurophysiol. 2002;88:1851–1858. doi: 10.1152/jn.2002.88.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Kan RK, Donough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chemico-Biol Inter. 2005;157–158:293–303. doi: 10.1016/j.cbi.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Efficacy of biperiden and atropine as anticonvulsant treatment for organophosphorus nerve agent intoxication. Arch Toxicol. 2000;74:165–172. doi: 10.1007/s002040050670. [DOI] [PubMed] [Google Scholar]
- Shih TM, Penetar DM, McDonough JH, Jr, Romano JA, King JM. Age-related differences in soman toxicity and in blood and brain regional cholinesterase activity. Brain Res Bull. 1990;24:429–436. doi: 10.1016/0361-9230(90)90097-j. [DOI] [PubMed] [Google Scholar]
- Silveira CL, Eldefrawi AT, Eldefrawi ME. Putative M2 muscarinic receptors of rat heart have high affinity for organophosphorus anticholinesterases. Toxicol Appl Pharmacol. 1990;103:474–481. doi: 10.1016/0041-008x(90)90320-t. [DOI] [PubMed] [Google Scholar]
- Sterri SH, Berge G, Fonnum F. Esterase activities and soman toxicity in developing rat. Acta Pharmacol Toxicol (Copenh) 1985;57:136–140. doi: 10.1111/j.1600-0773.1985.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Thiermann H, Kehe K, Steinritz D, Mikler J, Hill I, Zilker T, Eyer P, Worek F. Red blood cell acetylcholinesterase and plasma butyrylcholinesterase status: important indicators for the treatment of patients poisoned by organophosphorus compounds. Arh Hig Rada Toksikol. 2007;58:359–366. doi: 10.2478/v10004-007-0030-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP. Evidence for physiological growth of hippocampal mossy fiber collaterals in the guinea pig during puberty and adulthood. Hippocampus. 1995;5:329–340. doi: 10.1002/hipo.450050406. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Araki S, Murata K, Nishikitani M, Okumura T, Ishimatsu S, Takasu N, White RF. Chronic neurobehavioral effects of Tokyo subway sarin poisoning in relation to posttraumatic stress disorder. Arch Environ Health. 1998;53:249–256. doi: 10.1080/00039899809605705. [DOI] [PubMed] [Google Scholar]