Abstract
This case-series describes the 6 human infections with Onchocerca lupi, a parasite known to infect cats and dogs, that have been identified in the United States since 2013. Unlike cases reported outside the country, the American patients have not had subconjunctival nodules but have manifested more invasive disease (eg, spinal, orbital, and subdermal nodules). Diagnosis remains challenging in the absence of a serologic test. Treatment should be guided by what is done for Onchocerca volvulus as there are no data for O. lupi. Available evidence suggests that there may be transmission in southwestern United States, but the risk of transmission to humans is not known. Research is needed to better define the burden of disease in the United States and develop appropriately-targeted prevention strategies.
Keywords: Onchocerca lupi, emerging infectious diseases, zoonotic infection
Since the recognition of Onchocerca lupi as a common canine infection in parts of Europe and the United States [1–8], there has been growing interest in the parasite and its geographical distribution, range of natural definitive hosts, arthropod vectors, and pathology. Although the parasite’s life cycle is not fully understood, it should be similar to that of Onchocerca volvulus. Adult worms produce early stage larvae, called microfilariae, which migrate in the mammalian host’s skin. The microfilariae are ingested by blackflies and undergo several stages of larval development. Infectious stage larvae migrate to the mouth parts of the blackfly and enter the mammalian host’s skin during a blood meal, where development into the adult parasite is completed (for more details see http://www.cdc.gov/parasites/onchocerciasis/biology.html). Of particular interest is the seeming frequent infection of human hosts. Onchocerca lupi is now the most commonly reported zoonotic Onchocerca infection in humans. The first such case of human infection in the United States was reported in 2013 [9]. At the time of the report, there were approximately 20 case reports of zoonotic Onchocerca infection in the English-language world literature; 5 were attributed to O. lupi [10–13]. Since the report, 2 additional cases have been identified elsewhere [14, 15], and 5 additional cases have been identified in the United States.
Onchocerca lupi was first identified in a Caucasian wolf (cited in [16]) but since that time has been reported primarily in domestic dogs and cats in the United States [1, 9, 17, 18] and Europe [2]. The initial manifestation in animals can include excessive lacrimation, photophobia, conjunctivitis, exophthalmos, and periorbital swelling [17, 18]. Later manifestations may include subconjunctival or episcleral nodules. Infection may be asymptomatic; a study of asymptomatic dogs in Portugal and Greece found that 8% harbored microfilariae in their skin.
Human case reports of infections identified outside the United States have described patients with eye manifestations [10–15]. The patients presented with conjunctival redness that was painless, painful, or pruritic. A subconjunctival nodule containing the adult parasite was found in all patients [10–15]. These eye manifestations are similar to what has been seen in dogs but are distinct from O. volvulus (river blindness) infection. In O. volvulus infection, adult worms form subdermal nodules, often over bony prominences but not in the subconjunctiva [19], and the inflammation surrounding microfilariae results in damage to the cornea (eg, punctate keratitis and sclerosing keratitis) or the optic nerve. In contrast to the non-US cases, the US cases have had more severe manifestations and have had nodules appear in other areas of the body. These 6 US human infections will be described in this case series. Key findings and aspects of diagnosis and management of the cases are found in Table 1.
Table 1.
Key Features of the 6 Cases on Onchocerca lupi Infection
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age | 22 months old | 10 years old | 50 years old | 13 years old | 5 years old | 10 years old |
| Residence | Arizona | New Mexico | Arizona | Arizona | New Mexico | Texas |
| Location of nodule | Cervical spine | Scalp | Forearm | Cervical spine | Cervical spine | Superior rectus muscle |
| Additional evaluation for parasites | Skin snipsa | Eye examb | Eye exam | Eye exam, lumbar puncture | Eye exam, lumbar puncture | None |
| Method of confirming the diagnosis | Histology | Histology, PCR | Histology, PCR | Histology | Histology | Histology, PCR |
| Histologic findings | Gravid adult | Nongravid adult | Nongravid adult | Nongravid adult | Gravid adult | Multiple nongravid adults |
| Management | Biopsy and ivermectinc | Nodule excision | Nodule excision, ivermectin, doxycyclined | Partial excision, ivermectin, doxycycline | Nodule excision, ivermectin, Doxycycline | Nodule excision, ivermectin, doxycycline |
Abbreviation: PCR, polymerase chain reaction.
Skin snips are superficial skin biopsies.
Eye exam included a slit lamp examination of the anterior chamber of the eye, which can identify microfilariae.
For notes on the use of ivermectin during pregnancy or lactation and in pediatric patients please refer to http://www.cdc.gov/parasites/onchocerciasis/health_professionals/index.html#tx.
For notes on the use of doxycycline during pregnancy or lactation and in pediatric patients please refer to http://www.cdc.gov/parasites/onchocerciasis/health_professionals/index.html#tx.
Case 1
This case has been previously described [9]. In brief, a healthy 22-month-old girl residing in northeastern Arizona but born in New Mexico presented with a 4-week history of pain that limited the range of motion of her neck. Physical exam revealed decreased range of motion but no palpable masses. Magnetic resonance imaging (MRI) revealed a 19 mm enhancing extradural mass at the C2–C4 level with moderate to severe cord compression. A biopsy was performed, rather than excision, because of the unexpected finding of a rubbery, avascular mass. Examination of the tissue at the Centers for Disease Control and Prevention (CDC) resulted in the morphologic diagnosis of a gravid O. lupi. As the worm was gravid, 2 skin snip biopsies (superficial skin biopsies) were performed to determine whether microfilariae were present. The snips were negative, but because of the known low sensitivity of skin snips for low burden O. volvulus infections, the patient was treated with ivermectin to kill any microfilariae that might have been missed by the snips. No treatment was initiated to kill the adult form of the parasite, as the biopsy should have killed the worm. Seven weeks post-biopsy the size of the spinal lesion was markedly reduced on MRI. The patient has remained asymptomatic.
Case 2
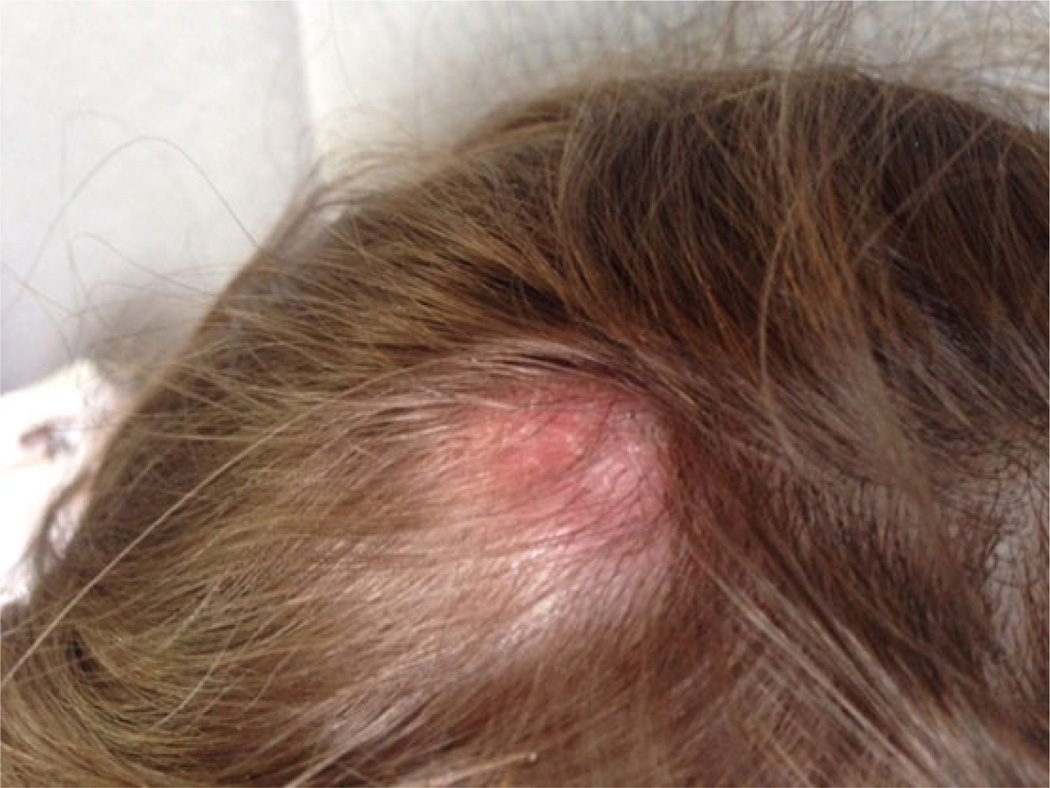
A healthy 10-year-old female from northwestern New Mexico complained in October 2013 of a minimally tender, erythematous swollen area on the right posterior-parietal scalp in that had been present for 2 months (Figure 1). She had been bitten on the head by a flying insect while boating on Navajo Lake, New Mexico 2 years earlier. The patient had traveled only in New Mexico and Colorado.
Figure 1.
Image of the minimally tender, erythematous swollen area on the right posterior-parietal scalp of the patient described in case 2.
The mass enlarged until its surgical excision in February 2014. Purulentmaterial was observed within the lesion, and a long, folded, strand-like structure was removed. It was yellow in color and 0.7 mm thick. The material was identified as nematode of the genus Onchocerca at the University of New Mexico. Microscopic review at CDC identified a nongravid female morphologically consistent with O. lupi (Figure 2); this was confirmed by polymerase chain reaction (PCR)molecular analysis. An ophthalmological evaluation for microfilariae was normal. Given the patient’s improvement since her surgery and the absence of a gravid nematode, no further diagnostic evaluation was performed. She has remained asymptomatic without antiparasitic therapy.
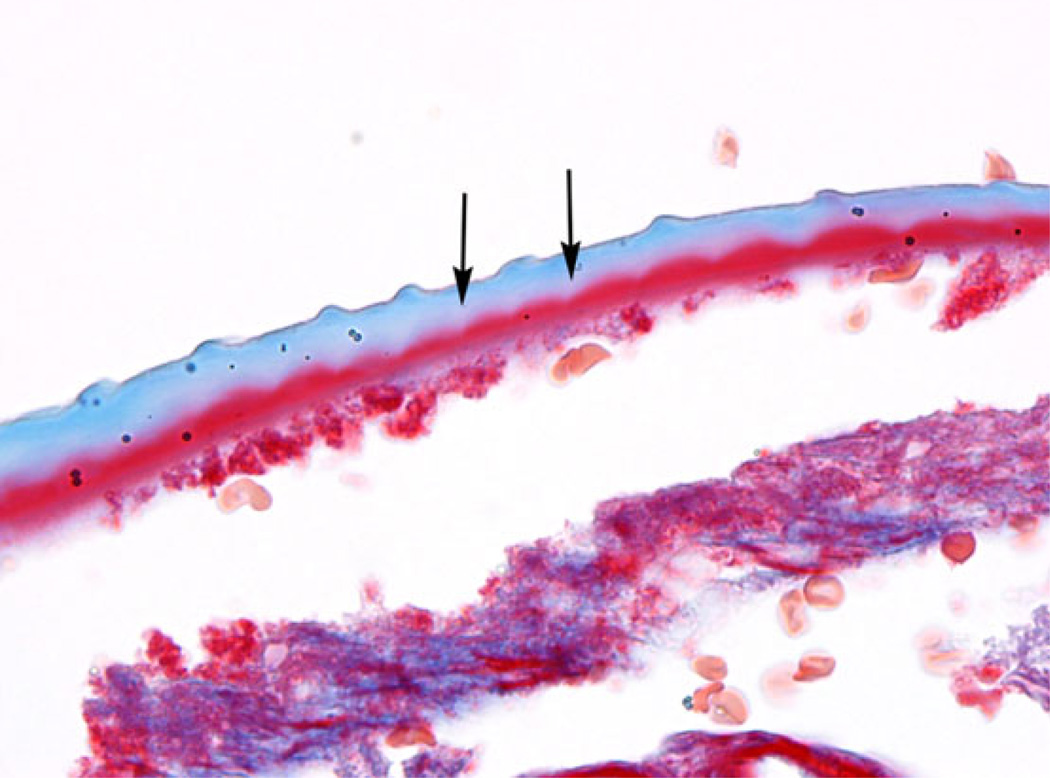
Figure 2.
Longitudinal section of the cuticle of O. lupi showing the characteristic transverse cuticular ridges, and the striae (arrows), that run between and below them. [Case 2, trichrome stain, 1000× magnification with oil].
Case 3
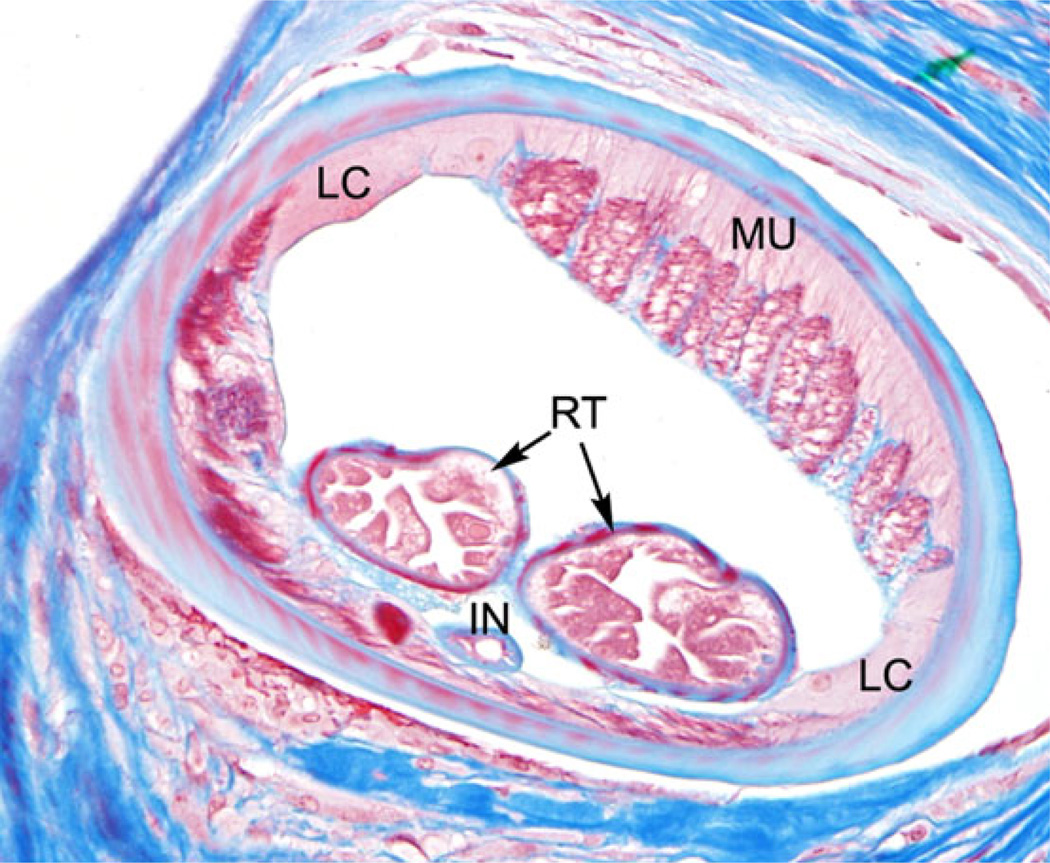
A healthy 50-year-old female from southern Arizona was evaluated during May 2014 following the removal of a subcutaneous granulomatous cyst on the dorsum of her right forearm that was discovered to contain O. lupi. The patient had first noticed a nickel-sized subcutaneous nodule on the dorsal aspect of her forearm in November 2013. It was rubbery, nontender, nonerythematous, and nonpruritic. She had travelled to Jamaica during May and June 2013 and to Monument Valley in Moab, Utah in 2008. During both trips she reported insect bites; in Moab she awoke one morning with pruritic bites on her right arm extending from shoulder to wrist. The pathology examination confirmed the presence of a helminth with surrounding fibrotic granuloma. Digital images of the organism were reviewed by CDC, which preliminarily identified the organism as Onchocerca. CDC parasitologists confirmed that the organism met morphological criteria for O. lupi (Figure 3) upon examination of the tissue; this diagnosis was confirmed by PCR.
Figure 3.
Cross-section of a female O. lupi, showing typical morphologic features of the genus, including reduced coelomyarian musculature (MU), short, yet conspicuous, lateral chords (LC), paired reproductive tubes (RT), and a small, simple intestine (IN). [Case 3, trichrome stain, 400×].
Ophthalmic slit lamp examination did not show microfilariae. Although the eye exam was normal and no gravid worm was seen on pathology, the patient was treated with a single dose of ivermectin to kill any undetected microfilariae and then with 6 weeks of doxycycline to kill any undetected adult worms. The patient has remained asymptomatic.
Case 4
A 13-year-old previously healthy male from northeastern Arizona presented April 2014 for evaluation with more than a week of worsening left-sided neck pain followed by development of sore throat, dysphagia, and headache. Initial evaluation suggested streptococcal pharyngitis. The patient was treated with a combination of anti-inflammatory medication, physical therapy, and antibiotics. Two days later, his pain and headache recurred and worsened, this time accompanied by meningismus on exam. Cerebrospinal fluid (CSF) studies after a lumbar puncture were consistent with bacterial meningitis. He was treated for presumptive bacterial meningitis with a 14-day course of ceftriaxone. Evaluation for bacterial and viral pathogens was negative. There was some improvement of the headache.
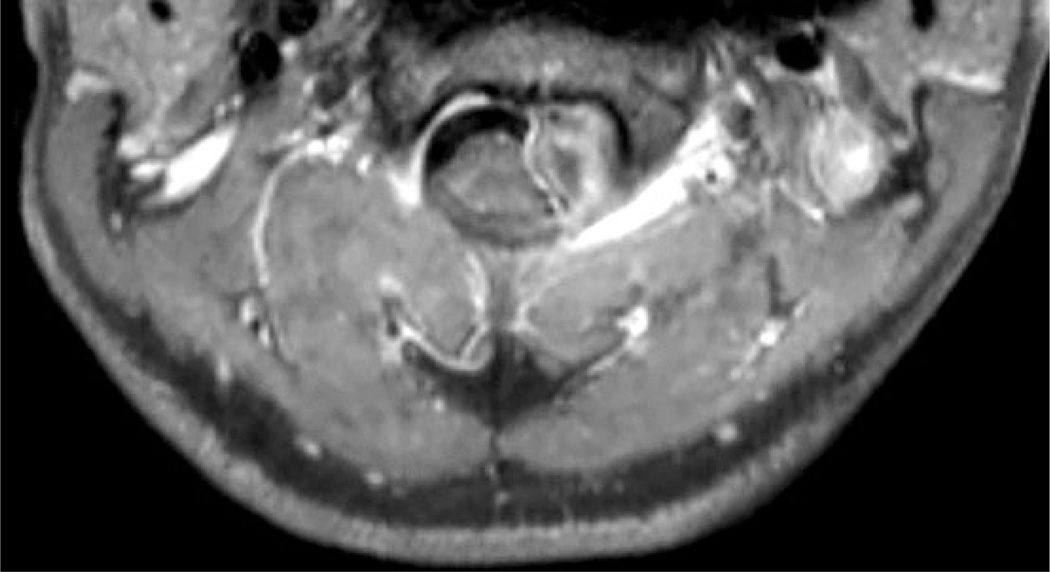
Four weeks later the patient’s symptoms recurred. A head computed tomography (CT) without contrast was normal. Lumbar puncture was repeated, and cebrebrospinal fluid (CSF) showed 2160 white blood cells (WBCs) (62% neutrophils, 38% monocytes), 43 red blood cells (RBCs), protein 252 mg/dL, and glucose 30 mg/dL. Gram stain showed no bacteria. Antibiotics were initiated, and the patient was transferred for further evaluation. An MRI of the brain and cervical spine revealed an intradural, extramedullary mass within the upper cervical spinal canal (Figure 4). The patient underwent posterior fossa decompression and partial resection of the mass and was discharged on corticosteroids while awaiting a diagnosis. The pathologists reported that the most likely diagnosis was O. lupi. This was confirmed by CDC. Because no gravid female worm was seen and ophthalmological examination was normal, the patient was treated with a 6-week course of doxycycline as recommended by CDC. Repeat MRI after completion of doxycycline showed interval decrease in size of the mass with decreased mass effect on the spinal cord.
Figure 4.
Magnetic resonance imaging of the cervical spine with contrast performed prior to the first surgery demonstrating an intradural, extramedullary mass within the leftward aspect of the upper cervical canal at the C2–C3 level.
Two months after his initial surgery, the patient’s symptoms recurred, followed by paresthesias and decreased sensation in his upper extremities. An emergent MRI showed an increase in the size of the cervical spinal canal mass with abscess. He underwent emergent resection of the cervical abscess. Intraoperative pathology of the mass showed multiple pieces of O. lupi. As the initial mass was not fully resected, it was felt that the increased size of the mass was most likely due to inflammation surrounding the parasite that was killed by the initial resection. Despite this, the patient was treated with a second course of doxycycline and a dose of ivermectin. He is now receiving ivermectin every 3 months and remains asymptomatic.
Case 5
A brief description of this case has been previously reported [20]. A 5-year-old girl with trisomy 21 from rural northwestern New Mexico presented in 2014 with occipital headaches and neck pain that occasionally woke her up. The pain increased in severity and frequency over several months. There was no associated vomiting, fever, or neurological symptoms. The patient had travelled only to Arizona and Colorado and frequently visited Navajo Lake near her home in New Mexico. She had a normal neurological examination, no limitation of neck range of motion, and no skin or eye findings. An MRI of the brain and spine showed an extradural soft tissue mass within the left anterolateral aspect of the C2–C3 spinal canal, extending through and remodeling the left C2–C3 neural foramen and causing moderate mass effect upon the underlying spinal cord.
A laminectomy was performed, and an intradural, extramedullary fusiform mass measuring 1.5 × 1.2 × 0.4 cm was resected. The mass was smooth, firm, and densely adherent to the dura, and complete excision of the portion extending through the neural foramen was unsuccessful due to intraoperative bleeding. Histologic examination of the mass revealed a fibroinflammatory nodule containing multiple cross-sections of an adult female nematode, which had a thickened cuticle with ridges on its surface. The nematode contained 2 paired uteri, which were filled with numerous microfilariae. CDC confirmed that the nematode was morphologically compatible with O. lupi; PCR confirmation was not done.
On postoperative day 18, lumbar puncture was performed; no microfilariae were identified. Ophthalmologic examination was normal. Due to concern for undetected microfilariae, medical treatment was initiated with ivermectin 1 dose every 3 months for a planned period of at least 5 years. Doxycycline daily for 6 weeks was added to the regimen to kill any adult worms not killed by the resection of the lesion. Three months after surgical excision, she remained asymptomatic. The family dogs had no evidence of O. lupi infection on eye examination or skin biopsy.
Case 6
A 10-year-old previously healthy boy from southern Texas developed progressive left upper eyelid drooping, periorbital edema, and conjunctival injection beginning 2 weeks prior to his presentation in 2014. A pet dog that had conjunctivitis and an eye lesion of unknown etiology had lived in the home prior to diagnosis. The patient went fishing in a fresh water lake near his home. His only travel was a car trip in the summer of 2012 from his home in Texas to South Dakota. During the trip he slept in tents and cabins in New Mexico and Colorado.
Magnetic resonance imaging revealed a 1.5 cm mass in the superior rectus muscle that compressed the globe. It had a thickened, enhancing capsule and a necrotic-appearing center. His laboratory results, including a complete blood count, were normal. The patient was taken to the operating room for a left anterior orbitotomy and exploration of the mass. Multiple nongravid adult helminths, identified preliminarily as Onchocerca spp, were extracted from the lesion. CDC confirmed the worms to be from the genus Onchocerca. PCR confirmed the worms as O. lupi. After complete resection of the mass, the patient received one dose of ivermectin and a 6-week course of doxycycline. The patient has remained asymptomatic.
DISCUSSION
Our series of cases with O. lupi infection highlights several important findings. An increasing number of cases caused by infection with O. lupi are being identified. We report 5 additional US cases since the first reported case in 2013. With one exception, all US infections have occurred in children. The US cases are unusual in their clinical presentation: only one patient had eye involvement, although it involved a periorbital rather than subconjunctival nodule. Two of the 6 US patients had palpable nodules, and 3 had spinal nodules. All patients with spinal nodules required neurosurgical intervention. One patient also had meningitis that was never explained by an alternate etiology though microfilariae were not found in the CSF. It is plausible that there was an inflammatory response to adult worm or nodule antigens that were present in the CSF because of the proximity of the nodule to the central nervous system. No other onchocercal parasite, including O. volvulus, is known to have a tropism for the spine or to invade the central nervous system. This potential to cause severe disease suggests that there should be heighted awareness of this emerging pathogen and that efforts are needed to better describe the spectrum of associated human disease.
Onchocerca lupi differs from other zoonotic filarial infections in several other important aspects. Usually humans with zoonotic filarial infections are infected with a single worm, and reproduction of the worm does not occur. In this case series, 2 patients were infected with gravid females, and a third patient with more than 1 female worm. There are 2 cases in the literature where multiple worms were found [14, 15].Onchocerca lupi appears to lack the host-specificity found in many other filarial parasites. It is known to reproduce in dogs and cats [17, 21] and has been found to do so in humans in these case-series. This finding could have serious implications for efforts to reduce the risk of infection in humans as there could be other, as yet not identified mammalian species that could serve as a reservoir to maintain risk of infection in humans. It also suggests that medical therapy could be required even when only 1 parasite is found on biopsy.
Much remains to be learned about where and how O. lupi is transmitted. The patients in this series lived in Arizona, New Mexico, and Texas. Several patients traveled to Colorado and Utah. Infected dogs or cats that originated from or travelled to Arizona, California, Colorado, Florida, Minnesota, Nevada, New Mexico, and Utah have been identified [1, 9, 17]. Infected blackflies have been identified in California [22]. This suggests that there is at least 1 area in the southwestern United States where a cycle of parasite transmission exists; there may be others. Identifying areas with infected mammalian species and vectors, particularly if human cases are also found in these areas, would help define areas of risk and inform the public health response. It is important to note that the parasite cannot be spread directly from animal to human as it needs to complete part of its life cycle in the blackfly. Interventions that target infected mammalian hosts or the blackfly vector should reduce the risk of infection in humans. In addition to the identification of foci of transmission, appropriately targeted interventions for this emerging parasitic infection will require the development of diagnostic tools and the demonstration of treatment efficacy.
The diagnosis of O. lupi infection is challenging. In dogs and cats, which harbor high-burden infections, there are 2 options. Subconjunctival nodules can be resected and examined for the presence of the adult worm, or skin snips can be performed and examined for the presence of microfilariae after incubation in normal saline for 24 hours. There is no blood test available, though 1 serologic test shows promise [23]. In humans, who have low burden or single worm infections, the diagnostic options are limited to the identification of the adult worm in an extracted nodule. Skin snips are not sensitive in low-burden infections and are of no use if gravid females are not present. In this case-series, all diagnoses were made after nodule resection; 4 of the 6 patients required invasive surgery. A serologic test for diagnosis in humans would be very helpful as it could identify individuals who could be treated medically rather than surgically (eg, patients with spinal nodules without neurologic symptoms) and would help public health officials identify areas of transmission in the United States.
There are no evidence-based treatment algorithms for human O. lupi infection. The treatments suggested for the cases in this series were based on what is known about other filarial parasites. Ivermectin is known to kill the microfilariae of O. volvulus and suppress the formation of new microfilariae for many months [24]. It may also accelerate the sterilization and death of adult female worms [25]. Its use in the cases presented, assuming that ivermectin has the same effect on O. lupi as O. volvulus, would be expected to rapidly kill microfilariae, preventing transmission to blackflies. Doxycycline is much more effective at killing and sterilizing the adult worms and should be used when not contraindicated. It kills Wolbachia, an endosymbiotic bacteria required for reproduction and long-term survival of O. volvulus [26] and zoonotic O. ochengi [27] which has also been identified in O. lupi [28]. The use of doxycycline in dogs in combination with ivermectin has been shown to kill Dirofilaria immitis and Dirofilaria repens [29–31], both of which are Wolbachia-containing nematodes. It is unclear whether or not ivermectin was necessary in the dogs, as the killing effect of doxycycline monotherapy in humans takes 21–27 months [26, 32] and the dogs were followed for 8 months. Based on the available evidence, the following strategy would be reasonable. Anyone diagnosed with O. lupi should have a slit-lamp eye examination to evaluate for the presence of microfilariae. Skin snips could be considered, but as they are more invasive than a slit lamp examination and are unlikely to be positive for infection unless the patient is harboring many gravid adult worms, they may not be necessary. If microfilariae are identified in the eye (or skin) or gravid worms are identified in a pathological specimen, a single dose of ivermectin (150 micrograms/ kg) should be given to kill any remaining microfilariae. For all patients (with or without microfilariae) it would be reasonable to treat with a 4–6 week course of doxycycline (4 mg/kg/day in 2 divided doses; maximum dose 100 mg twice daily) to kill any adult worms not found at the time of nodule resection. The resection of a single worm infection is curative, but as nodules may not be palpable, patients with multiple worm infections have been identified, and the presence of additional worms might require invasive surgical procedures, a more cautious approach might be to treat with doxycycline. It is important to note that this treatment should not be expected to kill O. lupi quickly. Studies will be needed to evaluate the effectiveness of this treatment strategy.
Onchocerca lupi is an emerging parasitic pathogen that is poorly understood. There appears to be a transmission cycle in the southwest United States that involves blackflies, dogs, and cats; humans may also be contributing to the transmission cycle. In the United States, the clinical manifestations, including cervical spine involvement, have been more severe than seen elsewhere. Given the significant morbidity that can be associated with O. lupi infection, continued investigation is needed to better describe the spectrum of human disease, develop approaches for the diagnosis and treatment of infection, and develop public health strategies to prevent transmission to humans.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflicts of interests. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Labelle AL, Maddox CW, Daniels JB, et al. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet Parasitol. 2013;193:297–301. doi: 10.1016/j.vetpar.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Otranto D, Dantas-Torres F, Giannelli A, et al. Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011–2012. Emerg Infect Dis. 2013;19:2000–2003. doi: 10.3201/eid1912.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komnenou A, Eberhard ML, Kaldrymidou E, Tsalie E, Dessiris A. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Vet Ophthalmol. 2002;5:119–126. doi: 10.1046/j.1463-5224.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 4.Komnenou A, Egyed Z, Sreter T, Eberhard ML. Canine onchocercosis in Greece: report of further 20 cases and molecular characterization of the parasite and its Wolbachia endosymbiont. Vet Parasitol. 2003;118:151–155. doi: 10.1016/j.vetpar.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Szell Z, Erdelyi I, Sreter T, Albert M, Varga I. Canine ocular onchocercosis in Hungary. Vet Parasitol. 2001;97:243–249. doi: 10.1016/s0304-4017(01)00397-1. [DOI] [PubMed] [Google Scholar]
- 6.Eberhard ML, Ortega Y, Dial S, Schiller CA, Sears AW, Greiner E. Ocular Onchocerca infections in two dogs in western United States. Vet Parasitol. 2000;90:333–338. doi: 10.1016/s0304-4017(00)00252-1. [DOI] [PubMed] [Google Scholar]
- 7.Orihel TC, Ash LR, Holshuh HJ, Santenelli S. Onchocerciasis in a California dog. Am J Trop Med Hyg. 1991;44:513–517. doi: 10.4269/ajtmh.1991.44.513. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner CH, Dick EJ, Jr, Meininger AC, Lozano-Alarcon F, Jackson P. Onchocerciasis in two dogs. J Am Vet Med Assoc. 1993;203:828–830. [PubMed] [Google Scholar]
- 9.Eberhard ML, Ostovar GA, Chundu K, et al. Zoonotic Onchocerca lupi infection in a 22-month-old child in Arizona: first report in the United States and a review of the literature. Am J Trop Med Hyg. 2013;88:601–605. doi: 10.4269/ajtmh.12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pampiglione S, Vakalis N, Lyssimachou A, Kouppari G, Orihel TC. Subconjunctival zoonotic Onchocerca in an Albanian man. Ann Trop Med Parasitol. 2001;95:827–832. doi: 10.1080/00034980120111163. [DOI] [PubMed] [Google Scholar]
- 11.Otranto D, Sakru N, Testini G, et al. Case report: First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am J Trop Med Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otranto D, Dantas-Torres F, Cebeci Z, et al. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasit Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilhan HD, Yaman A, Morishima Y, et al. Onchocerca lupi infection in Turkey: a unique case of a rare human parasite. Acta parasitol. 2013;58:384–388. doi: 10.2478/s11686-013-0152-8. [DOI] [PubMed] [Google Scholar]
- 14.Mowlavi G, Farzbod F, Kheirkhah A, Mobedi I, Bowman DD, Naddaf SR. Human ocular onchocerciasis caused by Onchocerca lupi (Spirurida, Onchocercidae) in Iran. J Helminthol. 2014;88:250–255. doi: 10.1017/S0022149X13000060. [DOI] [PubMed] [Google Scholar]
- 15.Bergua A, Hohberger B, Held J, Muntau B, Tannich E, Tappe D. Human case of Onchocerca lupi infection, Germany, August 2014. Euro Surveill. 2015:20. doi: 10.2807/1560-7917.es2015.20.16.21099. [DOI] [PubMed] [Google Scholar]
- 16.Sreter T, Szell Z. Onchocercosis: a newly recognized disease in dogs. Vet Parasitol. 2008;151:1–13. doi: 10.1016/j.vetpar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Labelle AL, Daniels JB, Dix M, Labelle P. Onchocerca lupi causing ocular disease in two cats. Vet Ophthalmol. 2011;14(suppl 1):105–110. doi: 10.1111/j.1463-5224.2011.00911.x. [DOI] [PubMed] [Google Scholar]
- 18.Otranto D, Giannelli A, Scotty Trumble N, et al. Clinical case presentation and a review of the literature of canine onchocercosis by Onchocerca lupi in the United States. Parasit Vectors. 2015;8:699. doi: 10.1186/s13071-015-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnham G. Onchocerciasis. Lancet. 1998;351:1341–1346. doi: 10.1016/S0140-6736(97)12450-3. [DOI] [PubMed] [Google Scholar]
- 20.Dudley RW, Smith C, Dishop M, Mirsky D, Handler MH, Rao S. A cervical spine mass caused by Onchocerca lupi. Lancet. 2015;386:1372. doi: 10.1016/S0140-6736(14)62255-8. [DOI] [PubMed] [Google Scholar]
- 21.Otranto D, Dantas-Torres F, Giannelli A, et al. Cutaneous distribution and circadian rhythm of Onchocerca lupi microfilariae in dogs. PLoS Negl Trop Dis. 2013;7:e2585. doi: 10.1371/journal.pntd.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan HK, Bolcen S, Kubofcik J, et al. Isolation of Onchocerca lupi in Dogs and Black Flies, California, USA. Emerg Infect Dis. 2015;21:789–796. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannelli A, Cantacessi C, Graves P, et al. A preliminary investigation of serological tools for the detection of Onchocerca lupi infection in dogs. Parasitol Res. 2014;113:1989–1991. doi: 10.1007/s00436-014-3844-6. [DOI] [PubMed] [Google Scholar]
- 24.Basanez MG, Pion SD, Boakes E, Filipe JA, Churcher TS, Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 25.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg. 2005;73:1159–1161. [PubMed] [Google Scholar]
- 26.Hoerauf A, Specht S, Buttner M, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol. 2008;197:295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert J, Nfon CK, Makepeace BL, et al. Antibiotic chemotherapy of onchocerciasis: in a bovine model, killing of adult parasites requires a sustained depletion of endosymbiotic bacteria (Wolbachia species) J Infect Dis. 2005;192:1483–1493. doi: 10.1086/462426. [DOI] [PubMed] [Google Scholar]
- 28.Egyed Z, Sreter T, Szell Z, Nyiro G, Marialigeti K, Varga I. Molecular phylogenetic analysis of Onchocerca lupi and its Wolbachia endosymbiont. Vet Parasitol. 2002;108:153–161. doi: 10.1016/s0304-4017(02)00186-3. [DOI] [PubMed] [Google Scholar]
- 29.Grandi G, Quintavalla C, Mavropoulou A, et al. A combination of doxycycline and ivermectin is adulticidal in dogs with naturally acquired heartworm disease (Dirofilaria immitis) Vet Parasitol. 2010;169:347–351. doi: 10.1016/j.vetpar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Giannelli A, Ramos RA, Traversa D, et al. Treatment of Dirofilaria repens microfilariaemia with a combination of doxycycline hyclate and ivermectin. Vet Parasitol. 2013;197:702–704. doi: 10.1016/j.vetpar.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Bazzocchi C, Mortarino M, Grandi G, et al. Combined ivermectin and doxycycline treatment has microfilaricidal and adulticidal activity against Dirofilaria immitis in experimentally infected dogs. Int J Parasitol. 2008;38:1401–1410. doi: 10.1016/j.ijpara.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Turner JD, Tendongfor N, Esum M, et al. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial. PLoS Negl Trop Dis. 2010;4:e660. doi: 10.1371/journal.pntd.0000660. [DOI] [PMC free article] [PubMed] [Google Scholar]