Abstract
Background and Purpose
Reduction of CT tube current is an effective strategy to minimize radiation load. However, tube current is also a major determinant of image quality. We investigated the impact of CTA tube current on spot sign detection and diagnostic performance for intracerebral hemorrhage expansion.
Methods
We retrospectively analyzed a prospectively collected cohort of consecutive patients with primary intracerebral hemorrhage from January 2001 to April 2015 who underwent CTA.The study population was divided in two groups according to the median CTA tube current level: low current (<350 milliampere) versus high current (≥350 milliampere).CTA first pass readings for spot sign presence were independently analyzed by two readers. Baseline and follow-up hematoma volumes were assessed by semi-automated computer-assisted volumetric analysis. Sensitivity, specificity, positive and negative predictive values and accuracy of spot sign in predicting hematoma expansion were calculated.
Results
709 subjects were included (288 and 421 in the low and high current group respectively).A higher proportion of low current scans identified at least one spot sign (20.8% versus 14.7%, p=0.034) but hematoma expansion frequency was similar in the two groups (18.4% versus 16.2%, p=0.434). Sensitivity, positive and negative predictive values were not significantly different between the two groups. Conversely, high current scans showed superior specificity (91% versus 84%, p=0.015) and overall accuracy (84% versus 77%, p=0.038).
Conclusions
CTA obtained at high levels of tube current showed better diagnostic accuracy for prediction of hematoma expansion using spot sign. These findings may have implications for future studies using the CTA spot sign to predict hematoma expansion for clinical trials.
Introduction
The CTA spot sign is a validated predictor of expansion in intracerebral hemorrhage (ICH) 1,2 but the optimal acquisition protocol for spot sign identification is still unknown. There is great heterogeneity in CTA imaging parameters across centers, especially in CTA tube current, with reported milliampere (mA) values ranging from 140 to 770 3-7. Furthermore, CT is an important source of radiation exposure8 and concerns remain regarding minimization of radiation delivery to acute stroke patients 9. Tube current reduction is a common and effective strategy to minimize the global radiation exposure 10. However, this parameter is also a major determinant of image noise and excessive reduction of the tube current level might negatively affect image quality 11. Defining the optimal CTA technical setting that predicts hematoma expansion might provide useful information for future clinical trials involving ICH patients. The main aim of our study was therefore to investigate the influence of different CTA tube current levels on spot sign detection and accuracy in predicting ICH expansion.
Methods
Patient selection
Institutional Review Board (IRB) approval was received for all aspects of our study and all the procedures comply with the Health Insurance Portability and Accountability Act. Informed written or verbal consent was obtained by patients or family members or waived by the IRB. We performed a single center, retrospective analysis of a previously described prospectively collected cohort of consecutive patients with primary ICH 12,13.
Patients were included if they presented from January 2001 to April 2015 with primary ICH and underwent CTA within 48 hours from symptom onset and follow-up noncontrast CT scan (NCCT). Patient exclusion criteria were the presence of (1) a vascular lesion or neoplastic lesion determined or suspected to be the cause of the ICH; 2) surgical evacuation of the hematoma; 3) traumatic intracranial bleeding 4) absence of thin slice axial CTA images (0.625 to 1.25 mm slice thickness); 5) unknown CTA acquisition protocol.
Both CTA tube current and voltage are important determinants of image quality 11. However, while there is great variability in the reported current values for CTA acquisition, this is not the case for voltage 3-7. Indeed, in our cohort and in most of the previous spot sign studies as well, the majority of CTA images for spot sign detection were acquired at a tube voltage level equal or above 120 kVp 3-7. For this reason, we decided to focus our analysis on the effects of tube current on diagnostic performance. Therefore, patients with CTAs obtained at low tube voltage level (< 120 kVp) were excluded from the final analysis.
Clinical Variables
Clinical information was collected from patients, families, or the medical record, and included age, sex, history of hypertension, treatment with antithrombotic medications including antiplatelet drugs or anticoagulant therapy. Time from symptom onset to baseline NCCT and CTA was also collected.
Image acquisition
Axial NCCT examinations were obtained with 5-mm slice thickness reconstruction. CTA was performed as part of standard clinical care by scanning from the base of the skull base to the vertex using an axial technique, 0.5 section pitch, 1.25-mm collimation, kVp 120 – 140. Prior publications of an overlapping cohort described that CTA scans at our institution were typically acquired at either 235 or 350 mA14,15. On detailed review we found that a wide range of mA (80 to 630) was used in clinical practice. Intravenous iodinated contrast material (65 to 85 mL), was administered by power injector with an infusion rate of 4-5 mL/s with Smart-Prep, a semiautomatic contrast bolus triggering technique. The contrast materials used were IsoVue 370 and IsoVue 300 (iopamidal, Bracco Diagnostics Inc, Milan, Italy). Volumetric Computed Tomography Dose Index (CTDI-vol) ranged from 34.7 to 89.4 mGy (mean 60.9, SD 16.6) and Dose-Length Product (DLP) ranged from 628.7 to 3763.4 mGy–cm (mean 1923.6, SD 957.5).
Image analysis
The subjects included in the study were divided into two groups: low current (< 350 mA, LmA) and high current (≥ 350 mA, HmA) scans. This cutoff was determined according to the median mA value. Illustrative spot sign positive CTA images acquired at LmA vs HmA are shown in figure 1. Baseline NCCT scans were reviewed to determine the ICH location (deep, lobar or infratentorial) and presence of associated intraventricular hemorrhage (IVH). Baseline and follow-up ICH volumes were calculated with semi-automated computer-assisted volumetric analysis (Analyze Direct 11.0 software) and hematoma expansion was defined a priori as a total volume increase greater than 6 mL or a relative volume increase greater than 30% from the baseline volume as previously described 5,16. For spot sign identification, first pass CTA images were independently reviewed by two experienced readers (AM, MJ), blinded to CTA acquisition protocol, clinical information, and results of the follow-up NCCT. Any disagreement in reader interpretation was adjudicated by consensus agreement, under the supervision of an expert neuroradiologist (JMR). Axial CTA source images were reviewed in “Spot Windows” (width 200, level 110) as previously described using the following radiological criteria for spot sign identification: (1) ≥1 focus of contrast pooling within the ICH, (2) with an attenuation ≥120 Hounsfield units (HU), (3) discontinuous from normal or abnormal vasculature adjacent to the hematoma, (4) of any size and morphology 16.
Figure 1.
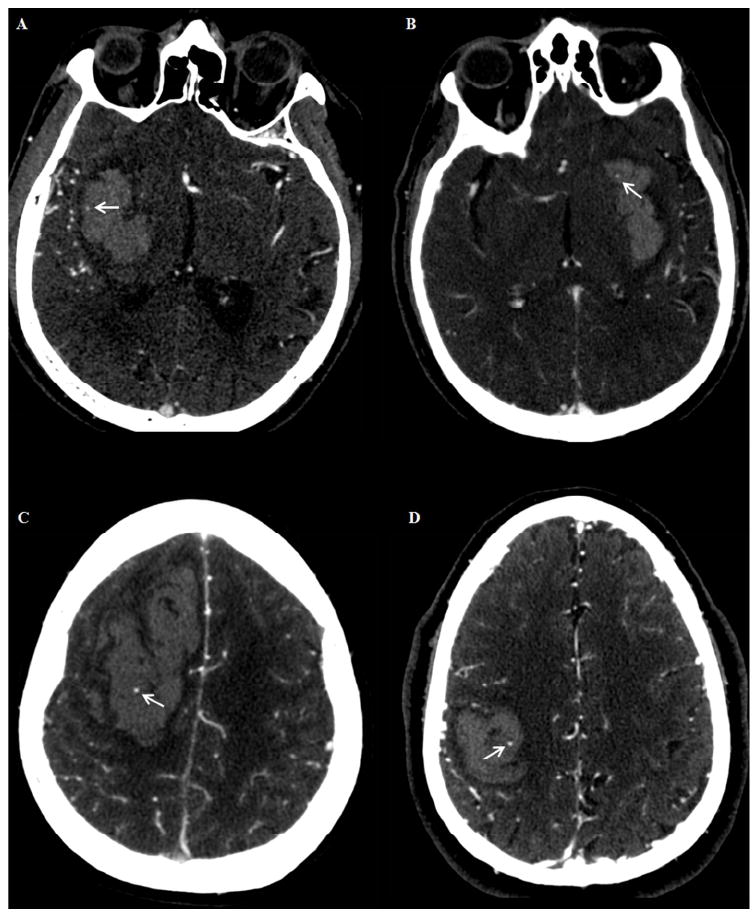
Appearance of the spot sign (arrows) on CTA images obtained at low tube current (A, 170 mA; B, 235 mA) versus high tube current (C, 350 mA; D, 350 mA). All the images were acquired on the same scanner at 120 kVp.
Statistical Analysis
All statistical analyses were performed with SPSS v. 21, 2012 (www.spss.com). Discrete variables are summarized as count (%). Normally distributed continuous variables are summarized as mean (SD) while continuous variables with non-normal distribution are expressed as median (interquartile range, IQR). Differences in the two study groups were examined with χ2 test for comparison between categorical variables, t-test for continuous variables with normal distribution and Mann–Whitney U test for continuous variables with non-normal distribution. Inter-rater and intra-rater reliability for the identification of any spot sign were determined using the Cohen’s kappa statistic. Subsequently, we calculated and compared sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for hematoma expansion. All 95 % confidence intervals were obtained using exact binomial methods. Comparison of the sensitivity, specificity, PPV, NPV and accuracy percentages between LmA and High mA was performed using the χ2 test. P value<0.05 was considered statistically significant.
Results
A total of 2381 consecutive patients with primary ICH were screened. After application of the eligibility and exclusion criteria 709 subjects were available for the analyses (Figure 2). The number of patients included in the LmA (<350) and HmA (≥350) group was 288, and 421 respectively. The baseline characteristics of the study population are listed in Table 1. Hematoma expansion occurred in 121/709 (17.1%) subjects and at least one spot sign was detected in 122/709 (17.2%) scans. Inter-rater and intra-rater reliability measures for spot sign detection were excellent (k = 0.85 and k > 0.90 respectively). Median time from symptom onset to CTA was 5 (IQR 3 - 10) hours. Table 2 illustrates the comparison between LmA and HmA demographic, clinical and imaging characteristics. We observed a higher number of spot sign positive scans in the LmA group compared with the HmA group (60/288, 20.8% versus 62/421, 14.7 %, p=0.038) while no differences were noted in the frequency of hematoma expansion (53/288, 18.4% versus 68/421, 16.2%, p = 0.434).
Figure 2.
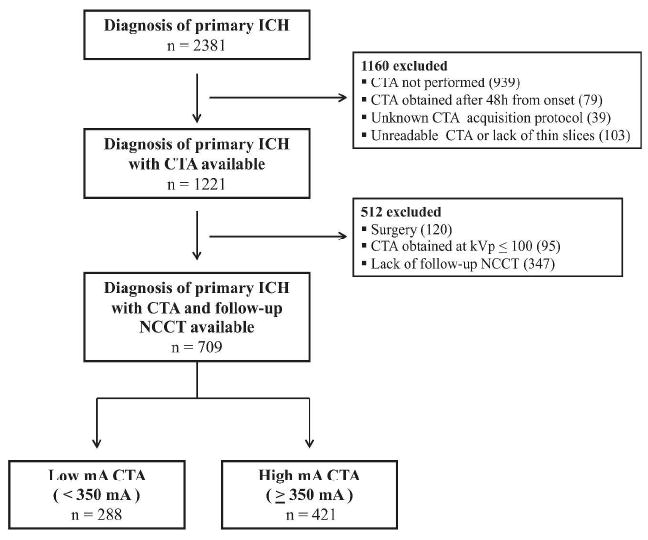
Cohort selection flowchart. ICH, intracerebral hemorrhage; CTA, computed tomography angiography; NCCT, non-contrast computed tomography;mA, milliampere; kVp, kilovoltage peak.
Table 1.
Baseline Study Cohort Characteristics (n = 709)
| Parameters | |
|---|---|
| Age, median (IQR), years | 74 (62 – 82) |
| Sex, male, n (%) | 396 (55.9) |
| History of hypertension, n (%) | 553 (78.0) |
| Antiplatelet treatment, n (%) | 314 (44.3) |
| Anticoagulant treatment, n (%) | 132 (18.6) |
| ICH Location | |
| Lobar | 346 (48.8) |
| Deep | 299 (42.2) |
| Infratentorial | 64 (9.0) |
| IVH presence, n (%) | 312 (44.0) |
| Baseline ICH volume, median (IQR), mL | 17 (6 – 39) |
| Baseline IVH volume, median (IQR), mL | 0 (0 – 4) |
| Time from symptom onset to CTA, median (IQR), h | 5 (3 – 10) |
| CTA spot sign presence, n (%) | 122 (17.2) |
| ICH expansion, n (%) | 121 (17.1) |
CTA indicates computed tomography angiography; IQR, interquartile range, ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage.
Table 2.
Patients’ characteristics stratified by tube current
| LmA (n = 288) | HmA (n = 421) | p value | |
|---|---|---|---|
| Age, median (IQR), y | 74 (62 – 82) | 73 (62 – 82) | 0.904 |
| Sex, male, n (%) | 163 (56.6) | 233 (55.3) | 0.741 |
| History of hypertension, n (%) | 219 (76.0) | 334 (79.3) | 0.299 |
| Antiplatelet treatment, n (%) | 123 (42.7) | 191 (45.4) | 0.484 |
| Anticoagulant treatment, n (%) | 49 (17.0) | 83 (19.7) | 0.364 |
| Admission INR, median (IQR) | 1.03 (1.00 – 1.20) | 1.10 (1.00-1.20) | 0.331 |
| ICH Location | 0.227 | ||
| Lobar | 130 (45.1) | 216 (51.3) | |
| Deep | 128 (44.4) | 171 (40.6) | |
| Infratentorial | 30 (10.4) | 34 (8.1) | |
| IVH presence, n (%) | 138 (47.9) | 174 (41.3) | 0.083 |
| Baseline ICH volume, median (IQR), mL | 18 (6 – 46) | 15 (6 – 36) | 0.018 |
| Baseline IVH volume, median (IQR), mL | 0 (0 – 7) | 0 (0 – 3) | 0.074 |
| Time from symptom onset to CTA, median (IQR), h | 5 (3 – 10) | 5 (3 – 10) | 0.342 |
| CTA spot sign presence, n (%) | 60 (20.8) | 62 (14.7) | 0.034 |
| ICH expansion, n (%) | 53 (18.4) | 68 (16.2) | 0.434 |
| CTDI-vol, mean ± SD, mGy | 43.3 ± 8.9 | 71.4 ± 9.8 | <0.001 |
| DLP, mean ± SD, mGy - cm | 1258.3 ± 618.3 | 2342.1 ± 864.7 | <0.001 |
ICH, Intracerebral hemorrhage; IQR, interquartile range; IVH, Intraventricular hemorrhage; CTA, computed tomography angiography. CTDI-vol, Volumetric Computed Tomography Dose Index; DLP, Dose-Length Product DLP.
The diagnostic performance of spot sign in predicting ICH expansion stratified by tube current levels is shown in table 3. The LmA setting was associated with a higher frequency of false positive cases (36/288, 12.5% versus 31/421, 7.4%, p = 0.022) while the false negative proportion was similar between the two groups (29/288, 10.1% versus 37/421, 8.9%, p = 0.564). At HmA level, spot sign showed significantly superior specificity than at LmA level (91% versus 84%, p=0.015). The overall accuracy was superior in HmA scans (84% versus 77%, p = 0.038).
Table 3.
Spot Sign prediction of hematoma expansion
| Variable | LmA (n = 288) | HmA (n = 421) | p value |
|---|---|---|---|
| Sensitivity (95% CI) | 0.45 (0.32 - 0.59) | 0.45 (0.34 - 0.58) | 0.973 |
| Specificity (95% CI) | 0.84 (0.79 - 0.89) | 0.91 (0.88 - 0.94) | 0.015 |
| Positive predictive value (95% CI) | 0.40 (0.28 - 0.53) | 0.50 (0.37 - 0.63) | 0.267 |
| Negative predictive value (95% CI) | 0.87 (0.82 - 0.91) | 0.90 (0.86 - 0.93) | 0.367 |
| Accuracy | 0.77 | 0.84 | 0.038 |
Significant expansion was defined as >30 % or >6 mL increase from baseline hematoma volume
As there are multiple definitions of ICH expansion, we repeated the analyses using another commonly used definition, absolute growth > 12.5 mL or relative growth > 33 % 17. We confirmed the superior specificity (91% versus 83%, p=0.004) and accuracy (84% versus 76%, p=0.008) of HmA scans, with no significant differences in sensitivity, PPV and NPV (all p values > 0.1).
Discussion
This study investigated the relationship between CTA tube current, spot sign detection and diagnostic accuracy for predicting ICH expansion. We found that the tube current level had a significant influence on spot sign detection and diagnostic accuracy of CTA spot sign. In particular CTA acquired with high tube current levels (≥ 350 mA) showed higher specificity.
Our results are consistent with previous findings on the relationship between CT tube current, radiation delivery and image quality. CTA is a commonly available tool for the emergency workup of ICH patients and additional radiation exposure is one of the main drawbacks of this technique18 . The CT tube current is directly associated with the radiation exposure, in a linear, dose-dependent relationship11,19 and, as expected, we observed a significantly higher radiation dose in the HmA group. Decreasing the CT tube current also results in increased image noise and inferior quality of CTA images 19,20.
In our study the presence of at least one spot sign was significantly more frequent in the LmA group. Baseline hematoma volume is a strong predictor of spot sign presence 21 and hematoma expansion13. Therefore this finding may simply reflect that patients in the LmA group had higher baseline ICH volumes. Another possible explanation is the well known inverse relationship between image noise and CT tube current 10,11,22. Severe background noise in the LmA group might lead to detection of false spot signs because of increased graininess of the scan. Indeed, despite the higher rate of spot sign detection, the LmA setting was not associated with a significant gain in sensitivity comparing the two current settings. Conversely, the specificity and overall diagnostic accuracy was significantly better in the HmA group. The observed difference between the diagnostic performances of the two current settings may be driven by the higher frequency of false positive cases in the LmA group. In other words, the fact that sensitivity was not affected suggests that if contrast extravasates into the hematoma, it can be successfully detected even with low current imaging. However, higher current may optimize the ability to distinguish such contrast from natural heterogeneity of the hematoma and avoid the detection of false spot signs. It may be that dual energy CT can help addressing this issue by distinguishing contrast from blood in a more robust way 23,24. Several CTA acquisition parameters can be varied in order to reduce in the radiation dose without compromising the image quality 25. Our results suggest that if one goal of CTA is to detect spot signs, such dose reduction comes at a cost.
CTA is widely used in the workup of ICH 26 and the CTA spot sign is a promising marker for early identification of ICH patients with the greatest opportunity to benefit from anti-expansion therapies27,28. Patients with a false positive spot sign may therefore be exposed to potentially harmful anti-expansion hemostatic treatments, despite having a low probability of hematoma expansion. The only multicenter study focused on spot sign as a predictor of hematoma expansion1 had an inferior diagnostic accuracy compared to single center studies 5,16,17. Heterogeneity in the CTA acquisition protocols and image quality across various institutions might have accounted for these differences. The results of our study and the above mentioned issues suggest the need to develop a standardized CTA acquisition protocol to optimize spot sign detection in ICH patients.
Some limitations of the present study should be mentioned. First, this was a non-randomized, single center, prospective observational study with retrospective analysis of the data. In addition, the number of subjects included in the LmA group was relatively small. Therefore, it is best interpreted as hypothesis generating, and the findings need to be confirmed by future studies. Second, the most relevant change in our Institution’s CTA protocol was the introduction of 90 seconds delayed CTA images. Such images are known to capture additional spot signs 29 and it may be that the influence of current on spot sign detection is different when such images are taken into account. Third, image noise and quality was not objectively measured so we can only speculate that image graininess and increased background noise are the mechanisms responsible for lower accuracy observed in the LmA group. Fourth, CTA tube current is not the only determinant of image quality and other factors not considered in this study, such as different scanner models and contrast types, may also influence diagnostic accuracy. Finally, our study was designed to explore the possibility that excessive tube current lowering reduces the diagnostic accuracy of spot sign, rather than to define the optimal balance between radiation exposure, image quality and clinical outcome. Therefore we are not able to evaluate the clinical impact of improving CTA specificity and accuracy.
Conclusion
CTA acquisition protocol influences spot sign detection and accuracy in predicting hematoma expansion. If confirmed, our findings may have important implications for future studies using the CTA spot sign to predict hematoma expansion. Further investigations are needed to establish the optimal balance between radiation delivery, image quality and diagnostic performance.
Acknowledgments
Sources of fundings
This study was supported by the following awards from the NINDS: 5R01NS073344, K23AG02872605, K23 NS086873, R01NS059727.
None of the funding entities had any involvement in study design; data collection, analysis, and interpretation; writing of the manuscript; or decision to submit the study for publication.
Abbreviations
- ICH
Intracerebral hemorrhage
- IVH
Intraventricular hemorrhage
- mA
Milliampere
- LmA
Low milliampere
- HmA
High milliampere
- kVp
Kilovoltage peak
- PPV
Positive predictive value
- NPV
Negative predictive value
- CTDI-vol
Volumetric Computed Tomography Dose Index
- DLP
Dose-Length Product DLP
Footnotes
DISCLOSURES: Andrea Morotti—RELATED: Grant: NINDS award: 5R01NS073344.* Anastasia Vashkevich—RELATED: Grant: NIH.* Christopher Anderson—RELATED: Grant: National Institutes of Health (NINDS K23 NS086873)*; UNRELATED: Grants/Grants Pending: National Institutes of Health (NINDS K23 NS086873).* Anand Viswanathan—RELATED: Grant: NIH (R01AG047975-02, K23 AG028726-05)*; UNRELATED: Consultancy: Roche Pharmaceuticals (served on Data Safety Monitoring Board as a consultant). Jonathan Rosand—RELATED: Grant: NIH*; UNRELATED: Grants/Grants Pending: NIH.* Joshua Goldstein—RELATED: Grant: NINDs*; Support for Travel to Meetings for the Study or Other Purposes: NINDS*; UNRELATED: Consultancy: CSL Behring, Boehringer Ingelheim; Grants/Grants Pending: NINDS.*
*money paid to institution
Contributor Information
Andrea Morotti, Department of Clinical and Experimental Sciences, Neurology Clinic, University of Brescia, Italy; Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Javier M. Romero, Neuroradiology Service, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Michael Jessel, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
H.Bart Brouwers, Department of Neurosurgery, Brain Center Rudolf Magnus University Medical Center, Utrecht, the Netherlands.
Rajiv Gupta, Neuroradiology Service, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Kristin Schwab, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Anastasia Vashkevich, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Christopher D. Anderson, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
M. Edip Gurol, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Anand Viswanathan, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School,Boston, MA.
Steven M. Greenberg, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Jonathan Rosand, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Division of Neurocritical Care and Emergency Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Joshua N. Goldstein, Hemorrhagic Stroke Research Group, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Division of Neurocritical Care and Emergency Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
References
- 1.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 2.Brouwers HB, Goldstein JN, Romero JM, et al. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage a review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havsteen I, Ovesen C, Christensen AF, et al. Showing no spot sign is a strong predictor of independent living after intracerebral haemorrhage. Cerebrovasc Dis. 2014;37:164–170. doi: 10.1159/000357397. [DOI] [PubMed] [Google Scholar]
- 4.Huynh TJ, Demchuk AM, Dowlatshahi D, et al. Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: Analysis from the PREDICT study. Stroke. 2013;44:972–977. doi: 10.1161/STROKEAHA.111.000410. [DOI] [PubMed] [Google Scholar]
- 5.Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 6.Romero JM, Bart Brouwers H, Lu J, et al. Prospective validation of the computed tomographic angiography spot sign score for Intracerebral hemorrhage. Stroke. 2013;44:3097–3102. doi: 10.1161/STROKEAHA.113.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 9.Mnyusiwalla A, Aviv RI, Symons SP. Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiology. 2009;51:635–640. doi: 10.1007/s00234-009-0543-6. [DOI] [PubMed] [Google Scholar]
- 10.Cohnen M, Fischer H, Hamacher J, Lins E, Kotter R, Modder U. CT of the head by use of reduced current and kilovoltage: Relationship between image quality and dose reduction. AJNR Am J Neuroradiol. 2000;21:1654–1660. [PMC free article] [PubMed] [Google Scholar]
- 11.Smith aB, Dillon WP, Gould R, et al. Radiation dose-reduction strategies for neuroradiology CT protocols. AJNR Am J Neuroradiol. 2007;28(9):1628–1632. doi: 10.3174/ajnr.A0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biffi A, Cortellini L, Nearnberg CM, et al. Body mass index and etiology of intracerebral hemorrhage. Stroke. 2011;42:2526–2530. doi: 10.1161/STROKEAHA.111.617225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting Hematoma Expansion After Primary Intracerebral Hemorrhage. JAMA Neurol. 2014;71:158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado Almandoz JE, Kelly HR, Schaefer PW, et al. CT angiography spot sign predicts inhospital mortality in patients with secondary intracerebral hemorrhage. J Neurointerv Surg. 2012;4:442–447. doi: 10.1136/neurintsurg-2011-010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Wang Y, Wang W, et al. Contrast extravasation on computed tomography angiography predicts clinical outcome in primary intracerebral hemorrhage: a prospective study of 139 cases. Stroke. 2011;42:3441–3446. doi: 10.1161/STROKEAHA.111.623405. [DOI] [PubMed] [Google Scholar]
- 18.Macellari F, Paciaroni M, Agnelli G, et al. Neuroimaging in intracerebral hemorrhage. Stroke. 2014;45:903–908. doi: 10.1161/STROKEAHA.113.003701. [DOI] [PubMed] [Google Scholar]
- 19.Kumamaru KK, Hoppel BE, Mather RT, et al. CT angiography: current technology and clinical use. Radiol Clin North Am. 2010;48:213–235. doi: 10.1016/j.rcl.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waaijer A, Prokop M, Velthuis BK, et al. Circle of Willis at CT angiography: dose reduction and image quality--reducing tube voltage and increasing tube current settings. Radiology. 2007;242:832–839. doi: 10.1148/radiol.2423051191. [DOI] [PubMed] [Google Scholar]
- 21.Radmanesh F, Falcone GJ, Anderson CD, et al. Risk factors for computed tomography angiography spot sign in deep and lobar intracerebral hemorrhage are shared. Stroke. 2014;45:1833–1835. doi: 10.1161/STROKEAHA.114.005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon JB, Li X, Samei E. Relating noise to image quality indicators in CT examinations with tube current modulation. AJR Am J Roentgenol. 2013;200:592–600. doi: 10.2214/AJR.12.8580. [DOI] [PubMed] [Google Scholar]
- 23.Gupta R, Phan CM, Leidecker C, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010;257:205–211. doi: 10.1148/radiol.10091806. [DOI] [PubMed] [Google Scholar]
- 24.Won S-Y, Schlunk F, Dinkel J, et al. Imaging of Contrast Medium Extravasation in Anticoagulation-Associated Intracerebral Hemorrhage With Dual-Energy Computed Tomography. Stroke. 2013;44:2883–2890. doi: 10.1161/STROKEAHA.113.001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raman SP, Mahesh M, Blasko RV, et al. CT scan parameters and radiation dose: practical advice for radiologists. J Am Coll Radiol. 2013;10:840–846. doi: 10.1016/j.jacr.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Khosravani H, Mayer SA, Demchuk A, et al. Emergency noninvasive angiography for acute intracerebral hemorrhage. AJNR Am J Neuroradiol. 2013;34:1481–1487. doi: 10.3174/ajnr.A3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein JN, Brouwers HB, Romero J, et al. SCORE-IT: the Spot Sign score in restricting ICH growth—an Atach-II ancillary study. J Vasc Interv Neurol. 2012;5:20–25. [PMC free article] [PubMed] [Google Scholar]
- 28.Meretoja A, Churilov L, Campbell BCV, et al. The spot sign and tranexamic acid on preventing ICH growth--AUStralasia Trial (STOP-AUST): protocol of a phase II randomized, placebo-controlled, double-blind, multicenter trial. Int J Stroke. 2014;9:519–524. doi: 10.1111/ijs.12132. [DOI] [PubMed] [Google Scholar]
- 29.Ciura Va, Brouwers HB, Pizzolato R, et al. Spot Sign on 90-Second Delayed Computed Tomography Angiography Improves Sensitivity for Hematoma Expansion and Mortality: Prospective Study. Stroke. 2014;45:3293–3297. doi: 10.1161/STROKEAHA.114.005570. [DOI] [PMC free article] [PubMed] [Google Scholar]


