Abstract
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide affecting over one-third of the population in the U.S. It has been associated with obesity, type 2 diabetes, hyperlipidemia, and insulin resistance and is initiated by the accumulation of triglycerides in hepatocytes. Isolated hepatic steatosis (IHS) remains a benign process, while a subset develops superimposed inflammatory activity and progression to nonalcoholic steatohepatitis (NASH) with or without fibrosis. However, the molecular mechanisms underlying NAFLD progression are not completely understood. Liver biopsy is still required to differentiate IHS from NASH as easily accessible noninvasive biomarkers are lacking. In terms of treatments for NASH, pioglitazone, vitamin E, and obeticholic acid have shown some benefit. All of these agents have potential complications associated with long-term use. Nowadays, a complex hypothesis suggests that multiple parallel hits are involved in NASH development. However, the ‘key switch’ between IHS and NASH remains to be discovered. We have recently shown that knocking out enzymes involved in S-adenosylmethionine (SAMe) metabolism, the main biological methyl donor in humans that is abundant in the liver, will lead to NASH development in mice. This could be due to the fact that a normal SAMe level is required to establish the proper ratio of phosphatidylethanolamine to phosphatidylcholine that has been found to be important in NAFLD progression. New data from humans have also suggested that these enzymes play a role in the pathogenesis of NAFLD and that some of SAMe cycle metabolites may serve as noninvasive biomarkers of NASH. In this review, we discuss the evidence of the role of SAMe in animal models and humans with NAFLD and how studying this area may lead to the discovery of new noninvasive biomarkers and possibly personalized treatment for NASH.
Keywords: SAMe, NASH, biomarkers, treatment, hepatocellular carcinoma
Introduction
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of diseases ranging from isolated hepatic steatosis (IHS) to nonalcoholic steatohepatitis (NASH), the progressive form of fatty liver disease associated with inflammation and cellular injury, which can lead to cirrhosis and liver-related mortality.1,2 NAFLD has become by far the most common chronic liver disease (CLD) in the United States, accounting for a steadily increasing percentage of CLD cases over the last quarter century.3 NAFLD accounted for 46.8% of CLD cases from 1988 to 1994; 62.8% from 1994 to 2004; and 75.1% from 2005 to 2008.3 These elevations occurred along with steady increases during the same time periods in obesity (21.7%, 30.0%, and 33.2%), visceral obesity (35.2%, 48.2%, and 51.4%), type II diabetes (5.6%, 7.9%, and 9.1%), and insulin resistance (23.3%, 32.5%, and 35.0%).3
Worldwide, results from NAFLD prevalence studies have varied substantially due to varying definitions, diagnostic methods used, and differences in the studied populations.4 According to a 2014 report by the World Gastroenterology Organization, prevalence estimates in the general population of Europe and the Middle East are 20–30%, in the Far East 15%, and in Pakistan 18%, with substantially higher prevalence in Western countries in populations with obesity or diabetes (75%) and with morbid obesity (90–95%), as well as in obese populations worldwide (40–90%).4 A prospective observational study of 4401 apparently healthy Japanese men and women found a baseline prevalence of 18%, and showed that people with metabolic syndrome at baseline were more likely to develop the disease during follow-up.5 Recent community-based studies from other Asian countries have reported overall NAFLD prevalence of 16.1% in Korea,6 15% in China (with prevalence approximately doubling in the last decade),7 11.5% in a rural population in Taiwan,8 23.1% in an urban population in Taiwan,9 32% in urban southern India,10 32.6% in urban Sri Lanka,11 and 27.3% in Hong Kong.12
Although studies based on National Health and Nutrition Examination Survey (NHANES) III data collected from 1988 to 1994 estimated that the prevalence of NAFLD in the United States ranged from 2.8%13 to 5.4%14 of the population, studies published in the last 10 years have reported substantially higher prevalence estimates. In a large (n = 2287), ethnically diverse (32.1% Caucasian, 48.3% African American, and 17.5% Hispanic), probability-based population sample from Dallas, Texas, the reported overall prevalence of hepatic steatosis was 34%.15 Substantial differences in prevalence were present among the three major ethnic groups (45% in Hispanics; 33% in Caucasians; 24% in African Americans). In Caucasians only, men had an approximately twofold higher prevalence of hepatic steatosis than women. A prospective study of adult outpatients without known liver disease recruited from Brooke Army Medical Center from January 2007 to March 2010 reported a prevalence of NAFLD of 46%, with the highest prevalence in Hispanics (58.3%), followed by Caucasians (44.4%) and African Americans (35.1%).1 The prevalence of NASH in this study was 12.2%. Although the frequency of obesity in this outpatient population was higher (45.4%) than the national prevalence estimates based on NHANES data collected during the same time period (33.8% in 2007–200816 and 35.9% in 2009–201017), which raises the concern that their findings do not represent the United States as a whole,18 it nevertheless indicates that the prevalence of NAFLD–NASH may be substantially higher than previously thought.
Currently, liver biopsy is required for differentiating simple steatosis from NASH. Although several biomarkers have been shown to be somewhat useful for differentiation, they have not been definitively validated and are not widely available; thus, new noninvasive biomarkers are urgently needed.19–21 There is no Food and Drug Administration (FDA)-approved treatment for NASH. Although weight loss and exercise are normally recommended, they are often difficult for patients to achieve and maintain, and new treatments are very much needed. This review summarizes current knowledge of the pathogenesis of NAFLD and NASH, available biomarkers, and treatments, and discusses the role of S-adenosylmethionine (SAMe) in these processes. Although our understanding of the role of SAMe stems largely from experimental animal models, there is accumulating evidence from human studies that supports the need for further study.
Pathogenesis of NAFLD and NASH
Hepatic fat accumulation leading to IHS
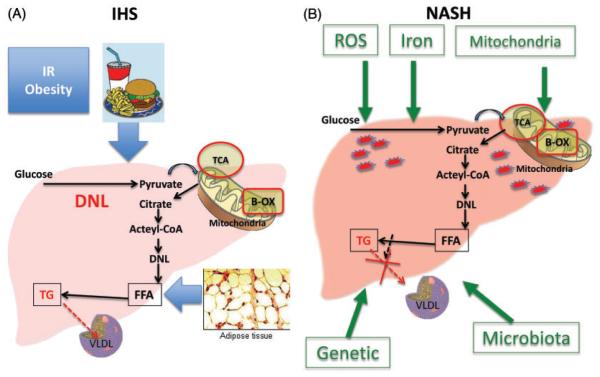
Although a number of theories have been proposed to explain the progression of NAFLD, we do not currently have a complete understanding of the mechanism(s) that underlie its pathogenesis.22–24 The generally accepted dogma in the pathogenesis of NAFLD is that liver fat accumulation and NAFLD occur when hyperinsulinemia and insulin resistance, commonly associated with obesity, lead to hepatic accumulation of triglycerides (TG), a process that usually results from an imbalance between increased free fatty acid (FFA) flux from adipose tissue to the liver, increased caloric intake, and increased de novo lipogenesis in the liver and the liver’s handling and export of the extra fat. The FFAs are usually either oxidized in the mitochondria (beta-oxidation) or esterified to TG, which in turn are either packaged as very low-density lipoproteins (VLDL) for export or are used for the production of lipids such as phospholipids (Figure 1).22,23 Factors that promote the progression of NAFLD are incompletely understood but include genetic and behavioral factors that may impair these processes.23
Figure 1.
Mechanisms involved in IHS and NASH development. Insulin resistance and obesity, increase caloric intake, increase de novo lipogenesis, increased free fatty acid (FFA) flux from adipose tissue to the liver, and impaired VLDL secretion lead to fat accumulation in the liver and HIS (A). Multiples hits are involved in the development of NASH including mitochondrial impairment, role of microbiota, iron accumulation, genetic factors, and release of reactive oxygen species (B). Abbreviations: IR, insulin resistance, DNL, de novo lipogenesis; TCA, citric acid cycle; B-OX, beta-oxidation; FFA, free fatty acids; TG, triglyceride; VLDL, very low density lipoprotein; ROS, reactive oxygen species; IHS, isolated hepatic steatosis; NASH, non-alcoholic steatohepatitis. (A color version of this figure is available in the online journal.)
Hyperglycemia also stimulates carbohydrate response element-binding protein, which in turn stimulates the liver-type pyruvate kinase (L-PK), a key enzyme in glycolysis. LPK stimulates the entry of pyruvate into the mitochondria and its conversion into citrate which forms acetyl-CoA. The acetyl-CoA enters the tricarboxylic acid cycle in the mitochondria and increases fatty acid synthesis22,25 via multiple reactions that include enzymes such as citrate lyase, acetyl-CoA carboxylase, fatty acid synthase, stearoyl-CoA desaturase-1 (SCD-1), and long-chain elongase-6.22,23 FFAs form monoglycerides, diglycerides, and eventually TG. Additionally, hyperinsulinemia activates a membrane-bound transcription factor, sterol regulatory element-binding protein-1 c (SREBP-1 c), which induces the expression of key lipogenesis genes and thus increases de novo fatty acid synthesis.26 The net result is an increased flow from FA to TG which are packaged into VLDL and then secreted into plasma.22 When the biosynthesis of TG exceeds the rate of TG secretion via VLDL, TG excess accumulates into lipid droplets in the liver resulting in steatosis. Conditions associated with a reduction of de novo lipogenesis, such as a diet rich in FA, may also result in steatosis if the rate of FA beta-oxidation cannot compensate for the increased flux of FA into the liver. Alternatively, steatosis may also originate when the packaging process of TG into VLDL particles is impaired due, for instance, to an abnormal supply of a class of phospholipids known as phosphatidylcholines (PC), which are rich in fatty acids.
In summary, TG accumulation results from an imbalance of TG synthesis, VLDL assembly and secretion, de novo lipogenesis, and FA beta-oxidation.27 This highlights the fact that NAFLD could have heterogeneous causes with one resulting from excessive de novo lipogenesis and mitochondrial exhaustion and another resulting from impaired VLDL secretion.
Progression of IHS to NASH
Steatosis develops once excessive TG are accumulated in the liver. However, to develop NASH, multiple pathways (multiple hits) are required to develop inflammation, cellular injury, and fibrosis. ‘Hits’ that may contribute include oxidative stress, iron accumulation, endotoxins, cytokines, changes in the gut–liver axis, and mitochondrial dysfunction,22,23 but these insults are thought to be secondary processes. Precisely why some patients with simple steatosis progress to NASH and others do not remains an unanswered question. Studies that investigate the key ‘switch’ pathways are needed. It is thought that lipotoxicity and increased reactive oxygen species (ROS) production are two of the main drivers of NASH development. An increase in ROS may result from some combination of iron overload, overburdened and dysfunctional mitochondria, proinflammatory cytokines, and the metabolism of FFAs via peroxisomes and cytochromes P450 (CYPs). FA catabolism in liver takes place mainly via mitochondrial beta-oxidation, a process that can lead to the generation of ROS, including superoxide, hydrogen peroxide, and hydroxyl radicals, if there is an excessive load of FA.28,29 Once the mitochondria are exhausted or if their function is impaired, FFAs are metabolized at other sites in hepatocytes, including the CYP enzymes of the smooth endoplasmic reticulum (omega-oxidation) and peroxisomes (beta-oxidation).29,30 FA oxidation at these sites also generates ROS as well as lipotoxic products, a process that occurs in the lysosomes, leading to production of proinflammatory cytokines and inflammatory status associated with cellular injury and hence NASH development.31,32 As a rule, saturated fatty acids, such as stearic and palmitic acid, are more lipotoxic than unsaturated FA. Storing FA as TG into lipid droplets may actually be protective. It is therefore the mechanism leading to TG accumulation (i.e. impaired FA oxidation and phospholipid metabolism) rather than the accumulation of TG per se that leads to liver injury.
Noninvasive biomarkers in NAFLD and NASH
Liver enzymes are usually the first clinical indication for work up of NAFLD and referral to a hepatologist. However, liver enzymes are normal in up to half of NAFLD patients.20,33–35 Other etiologies of liver disease should be excluded and radiological evidence of steatosis should be established.19,20 To distinguish NASH from IHS and stage the degree of fibrosis, liver tissue sampling via biopsy is usually needed.19,36 However, because liver biopsy is an invasive test that is associated with adverse events and sampling variability,37 it is associated with both patient dissatisfaction and, in some cases, substantial misdiagnosis and staging inaccuracies.37 Ultrasonography (US) and CT have shown low sensitivity and specificity in diagnosis and follow-up and are associated with underestimating or missing steatosis, especially when it is less than 3038–40 Although US is easy to perform, it cannot quantify fat, assess disease severity or stage, and has no role in longterm follow-up. CT scan requires radiation and has been found to be less accurate than US.41 MRI imaging techniques were promising when first utilized to diagnose NAFLD and with the evolution of MR spectroscopy (MRS)20,42 and MRI-determined proton density fat fraction (MRI-PDFF), the diagnosis of NAFLD became much more precise.43,44 While MRS remains a research method, requiring highly trained individuals and limited to a few centers, MRI-PDFF has been shown to reduce the biases seen with MRS and to correlate highly with MRS in quantifying liver fat in patients enrolled in a clinical trial for NASH,43,45 highlighting its potential role as an outcome measurement in clinical trials and its diagnostic value in clinical practice.20,46 MR elastography, and US elastography are techniques that have been shown to potentially have some promise in detecting fibrosis in NAFLD patients;47 however, more research is needed to explore clinical implications.
While imaging techniques may prove to be effective as noninvasive biomarkers, serum biomarkers are still under investigation. Although in the past neither imaging nor blood biomarkers has been shown to be reliable for distinguishing HIS from NASH,19,47 our group’s lipidomics studies have shown promising results.48,49 Biomarkers can be divided into those that help with steatosis diagnosis, those that may differentiate IHS from NASH, and those that may be useful for detecting fibrosis and staging the disease. As discussed above, liver enzymes play only a modest role in NAFLD diagnosis since they are often normal in NAFLD patients. Other scoring systems have been used to optimize IHS diagnosis including steatosis test, liver fat score, hepatic steatosis index, and fatty liver index.35 Markers that differentiate IHS from NASH are of greatest importance as imaging markers are now accurate in detecting the other two spectrums of the disease. Many scores have been developed including HAIR (includes hypertension, alanine aminotransferase [ALT] and insulin resistance), NASH test, NASH clinical scoring system, and others.35 These biomarkers have been shown to lack accuracy and include tests that are not commercially available.19,47 Although cytokeratin 18 correlates with the presence of NASH and has been shown to be promising, it lacks sensitivity to stage NASH.19 Using serum from 465 individuals with biopsy-proven NAFLD, IHS and NASH patients demonstrated distinguishing lipid serum biomarkers which accurately differentiated between IHS and NASH.49 These lipid biomarkers should be further confirmed in other cohorts and made commercially available. Noninvasive biomarkers that can distinguish steatosis from NASH and assess response to treatment will lead to significant change in our current practice and research outcomes.
Treatment of NASH
Weight loss and exercise are the measures currently recommended by the FDA for NAFLD–NASH, with at least 10% weight loss required for histological improvement.19 Unfortunately, because for many patients consistent exercise and weight loss are difficult to achieve and maintain, additional therapies are needed. Therapies studied to date that have shown some benefit are included in Table 1.50–59
Table 1.
Therapeutic agents that have shown benefits in NASH
| Therapeutic agent | Indication | Summary of benefits | Adverse effects and concerns |
|---|---|---|---|
| Metformin50,51 | No current indication | Improved aminotransferases, BMI, and insulin resistance; possible positive effect on ballooning in those who lose weight |
AEs: diarrhea, nausea, and vomiting Concerns: Contraindicated in patients with renal failure |
| Orlistat53,53 | No current indication | Two trials; one showed improvement in ALT, BMI, and hepatic steatosis based on ultrasound; the other failed to show this |
AEs: fatty diarrhea, abdominal pain–discomfort flatulence and fecal urgency |
| Pentoxifylline57–59 | No current indication | One trial showed improvements in steatosis and lobular inflammation, but not in ballooning; another showed improvements in steatosis and ballooning |
AEs: nausea, vomiting |
| Ursodeoxycholic acid54,55 | No current indication | Borderline benefits in aminotrans- ferases; only one trial showed histological benefit |
AEs: GI upset, headache, and dizziness |
| Pioglitazone56 | Biopsy proven NASH with or without DM |
Improved histology of NASH; one trial showed significant reductions in steatosis, lobular inflammation, and aminotransferases |
AEs: weight gain, GI upset, edema, and fatigue Concerns: contraindicated in patients with bladder cancer and postmenopausal women with increase risk of fracture, long-term efficacy, and safety |
| Vitamin E56 | Biopsy proven NASH without DM and without cirrhosis |
Improved histology of NASH; one trial showed significant reductions in steatosis, lobular inflammation, and aminotransferases, and significant improvement in ballooning |
Concerns: Long-term efficacy and safety, lack of studies in patients with DM, cirrhosis, and posttransplant |
| Obeticholic acid63 | (Not currently available) Biopsy proven NASH without cirrhosis |
Improved histology of NASH | AEs: itching (23%), increased total cholesterol and LDL cholesterol, and a modest decrease in HDL cholesterol Concerns: Dyslipidemia, long-term efficacy and safety, lack of studies in patients with cirrhosis, and posttransplant |
Note: BMI, body mass index; ALT, alanine aminotransferase; AEs, adverse effects; DM, diabetes mellitus; GI, gastrointestinal; NASH, nonalcoholic steatohepatitis; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Although one small trial in which 26 NASH patients completed 48 weeks of metformin therapy (2000 mg/day) showed improvements in liver histology and ALT levels in 30% of patients, probably due to its effects in causing weight loss,50 other studies failed to show such benefits. In one study, neither vitamin E (800 international units [IU] daily) nor metformin (1000 mg daily) was superior to placebo in attaining the primary outcome of sustained reduction in ALT level in patients with pediatric NAFLD.51 A 2014 meta-analysis and review concluded that although metformin improves AST, ALT, insulin resistance, and body mass index to some extent, it does not yield histological improvement (steatosis, inflammation, hepatocellular ballooning, or fibrosis) in NAFLD patients.60
Treatment with pentoxifylline has been assessed in small trials.54,55,61 In one study with adults with NASH, 1200 mg daily for one year resulted in statistically significant improvement compared to placebo in steatosis and lobular inflammation.55 In another trial that compared the same 1200 mg dose to placebo, steatosis, and cellular ballooning improved in the pentoxifylline group (P < 0.05); however, pentoxifylline failed to reduce transaminases compared to placebo and did not positively affect any of the metabolic markers postulated to contribute to NASH.54 A study showing that pentoxifylline was associated with a significant reduction of oxidized fatty acids supports the idea that its beneficial effects in patients with NASH may be mediated through decreasing lipid oxidation.61
Vitamin E has shown biochemical and histological benefits in the PIVEN trial for treatment of NASH in which the primary outcome was an improvement in histologic features of NASH defined as improvement by 1 or more points in the hepatocellular ballooning score; no increase in the fibrosis score; and either a decrease in the NAFLD activity score (in which steatosis is scored 0–3, ballooning 0–2, and lobular inflammation 0–3) to a score of 3 or less or a decrease in the activity score of at least 2 points, with at least a 1-point decrease in either the lobular inflammation or steatosis score.56 In this trial in which NASH patients received pioglitazone at a dose of 30 mg daily (80 subjects), vitamin E at a dose of 800 IU daily (84 subjects), or placebo (83 subjects) for 96 weeks, vitamin E therapy was associated with a significantly higher rate of improvement in NASH compared to placebo (43% vs. 19%); the difference in the rate of improvement with pioglitazone as compared with placebo was also significant (34% and 19%, respectively). Both vitamin E and pioglitazone were associated with secondary outcome improvements, including reductions in serum alanine and aspartate aminotransferase levels, hepatic steatosis, and lobular inflammation, but not with improvement in fibrosis scores. Because the long-term effects of vitamin E are unknown, it has not been used in clinical practice. Pioglitazone is associated with increased adiposity and weight gain, which is a major concern for use over the long term.2
The bile acid derivative obeticholic acid (OCA) is an activator of the farnesoid X nuclear receptor that has been shown to reduce liver fat and fibrosis in animal models. In a small proof-of-concept study, OCA given in a dose of 50 mg once daily for six weeks was reported to increase insulin sensitivity and reduce liver inflammation markers.62 In the 72-week multicenter Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) trial in patients with noncirrhotic NASH, 141 patients were randomly assigned to receive OCA in a dose of 25 mg daily and 142 to receive placebo. Inclusion criteria for patients were histological evidence of definite or borderline NASH based upon a liver biopsy obtained within 90 days of randomization, and a histological NAFLD activity score of 4 (possible NASH) or 5 or more (definite NASH) with a score of 1 or more in each component of the score (steatosis scored 0–3, ballooning 0–2, and lobular inflammation 0–3). The primary outcome was improvement in liver histology defined as a decrease in the NAFLD activity score by at least 2 points without worsening of fibrosis. As the result of a planned interim analysis showing improved efficacy of OCA, treatment was discontinued early in 64 patients; 50 (45%) of 110 patients in the OCA group who were meant to have biopsies at both baseline and 72 weeks had improved liver histology compared with 23 (21%) of 109 such patients in the placebo group; 33 (23%) of 141 patients in the OCA group developed pruritus compared with 9 (6%) of 142 in the placebo group.63 There was also a statistically significant increase in TG and low-density lipoprotein (LDL) and decrease in high-density lipoprotein (HDL) in the OCA group compared to placebo. Although these changes were small, long-term effects are unknown. In addition, the improvement in liver enzymes disappeared once the medication was stopped, suggesting that the treatment may require long-term administration. Clearly, further study will be required to determine long-term benefits, safety, and patient tolerance; itching has limited its use in other liver diseases.64 While the FLINT trial has shown a slight improvement in fibrosis with OCA treatment, results with OCA are somewhat comparable to those seen in the PIVEN trial with vitamin E (45% improvement in histology with OCA vs. 43% with vitamin E); the PIVEN trial’s primary outcome included improvement in hepatocellular ballooning, whereas the FLINT trials did not.
The magnitude of improvement seen with any of the therapies tested to date is small, and additional and more effective therapies are clearly needed.19,20
SAMe metabolism in the liver
The liver plays a major role in metabolism of SAMe, the principal biological methyl donor made in all mammalian cells.65,66 In the hepatocyte, SAMe is produced as the result of an interaction between methionine and adenosine triphosphate via the enzyme methionine adenosyltransferase (MAT), while its degradation is dependent on the glycine-N-methyltransferase (GNMT) enzyme.67 The MAT isoenzymes consist of catalytic subunits a1 and α2 encoded by MAT1A and MAT2A, respectively. MAT1A is expressed mostly in differentiated liver (mainly hepatocytes), while MAT2A is widely distributed and has been shown to play a role in hepatocellular carcinoma (HCC).66
SAMe is made in all mammalian cells, is widely distributed throughout the body and plays an essential role in a number of biochemical reactions involving enzymatic transmethylation, transsulfuration, and polyamine synthesis.65,66 Up to 85% of all transmethylation reactions occur in the liver.68 In these reactions, the methyl group is transferred from SAMe to hormones, neurotransmitters, nucleic acids, proteins, phospholipids, and certain drugs.65 Of importance, the methylation of phospholipids plays a role in lipid metabolism and may be responsible for membrane fluidity and establishing the proper ratio of phosphatidylethanolamine (PE) to PC.69 This ratio of PE to PC has been found to be important in NAFLD development. In mice, the PC/PE ratio may be a key regulator of cell membrane integrity and play a role in the progression of steatosis to NASH.70 In hepatocytes, the key methyltransferase that is largely responsible for ‘degrading’ SAMe is GNMT, which accounts for 1% of cytosolic protein. All methyltransferase reactions generate S-adenosylhomocysteine (SAH), a potent competitive inhibitor of methyltransferases that needs to be promptly hydrolyzed to homocysteine (Hcy) and adenosine by SAH hydrolase. Hcy can be remethylated to form methionine through two enzymes: methionine synthase, which requires normal levels of folate and vitamin B12, and betaine homocysteine methyltransferase, which requires betaine, a choline metabolite.
Methionine metabolism has been found to be altered in CLD, likely as the result of decreased MAT and PEMT (phosphatidylethanolamine N-methyltransferase) activity.71 The formation of PC, the most abundant phospholipid in liver membrane, is reduced as well.68 PEMT catalyzes the methylation of PE to PC (the main membrane phospholipid) via a metabolic pathway that utilizes SAMe as a methyl donor.69 The transsulfuration pathway converts Hcy to cysteine and ultimately to sulfates and reduced glutathione (GSH), an important intracellular antioxidant.65,66 GSH binds and detoxifies various undesirable compounds. In the aminopropylation pathway, SAMe is metabolized to decarboxylated SAMe and the aminopropylation group is transferred to putrescine. The polyamines spermidine and spermine, major elements in cell growth, are then formed.69
Patients with alcoholic hepatitis and fibrosis have diminished MAT1A expression and hepatic SAMe levels,72 which contributes to decreased hepatic GSH levels in these patients. SAMe administration has been shown to normalize GSH levels in patients with either alcoholic or nonalcoholic liver disease.73 Many studies done mostly in alcoholic liver disease, cholestasis of pregnancy, and primary biliary cirrhosis have shown significant improvement in liver test abnormalities during therapy with SAMe.74
SAMe treatment in CLDs
SAMe has been shown to have differing effects in several CLDs including intrahepatic cholestasis (IHC), cholestasis of pregnancy, and alcoholic liver disease.74 There have been few randomized controlled trials to assess the efficacy of SAMe in IHC.75,76 In these studies, IHC was attributed to different etiologies including cirrhosis, viral hepatitis, and primary biliary cirrhosis. SAMe treatment, administered either orally or parentally for a duration of two to four weeks, resulted in improvements in pruritus. In a subsequent meta-analysis that included several randomized clinical trials SAMe was shown to be superior to placebo in improving pruritus and serum bilirubin.77
Cholestasis of pregnancy leads to adverse perinatal outcomes such as preterm birth, meconium passage, fetal distress, and death.78 Both SAMe and ursodeoxycholic acid (UCDA) have been used in this condition.75,78–83 The largest randomized clinical trial enrolled 46 patients with cholestasis of pregnancy and randomized patients to either oral SAMe (1 g/day) or UDCA (600 mg/day) starting before 36 weeks of pregnancy and continuing until delivery. In this study, UDCA was found to be more effective than SAMe in lowering bile acid levels, but both improved pruritus.83 A more recent meta-analysis concluded that UCDA is effective in reducing pruritus and may reduce fetal mortality, while SAMe was less effective.84
Promising results with SAMe in animal models of alcoholic liver disease73,85–89 led to multiple clinical trails using SAMe in alcoholic liver disease. The largest trial was a Spanish multicenter study led by Dr. Mato that randomized 62 patients with cirrhosis due to alcoholic liver disease to 1.2 g/day of SAMe and 61 to placebo, with treatment continued for up to two years. The combined all-cause mortality–transplantation end point was 30% in the placebo arm compared to 16% in those treated with SAMe; however, this did not reach statistical significance (P = 0.077).89 However, when the authors excluded those with advanced cirrhosis (Child-Pugh score C) the results were significant, 29% versus 12% (P = 0.025). Other studies, including a meta-analysis, did not support this study’s findings; however, these studies were smaller and SAMe treatment was for a shorter duration of time (up to 24 weeks).90 SAMe has been also used in other liver diseases, including hepatitis C, where SAMe addition to peginterferon and ribavirin improved early viral kinetics and increased interferon-stimulated gene induction in nonresponders to previous therapy.91
Evidence of SAMe’s role in the pathogenesis of NAFLD and NASH
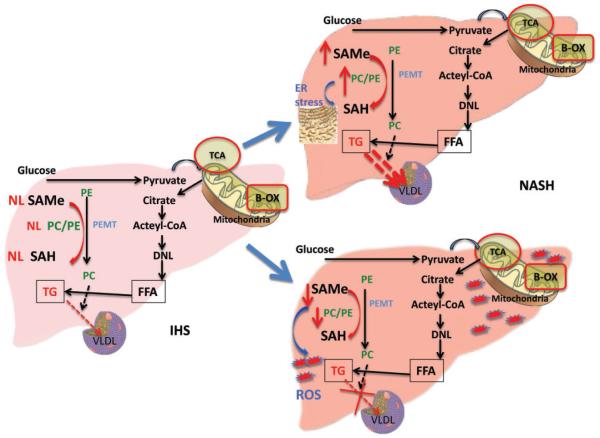
Our group has shown that mice deficient in MAT1A or GNMT (key enzymes in SAMe biosynthesis and degradation, respectively) develop NASH and HCC.66 These findings have led us to hypothesize that a chronically altered hepatic SAMe level may be a trigger that converts simple steatosis to NASH. Thus, SAMe metabolites may be useful biomarkers and may help to personalize NASH treatment. In the setting of chronic hepatic SAMe deficiency as in the MAT1A knockout model, we have shown that PC biosynthesis is a key determinant in NASH development.92,93 PC is produced in the liver via two main pathways. The CTP:phosphocholine cytidylyltransferase pathway is responsible for approximately 70% of PC synthesis. The PEMT pathway is responsible for the remaining 30%. The PEMT pathway is dependent on SAMe methylation to catalyze PE to form PC, which is essential for assembly and export of VLDL.94 The MAT1A knockout mice have shown decreased PC biosynthesis via PEMT.92,93 With the alteration of the PC/PE ratio, the liver adjusts to restore it to normal by inhibiting PC secretion which in turn impairs VLDL export resulting in increased hepatic TG.92,93 As a result of this impaired TG export via VLDL, the abnormal SAMe and PC synthesis decreases FA esterification by reducing SCD-1 expression and de novo lipogenesis, decreasing SREBP-1 expression, and activating adenosine monophosphate-activated protein kinase (AMPK). As a consequence of AMPK activation, FA beta-oxidation and uptake are increased.95,96 The decreased PC–PE ratio increases membrane permeability leading to leakage of cellular components, activation of Kupffer cells, and cytokine release leading to liver cell injury.97,98 In addition, the reduced SAMe level sensitizes the liver to lipopolysaccharide-induced injury and promotes expression and release of pro-inflammatory cytokines.98,99
In contrast, GNMT knockout mice exhibit increased SAMe levels which in turn activate natural killer cells in the liver and the PEMT pathway resulting in more PC synthesis.100 As a response, the liver stimulates VLDL and HDL export to restore a normal PC–PE ratio, and increases PC catabolism via phospholipase D or C, leading to increased DG production, which leads to increased TG and PC mobilization via VLDL export.101 Interestingly, GNMT knockout mice have an increased PC–PE ratio and this has been shown to induce ER stress.99 The above findings clearly show that high and low SAMe levels lead to NASH development, with PC–PE ratio being a key player.
In a comparison, human data has shown that diacylglycerol, TG, and phospholipid contents were increased significantly in humans with NAFLD with a stepwise increase between simple steatosis and NASH.102 Interestingly, PC levels were decreased in both simple steatosis and NASH patients without a statistically significant difference in PC level between simple steatosis and NASH; however, the study was small and included only 18 patients with simple steatosis and NASH so this should be explored further. In another human study, VLDL hepatic secretion was lower in NASH patients compared to simple steatosis, which was attributed to a greater decrease in PC.103 Another group has shown that the hepatic PC–PE ratio was significantly lower in NASH patients compared to healthy controls.70 An important study has found that a decrease in the PC–PE ratio in hepatocytes is dependent on SAMe and leads to lipogenesis and fatty liver.93 Finally, Diehl and colleagues recently investigated whether hepatic gene expression can distinguish between patients with mild versus advanced NAFLD.104,105 The researchers have found that MAT1A was underexpressed in patients with advanced NAFLD but not mild NAFLD, clearly emphasizing a key role for the SAMe cycle in NAFLD pathogenesis in humans.
Collectively, these human data show a decrease in MAT1A and PC–PE ratio pointing toward similarities with the MAT1A knockout model; however, SAMe levels and its metabolites still need to be investigated. Also, the role of GNMT in humans is unknown and requires further study. Indeed, the different phenotypes and underlying mechanisms seen in MAT1A and GNMT knockout mice may offer an explanation for the heterogeneous phenotypes seen in humans with NAFLD and why it may be difficult to find one treatment that will benefit all. Our current work concentrates on the hypothesis that low SAMe levels will lead to a reduced PC–PE ratio and impaired VLDL secretion as well as proinflammatory cytokine release and NASH development, while patients with simple steatosis have normal MAT1A and SAMe levels. On the other hand, high SAMe levels will lead to activation of the PEMT pathway resulting in more PC synthesis and consequently the liver’s stimulating VLDL and HDL export to restore a normal PC–PE ratio. Increasing PC synthesis in turn results in increased DG and TG and accumulation in the liver. This eventually leads to increased ER stress and inflammatory milieu causing NASH development. This hypothesis focusing on alteration of the SAMe level remains to be studied in humans with IHS and NASH (Figure 2).
Figure 2.
Effect of SAMe level on hepatic lipid metabolism and NASH development. In IHS, SAMe levels and PC–PE ratio are normal. NASH can occur when hepatic SAMe level is chronically elevated (i.e. GNMT knockdout mice) or low (i.e. MAT1A knockout mice). High SAMe level increases PC–PE ratio, activating VLDL and HDL export and increase DG production. High PC–PE ratio causes ER stress and high SAMe level activates natural killer cells in the liver. This may be a mechanism of how high SAMe level converts steatosis to NASH. Conversely, low SAMe level results in low PC–PE ratio, leading to impaired VLDL export and TG accumulation. Low PC–PE ratio increases membrane permeability and low SAMe level sensitizes the liver to LPS-induced expression and release of pro-inflammatory cytokines. These may cooperate to convert steatosis to NASH. Abbreviations: SAMe, S-adenosylmethionine; SAH, S-adenosylhomocysteine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; TCA, citric acid cycle; B-OX, beta-oxidation; FFA, free fatty acids; TG, triglyceride; VLDL, very low-density lipoprotein; IHS, isolated hepatic steatosis; ROS, reactive oxygen species; ER, endoplasmic reticulum; NASH, nonalcoholic steatohepatitis. (A color version of this figure is available in the online journal.)
Evidence of potential role of SAMe for treatment and its metabolites as biomarkers
In an ongoing search for noninvasive biomarkers to distinguish IHS from NASH, we have reexplored previous findings showing that the rates of transmethylation of methionine and methylation of Hcy were lower in a group of NASH patients.106 Interestingly, we have found that human NASH, but not simple steatosis, is associated with increased blood levels of methionine (1.37-fold increase in NASH compared to normal).48 This suggests that methionine can be used as a noninvasive biomarker in NASH patients. However, increased methionine level will not differentiate low hepatic MAT1A from low GNMT activity, as they both raise blood methionine level. Instead, blood SAMe level will be low in MAT1A deficiency but high in GNMT deficiency. Thus, exploring the other metabolites of the SAMe cycle may offer other potentially highly insightful noninvasive biomarkers. Another important component of the SAMe cycle is betaine, which is required for the generation of methionine from Hcy. Betaine has been investigated as a treatment agent for NASH in two studies, a pilot study in 10 NASH patients followed by a randomized controlled clinical trial that assessed histological outcome in 50 patients.107,108 Although the pilot study was able to show improvement in liver enzymes with betaine treatment, the randomized trial did not show improvement in histology but rather stabilization in steatosis compared to controls. A major concern of the randomized trial is the rate of dropout as approximately 32% of the patients did not undergo the exit liver biopsy. In addition, betaine might not have worked because it requires the MAT1A-encoded enzyme to generate SAMe and those with severe NASH have reduced MAT1A expression.87 The first evidence of the beneficial effect of SAMe in animal models of NASH came from the methionine–choline deficient animal dietary model of NASH. In these mice, SAMe treatment has been shown to protect against NASH, improving liver enzymes, inflammatory and fibrosis markers, and liver histology.109 This ‘key role’ of SAMe is likely due to the fact that a reduced hepatic SAMe level sensitizes the liver to release proinflammatory cytokines and this may be prevented by SAMe treatment.110 However, SAMe treatment will likely benefit only NASH that develops when the liver SAMe level is low (like the methionine–choline deficient diet and also in MAT1A knockout mice, unpublished observation). However, unlike many treatments listed in Table 1, SAMe is well tolerated (used as a supplement in the United States) with little to no toxicity, making it particularly attractive as a long-term treatment strategy.66
Taken together, SAMe treatment is a potentially effective therapy in some patients with NASH, particularly those who have reduced hepatic SAMe levels (due to decreased MAT1A expression). Its mechanism likely involves improving the PC–PE ratio, correcting the impaired VLDL export from the liver, and decreasing the release of proinflammatory cytokines. Serum SAMe metabolites may serve as useful biomarkers to identify individuals who may benefit from its use.
SAMe, NASH, and HCC
Recently, a role for abnormal levels of SAMe in the development of NASH and HCC has been suggested.66 Animal studies show that both chronic hepatic SAMe deficiency (MAT1A knockout) and excess (GNMT knockout) can result in NASH and HCC. However, the underlying mechanisms are distinct. While activation of multiple oncogenic pathways and expansion of liver progenitor cells including cancer stem cells play key roles in MAT1A knockout mice, aberrant hypermethylation resulting in silencing of inhibitors of the JAK/STAT pathway is implicated in GNMT knockout mice. Since SAMe has been shown by our group and others to exhibit chemopreventive action against HCC, it becomes even more important to identify those who would benefit from its use.111
Conclusion
There is increasing evidence that SAMe plays a role in the pathogenesis of NAFLD including the development of IHS and NASH. Most of the published data to date comes from animal models, although human data is now emerging. In addition, there is evidence that SAMe metabolites may be able to differentiate IHS from NASH in humans. Due to the lack of effective treatments for NASH and SAMe’s effectiveness in animal models of NASH, we believe that SAMe metabolites may serve as useful biomarkers to identify those NASH patients who will benefit from SAMe treatment. In NASH patients who have a reduced SAMe level (presumably due to reduced MAT1A expression), SAMe may also be effective in preventing the development of HCC. Further studies are very much needed to explore these novel areas.
ACKNOWLEDGMENT
This work was supported by NIH grants R01AT001576 (S. C. Lu and J. M. Mato) and Plan Nacional of I+D SAF 2011-29851, and Departamento de Educación del Gobierno Vasco (J. M. Mato).
Footnotes
Authors’ contributions: M.N. carried out the bulk of the literature searches and writing of the manuscript and preparation of the figures. J.M.M. and S.C.L. were involved in the writing and editing of the manuscript. M.N. and S.C.L share corresponding authorship.
REFERENCES
- 1.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 2.LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Penaranda MM, Ramos JF, Sarin S, Stimac D, Thomson AB, Umar M, Krabshuis J, LeMair A, Review Team World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–73. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Review T, LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Penaranda MM, Ramos JF, Sarin S, Stimac D, Thomson AB, Umar M, Krabshuis J, LeMair A. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–73. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 5.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–43. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 7.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204–10. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of Taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745–52. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Tsai CH, Li TC, Lin CC. Metabolic syndrome as a risk factor for non-alcoholic fatty liver disease. South Med J. 2008;101:900–5. doi: 10.1097/SMJ.0b013e31817e8af9. [DOI] [PubMed] [Google Scholar]
- 10.Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Dassanayake AS, Kasturiratne A, Rajindrajith S, Kalubowila U, Chakrawarthi S, De Silva AP, Makaya M, Mizoue T, Kato N, Wickremasinghe AR, de Silva HJ. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol. 2009;24:1284–8. doi: 10.1111/j.1440-1746.2009.05831.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, Chan FK, Chan HL. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 14.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 15.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 16.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 18.Charlton MR. Fibrosing NASH: on being a blind man in a dark room looking for a black cat (that isn’t there) Gastroenterology. 2011;140:25–8. doi: 10.1053/j.gastro.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 20.Rinella ME, Loomba R, Caldwell SH, Kowdley K, Charlton M, Tetri B, Harrison SA. Controversies in the diagnosis and management of NAFLD and NASH. Gastroenterol Hepatol. 2014;10:219–27. [PMC free article] [PubMed] [Google Scholar]
- 21.Mato JM, Lu SC. Where are we in the search for noninvasive nonalcoholic steatohepatitis biomarkers? Hepatology. 2011;54:1115–7. doi: 10.1002/hep.24642. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Investig. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan K, Bhalla V, El Regal ME, HH AK. Nonalcoholic fatty liver disease: a comprehensive review of a growing epidemic. World J Gastroenterol. 2014;20:12082–101. doi: 10.3748/wjg.v20.i34.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/ dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci USA. 2001;98:13710–5. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide micro-array data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–32. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–65. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- 28.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–31. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabet Metabol. 2004;30:121–38. doi: 10.1016/s1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 30.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metabol. 2011;14:804–10. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 32.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–76. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 34.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for non-alcoholic steatohepatitis. Hepatology. 2011;54:344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noureddin M, Loomba R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis. 2012;1:104–7. doi: 10.1002/cld.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 38.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of non-alcoholic fatty liver disease (NAFLD) Am J Gastroenterol. 2007;102:2716–7. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–45. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560–6. doi: 10.3748/wjg.v16.i13.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C, Loomba R. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–4. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–31. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C, Loomba R. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–75. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 48.Barr J, Vazquez-Chantada M, Alonso C, Perez-Cormenzana M, Mayo R, Galan A, Caballeria J, Martin-Duce A, Tran A, Wagner C, Luka Z, Lu SC, Castro A, Le Marchand-Brustel Y, Martinez-Chantar ML, Veyrie N, Clement K, Tordjman J, Gual P, Mato JM. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J Proteome Res. 2010;9:4501–12. doi: 10.1021/pr1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barr J, Caballeria J, Martinez-Arranz I, Dominguez-Diez A, Alonso C, Muntane J, Perez-Cormenzana M, Garcia-Monzon C, Mayo R, Martin-Duce A, Romero-Gomez M, Lo Iacono O, Tordjman J, Andrade RJ, Perez-Carreras M, Le Marchand-Brustel Y, Tran A, Fernandez-Escalante C, Arevalo E, Garcia-Unzueta M, Clement K, Crespo J, Gual P, Gomez-Fleitas M, Martinez-Chantar ML, Castro A, Lu SC, Vazquez-Chantada M, Mato JM. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11:2521–32. doi: 10.1021/pr201223p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Therapeut. 2009;29:172–82. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Nonalcoholic Steatohepatitis Clinical Research N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA: J Am Med Assoc. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assy N, Hussein O, Abassi Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with non-alcoholic steatohepatitis. Gut. 2007;56:443–4. doi: 10.1136/gut.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–6. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 54.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–86. [PubMed] [Google Scholar]
- 55.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Eng J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 58.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L, Bonnefont-Rousselot D, Bastard JP, Riviere M, Spenard J, FRESGUN A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–9. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 59.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, Zeuzem S, Hein J, Berg T, Group NS. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a doubleblind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–9. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–9. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82. doi: 10.1053/j.gastro.2013.05.042. e1. [DOI] [PubMed] [Google Scholar]
- 63.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silveira MG, Lindor KD. Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis. Expert Opin Pharmacother. 2014;15:365–72. doi: 10.1517/14656566.2014.873404. [DOI] [PubMed] [Google Scholar]
- 65.Mato JM, Martinez-Chantar ML, Lu SC. S-adenosylmethionine metabolism and liver disease. Ann Hepatol. 2013;12:183–9. [PMC free article] [PubMed] [Google Scholar]
- 66.Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92:1515–42. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–12. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 68.Friedel HA, Goa KL, Benfield P. S-adenosyl-L-methionine. A review of its pharmacological properties and therapeutic potential in liver dysfunction and affective disorders in relation to its physiological role in cell metabolism. Drugs. 1989;38:389–416. doi: 10.2165/00003495-198938030-00004. [DOI] [PubMed] [Google Scholar]
- 69.Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside—molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–7S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–31. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Duce AM, Ortiz P, Cabrero C, Mato JM. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 1988;8:65–8. doi: 10.1002/hep.1840080113. [DOI] [PubMed] [Google Scholar]
- 72.Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2004;28:173–81. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- 73.Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, Carrieri V, Albano O. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989;24:407–15. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- 74.Anstee QM, Day CP. S-adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. J Hepatol. 2012;57:1097–109. doi: 10.1016/j.jhep.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 75.Frezza M, Centini G, Cammareri G, Le Grazie C, Di Padova C. S-adenosylmethionine for the treatment of intrahepatic cholestasis of pregnancy. Results of a controlled clinical trial. Hepato-gastroenterology. 1990;37(Suppl 2):122–5. [PubMed] [Google Scholar]
- 76.Qin B, Guo S, Zhao Y, Zou S, Zhang Q, Wang Z, Zeng W, Zhang D. A trial of ademetionine in the treatment of intrahepatic biliary stasis viral hepatitis. Zhonghua gan zang bing za zhi ¼ Zhonghua ganzangbing zazhi ¼ Chin J Hepatol. 2000;8:158–60. [PubMed] [Google Scholar]
- 77.Hardy ML, Coulter I, Morton SC, Favreau J, Venuturupalli S, Chiappelli F, Rossi F, Orshansky G, Jungvig LK, Roth EA, Suttorp MJ, Shekelle P. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease. Evid Rep Technol Assess (Summ) 2003;64:1–3. [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed KT, Almashhrawi AA, Rahman RN, Hammoud GM, Ibdah JA. Liver diseases in pregnancy: diseases unique to pregnancy. World J Gastroenterol: WJG. 2013;19:7639–46. doi: 10.3748/wjg.v19.i43.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Floreani A, Paternoster D, Melis A, Grella PV. S-adenosylmethionine versus ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: preliminary results of a controlled trial. Eur J Obstetr Gynecol Reproduct Biol. 1996;67:109–13. doi: 10.1016/0301-2115(96)02442-6. [DOI] [PubMed] [Google Scholar]
- 80.Frezza M, Pozzato G, Chiesa L, Stramentinoli G, di Padova C. Reversal of intrahepatic cholestasis of pregnancy in women after high dose S-adenosyl-L-methionine administration. Hepatology. 1984;4:274–8. doi: 10.1002/hep.1840040217. [DOI] [PubMed] [Google Scholar]
- 81.Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstetr Gynaecol. 1998;105:1205–7. doi: 10.1111/j.1471-0528.1998.tb09976.x. [DOI] [PubMed] [Google Scholar]
- 82.Ribalta J, Reyes H, Gonzalez MC, Iglesias J, Arrese M, Poniachik J, Molina C, Segovia N. S-adenosyl-L-methionine in the treatment of patients with intrahepatic cholestasis of pregnancy: a randomized, double-blind, placebo-controlled study with negative results. Hepatology. 1991;13:1084–9. [PubMed] [Google Scholar]
- 83.Roncaglia N, Locatelli A, Arreghini A, Assi F, Cameroni I, Pezzullo JC, Ghidini A. A randomised controlled trial of ursodeoxycholic acid and S-adenosyl-l-methionine in the treatment of gestational cholestasis. BJOG: Int J Obstetr Gynaecol. 2004;111:17–21. doi: 10.1046/j.1471-0528.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 84.Gurung V, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochr Database Syst Rev. 2013;6:CD000493. doi: 10.1002/14651858.CD000493.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem. 2003;14:591–7. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 86.Colell A, Garcia-Ruiz C, Morales A, Ballesta A, Ookhtens M, Rodes J, Kaplowitz N, Fernandez-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-L-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 87.Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF, 3rd, Albanese A, Bowlus CL, Devaraj S, Panacek EA, Richards JR, Halsted CH. S-adenosyl-L-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960–5. doi: 10.1111/j.1530-0277.2011.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trespi E, Panizza P, Colla C, Bottani G, De Vecchi P, Matti C. Efficacy of low dose mesalazine (5-ASA) in the treatment of acute inflammation and prevention of complications in patients with symptomatic diverticular disease. Preliminary results. Minerva gastroenterologica e dietologica. 1997;43:157–62. [PubMed] [Google Scholar]
- 89.Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Benita V, Caballeria J, Sola R, Moreno-Otero R, Barrao F, Martin-Duce A, Correa JA, Pares A, Barrao E, Garcia-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodes J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 90.Rambaldi A, Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochr Database Syst Rev. 2006;2:CD002235. doi: 10.1002/14651858.CD002235.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Feld JJ, Modi AA, El-Diwany R, Rotman Y, Thomas E, Ahlenstiel G, Titerence R, Koh C, Cherepanov V, Heller T, Ghany MG, Park Y, Hoofnagle JH, Liang TJ. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology. 2011;140:830–9. doi: 10.1053/j.gastro.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cano A, Buque X, Martinez-Una M, Aurrekoetxea I, Menor A, Garcia-Rodriguez JL, Lu SC, Martinez-Chantar ML, Mato JM, Ochoa B, Aspichueta P. Methionine adenosyltransferase 1A gene deletion disrupts hepatic very low-density lipoprotein assembly in mice. Hepatology. 2011;54:1975–86. doi: 10.1002/hep.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, Vance DE, Tzoneva M, Hart AC, Naar AM. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–52. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vance DE. Physiological roles of phosphatidylethanolamine N-methyltransferase. Biochim Biophys Acta. 2013;1831:626–32. doi: 10.1016/j.bbalip.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, Kennedy BP, Dyck JR, Vance DE. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from dietinduced obesity. J Biol Chem. 2010;285:22403–13. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–44. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, Caballeria J, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–36. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chawla RK, Bonkovsky HL, Galambos JT. Biochemistry and pharmacology of S-adenosyl-L-methionine and rationale for its use in liver disease. Drugs. 1990;40(Suppl 3):98–110. doi: 10.2165/00003495-199000403-00010. [DOI] [PubMed] [Google Scholar]
- 99.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomez-Santos L, Luka Z, Wagner C, Fernandez-Alvarez S, Lu SC, Mato JM, Martinez-Chantar ML, Beraza N. Inhibition of natural killer cells protects the liver against acute injury in the absence of glycine N-methyltransferase. Hepatology. 2012;56:747–59. doi: 10.1002/hep.25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez-Una M, Varela-Rey M, Cano A, Fernandez-Ares L, Beraza N, Aurrekoetxea I, Martinez-Arranz I, Garcia-Rodriguez JL, Buque X, Mestre D, Luka Z, Wagner C, Alonso C, Finnell RH, Lu SC, Martinez-Chantar ML, Aspichueta P, Mato JM. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology. 2013;58:1296–305. doi: 10.1002/hep.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 103.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–80. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 104.Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, Garrett ME, Ashley-Koch A, Suzuki A, Tillmann HL, Hauser MA, Diehl AM. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076–87. doi: 10.1053/j.gastro.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, Murphy SK, Ashley-Koch AE, Choi SS, Michelotti GA, Hampton DD, Chen Y, Tillmann HL, Hauser MA, Abdelmalek MF, Diehl AM. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–82. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalhan SC, Edmison J, Marczewski S, Dasarathy S, Gruca LL, Bennett C, Duenas C, Lopez R. Methionine and protein metabolism in non-alcoholic steatohepatitis: evidence for lower rate of transmethylation of methionine. Clin Sci (Lond) 2011;121:179–89. doi: 10.1042/CS20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711–17. doi: 10.1111/j.1572-0241.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- 108.Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ, Lindor KD. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818–26. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 109.Oz HS, Im HJ, Chen TS, de Villiers WJ, McClain CJ. Glutathione-enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol. 2006;20:39–47. doi: 10.1002/jbt.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine deficiency and TNF-alpha in lipopolysaccharide-induced hepatic injury. Am J Physiol. 1998;275:G125–9. doi: 10.1152/ajpgi.1998.275.1.G125. [DOI] [PubMed] [Google Scholar]
- 111.Lu SC, Ramani K, Ou X, Lin M, Yu V, Ko K, Park R, Bottiglieri T, Tsukamoto H, Kanel G, French SW, Mato JM, Moats R, Grant E. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–71. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]