Abstract
This review highlights the most important discovery in the reticular activating system (RAS) in the last 10 years, the manifestation of gamma band activity in cells of the RAS, especially in the pedunculopontine nucleus (PPN), which is in charge of the high frequency states of waking and REM sleep. This discovery is critical to understanding the modulation of movement by the RAS and how it sets the background over which we generate voluntary and triggered movements. The presence of gamma band activity in the RAS is proposed to participate in the process of preconscious awareness, and provide the essential stream of information for the formulation of many of our actions. Early findings using stimulation of this region to induce arousal, and also to elicit stepping, are placed in this context. This finding also helps explain the novel use of PPN deep brain stimulation (DBS) for the treatment of Parkinson’s disease (PD), although considerable work remains to be done.
Keywords: Arousal, calcium channels, deep brain stimulation, mu rhythm, Parkinson’s disease, P13 potential, P50 potential, Readiness Potential
Introduction
What happens when we respond to a sudden stimulus? What is required to execute voluntary movements, and what leads to involuntary movements, such as those in Parkinson’s disease (PD). This review will address an issue not normally emphasized in the motor control and movement disorder literature, the background of information necessary for the execution of movements, both voluntary and unplanned. In his book, I of the Vortex, Rodolfo Llinas proposed that what we call thinking is the evolutionary internalization of movement [1]. Llinas explored how the mind arose in evolution, and concluded that the brain's control of organized movement gave birth to the generation of the mind. The simple but crucial point is that the evolutionary development of a nervous system is a property of actively moving creatures. Llinas suggested that prediction is the ultimate function of the brain and that the self is the centralization of prediction. The argument was advanced in linear fashion, supported by a fountain of information on the physiology of motor control [1]. One reason why this rationale is so persuasive is because the motor "card" is precisely what has been missing from play in a number of theories of consciousness and free will. The unsatisfactory nature of concepts of mindness based only on sensory perception, the uneasy discomfort generated by such hypotheses, is laid bare by this patently evident idea. While the argument is complex, the basic idea is simple, almost obvious. The theory advanced by Llinas is based on oscillations, from the oscillations of graded potentials along a nerve cell's membrane, to the rhythmic activity of groups of nerve cells firing in phase, to the synchronization of rhythms in what are effectively analog brain systems.
If groups of neurons oscillate in phase or resonate, a global activity pattern results that provides the components for a transient construct of the external world [1]. This coherence in time is believed to form the neurological mechanism for perceptual binding. That is, to bring together the separate sensory components of a stimulus, say color, size, motion, and so on, that are processed in different regions of the cortex. This process not only serves to provide binding of sensory events, but there are coherent oscillations that subserve the precise temporal activation of muscles needed to execute a movement accurately. There is ample evidence for the coherent activation of neurons during sensory activation. For example, a visual stimulus produces coherent gamma band oscillations in the visual cortex [2,3]. These oscillations are evident in widely separated cortical regions but they are coherent in time and frequency. That is, they are ideally manifested to perform a binding function.
Both the cortex and the thalamocortical system are endowed with intrinsic membrane properties such as sodium-dependent subthreshold oscillations or high threshold voltage-sensitive calcium channels, both of which can resonate at gamma frequencies [4–6]. This means that sensory information arriving in an awake cortex and thalamus is superimposed on ongoing gamma band oscillations. Basically, “specific” thalamic nuclei receive the “content” of sensory experience from primary sensory pathways and relay it to layer IV of the cortex, while “non-specific” thalamic nuclei receive the “context” of sensory experience from the RAS, and relay it to layer I of the cortex. Temporal coherence is due to the coincidence in firing of the two pathways which, when activated together, will summate to trigger cortical output to the thalamus. The cortical output flows back down to “specific” and “non-specific” thalamic nuclei to set up a thalamocortical resonance. It is this resonance that is thought to underlie the process of perception. A common definition of consciousness is awareness, such that if there is no awareness, there is no consciousness [7]. This is fundamentally intertwined with the concept of free will. When choosing to make a movement, the implication is that there is a will that decides to engage the motor system to then induce the movement. But what happens before we make a movement?
We will pursue a new line of evidence that suggests that the preparation for movement begins at much lower levels than previously thought. The preamble to volition and response time is subcortical in origin, and arises from the moment we awaken until the moment we fall asleep [8].
The Readiness Potential, the mu rhythm, and the P50 Potential
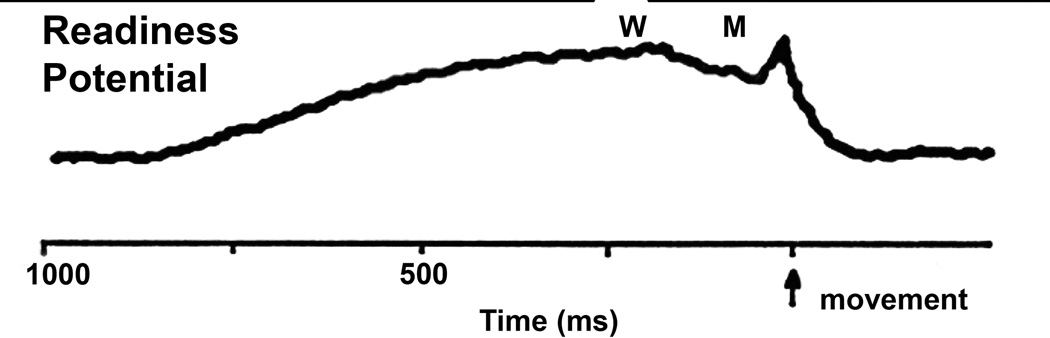
What do these three well-known waveforms have in common? The readiness Potential (RP), the mu rhythm, and the P50 midlatency auditory evoked potential all are recorded in the same region of the cortex. The RP was originally described by Kornhuber as a negative DC shift that began 600–800 msec before a voluntary, uncued movement [9]. The RP was present at maximal amplitude at the vertex in the region of the supplementary motor cortex and precentral cortex [9,10]. Pioneering studies by Libet first showed that when people consciously set a goal to engage in a behavior, their conscious will to act begins “unconsciously” [11]. The studies of Libet employed the RP, which is known to have an early component that precedes the movement by as much as 1 sec, and a late component that precedes the movement by 400 msec. Libet’s subjects were asked to move voluntarily, and were also asked to subjectively time the moment at which they felt the “will” to move as well as the onset of the actual movement. Figure 1 is a diagram of the vertex-recorded RP with the timing of the subjectively perceived “will” and perception of actual “movement”. The early and late phases of the RP preceded the “consciously” determined will to move by hundreds of milliseconds. These authors concluded that cerebral initiation of spontaneous, freely voluntary acts can begin “unconsciously”, before there is any subjective “conscious” awareness that a decision to act was initiated cerebrally [11].
Figure 1. The human readiness potential (RP).
Representation of a vertex-recorded RP and the timing reported by subjects performing an uncued voluntary movement. The estimation of the sense of will (signified by “W”) or intent to move occurred well after the beginning of the RP, and the sensation of movement (signified by “M”) occurred even later, and well after the beginning of the RP as in the Libet study [11].
Even simple movements appear to be generated “subconsciously”, and the “conscious” sense of volition comes later [12]. Hallett described the details of studies showing that voluntary movements can be triggered with stimuli that are not “consciously” perceived, that movement may well occur prior to the apparent planning of the movement, and that not only the sense of the movement having occurred, but also the sense of willing the movement, happen before the actual movement [12]. However, Libet suggested that voluntary acts begin “unconsciously”, before there is subjective “conscious” awareness that a decision to act was initiated by the brain. This conclusion has been extrapolated to suggest that there is no free will. In response, Libet suggested that, although the movement was indeed initiated “unconsciously”, it was subject to veto once it reached consciousness [13]. This has been regarded as unsatisfactory and not answering the question of whether there is free will or not. The question is complex because so many factors influence the sense of volition such as the perception of time, the conditions under which the movement is executed, the perception of volition, etc. [12].
We proposed an alternative view simply by concluding that it is the interpretation of the results that assumes that the process preceding the movement is “unconscious” [8]. There is no evidence that this is the case. The preparation for movement generates brain processes that are clearly related to the intent, but these occur during waking, and therefore should be labeled “preconscious”, not “subconscious”. The replacement of the word “preconscious” for the word “subconscious” significantly alters the conclusion of these studies. That is, the conclusion should have been: “voluntary acts begin preconsciously, before there is subjective conscious awareness that a decision to act was initiated by the brain”. We will see below how the RAS is an ideal candidate for the process of preconscious awareness.
The mu rhythm was first described by Gastaut [14] as a 8–12 Hz wave present over the vertex and bilaterally across the precentral motor cortex, basically at the EEG C3, Cz (vertex), and C4 electrode placements. The mu rhythm is present when the body is at rest, but the rhythm is “suppressed” or “blocked” when the person performs a motor action, or, after practice, when the person views another or visualizes a motor action. That is, it is more likely that, just as with the alpha rhythm, the mu rhythm is merely replaced by faster activity when the function of that region is called for; that the mu rhythm over the vertex and precentral cortex is shifted to higher frequencies, not “suppressed”, from idle speed to higher frequencies, when motor events are called for.
The mu rhythm is also suppressed during tactile stimulation [15], as is the alpha rhythm [16]. Both the occipital alpha rhythm and the precentral mu rhythm have been referred to as “idle rhythms” or “resting state” activity [17]. The mu rhythm has been associated with somatosensory information, while a faster beta rhythm in the precentral region has been associated with actual motor processing [18]. That is, these “idle” or “resting” rhythms are of slower frequencies and are “blocked” or “suppressed” (actually replaced) by higher frequencies when sensory or motor events are called for.
We should emphasize that the mu rhythm, like the RP, is present at the vertex (EEG Cz electrode location and also laterally at C3 or C4 depending on which hand or arm movement is being performed contralaterally). The vertex is also where the P50 potential is maximal. The human P50 potential is a midlatency auditory evoked response evoked by a click stimulus and recorded at the vertex [19]. The P50 potential peaks at a latency of 40–70 msec. The P50 potential was also referred to as the P1 potential because it is the first positive wave following the early brainstem auditory evoked response (BAER) that occurs at a 5–10 msec latency, and the primary auditory response, called Pa, at the superior temporal gyrus, that occurs at a 25 msec latency. The Pa response is directly related to the auditory lemniscal, “specific” thalamic nuclei, and the auditory cortex, while the P50 potential is related to the non-lemniscal, reticular, intralaminar or “non-specific” thalamic nuclei ascending pathways [19].
The human P50 potential has three main characteristics, 1) it is sleep state-dependent, that is, it is present during waking and REM sleep, but is absent during deep SWS [20,21], so that it is present when the cortex is activated and the EEG shows high frequency activity and, of course, when the PPN is active, 2) it is blocked by muscarinic cholinergic antagonists, such as scopolamine, so that it may be mediated, at least in part, by cholinergic neurons such as those in the PPN [22], and, 3) it undergoes rapid habituation at stimulation rates greater than 2 Hz, indicative of a “reticular” pathway. For example, the primary auditory cortex Pa potential can follow stimulus frequencies close to 20 Hz. However, the P50 potential cannot follow such high frequencies of stimulation, implying that it is not generated by a primary afferent pathway, but by a multi-synaptic, low security synaptic elements of the RAS [20–22].
The P50 potential decreases and disappears with progressively deeper stages of sleep and then reappears during REM sleep at full amplitude [20,23]. None of the earlier latency auditory evoked potentials (BAER or Pa primary auditory cortex potentials) possess this sleep-dependent characteristic. This suggests that the P50 potential is functionally related to states of arousal. In addition, the P50 potential is most likely generated, at least in part, by a cholinergic mesopontine cell group such as the PPN, since its cells are preferentially active during waking and REM sleep, but inactive during slow wave sleep [19,24–26]. The P50 potential has been localized to the frontal lobes in the region of the vertex, a source we confirmed using magnetoencephalography [27]. Studies in the cat [20,21] and the rat [28–30] showed that the feline “wave a” and the rodent P13 potential are the animal equivalents of the human P50 potential. These waveforms can be blocked by agents injected locally into the PPN to decrease its output, so that there is little doubt that these potentials are state-dependent, reticular in origin, and modulated in the same manner as the PPN, and therefore the equivalent of the human P50 potential. The P50 potential is, therefore, a measure of PPN output, an arousal-related waveform recorded at the level of the vertex in the human. Moreover, changes in its manifestation will inform about the function of the PPN in disease.
We hypothesize that the RP (in particular the early component), mu rhythm and P50 potential are all at least in part generated by PPN activity, activity that sets the background of activity for assessing sensory events, and precedes the execution of voluntary movements. The pedunculopontine nucleus (PPN), located at the transition between the midbrain and pons, is part of the reticular activating system (RAS) and is in charge of the EEG high frequency states of waking and REM sleep (as opposed to low frequency activity during slow wave sleep). The PPN, however, has become a target for deep brain stimulation (DBS) for the treatment of PD in human subjects. We suggested this possibility based on animal studies using stimulation in the region of the PPN, which at the time was thought to be part of the mesencephalic locomotor region (MLR) [24,25,31]. As we will see below, it took some 20 years for these findings to be translated to the bedside.
The Mesencephalic Locomotor Region and the Pedunculopontine Nucleus
The MLR was originally described as a region that required very specific parameters in order to induce locomotion on a treadmill in the decerebrate cat [32]. That is, stimulation at low levels (typically <100 µA) needed to be localized to the lateral cuneiform, of increasing current amplitude, of long duration (0.5–1.0 msec) pulses, and delivered at 40–60 Hz. We became interested in the PPN since it is a descending target of basal ganglia structures [33,34]. We carried out experiments to determine if the PPN and the MLR represented overlapping structures. We were able to use electrical stimulation as well as injection of neuroactive agents into the region of the PPN to induce locomotion [35], and to record from locomotion-related PPN neurons, with some cells being active in relation to limb alternation, while others were related to the duration of the locomotor episode [36]. We then addressed why, in order to induce locomotion, lateral, but not medial, cuneiform nucleus stimulation was required, ramping up of the current was required, stimulation at 40–60 Hz, but not higher or lower was required, and why long duration pulses were required. On a practical basis, two other peculiarities applied to these parameters. When current was ramped up and locomotion was initiated, further increase in the current would lead to cessation of stepping, therefore, current had to be backed down in order to maintain locomotion. The other issue was that stepping was never immediate, and always ensued but only after 1–2 sec of stimulation. These requirements have finally been satisfactorily resolved.
We localized electrical stimulation sites within the region of NADPH-diaphorase labeled cells (cholinergic neurons in the PPN are selectively labeled by this histochemical method) to induce locomotion on a treadmill in the precollicular-postmamillary transected cat. We also used neuroactive agents such as NMDA injected into the region of cholinergic cell labeling in transected cats. This ensured that activation of cell bodies by these agents, and not merely fibers of passage as was possible using electrical stimulation, was responsible for the stepping. Alternation between antagonists in the same limb, between agonists in opposite limbs, and a proximodistal delay of agonists in the same limb signaled that an appropriate locomotor step cycle was being induced following PPN stimulation chemically or electrically. Moreover, we found similar responses in the cat and the rat [24,31–33,35–43]. It is critical to realize that the reason such localization was possible was the use of very low current levels, below 100 µA, usually ~50 µA. By applying higher current levels, of course, locomotion can be induced following stimulation of more distant locations such as in medial cuneiform, etc. by current spread to truly active sites.
Since we were recording or injecting agents into a region that we had first physiologically identified to induce locomotion, we used the term “MLR” in most of our studies to describe our findings. However, we became convinced that the lowest threshold sites for inducing locomotion were located within the PPN [36]. We questioned the interpretation of the MLR as a locomotion-specific area, showing instead that the region stimulated was a “rhythmogenic” region that was part of the RAS [24–26,36,39–42]. The term “MLR” needs to be retired.
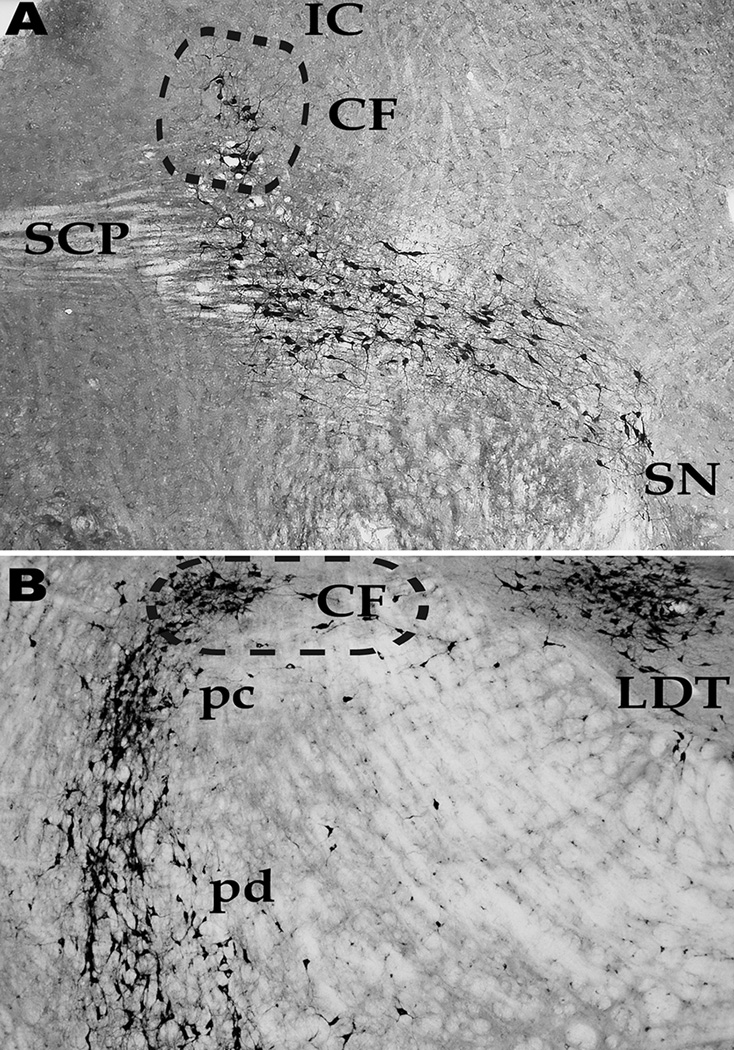
The PPN has long been identified as a cholinergic cell group [44], but recent studies established that this nucleus includes non-overlapping populations of cholinergic, glutamatergic and GABAergic neurons [45]. Sagittal sections are optimal for visualizing the PPN, since it has a wedge shape extending from the dorsolateral to the ventrolateral mesopontine region (Figure 2A). The pars compacta of the PPN is located in the lateral cuneiform (CF), posteriorly and dorsal to the lateral portion of the superior cerebellar peduncle (SCP), in the optimal site for inducing locomotion on a treadmill at low thresholds [37]. As the nucleus ranges anteroventrally, it is embedded in the superior cerebellar peduncle and ends in the posterior substantia nigra. The lowest threshold sites for inducing locomotion were located within the PPN pars compacta in the lateral part of the cuneiform nucleus [37,39]. Stimulation at more ventral sites did not induce stepping, but rather changes in muscle tone [46], an effect that may also have been due to suddenly switching on the stimulus (see below). Stimulation of more medial regions such as the medial cuneiform, laterodorsal tegmental nucleus (LDT), the medial partner of the PPN, or anteroventrally in the region of the substantia nigra, did not induce locomotion [24,26,40–42]. This explains why stimulation of only lateral, but not medial, cuneiform, in which the PPN is embedded, produces reliable stepping on a treadmill.
Figure 2. The pedunculopontine nucleus.
A. Sagittal. Histochemical NADPH-diaphorase labeling of only the cholinergic neurons in the PPN revealed the wedge-shaped structure of this nucleus. Posterodorsally is located the pars compacta of the PPN, which is located within the lateral cuneiform nucleus (CF) ventral to the inferior colliculus (IC). As the nucleus descends, its cells are intermixed with the fibers of the superior cerebellar peduncle (SCP). The body or pars dissipata of the PPN is located more ventrally and extends to the posterior edge of the substantia nigra (SN). Anterior is to the right. B. Semi-horizontal. This section is angled along the long axis of the PPN from the posterodorsal to the medioventral direction (see Figure 2A for sagittal orientation). Histochemical NADPH-diaphorase labeling of cholinergic cells shows that PPN neurons of the pars compacta (pc) are located dorsally, are found within the cuneiform nucleus (CF), and laterally to the locus coeruleus. Medial to the locus coeruleus are cells of the laterodorsal tegmental nucleus (LDT) embedded within the central gray. Laterally, the pars dissipata (pd) PPN descends in an arc towards the posterior edge of the subtantia nigra.
Figure 2B is a semi-horizontal section taken along the long axis of the PPN. The section is from the parabrachial area dorsally and posteriorly, angling downwards and anteriorly towards the posterior end of the substantia nigra. Basically, it is a view looking down from the top of the midbrain at the cholinergic neurons of the PPN and LDT. Medially and dorsally within the central gray is the aggregation of cholinergic cells of the LDT. More laterally to the LDT is the space is occupied by the locus coeruleus (LC), whose cells are not labeled in this section. Dorsal to the LC is the CF, which contains the remainder of the pars compacta of the PPN in its lateral side. More ventrally and lateral to the LC is located the ventral edge of the pars compacta of the PPN, and the curvature of the PPN as it descends towards the posterior pole of the substantia nigra. Note the more compact nature of the cells in the pars compacta posteriorly (top of the view) compared to the pars dissipata anteriorly (bottom of the view). This section makes clear the reason why the optimal target for inducing locomotion at low current levels is so small, containing the aggregation of cells of the pars compacta of the PPN, which is embedded in the lateral cuneiform, but not in the medial cuneiform. In keeping with these findings, a recent study on a rodent model of PD showed that stimulation of anterior PPN induced freezing and worsened gait, but gait was improved by posterior PPN stimulation [47].
The bottom line is that we believe the MLR is NOT a locomotion-specific region but rather an effect elicited by PPN stimulation. Further evidence that the PPN is responsible for these effects lies in the physiology of PPN neurons.
PPN Physiology
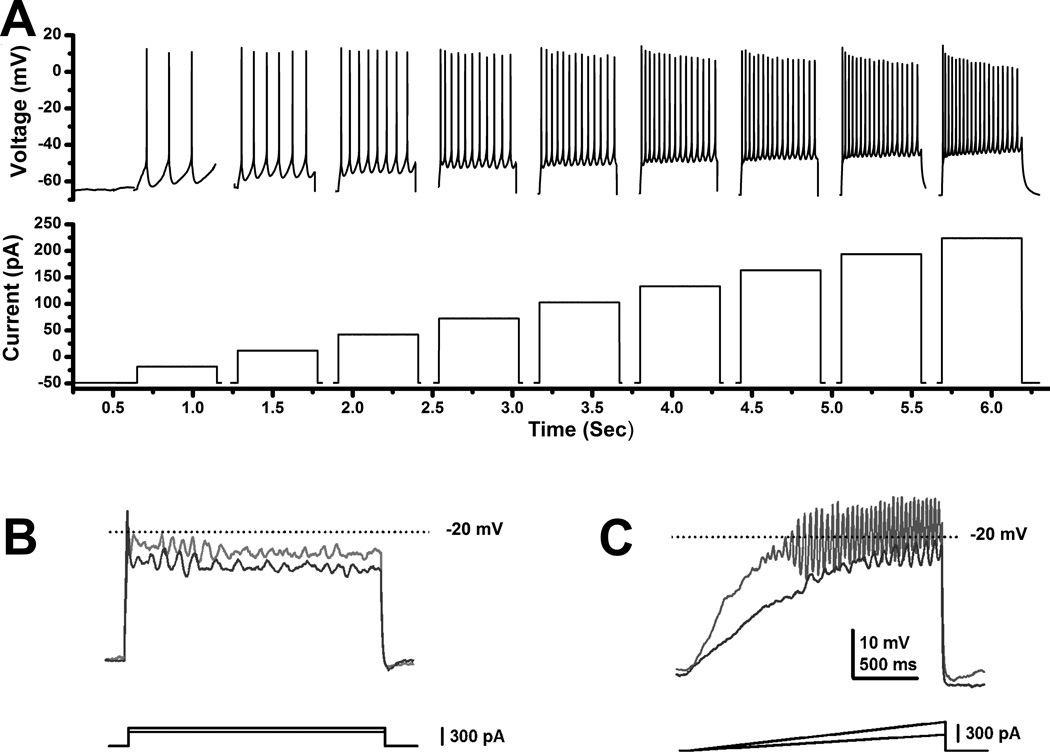
Why does stimulation of the PPN need to be applied at 40–60 Hz to induce stepping? We found that, when we applied depolarizing steps, all PPN cells increased firing frequency and then plateaued at 40–60 Hz as current levels were increased [48]. That is, these neurons could not be induced to fire any faster than 40–60 Hz, regardless of the level of current delivered intracellularly. This property was further investigated to determine the mechanism responsible for such a unique characteristic by recording patch clamped neurons in the presence of synaptic blockers (to prevent afferent signals) and tetrodotoxin (to prevent action potential generation). In our investigation of the intrinsic properties of these cells, we applied current steps to attempt to drive high threshold channels, but the steps were unable to depolarize the membrane sufficiently to maintain depolarization to reveal membrane oscillations, probably due to the activation of potassium channels by the sudden depolarization [49]. However, when we applied ramps (instead of steps) to gradually depolarize the membrane to activate high threshold channels, we were able to elicit membrane oscillations in the beta and gamma band range. We found that all PPN neurons, regardless of transmitter or electrophysiological type, manifested intrinsic membrane oscillatory activity at beta/gamma frequencies (20–60 Hz) [49]. We used specific calcium channel blockers to identify both P/Q- and N-type high threshold, voltage-dependent channels as responsible for PPN neuron beta/gamma band oscillations. It should be noted that cells outside of the PPN do not show similar properties, making the cellular boundaries of the nucleus specific to this kind of activity. The presence of a frequency plateau in PPN cells explains the requirement to stimulate this region at 40–60 Hz to induce locomotion.
Early locomotion studies revealed that suddenly switching on the current at previously determined threshold levels elicited only decrements in muscle tone instead of stepping [24,46,50]. Pulses delivered to the PPN had to be increased gradually until locomotion was induced after 1–2 sec, in many cases then backing off the current, and only then was continuous stepping evident [24,26,50]. We referred to this characteristic as “recruiting” locomotion. Single cells in the PPN also need to be “recruited” to fire at gamma band frequencies. The requirement to use ramps to elicit oscillations helps explain why current has to be gradually increased to depolarize PPN neurons slowly, i.e. to avoid activating potassium channels, and thus induce stepping. Sudden application of high amplitude currents would activate potassium channels and fail to sufficiently depolarize neurons. This would instead lead to inactivation of PPN neurons, explaining the decrement in muscle tone evident with sudden onset stimulation. This accounts for the need for ramping up current, instead of suddenly switching it on, in order to “recruit” stepping. In order to maintain such depolarization, the current needs to be reduced slightly, otherwise the depolarization will exceed the window for high threshold calcium channels. This explains the characteristic need to back off the current once locomotion is induced.
Why the need to use long duration pulses to elicit stepping? We hypothesize that the need to use long duration pulses is related to the high threshold calcium channels present in PPN neurons. We used calcium imaging to visualize ramp-activated calcium channels in PPN cells [51]. These voltage-dependent high threshold P/Q- and N-type calcium channels are located in the dendrites of PPN neurons. This explains the need to apply high levels of depolarization of the cell body in order to ultimately depolarize the higher resistance dendrites sufficiently to activate the high threshold calcium channels and promote gamma band oscillations. Long duration pulses, along with ramping up current levels, would be effective methods for activating dendritic calcium channels that would in turn allow PPN neurons to fire maximally at gamma band frequencies.
Figure 3A shows the responses elicited in a representative PPN neuron to intracellular application of current steps. PPN cells fired maximally at 40–60 Hz when intracellular steps were applied [48]. We then identified the intrinsic membrane properties that caused PPN neurons to fire at these frequencies and found that they were due to the presence of high threshold voltage-dependent calcium channels [49]. Figure 3B,C shows that these channels required that ramps be used to depolarize the cell sufficiently to reach the high thresholds, since sudden current steps activated potassium channels that prevented the maintenance of such depolarization. These properties explain the need to stimulate the PPN at specific frequencies, ramping up current up to certain levels, then backing off to maintain depolarization, etc.
Figure 3. PPN neuronal properties.
A. Increasing steps of current (increase of 30 pA per step, each step was 500 ms in duration, 2.5 sec latency between each step, and the record was truncated between current steps and spliced to show only the current steps) caused cells to fire action potentials at higher frequencies until reaching a plateau at 40–60 Hz. This cell fired maximally at 54 Hz, which is within the gamma frequency range [48]. B. Current clamp recording of a PPN neuron in the presence of fast synaptic blockers and TTX, to which were applied current steps of increasing amplitude (dark gray record represents the response to lower amplitude square current while light gray record represents the response to higher amplitude square current steps). Note that the membrane potential failed to be maintained and repolarized below the window for high threshold, voltage-dependent calcium channels (−20 mV). C. Recordings in the same neuron but using ramps of increasing amplitude (dark gray record represents the response to lower amplitude current ramp while the light gray record represents the response to higher amplitude current ramp). Note that the membrane potential could be gradually increased to induce membrane oscillations that could be maintained within the window for activation of high threshold calcium channels (around −20 mV in the soma). Further studies showed that these oscillations were mediated by N- and P/Q-type voltage-dependent calcium channels [49].
Years before the MLR was described, in the initial identification of the RAS, investigators stimulated the region of the PPN to transform the EEG from slow wave activity and sleep to fast activity and arousal [52]. They used chloralose-anesthetized or midbrain-transected (decerebrate) cats and stimulated the region of the mesencephalic reticular formation. They also performed lesions immediately anterior to the PPN that eliminated the effects of such stimulation. These studies typically used stimulation frequencies of 300 Hz, but established that 50 Hz stimulation was close to the lowest effective frequency. Interestingly, the effects of stimulation had a latency, typically 1–2 sec, that is, the effect of stimulation was not instantaneous, but ultimately effective in “recruiting” high frequency EEG activity. The frequency of stimulation and latency used in these RAS studies are remarkably similar to those used in the MLR studies.
But why does the RAS modulate arousal as well as postural and locomotor pathways? The RAS is a phylogenetically conserved system that modulates arousal and fight-or-flight responses. During waking, man’s ability to detect predator or prey is essential to survival. Under these circumstances, it is not surprising that the RAS can also modulate muscle tone and locomotion. This system is thus intrinsically linked to the control of the motor system in order to optimize attack or escape. During REM sleep, the RAS generates the atonia keeps us from acting out our dreams. In fact, only our diaphragm and eye muscles appear to be acting out dream content. Therefore, during both waking and REM sleep, two states modulated by the PPN, the RAS can influence muscle tone and locomotion via the same reticulospinal systems, as well as arousal through ascending pathways to the intralaminar thalamus.
Outputs from the PPN activate reticulospinal systems that lead to profound hyperpolarization of motoneurons, which is the mechanism responsible for the atonia of REM sleep [53]. However, cholinergic projections from the PPN to the medioventral medulla elicit locomotion [54]. Outputs from this medullary region in turn activate reticulospinal systems that lead to the triggering of spinal pattern generators to induce stepping [24,26,50]. In general, electrical stimulation of the pontine and medullary reticular formation is known to induce decreased muscle tone at some sites, while producing stepping movements at other sites. This suggests the presence of a heterogeneous, distributed system of reticulospinal motor control. The required parameters of stimulation for eliciting these differing effects are important such that instantaneous, high frequency trains (similar to high frequency bursting activity in the range of PGO burst neurons that may drive the atonia of REM sleep) trigger pathways which lead to decreased muscle tone, while lower frequency tonic stimulation leads gradually to the “recruitment” of locomotor movements [24,26,50]. Therefore, given the extensive evidence, it is to be expected that the PPN should modulate both posture and locomotion through descending projections, in addition to modulating arousal through ascending projections. Stimulation of the PPN would be expected to affect both postural and locomotor status, as well as arousal and wake-sleep functions.
Parkinson’s disease (PD)
PD patients, in addition to tremor, bradykinesia and akinesia, show sleep disturbances that include increased REM sleep drive, decreased slow wave sleep, frequent awakenings leading to daytime sleepiness, all resulting in insomnia [55]. These observations suggest that the RAS, especially the PPN that is in charge of waking and REM sleep, is overactive in PD. We carried out a study of the P50 potential in PD patients [56]. We showed that P50 potential habituation (the amplitude of the second response as a percent of the first response of a pair) decreased significantly in the PD group. In addition, there was a statistically significant decrease in habituation with disease severity. These findings provided convincing evidence of a decrease in habituation (indicative of a decrease in sensory gating) and a trend towards increased amplitude (indicative of an increase in level of arousal) in a population of PD subjects. Also, we should note that animal studies subsequently confirmed that the PPN is overactive in rodent models of PD [57,58]. In addition, primate models of PD exhibit cell loss in the PPN, and lesions of the PPN in monkeys induce gait and postural deficits [59,60]. Moreover, primate models of PD show sleep disorders that are ameliorated by l-DOPA therapy [61]. These findings point to the confluence of motor and arousal disturbances in PD and its animal models.
We also measured the P50 potential in PD patients who received bilateral pallidotomy that alleviated their motor symptoms, and we found that the amplitude and habituation of the P50 potential were within normal levels after surgery [62]. We speculated that the increased PPN output usually present in PD was instantly re-inhibited or down regulated by the surgery to normalize sensory gating and PPN output. Interestingly, after surgery these patients still needed l-DOPA [59].
As mentioned above, the PPN recently has become a target for deep brain stimulation (DBS) in PD. The clinical approaches, procedures, measures, and outcomes of this therapy have been discussed in detail in a recent review and will not be reiterated [63]. Significantly, DBS of the PPN induces the greatest changes in cortical blood flow in the region of the vertex [64]. This is also the region from which the P50 potential, the mu rhythm, and the RP are recorded, and, significantly, the RP is reduced or absent in PD [65,66]. This emphasizes the fact that the output of the PPN is reflected in activity in the region of the vertex, suggesting that the PPN provides the background on which we base many of our movements. In PD, this background is dysregulated. This process has been termed preconscious awareness, and PPN DBS thus restores a continuous background of activity dysregulated by the disease.
Briefly, patients subjected to PPN DBS at beta/gamma frequencies showed improvements in gait [67], falls [68], and sleep patterns [69,70], sleep scores and executive function also were improved [71]. The take home lesson of these findings is that electrical stimulation of the brain, like that applied using DBS, must take into account the intrinsic membrane properties of neurons in the region being stimulated. If indeed activation is required, it must be within the optimal frequency manifested by those cells, i.e. beta/gamma. If it is inactivation that is required, it may require that stimulation drive neurons beyond their effective firing frequency, i.e. >100 Hz. Therefore, the selection of stimulus parameters is critical to the desired action. Presumably, the continuous stimulation used during DBS helps maintain beta/gamma band activity in the PPN, but does not produce hyperarousal because the system is characteristically rapidly habituating. That may be why continuous PPN DBS (20/24 hr) does not produce undesirable arousal side effects, and may in fact help stabilize PPN output. Thus, it is possible that continuous PPN DBS provides a persistent signal, regulating and stabilizing the circuits at physiological (beta/gamma band) frequencies [34].
Despite the improved outcomes and lack of adverse events, the field still needs further study. It would be advantageous to determine the effects of PPN DBS at 40–60 Hz on the generation and maintenance of gamma band activity in the EEG, on the RP, and on the P50 potential. These would represent more direct measures of PPN output. A more difficult endeavor is the assessment of the role of the PPN in preconscious awareness. Those studies would require perhaps the use of masking experiments and the like.
Preconscious Awareness
The “preconscious” mind includes those things of which we are aware, but we are not paying attention to them. If we choose to pay attention, we bring them to the “conscious” mind [72]. We proposed that it is the activation of the RAS during waking that induces coherent activity (through electrically coupled cells) and high frequency oscillations (through P/Q-type calcium channel and subthreshold oscillation activity), and lead to the maintenance (through activation of G-proteins) of the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis [73,74]. Our results suggest that a similar mechanism to that in the cortex for achieving temporal coherence at high frequencies is present in the PPN, and in its subcortical targets. We suggested that gamma band activity and electrical coupling generated in the PPN may help stabilize coherence related to arousal, providing a stable activation state during waking that participates in preconscious awareness [73,74].
Functionally, the process of preconscious awareness would need to be fairly continuous during waking in order to provide the sensory foundation for planned behavior. Importantly, this “stream of preconsciousness” would need to begin upon waking. It has been shown that increases in blood flow in the thalamus and brainstem begin within 5 min of waking, but as much as 15 min elapse before significant changes in frontal cortex blood flow are observed [75]. This is more surprising when considering that waking follows the last REM sleep episode of the night, during which frontal cortex blood flow is low [76]. The sudden onset of waking after the last REM sleep episode of the night does not instantly increase frontal lobe blood flow. Instead, upon waking, significant increases in the brainstem and thalamus precede restoration of blood flow to the frontal lobes.
The importance of subcortical structures in the determination of states of awareness is being growingly emphasized. Damasio proposed that the brainstem is critical to the formulation of the self, which is critical to the formulation of feelings [77]. Wilder Penfield extensively described the responses to cerebral cortex stimulation in a large number of epilepsy patients. Penfield arrived at the conclusion that, “There is no place in the cerebral cortex where electrical stimulation will cause a patient to believe or to decide” [78]. In addition, he emphasized that, while cortical seizures localized to specific cortical regions elicit sensory or motor effects but maintain consciousness, petit mal seizures in “mesothalamic” (midbrain and thalamus) regions always eliminate consciousness. Based on these results, Penfield proposed the presence of a “centrencephalic integrating system” that fulfills the role of sensorimotor integration necessary for consciousness.
Conclusion
The presence of gamma band activity in the RAS, the most important discovery in the RAS in 10 years, participates in the process of preconscious awareness, and provides the essential stream of information for the formulation of many of our actions. The clinical implications of such a system are far reaching and modulate such functions as fight-or-flight, arousal, wake-sleep control, posture and locomotion, and even volition and free will.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill.
Footnotes
The authors have no conflicts of interest.
References
- 1.Llinas RR. I of the Vortex, from neurons to self. Cambridge: MIT Press; 2001. [Google Scholar]
- 2.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol. Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 3.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llinas RR, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos. Trans. R. Soc. London B. Biol. Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc. Natl. Acad. Sci. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 327–347. [Google Scholar]
- 7.Searle JR. How to study consciousness scientifically. Philos. Trans. Roy. Soc. London B. Biol. Sci. 1998;353:1935–1942. doi: 10.1098/rstb.1998.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Rill E. Waking and the Reticular Activating System in Health and Disease. New York: Academic Press; 2015. [Google Scholar]
- 9.Kornhuber HH, Deecke L. Hirnpotentialänderungen bei Willkürbewe-gungen und passiven bewegungen des menschen: bereitschaftspotential und reafferente potential. Pflug. Arch. 1995;284:1–17. [PubMed] [Google Scholar]
- 10.Deecke L, Grozinger B, Kornhuber HH. Voluntary finger movement in man: cerebral potentials and theory. Biol. Cyber. 1976;23:99–119. doi: 10.1007/BF00336013. [DOI] [PubMed] [Google Scholar]
- 11.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 12.Hallett M. Volitional control of movement: the physiology of free will. Clin. Neurophysiol. 2007;118:1179–1192. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libet B. Do we have free will? J. Consc. Studies. 1999;9:47–57. [Google Scholar]
- 14.Gastaut H, Dongier M, Courtois G. On the significance of “wicket rhythms” (“rhythmes en arceau”) in psychosomatic medicine. Electroenceph. Clin. Neurophysiol. 1954;6:687–688. [Google Scholar]
- 15.Cheyne D, Gaetz W, Garnero L, Lachaux JP, Ducorps A, Schwartz D, et al. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Res. Cog. Brain Res. 2003;17:599–611. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- 16.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J. Neurophysiol. 1954;17:533–537. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 17.Pfurtscheller G. Event-related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroenceph. Clin. Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- 18.Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Map. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Rill E, Skinner RD. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong T, Carskadon MA, M J, editors. Sleep Medicine. Philadelphia, PA: Hanley & Belfus; 2001. pp. 697–704. [Google Scholar]
- 20.Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: Differential effects of sleep in the human. Electroenceph. Clin. Neurophysiol. 1986;65:383–392. doi: 10.1016/0168-5597(86)90017-1. [DOI] [PubMed] [Google Scholar]
- 21.Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: Differential recovery cycle characteristics. Electroenceph. Clin. Neurophysiol. 1986;64:417–423. doi: 10.1016/0013-4694(86)90075-1. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald JS, Rubinstein EH, Schwafel J, Strandburg RJ. Midlatency auditory evoked responses: Differential effects of a cholinergic agonist and antagonist. Electroenceph. Clin. Neurophysiol. 1991;80:303–309. doi: 10.1016/0168-5597(91)90114-d. [DOI] [PubMed] [Google Scholar]
- 23.Kevanishvili Z, von Specht H. Human auditory evoked potentials during natural and drug-induced sleep. Electroenceph. Clin. Neurophysiol. 1979;47:280–288. doi: 10.1016/0013-4694(79)90280-3. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rill E. The pedunculopontine nucleus. Prog. Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Rill E. Disorders of the Reticular Activating System. Med. Hypoth. 1997;49:379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- 26.Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog. Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Rill E, Moran K, Garcia J, Findley WM, Walton K, Strotman B, et al. Magnetic sources of the M50 response are localized to frontal cortex. Clin. Neurophysiol. 2008;119:388–398. doi: 10.1016/j.clinph.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazato H, Skinner RD, Reese NB, Boop FA, Garcia-Rill E. A middle latency auditory evoked potential in the rat. Brain Res. Bull. 1995;37:265–273. doi: 10.1016/0361-9230(95)00003-w. [DOI] [PubMed] [Google Scholar]
- 29.Miyazato H, Skinner RD, Reese NB, Garcia-Rill E. Midlatency auditory evoked potentials and the startle response in the rat. Neurosci. 1996;25:289–300. doi: 10.1016/0306-4522(96)00176-5. [DOI] [PubMed] [Google Scholar]
- 30.Miyazato H, Skinner RD, Garcia-Rill E. Sensory gating of the P13 midlatency auditory evoked potential and the startle response in the rat. Brain Res. 1999;822:60–71. doi: 10.1016/s0006-8993(99)01074-4. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. Rev. 1986;11:47–63. [PubMed] [Google Scholar]
- 32.Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- 33.Garcia-Rill E, Skinner RD, Gilmore SA. Pallidal projections to the mesencephalic locomotor region (MLR) in the cat. Amer. J. Anat. 1981;161:311–322. doi: 10.1002/aja.1001610305. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Rill E, Skinner RD, Gilmore SA, Owings R. Connections of the mesencephalic locomotor region (MLR). II. Afferents and efferents. Brain Res. Bull. 1983;10:63–71. doi: 10.1016/0361-9230(83)90076-x. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neurosci. 1988;17:639–654. doi: 10.1016/0306-4522(88)90295-3. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Rill E, Skinner RD. Modulation of rhythmic functions by the brainstem. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiology of Human Locomotion. Tokyo: Japan Sci. Soc. Press; 1991. pp. 137–158. [Google Scholar]
- 39.Garcia-Rill E, Reese NB, Skinner RD. Arousal and locomotion: from schizophrenia to narcolepsy. In: Holstege G, Saper C, editors. The Emotional Motor System, Prog. Brain Res. Vol. 107. 1996. pp. 417–434. [DOI] [PubMed] [Google Scholar]
- 40.Skinner RD, Kinjo N, Ishikawa Y, Biedermann JA, Garcia-Rill E. Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuro Rep. 1990;1:207–210. doi: 10.1097/00001756-199011000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Skinner RD, Garcia-Rill E. Brainstem modulation of rhythmic functions and behaviors. In: Klemm WR, Vertes RP, editors. Brainstem Mechanisms of Behavior. New York: John Wiley & Sons; 1990. pp. 419–445. [Google Scholar]
- 42.Skinner RD, Garcia-Rill E. Mesolimbic interactions with mesopontine modulation of locomotion. In: Kalivas P, Barnes C, editors. Limbic Motor Circuits and Neuropsychiatry. New York: CRC Press; 1994. pp. 155–191. [Google Scholar]
- 43.Skinner RD, Kinjo N, Henderson V, Garcia-Rill E. Locomotor projections from the pedunculopontine nucleus to the spinal cord. Neuro Rep. 1990;1:183–186. doi: 10.1097/00001756-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neurosci. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neurosci. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 47.Gut NK, Winn P. Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J. Neurosci. 2015;35:4692–4803. doi: 10.1523/JNEUROSCI.3646-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, et al. Gamma band unit activity and population responses in the pedunculopontine nucleus (PPN) J. Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur. J. Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Rill E, Skinner RD. The basal ganglia and the mesencephalic locomotor region. In: Grillner S, G PS, Stein D, Stuart R, Herman H, editors. Neurobiology of Vertebrate Locomotion. London: MacMillan Press; 1986. pp. 77–103. [Google Scholar]
- 51.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J. Appl. Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph. Clin. Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 53.Chase MH, Morales FR. The control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. London: WB Saunders; 1994. pp. 163–176. [Google Scholar]
- 54.Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res. Bull. 1990;24:509–516. doi: 10.1016/0361-9230(90)90104-8. [DOI] [PubMed] [Google Scholar]
- 55.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiat. 2007;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 56.Teo C, Rasco Al, Al-Mefty K, Skinner RD, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson's disease. Movement Disord. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- 57.Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, et al. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neurosci. 2001;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 58.Breit S, Bouali-Benazzouz R, Benabid A, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur. J. Neurosci. 2000;14:1833–1842. doi: 10.1046/j.0953-816x.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 59.Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Inv. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grabli D, Karachi C, Folgoas E, Monfort M, Tande D, Clark S, et al. Gait disorders in parkinsonian monkeys with pedunculopontine nucleus lesions: a tale of two systems. J. Neurosci. 2013;33:11986–11993. doi: 10.1523/JNEUROSCI.1568-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belaid H, Adrein J, Laffrat E, Tande D, Karachi C, Grabli D, et al. Sleep disorders in parkinsonian macaques: effects of L-DOPA treatment and pedunculopontine nucleus lesion. J. Neurosci. 2014;34:9124–9133. doi: 10.1523/JNEUROSCI.0181-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teo C, Rasco Al, Skinner RD, Garcia-Rill E. Disinhibition of the sleep state-dependent P1 potential in Parkinson’s disease-improvement after pallidotomy. Sleep Res. Online. 1998;1:62–70. [PubMed] [Google Scholar]
- 63.Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E. The physiology of the pedunculopontine nucleus-implications for deep brain stimulation. J. Neural Transm. 2014;122:225–235. doi: 10.1007/s00702-014-1243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, Cilia R, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: A[15O] H2O PET study. Human Brain Map. 2009;30:3901–3909. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dick JP, Rothwell JC, Day BL, Cantello R, Buruma O, Gioux M, et al. The Beresitschaftspotential is abnormal in Parkinson’s disease. Brain. 1989;112:233–244. doi: 10.1093/brain/112.1.233. [DOI] [PubMed] [Google Scholar]
- 66.Simpson JA, Khurabeit AJ. Readiness potential of cortical area 6 preceding self paced movement in Parkinson’s disease. J. Neurol. Neurosurg. Psychiat. 1987;50:1184–1191. doi: 10.1136/jnnp.50.9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- 68.Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 69.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Troppei D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 70.Stefani A, Peppe A, Galati S, Stampanoni A, Bassi M, D’Angelo V, et al. The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognitive improvement. Frontiers Neurol. 2013;4 doi: 10.3389/fneur.2013.00068. 68(1–12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive problems. J. Neurol. Sci. 2010;289:44–48. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Civin M, Lombardi KL. The preconscious and potential space. Psychoanal. Rev. 1990;77:573–585. [PubMed] [Google Scholar]
- 73.Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, Bisagno V, et al. Gamma band activity in the RAS-intracellular mechanisms. Exptl. Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bisagno V, Garcia-Rill E. Pedunculopontine nucleus gamma band activity-preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, et al. The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Rill E. Reticular activating system. In: Stickgold R, Walker M, editors. The Neuroscience of Sleep. London, England: Academic Press; 2010. pp. 133–138. [Google Scholar]
- 77.Damasio A. Self Comes to Mind: constructing the conscious brain. New York, NY: Pantheon Books; 2010. [Google Scholar]
- 78.Penfield W. The Mystery of the Mind. Princeton, New Jersey: Princeton University Press; 1975. [Google Scholar]