Abstract
Recent advances have identified a signaling cascade involving receptor interacting protein kinase 1 (RIPK1), RIPK3 and the pseudokinase mixed lineage kinase domain-like (MLKL) that is crucial for induction of necroptosis, a non-apoptotic form of cell death. RIPK1–RIPK3–MLKL-mediated necroptosis has been attributed to cause many inflammatory diseases through the release of cellular damage-associated molecular patterns (DAMPs). In addition to necroptosis, emerging evidence suggests that these necroptosis signal adaptors can also facilitate inflammation independent of cell death. In particular, the RIP kinases can drive NF-κB and inflammasome activation independent of cell death. In this review, we will discuss recent discoveries that led to this realization and present arguments why cell death-independent signaling by the RIP kinases may have a more important role in inflammation than necroptosis.
Keywords: RIPK1, RIPK3, IL-1β, NF-κB, RelB
Introduction
Organismal homeostasis is achieved through an intricate balance of cellular proliferation, senescence and cell death. Apoptosis is an evolutionary conserved process for complex organisms to eliminate unwanted or damaged cells. Early during the process, apoptotic cells express the “eat-me” signal phosphatidyl serine (PS) on the cell surface, which prompts their clearance by macrophages or phagocytes. This process is normally efficient, which explains why apoptotic cells are hard to detect in situ. Immunologists have long recognized the anti-inflammatory nature of apoptosis. This is an important characteristic since apoptosis is prevalent during metazoan development and inflammation would not be a desired outcome. However, excessive apoptosis can occur in certain pathological conditions such as infections. In this situation, apoptosis can progress to secondary necrosis, leading to plasma membrane leakage, release of immunogenic cellular contents and inflammation. In mouse models, inhibition of apoptotic cell clearance often promotes autoinflammatory disease-like symptoms. These observations highlight the intimate link between cell death and inflammation.
In contrast to apoptosis, necrosis is generally considered to be pro-inflammatory and immunogenic. Although necrosis was thought to be the consequence of non-specific trauma to the cells, recent advances demonstrate that necrosis can also be executed in a regulated manner. The receptor interacting protein kinases (RIPKs) are key drivers for a form of regulated necrosis termed necroptosis. The regulation of necroptosis is extensively discussed in other reviews in the same issue and will not be the focus of this review. Instead, we will discuss the emerging evidence that points to a necroptosis-independent role for the RIPKs in inflammation. We present an alternative viewpoint that the RIPKs predominantly drive inflammation through necroptosis-independent mechanisms, and that necroptosis is a fallback option when the inflammatory process goes awry.
The RIPK1 and NF-κB activation
The serine/threonine kinases RIPK1 and RIPK3 are essential adaptors for TNF-induced necroptosis. RIPK1 was originally identified in a yeast two-hybrid screen as a Fas/CD95-interacting adaptor [1]. Although early studies showed that overexpression of RIPK1 could lead to cell death, subsequent studies revealed that RIPK1 predominantly signals for NF-κB activation downstream of TNF receptor 1 (TNFR1) [2–4]. Because NF-κB is a transcription factor that drives expression of many inflammatory genes, it is clear from the early days that RIPK1 can promote cell death-independent inflammation. However, it is noteworthy that studies from different groups have not consistently detected defects in TNF-induced NF-κB activation in Ripk1−/− MEFs [5–7]. In the RIPK1-deficient T cell leukemia cell line Jurkat, early TNF-induced phosphorylation and degradation of IκBα (5 min) was abolished compared with wild-type Jurkat cells. However, by 15 min after TNF stimulation, phosphorylation of IκBα was comparable between wild-type and RIPK1-deficient Jurkat cells [8]. Given the multi-phasic nature of NF-κB-dependent gene expression [9], it is possible that the moderate delay in NF-κB activation in Ripk1−/− cells can result in altered gene expression pattern. Regardless of the extent of its impact on gene expression, it is safe to say that RIPK1 plays only an accessory role in NF-κB activation by modulating the kinetics of IκBα phosphorylation and degradation. Since the majority of signal adaptors of the TNFR1 pathway are ubiquitinated species, they may compensate for the loss of RIPK1 to facilitate IKK complex and NF-κB activation in Ripk1−/− cells [10].
The kinase activity of RIPK1, which is crucial for death receptor-mediated apoptosis and necroptosis, is dispensable for NF-κB activation [2, 3]. Interestingly, while germline Ripk1−/− mice suffer from post-natal lethality [4], knock-in mice expressing kinase-inactive RIPK1 are viable [11–13]. This indicates that RIPK1 has a unique function in organismal survival that is scaffold dependent, but kinase independent [14–17]. This is an emerging theme for RIPK1 and RIPK3 (see below): that they can promote inflammation through scaffold-dependent and necroptosis-independent mechanisms. In contrast to Ripk1−/− mice, mice lacking the canonical NF-κB subunit RelA/p65 die in utero on e15.5 [18], and deficiency of the non-canonical NF-κB subunit RelB did not compromise post-natal survival [19]. As such, RIPK1 mediates post-natal survival independent of NF-κB.
Scaffold-dependent signaling by RIPK1 is critical for survival of intestinal and skin epithelial cells and hematopoietic stem cells (HSCs) [6, 7, 16, 20]. Although RIPK1 is crucial for HSCs survival [16, 20], it is dispensable for survival of fully differentiated mature bone marrow-derived dendritic cells (BMDCs) ([21] and unpublished observation). Similarly, mature T cells from Ripk1−/−Fadd−/− mice proliferated normally in response to viral pathogen challenge [22]. Hence, RIPK1 is dispensable once HSCs differentiate beyond a certain developmental checkpoint. A similar function for RIPK1 may also protect rapidly dividing tissues such as the skin and intestinal epithelium from cell death-induced inflammation.
RIPK3 and NF-κB activation
Because of its homology to RIPK1, early studies on RIPK3 also focused on its ability to induce apoptosis and NF-κB. Results from overexpression studies were confusing, with reports showing both an activating and inhibitory role for RIPK3 in NF-κB activation. For instance, RIPK3 inhibited NF-κB activation by the toll-like receptor 3 (TLR3) and TLR4 signal adaptor TRIF, TNFR1 and DNA activator of interferon (DAI) [23–26], but enhanced NF-κB activation in other studies [27, 28]. In mouse embryonic fibroblasts (MEFs) and bone marrow-derived macrophages (BMDMs), RIPK3 was reported to be dispensable for TNF-or TLR4-induced NF-κB activation [29, 30]. Hence, it was widely accepted that RIPK3 plays no major role in NF-κB activation. However, closer examination of the published results showed that although TNF, TLR2 and TLR4-induced IκBα phosphorylation and degradation was normal in Ripk3−/− cells, LPS-induced TNF, IL-6 and IL-1β expression and hypothermia were reduced in Ripk3−/−mice [30–32].
We re-evaluated the role of RIPK3 in NF-κB activation and found that RIPK3 expression in BMDCs is crucial for LPS-induced and NF-κB-dependent cytokine expression [33]. Consistent with results from MEFs and BMDMs, the initial LPS-induced IκBα phosphorylation and degradation was normal in Ripk3−/− BMDCs [30, 33]. However, LPS-induced nuclear translocation of the RelB-p50 heterodimer was severely impaired in Ripk3−/− BMDCs [33]. Strikingly, nuclear translocation of other NF-κB subunits was not affected. These results indicate that while RIPK1 facilitates the early phosphorylation and degradation of IκBα, RIPK3 regulates NF-κB activation downstream of IκBα in a cell type-specific manner. Thus, although RIPK1 and RIPK3 often act in synergy to promote cell death, they regulate NF-κB activation independently through distinct mechanisms.
How might RIPK3 regulate RelB-p50 nuclear translocation? Curiously, a recent report shows that RIPK1, RIPK3 and MLKL translocate to the nucleus during necroptosis [34]. Nuclear RIPK3 has been detected in damaged neurons after ischemia–reperfusion-induced injury, a process believed to involve necroptosis [35]. This raises the tantalizing possibility that nuclear RIPK3 may control cell death. Several early studies showed that RIPK3 contains both nuclear localization and nuclear export signal sequences and could shuttle between the cytosol and the nucleus [36, 37]. Hence, RIPK3 may directly chaperone RelB-p50 dimer into the nucleus in response to TLR4 stimulation. Alternatively, RIPK3 may indirectly control RelB-p50 nuclear translocation through its molecular chaperone Hsp90, which binds to and regulates RIPK3-dependent necroptosis [38, 39]. In this regard, Hsp90 has been shown to regulate NF-κB nuclear translocation by stabilizing the upstream activators IKKs and IRAK-1 [40]. Hsp90 inhibitors are promising anti-cancer agents [41]. It will be interesting to determine whether Hsp90 inhibitors exert their anti-tumor effects by suppressing RIPK3-dependent necroptosis and NF-κB-dependent inflammatory gene expression.
Context is important: the mechanism of RIPK3-mediated inflammasome activation
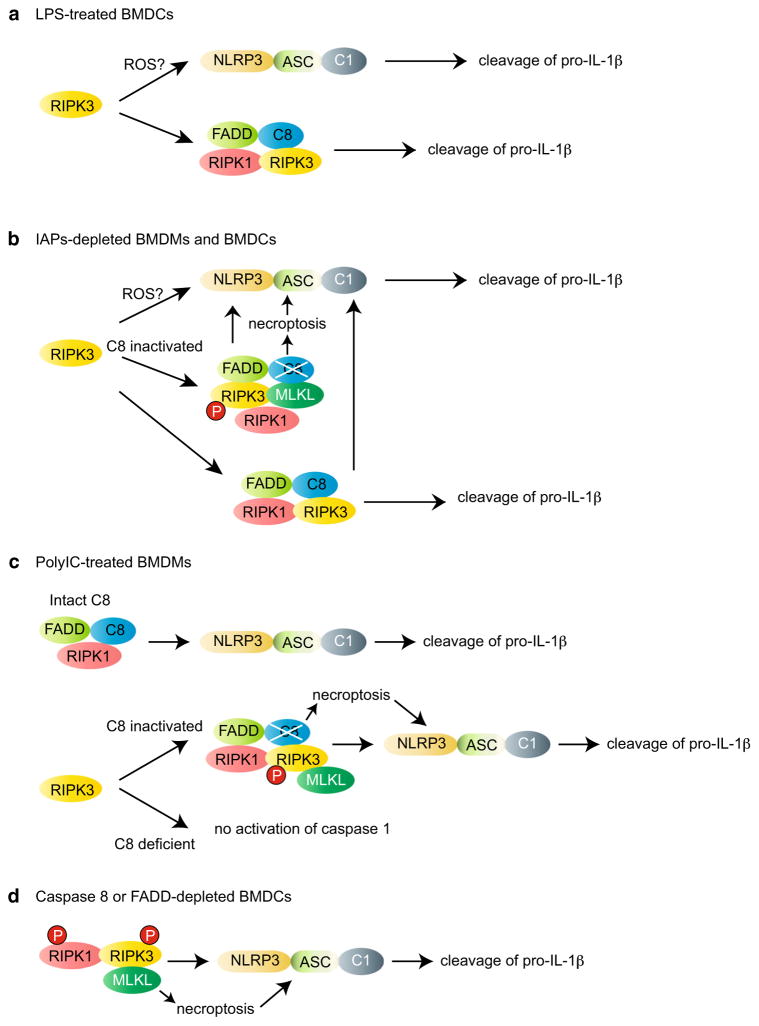
Much of the work on necroptosis-independent signaling by RIPK3 has focused on its role in NLRP3 inflammasome activation. The inflammasome is a macromolecular complex composed of the adaptor protein ASC, the IL-1β converting enzyme (ICE) caspase 1, and a sensor molecular such as NLRP3. Inflammasome activation results in caspase 1-mediated cleavage of pro-IL-1β and pro-IL-18, secretion of the mature cytokines, and a non-apoptotic form of cell death called pyroptosis [42, 43]. In contrast to LPS-primed BMDMs, which require a second inflammasome signal to secrete mature IL-1β, LPS alone is sufficient to induce IL-1β secretion in BMDCs [44]. This LPS-induced IL-1β secretion was completely abolished in Ripk3−/−BMDCs [21, 44]. Since pro-IL-1β protein expression was normal in LPS-primed Ripk3−/− BMDCs, RIPK3 regulates processing, but not de novo synthesis of IL-1β. RIPK3 promotes pro-IL-1β cleavage through the NLRP3 inflammasome as well as the ripoptosome, a macromolecular apoptosis and necroptosis-inducing complex consisting of RIPK1, RIPK3, FADD and caspase 8 [45–47]. Importantly, kinase activities of RIPK1 and RIPK3 are dispensable and cell death was not detected under these conditions (Fig. 1a). Hence, in contrast to ripoptosome assembly during cell death, RIPK3 acts as a positive activator for caspase 8 during pro-IL-1β processing.
Fig. 1.
The different modes of RIPK3-mediated NLRP3 inflammasome activation. a In BMDCs, RIPK3 promotes activation of caspase 1 (C1) in response to LPS alone, possibly through ROS production. In addition to caspase 1, RIPK3 promotes caspase 8 activation through the ripoptosome, which directly cleaves pro-IL-1β. b Depletion of IAP proteins induces IL-1β secretion through robust activation of caspase 1 and caspase 8 in LPS-primed BMDMs and BMDCs. In contrast to necroptosis, the kinase activities of RIPK1 and RIPK3 are dispensable for pro-IL-1β processing through caspase 1 and caspase 8. However, when caspase 8 (C8) activity is blocked, the RIPK3 kinase activity and MLKL becomes essential to stimulate the NLRP3 inflammasome activation. c In BMDMs, poly(I:C) treatment stimulates TLR3 and TRIF, leading to FADD, RIPK1 and caspase 8 (C8)-dependent NLRP3 inflammasome activation. RIPK3 is not required for this response. However, when caspase 8 activity is blocked, RIPK3 kinase activity and MLKL phosphorylation become essential for NLRP3 inflammasome activation. In addition, RIPK3-dependent NLRP3 inflammasome activation requires caspase 8 scaffold function, since TLR3 can no longer stimulate NLRP3 inflammasome when caspase 8 is missing. d LPS-primed caspase 8 or FADD-deficient BMDCs produce increased levels of IL-1β through enhanced caspase 1 activation. This response requires RIPK1 and RIPK3 kinase activities and MLKL. MLKL activation through the RIPK3 kinase activity may directly activate the NLRP3 inflammasome. Alternatively, MLKL might enhance necroptosis, leading to DAMPs release or K+ efflux [102] and subsequent NLRP3 inflammasome activation. P in a circle indicates that the kinase activity of RIPK1 or RIPK3 is required
As in the case of necroptosis, RIPK3 and ripoptosome-mediated pro-IL-1β processing is tightly controlled by FADD, caspase 8 and the E3 ligase IAPs, cIAP1, cIAP2 and X-linked IAP (XIAP). Genetic inactivation or pharmacological depletion of the IAPs, especially XIAP, greatly enhanced IL-1β secretion in LPS-primed BMDMs [48, 49]. As in the case of LPS-induced BMDCs, an intact RIPK3, but not its kinase activity, is essential for IL-1β release under this condition (Fig. 1b). RIPK3 and the ripoptosome stimulate pro-IL-1β processing by turning on the NLRP3 inflammasome [50]. When caspase 8 activity is inhibited by caspase inhibitors, the ripoptosome recruits an additional component, the necroptosis effector MLKL, to promote IL-1β secretion (Fig. 1b). Strikingly, RIPK3 kinase activity is required for optimal IL-1β secretion when caspase 8 activity is compromised [50]. These results highlight the highly intertwined nature of the machineries that mediate necroptosis and NLRP3 inflammasome activation. However, Asc−/− and Casp1−/− macrophages are equally sensitive to LPS and zVAD-fmk-induced necroptosis as wild-type macrophages (unpublished observation). Thus, the ripoptosome and NLRP3 inflammasome are not interchangeable in function.
In addition to determining the mechanism of ripoptosome-induced NLRP3 inflammasome activation, caspase 8 also has a scaffolding function in inflammasome activation. In Ripk3−/−Fadd−/− and Ripk3−/−casp8−/− BMDMs, ASC oligomerization, caspase 1 activation, and IL-1β secretion in response to the synthetic double-strand RNA poly(I:C), a TLR3 ligand, and ATP were abrogated [51]. This response was also abrogated in Ripk1−/−, but not Ripk3−/− BMDMs. Hence, RIPK1, FADD and caspase 8 can drive NLRP3 inflammasome activation without RIPK3 (Fig. 1c). Several reports have indicated a role for RIPK3 in NLRP3 inflammasome activation in response to RNA virus infection [51, 52]. However, we have not been able to detect RIPK3-dependent IL-1β secretion in response to vesicular stomatitis virus infection (unpublished observation). Therefore, in BMDMs with intact caspase 8, RIPK3 appears to have minimal role in RNA-induced IL-1β release. When caspase 8 activity is inhibited by pharmacologic inhibitors, RIPK3 becomes an essential component for TLR3-induced NLRP3 inflammasome assembly (Fig. 1c). In addition to RIPK3, RIPK1 and MLKL are also required for this assembly. Although caspase 8 protease activity is dispensable for this activity, TLR3-induced NLRP3 inflammasome assembly was completely abolished in caspase 8-deficient cells. Hence, caspase 8 scaffold function is crucial for RIPK1–RIPK3–MLKL-mediated NLRP3 inflammasome activation (Fig. 1c).
In contrast to TLR3, TLR4-induced caspase 1 activation and IL-1β secretion were highly elevated in LPS-primed Fadd−/− or caspase 8−/− BMDCs [53, 54]. This is especially surprising given that FADD and caspase 8 have been implicated in transcriptional priming of pro-IL-1β and NLRP3 in Fadd−/−Ripk3−/− and Casp8−/−Ripk3−/− BMDMs [31, 55]. Increased IL-1β secretion by Fadd−/−and casp8−/− BMDCs requires RIPK1 kinase activity, RIPK3 and MLKL [53]. Thus, RIPK1–RIPK3–MLKL-driven inflammasome activation and IL-1β secretion can occur in the absence of FADD and caspase 8 in BMDCs (Fig. 1d). Taken together, these studies reveal the complex interplay between RIPK1/RIPK3 and FADD/caspase 8. In the context of necroptosis, caspase 8 acts as a natural inhibitor of RIPK1 and RIPK3 through its proteolytic activity. On the other hand, RIPK3 drives caspase 8 activation through assembly and activation of the ripoptosome in response to TLR3 and TLR4 stimulation [56]. The molecular basis that dictates cell type- and context-dependent ripoptosome and inflammasome activation is unknown at present, but is likely to be related to different expression and wiring of ripoptosome components in BMDMs versus BMDCs.
How does RIPK3 turn on the NLRP3 inflammasome?
Although the role of RIPK3 in NLRP3 inflammasome activation is well established, the underlying mechanism is undefined at present. Direct physical interaction between the ripoptosome and inflammasome components has not been reported, suggesting that RIPK3 regulates inflammasome activation in an indirect manner. Interestingly, reactive oxygen species (ROS) scavengers such as N-acetyl cysteine inhibit caspase 1, but not caspase 8 activation [33, 49]. Since mitochondrial ROS has been implicated in NLRP3 inflammasome activation [57], RIPK3 may indirectly promote NLRP3 inflammasome activation through stimulating mitochondrial ROS production.
As we have already discussed in previous sections, in the presence of intact FADD and caspase 8, RIPK1 and RIPK3 kinase activities are dispensable for ripoptosome-mediated pro-IL-1β processing. For example, BMDCs that express kinase-inactive RIPK1 or RIPK3 produced normal levels of IL-1β in response to LPS [21]. This is distinct from necroptosis, which critically depends on the kinase function of RIPK3. By contrast, an intact RHIM is required for RIPK3-dependent necroptosis, ripoptosome formation and NLRP3 inflammasome activation. The RHIM, or RIP homotypic interaction motif [58], is found in a select group of cell death/innate immune signal adaptors including TRIF, RIPK1, RIPK3, DAI, herpesvirus-encoded necroptosis inhibitors, and certain Drosophila immune deficiency (IMD) pathway adaptors [59–64]. During necroptosis, the RHIM mediates conformational change that leads to amyloid-like filament formation. This process is crucial for nucleating the ripoptosome complex [65]. Mutations in the tetra-peptide core of the RIPK3 RHIM domain abolished amyloid formation and TNF-induced necroptosis [29]. An intact RHIM is also required for LPS-induced ripoptosome activation and IL-1β secretion by BMDCs (unpublished observation), although it is not clear if amyloid conversion is also involved.
RIPK1 as an inhibitor of RIPK3
Although RIPK1 is widely known to act in synergy with RIPK3 to promote apoptosis, necroptosis and NLRP3 inflammasome activation, recent evidence indicates that RIPK1 can surprisingly inhibit RIPK3 activity in certain situations. Deletion of Ripk1 in intestinal epithelium or skin epidermal tissues led to spontaneous cell death and inflammation [6, 7]. Inactivation of Ripk3 in the skin epidermis rescued the Ripk1 deficiency-induced inflammation, while dual inactivation of RIPK3 and FADD restored normal intestinal integrity in Ripk1-deficient mice [6, 7]. These results indicate that RIPK1 enforces barrier integrity by limiting RIPK3 activity. Germline Ripk1−/− mice suffer from post-natal mortality due to multi-organ cell injury and inflammation [4]. In contrast to the germline Ripk1−/−mice, knock-in mice expressing kinase-inactive RIPK1 are viable and do not exhibit increased cell death and inflammation [11–13]. Hence, while RIPK1 kinase activity is responsible for apoptosis and necroptosis, it is dispensable for its RIPK3 inhibitory effect. RIPK1-independent but RIPK3-dependent necroptosis has been observed in tissue culture experiments [66–68], although the precise mechanism by which RIPK1 inhibits RIPK3 activation is unknown at present.
Does necroptosis-independent signaling matter in tissue inflammation?
As discussed in other reviews in this issue, the current dogma predicates that RIPK3 drives tissue inflammation mainly through necroptosis-associated release of DAMPs, which subsequently trigger an inflammatory cytokine storm. Evidence that supports this model mainly comes from mouse studies in which multiple IAPs, FADD or caspase 8 are inactivated [69–72]. In these models, germ-line inactivation of RIPK3 was often sufficient to rescue the inflammatory conditions. With the realization that RIPK3 can promote inflammation through scaffold-dependent and kinase-independent mechanisms, it is high time for researchers to re-evaluate the relative contribution of these mechanisms to physiological inflammation [73].
One of the first examples of physiological/pathological necroptosis is found in vaccinia virus infection. Poxviruses such as vaccinia virus encode caspase inhibitors that can skew the response from apoptosis to necroptosis (reviewed in [74]). Like other poxviruses, vaccinia virus causes tissue necrosis and inflammation that are eventually resolved in wild-type mice. Surprisingly, tissue necrosis and inflammation was significantly reduced in infected Ripk3−/−mice. This led to highly elevated levels of viral load and eventual death of Ripk3−/− mice [29]. In contrast to vaccinia virus, a growing number of studies show that herpes viruses actively suppress RIPK3-dependent necroptosis through the viral inhibitor of RIP kinase activation (vIRA) [59, 60, 75, 76]. The equine herpesvirus encoded inhibitor E8 and Kaposi sarcoma virus encoded K13, which are viral inhibitors of caspase 1 and caspase 8, also inhibited TNF-induced necroptosis [77]. Hence, herpesviruses have developed multiple strategies to counteract the anti-viral effects of necroptosis. Interestingly, the human poxvirus cell death inhibitor MC159 also potently inhibited TNF-induced necroptosis [77, 78]. Therefore, sensitization to necroptosis may not be a common feature for all poxviruses.
Inhibition of necroptosis is not restricted to virus infections. In tissue culture experiments, necroptosis induction requires inhibition of the cIAPs and FADD/caspase 8. In addition, the TNF receptor signal adaptors TRAF2, TAK1, IKKs and NEMO have also been shown to restrict necroptosis [79–83]. The multiple inhibitory mechanisms argue that physiological necroptosis requires the “perfect storm” in which all these regulatory checkpoints are compromised. This is certainly a high bar to reach under normal circumstances. Genetic studies tell us that these cellular necroptosis inhibitors are critical for organismal survival. In fact, deficiency of these molecules in specific tissues is often sufficient to cause deleterious inflammation. These observations argue that physiological necroptosis is a rare occurrence.
An examination of the role of RIPK1 and RIPK3 in lymphocytes also suggests that necroptosis may not always result in inflammation. Mice lacking FADD or caspase 8 and RIPK3 developed systemic autoimmune lymphoproliferation that resembles human lupus and mice with Fas or Fas ligand (FasL) mutations [22, 84–87]. Hence, caspase-dependent apoptosis and RIP kinase-mediated necroptosis cooperate to regulate lymphocyte homeostasis. By eliminating activated, cytokine-producing lymphocytes, one can consider RIPK1/RIPK3-dependent necroptosis as an anti-inflammatory response [88]. As such, we propose an alternative model in which the main mechanism by which RIPK3 promotes inflammation is through NF-κB-dependent cytokine gene transcription and ripoptosome/ inflammasome-mediated pro-IL-1β processing. When key components of this pathway are disrupted, such as that found in certain virus infections and mice lacking FADD, caspase 8 or cIAPs, necroptosis is activated as a last resort to tamp down the collateral damage from a hyperactive inflammatory response. The concept that RIPK1 and RIPK3 have “day jobs” other than necroptosis is not new among cell death signal adaptors. Members of the Bcl-2 family, for instance, have been shown to regulate diverse cellular functions such as glucose and mitochondrial metabolism [89], regulation of calcium signaling [90] and autophagy [91].
Concluding remarks
To distinguish the contribution of necroptosis-dependent and independent signaling by RIPK1 and RIPK3 in inflammation, we need better knowledge on how these kinases are activated under different conditions. Phosphorylation sites on RIPK1 and RIPK3 that are important for necroptosis have been identified [8, 92–94]. In addition, phospho-MLKL and phospho-RIPK3 antibodies have been developed [94–97]. These reagents will be useful tools in distinguishing the mode of RIPK3 activation during necroptosis-independent signaling. In addition to benefiting basic science, a holistic understanding of the biology and mechanism of RIP kinase activation is important for potential therapeutic targeting of these molecules in inflammatory diseases. In this regard, RIPK1 and RIPK3 inhibitors have been developed [56, 95, 98–101]. Developing inhibitors that can target additional inflammatory pathways beyond necroptosis may magnify therapeutic potential of RIP kinase-targeted therapies.
Acknowledgments
This work is supported by NIH grant AI119030. F. K. M. C. is supported by a Senior Research Award from the Crohn’s & Colitis Foundation of America. K. M. was supported by fellowships from the Uehara Memorial Foundation and the Japan Society for the Promotion of Science.
References
- 1.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/ APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 2.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4(4):387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 3.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15(22):6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 4.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 5.Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17(3):482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, Goossens V, Lefebvre S, Gunther C, Becker C, Bertin J, Gough PJ, Declercq W, van Loo G, Vandenabeele P. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513(7516):95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 7.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513(7516):90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuade T, Cho Y, Chan FK. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem J. 2013;456(3):409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen R, Smale ST. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect Biol. 2010;2(4):a000257. doi: 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmukle AC, Walczak H. No one can whistle a symphony alone—how different ubiquitin linkages cooperate to orchestrate NF-kappaB activity. J Cell Sci. 2012;125(Pt 3):549–559. doi: 10.1242/jcs.091793. [DOI] [PubMed] [Google Scholar]
- 11.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192(12):5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FK, Pasparakis M, Kelliher MA. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193(4):1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343(6177):1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA. 2014;111(21):7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Dowling JP, Nair A, Zhang J. A novel function of RIP1 in postnatal development and immune homeostasis by protecting against RIP3-dependent necroptosis and FADD-mediated apoptosis. Front Cell Dev Biol. 2015;3:12. doi: 10.3389/fcell.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 19.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 20.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, Kelliher MA. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci USA. 2014;111(40):14436–14441. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J Immunol. 2015;194(4):1938–1944. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471(7338):373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5(5):503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274(24):16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 25.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10(8):916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181(9):6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pazdernik NJ, Donner DB, Goebl MG, Harrington MA. Mouse receptor interacting protein 3 does not contain a caspase-recruiting or a death domain but induces apoptosis and activates NF-kappaB. Mol Cell Biol. 1999;19(10):6500–6508. doi: 10.1128/mcb.19.10.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, Nolan GP, Payan DG, Luo Y. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9(10):539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 29.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24(4):1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, Mason KD, White MJ, Stacey KJ, Strasser A, O’Reilly LA, Alexander W, Kile BT, Vaux DL, Vince JE. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15(9):982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA. 2014;111(31):E3206–E3213. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41(4):567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon S, Bogdanov K, Kovalenko A, Wallach D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin B, Xu Y, Wei RL, He F, Luo BY, Wang JY. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/ reperfusion injury. Brain Res. 2015;1609:63–71. doi: 10.1016/j.brainres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Ma J, Chen Y, Wu M. Nucleocytoplasmic shuttling of receptor-interacting protein 3 (RIP3): identification of novel nuclear export and import signals in RIP3. J Biol Chem. 2004;279(37):38820–38829. doi: 10.1074/jbc.M401663200. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Feng S, Wu M. Multiple roles for nuclear localization signal (NLS, aa 442-472) of receptor interacting protein 3 (RIP3) Biochem Biophys Res Commun. 2008;372(4):850–855. doi: 10.1016/j.bbrc.2008.05.144. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA. 2015;112(16):5017–5022. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SY, Shim JH, Chae JI, Cho YS. Heat shock protein 90 inhibitor regulates necroptotic cell death via down-regulation of receptor interacting proteins. Pharmazie. 2015;70(3):193–198. [PubMed] [Google Scholar]
- 40.Lee KH, Jang AH, Yoo CG. 17-Allylamino-17-Demethoxygeldanamycin and the Enhancement of PS-341-Induced Lung Cancer Cell Death by Blocking the NF-kappaB and PI3K/Akt Pathways. Am J Respir Cell Mol Biol. 2015;53(3):412–421. doi: 10.1165/rcmb.2014-0186OC. [DOI] [PubMed] [Google Scholar]
- 41.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 44.He Y, Franchi L, Nunez G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190(1):334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205(9):1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwalder M, Strasser A, Gross O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell reports. 2014;7(6):1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36(2):215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, Rashidi M, Wicks IP, Alexander WS, Mitsuuchi Y, Benetatos CA, Condon SM, Wong WW, Silke J, Vaux DL, Vince JE. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, Roback L, Kaiser W, Oberst A, Sagara J, Fitzgerald KA, Green DR, Zhang J, Mocarski ES, Alnemri ES. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15(12):1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 53.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Young JA, He TH, Reizis B, Winoto A. Commensal microbiota are required for systemic inflammation triggered by necrotic dendritic cells. Cell Rep. 2013;3(6):1932–1944. doi: 10.1016/j.celrep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. RIP3 Induces Apoptosis Independent of Pronecrotic Kinase Activity. Mol Cell. 2014;56(4):481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277(11):9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 59.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17(2):243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17(2):229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111(43):15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleino A, Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev Comp Immunol. 2014;42(1):25–35. doi: 10.1016/j.dci.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7(7):715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 64.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Ann Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kearney CJ, Cullen SP, Clancy D, Martin SJ. RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J. 2014;281(21):4921–4934. doi: 10.1111/febs.13034. [DOI] [PubMed] [Google Scholar]
- 68.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21(10):1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477(7364):330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 70.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35(4):572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477(7364):335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O’Reilly L, Jost PJ, Murphy JM, Alexander WS, Strasser A, Vaux DL, Silke J. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123(16):2562–2572. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- 73.Wallach D, Kovalenko A, Kang TB. ‘Necrosome’-induced inflammation: must cells die for it? Trends in immunology. 2011;32(11):505–509. doi: 10.1016/j.it.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Upton JW, Chan FK. Staying alive: cell death in antiviral immunity. Mol Cell. 2014;54(2):273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 78.Challa S, Woelfel M, Guildford M, Moquin D, Chan FK. Viral cell death inhibitor MC159 enhances innate immunity against vaccinia virus infection. J Virol. 2010;84(20):10467–10476. doi: 10.1128/JVI.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Donnell MA, Hase H, Legarda D, Ting AT. NEMO inhibits programmed necrosis in an NFkappaB-independent manner by restraining RIP1. Plos One. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33(3):582–595. doi: 10.1128/MCB.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ. NF-kappaB-independent role of IKKalpha/ IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015 doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 83.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ. NF-kappaB-independent role of IKKalpha/ IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60(1):63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 84.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, Walsh CM. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci USA. 2011;108(37):15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208(4):633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, Martin SJ. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ. 2015;22(8):1313–1327. doi: 10.1038/cdd.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424(6951):952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 90.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13(8):1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 91.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20(2):366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288(23):16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, Chen T, Green DR. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci USA. 2015;112(35):11007–11012. doi: 10.1073/pnas.1514730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M, Gridling M, Parapatics K, Colinge J, Bennett KL, Kubicek S, Krautwald S, Linkermann A, Superti-Furga G. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Najjar M, Suebsuwong C, Ray SS, Thapa RJ, Maki JL, Nogusa S, Shah S, Saleh D, Gough PJ, Bertin J, Yuan J, Balachandran S, Cuny GD, Degterev A. Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep. 2015;10(11):1850–1860. doi: 10.1016/j.celrep.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]