Abstract
African American participation in Alzheimer’s disease (AD) research studies has been historically low. To determine if older African Americans and Caucasians had different knowledge or attitudes related to AD, we administered the Alzheimer’s Disease Knowledge Scale (ADKS) to 67 older African Americans and 140 older Caucasians in the greater Atlanta area, as well as questions targeting locus of control over general health and AD risks. Older African Americans scored slightly lower on ADKS than older Caucasians, with race only accounting for 1.57 (95% CI 0.57–2.61, p < 0.001) points of difference in a mutli-variate model. Attitudes towards AD were also similar between the two groups, but one in three (35.7%) adults reported control over general health but not AD risks. In addition to enhancing education content in outreach efforts, there is an urgent need to address the perception that future AD risks are beyond one’s own internal control.
Keywords: African American, Caucasian, education, locus of control, minority, race, survey
INTRODUCTION
Alzheimer’s disease (AD) is the leading cause of dementia and a major cause of death in the United States.1 Natural history and biomarker studies in AD have provided significant insight into shared mechanisms of disease,2, 3 but racial minority participation has historically been limited for the purpose of identifying endophenotypes and disease mechanisms unique to certain populations.4–8 Previous studies have shown that minority participation in AD research is influenced by knowledge, both positive and negative, about general research issues and specific study protocols.8–10 However, it is not clear if race directly contributes to a difference in AD-related knowledge, as knowledge can be influenced by other factors such as age, gender, education, and personal exposure to AD. It is also not known if attitudes towards AD differ between racial groups and are influenced by race-independent factors.
The Alzheimer’s Disease Knowledge Scale (ADKS) was developed to assess an individual’s AD-related knowledge, including risk factors, diagnosis, symptoms, course, impact, and management.11 The English version has been administered in the US, UK, and Australia,12–15 and non-English versions have also been created.16, 17 Analysis of ADKS scores from several US regions has yielded interesting findings. In a multiracial cohort from St. Louis, Caucasians had higher ADKS scores than Asians and those identifying as other races or multiracial, but had similar scores as African Americans.13 However, over half of the respondents were undergraduate students who were overall less knowledgeable about AD, and only 11% of respondents were community-dwelling older adults. Another study using paid online participants (average age 34.5 yrs) did not report any difference in ADKS scores according to demographic variables.18 A third study administered ADKS to older African Americans in an urban senior community center in Philadelphia.14 These older adults scored much lower as a group (18.7) than older adults from St. Louis (24.1), but no demographic variables were available in the Philadelphia study to determine whether ADKS score is influenced by gender, education, income, and other demographic variables. While no race information was specifically reported for the older adult group in St. Louis, these results together suggest that race may account for up to 6 points difference in ADKS. This gap can be due to differences in education, household income, and recruitment bias associated with the two racial groups. It is also unknown whether the ADKS score difference between the two studies was influenced by factors beyond race.
Among U.S. metropolitan areas, Atlanta has the second highest number of African Americans who account for over 30% of the Atlanta population,19 and was involved in a previous study on race and AD knowledge.20 As part of an on-going biomarker study on African Americans and non-Hispanic Caucasians, we have targeted older adults with no cognitive impairment for participation through community events, research registries, and primary care clinics of a large academic medical center. Here we examined the relationship between ADKS scores, demographic factors, and recruitment source in a large multi-racial cohort of older adults to determine predictors of ADKS score. Furthermore, we assessed participants’ attitudes toward AD using an established scale14 to determine factors which influence attitudes towards AD, and whether there is a relationship between knowledge and attitudes in AD.
DESIGN AND METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
Because no protected health or other identifying information (other than categories) was collected, the Emory Institutional Review Board reviewed the study and determined the work to not require formal approval.
Study Participants
Potential participants over the age of 50 in the greater Atlanta area were recruited from September, 2014 to April, 2015 from three sources: 1) Research: research participants in an NIH-funded study on biomarkers on aging, endothelial dysfunction, and AD among African American and Caucasian seniors; 2) Community: family or friends who accompanied participants in the research study in 1, or community members attending community outreach and education events; and 3) Primary Care: patients presenting to the Emory Primary Care Internal Medicine Clinic for annual physical examination or illness visits.
Study Design
Participants were asked by study staff to complete a brief survey related to AD. They were also informed that their decision to complete or not complete the survey would not influence their research participation or clinical care. Among 300 potential respondents contacted, 218 (73%) completed surveys were returned in person for analysis (Table 1).
Table 1.
Demographic information for all survey respondents. Respondents who did not report African American or Caucasian race were excluded from subsequent analysis due to their small number.
| African American (n=67) | Caucasian (n=140) | Other (n=11) | p | |
|---|---|---|---|---|
| Male | 26 (39%) | 69 (49%) | 3 (27%) | 0.178 |
| Age | 0.238 | |||
| <55 | 13 (19%) | 33 (24%) | 5 (45%) | |
| 55–64 | 19 (28%) | 21 (15%) | 1 (9%) | |
| 65–74 | 24 (36%) | 49 (35%) | 3 (27%) | |
| 75–84 | 10 (15%) | 29 (21%) | 2 (18%) | |
| >84 | 1 (2%) | 8 (6%) | 0 (0%) | |
| Education | 0.143 | |||
| High school or less | 18 (27%) | 20 (14%) | 2 (18%) | |
| Associate degree | 11 (16%) | 22 (16%) | 1 (9%) | |
| Bachelor’s degree | 17 (25%) | 47 (34%) | 2 (18%) | |
| Master’s degree or more | 21 (31%) | 51 (36%) | 6 (55%) | |
| Self-reported health | <0.001* | |||
| Fair or poor | 12 (18%) | 8 (6%) | 0 (0)% | |
| Good | 43 (64%) | 84 (60%) | 3 (27%) | |
| Excellent | 12 (18%) | 48 (34%) | 8 (73%) | |
| Annual household income | <0.001† | |||
| < $20,000 | 9 (13%) | 10 (7%) | 0 (0%) | |
| $20,000–$38,000 | 8 (12%) | 6 (4%) | 0 (0%) | |
| $38,001–$60,000 | 11 (16%) | 22 (16%) | 6 (55%) | |
| $60,001–$100,000 | 23 (34%) | 26 (19%) | 2 (18%) | |
| > $100,000 | 12 (18%) | 66 (47%) | 3 (27%) |
p = 0.003 for African Americans compared to Caucasians;
p < 0.001 for African Americans compared to Caucasians.
The survey consisted of three parts: 1) basic demographic information including age (<55, 55–64, 65–74, 75–84, >85), gender, self-identified race (Caucasian, African American, Asian, Other), ethnicity (Hispanic, non-Hispanic), household income range (according to quintiles from US census),21 education (high school or less, associate degree, bachelor’s degree, master’s degree or more), and self-reported health status (excellent, good, fair, poor); 2) the ADKS,11 which consists of 30 true/false questions assessing AD-related knowledge in the following seven subcategories: life impact, risk factors, symptoms, treatment and management, caregiving, assessment and diagnosis, and disease course (test-retest reliability coefficient 0.81, correlation with self-reported knowledge 0.50, correlation with AD Knowledge Test22 0.65); and 3) respondents’ attitudes towards six AD-related statements in Likert scale format (0, 1, 2, 3) with 0 representing “strongly disagree” and 3 representing “strongly agree.”14 The Likert scale was chosen to maximize sensitivity,23 and subjects’ responses were subsequently dichotomized (agree for 0 and 1, and disagree for 2 and 3). Statements reflecting attitudes were previously used to assess health locus of control (LOC), with internal LOC associated with health-related events occurring under one’s own control, and external LOC associated with events determined by external factors such as powerful others –including physicians – and chance (for exact wording of the survey on AD attitudes, please refer to work by Rovner and co-workers).24, 25 Each participant was asked to complete the entire survey in approximately 10 minutes. Because of the small number of respondents whose self-reported race was neither Caucasian nor African American, these subjects (n=11) were excluded from subsequent analysis. None of the remaining subjects (n=207) reported Hispanic or Latino ethnicity.
Statistical Analysis
All statistical analysis was performed in IBM SPSS 21 (Chicago, IL). Baseline characteristics were analyzed between racial groups (African American, Caucasian, Other) using Chi-squared test for categorical variables or analysis of variance (ANOVA) for continuous variables. Respondents who were African American (n=67) or Caucasian (n=140) then underwent univariate analysis as a group to identify factors influencing ADKS scores by Student’s T-test or ANOVA. Factors associated with ADKS scores in univariate models (p < 0.05) were then entered into a multivariate linear regression model, with backwards elimination for variables with alpha value greater than 0.10. Interactions between variables in the final model were further analyzed, but no significant interaction was identified.
For attitudes towards AD, we analyzed the survey responses in dichotomous form (disagree for 0 and 1, agree for 2 and 3). Chi-squared tests were first used to identify differences in responses from the current study and a prior study in Philadelphia.14 Within the Atlanta cohort, univariate analysis using Chi-squared tests was used to identify demographic factors associated with agreement for each statement with alpha value of 0.01 to adjust for multiple comparisons. To determine the association between AD knowledge and attitudes, we also compared respondents’ ADKS scores according to response to each statement. Attitudes associated with ADKS score differences with alpha value of 0.01 were then entered into the previous linear regression model for ADKS scores to determine the association between knowledge, attitude, and demographic variables.
RESULTS
A total of 207 completed surveys were included in the analysis, with 63 from Research, 69 from Community, and 75 from Primary Care. Compared to Research and Community, respondents from Primary Care were more likely to be Caucasian (88.0% vs. 52.4% and 59.4%, χ2=23.03, p < 0.001), younger in age (56.0% under 65 vs. 30.2% and 36.2%, χ2=11.33, p = 0.001), and have household income greater than $100,000 (69.3% vs. 22.2% and 17.4%, χ2=50.51, p < 0.001). Across the entire cohort, African American older adults were much more likely to be contacted through Research or Community (86.6% vs. 52.9%, χ2=23.03, p < 0.001), less likely to have excellent health (17.9% vs. 34.3%, χ2=5.90, p = 0.015) and more likely to have fair or poor health (17.9% vs. 5.7%, χ2=7.72, p = 0.005) than Caucasian older adults.
Knowledge about AD
ADKS scores were similar between the three sources, with the average score being 22.5 in Research, 23.4 in Community, and 23.6 in Primary Care. Univariate analysis showed higher scores to be associated with Caucasian race (t=3.22, p=0.001, Figure 1A) and higher education (F=12.09, p<0.001, Figure 1B), but not age (F=2.15, p=0.062), gender (t=0.698, p=0.486), income (F=1.82, p=0.204), or recruitment source (F=1.59, p=0.206). Multivariate analysis showed education (p<0.001) and race (p=0.005) to independently associate with higher ADKS scores (Table 2, Model 1).
Figure 1. ADKS score distribution according to A) race and B) education level.
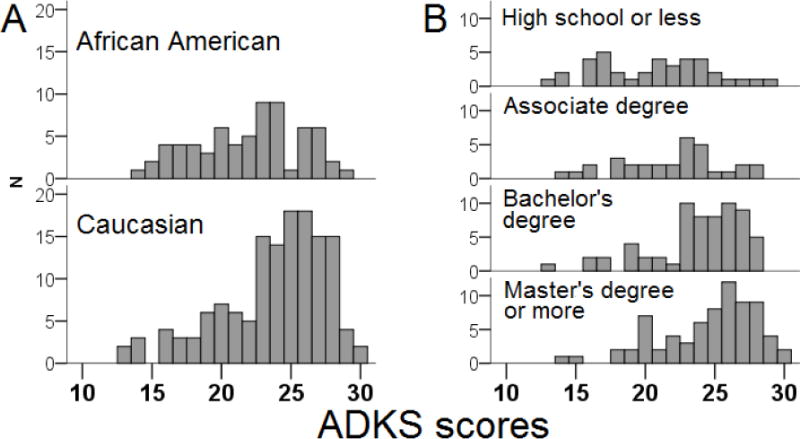
Higher education (p<0.001) and white race (p=0.001) were associated with higher ADKS scores in univariate analyses and were the only significant factors at an alpha-value of 0.05 in multivariate analysis. Each increase in educational level was associated with an average 1.3-point increase in ADKS score.
Table 2.
Multi-variate linear regression model for ADKS scores including demographic variables only (Model 1) or including demographic variables as well as attitudes toward AD (Model 2). Control 1: disagreeing with the statement “I have little control over my own health”; Control 2: disagreeing with the statement “There is nothing I can do about getting Alzheimer’s disease”; 95% C.I.: 95% confidence interval.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| B | 95% C.I. | p | B | 95% C.I. | p | |
| Constant | 11.46 | 6.412 – 16.51 | <0.001 | 12.54 | 7.53 – 17.56 | <0.001 |
| Race | 1.46 | 0.400 – 2.51 | 0.007 | 1.57 | 0.54 – 2.61 | <0.001 |
| Education | 1.28 | 0.83 – 1.73 | <0.001 | 1.09 | 0.65 – 1.54 | <0.001 |
| Control 1 | – | – | – | 1.25 | 0.02 – 2.00 | 0.05 |
| Control 2 | – | – | – | 1.05 | 0.06 – 2.04 | 0.04 |
Attitudes towards AD
We first compared AD attitudes between older adults in Atlanta and Philadelphia.14 Whereas Caucasian and African American older adults in Atlanta showed similar attitudes towards AD, there were some significant geographic differences (Figure 2).14 Fewer older adults in Atlanta believed they could prevent AD (52.2% vs. 67.8%, p < 0.001) or “it’s God’s will” whether they get AD (27.9% vs. 49.4%, p < 0.001), despite older adults from both cities reporting control over their general health (79% vs. 70.5%). Within the Atlanta cohort, univariate analysis additionally showed three factors which influence AD attitudes (eFigure 1, eFigure 2): good or excellent self-reported health was associated with greater control over one’s own health; Research or Community referral was associated with greater memory concern, agreement with greater AD risks in African Americans, and likelihood to approach physicians with memory loss; and higher annual household income was also associated with greater likelihood to approach physicians with memory loss.
Figure 2. Attitudes about AD among Caucasians and African Americans from Atlanta and Philadelphia.
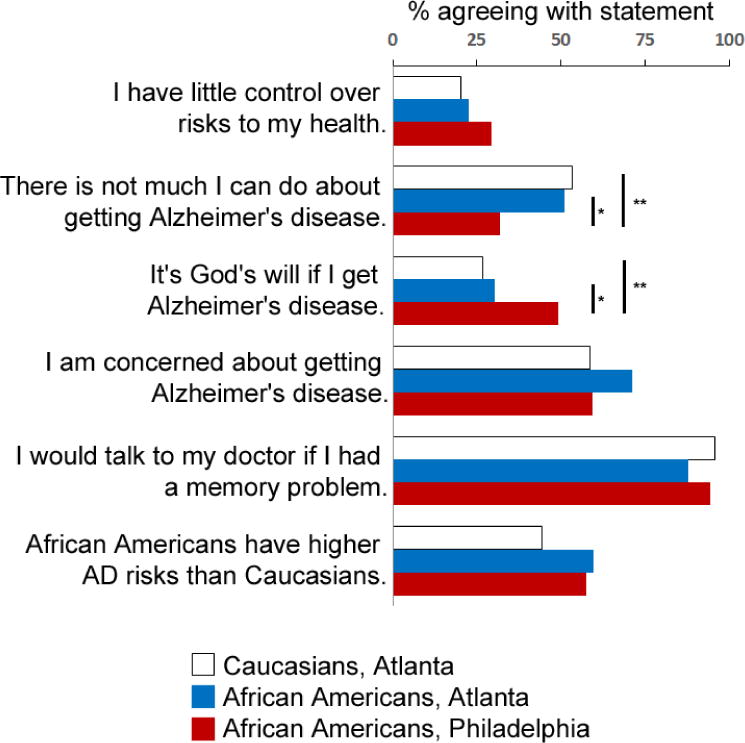
Shown are percent of respondents agreeing with each statement (data from Philadelphia derived from Rovner et al., 2013). Agreeing with the first two statements translates into less control over general health risks and AD risks. * p < 0.007; ** p < 0.0001
Relationship between AD knowledge and AD attitudes
We hypothesized that knowledge is linked to attitudes in AD. Subjects who reported some control over general health (23.7) scored higher on ADKS than those who reported little control (21.5, t=3.17, p = 0.002), and subjects who reported some control over AD (24.1) scored higher on ADKS than those reported little control (22.6, t=2.82, p = 0.005). Introducing these two factors into the previous multivariate regression model for ADKS score showed race and education to significantly influence ADKS scores even after adjustment for attitudes toward AD (Table 2, Model 2).
We also noted that a significant number of respondents in Atlanta expressed control over their own health but not their chances of getting AD (n=74, or 35.7%). Compared to respondents who expressed control over both general health and AD risks, these older adults scored slightly lower on ADKS (23.1 vs. 24.3, t=2.04, p < 0.05), but were otherwise similar in age, gender, race, education, self-reported health, income, and their responses to the other attitude questions.
DISCUSSION
A better understanding of the knowledge and attitudes towards AD among older adults is critical in enhancing research participation, designing education programs, and accelerating AD prevention and cure. Here we collected and analyzed data regarding AD knowledge and attitudes from a large multiracial group of older adults in the Atlanta area. We found African Americans to have slightly lower ADKS scores compared to Caucasians, but ADKS scores were also influenced by education and attitudes. In contrast to prior studies,20, 26 we found no clear relationship between race and attitudes towards AD. At the same time, an external LOC towards AD despite an internal LOC towards general health is prevalent in both races. Thus, race only modestly influences AD knowledge without impacting AD attitudes among older adults, and efforts aimed at improving AD knowledge in African American and Caucasian older adults alike can potentially influence attitudes towards AD prevention and treatment.
African American participation in AD-related studies has historically been limited.2, 3, 27 Many explanations have been proposed, including general knowledge and attitudes towards medical research and specific knowledge and attitudes towards AD. We had chosen the ADKS to assess general knowledge about AD because of its high test-retest reliability and demonstrated differences in people predicted to have different AD knowledge.11 At the group level, we identified a significant but small difference in ADKS scores between African Americans and Caucasians. This difference was not identified in a previous study conducted in St. Louis, but that study was likely not powered (89 older adults) to detect a subtle difference. It is important to note that the ADKS score difference attributable to race (95% confidence interval: 0.537 – 2.607) in our study is smaller in magnitude than the difference between African American respondents between multi-racial studies (21.9 in Atlanta, 21.4 in St. Louis) and the African American only Philadelphia study (18.7). African Americans participating in an AD biomarker study or attending AD-related activities may have higher interest and knowledge levels than average, and this bias may have underestimated the difference in knowledge between the two racial groups. This may in part explain the relatively large difference in knowledge between the races identified by one study,20 but recruitment bias (African Americans from Atlanta vs. Caucasians from Boston) and enhanced exposure to AD over the past decade may also explain the different findings. African Americans in Atlanta also have higher median household income and education achievements than those in Philadelphia or St. Louis.28, 29 At the same time, a prior study using group discussion to enhance knowledge about AD research among African Americans in Boston also resulted in no clear improvement in research participation rates.30 Therefore, while future studies incorporating ADKS will need to account for geographic differences as well as those attributable to race and other factors, it is not clear that a knowledge difference can account for minority under-representation in research participation, or narrowing the small knowledge gap between races would encourage minority research participation.
We also found geographical differences in attitudes towards AD.14 High proportions of African American older adults in Philadelphia believed they could control risks to their own health (70.5%) and risks to developing AD (68.0%), but this was not the case in Atlanta. To our surprise, over one third of the older adults in Atlanta reported control over their general health risks but not their AD risks. It is tempting to call these older adults AD pessimists (compared to optimists identified in two previous surveys),20, 26 but it may be more appropriate to consider them as having an external health LOC.25 Prior work in Parkinson’s disease has shown that optimism was correlated with quality of life while external LOC was correlated with greater disability,31 and work in gerontology showed increasing number of chronic conditions associated with external LOC.32 Older adults in Atlanta had higher ADKS scores than those in Philadelphia, and it is possible that the greater general knowledge translates into more external LOC. Alternatively, it is possible that attitudes of older adults in the current study are shaped by recent failures of large AD clinical trials to reach primary endpoints,33 but our finding of external LOC may reflect more than just pessimism in AD. Further qualitative research is necessary to disentangle the locus of control from optimism/pessimism related to AD among older adults, as the pace of AD research can be accelerated by the belief that medical research can help stave off AD, or halted by pessimism related to failed AD trials. Determining which external LOC (powerful others vs. chance) is most relevant in one in three older adults will also guide outreach and education efforts to highlight innovative AD treatments,34, 35 non-pharmacological preventive strategies,36 or both.
We believe that the sample size in this cohort of older African Americans and Caucasians allowed us to make some thought-provoking observations. At the same time, this study has some limitations, including those inherent to survey-based studies: it is not known how survey respondents compare with non-respondents according to AD knowledge and attitudes; those who attend community events may be more informed than the average older adults; survey respondents’ attitudes may differ according to their prior experience with AD which was not explored. There were uneven recruitment along racial and income categories between the three recruitment sources which may have confounded the analysis. Because some knowledge and attitude differences between African Americans and Caucasians may not be detectable using the survey tools available, more subjects recruited from each site could allow for more detailed analysis such as examining individual responses and their association with prior exposure to AD.13 Nevertheless, future work should identify factors other than knowledge and attitude to explain differences in research participation between African American and Caucasian older adults, such as trust in research and shared identity with researchers.37 Just as important is the need to explore internal and external LOC issues related to AD, as they may influence how older adults perceive AD research findings (positive and negative) and participate in research.
Acknowledgments
The authors thank Allan Levey, MD, PhD for critical review of the manuscript. This work was supported by the National Institutes of Health [AG43885 to WTH, AG42856 to WTH, AG25688 to MP and CDD), the O. Wayne Rollins Fund for Clinical Excellence, and Emory Primary Care Internal Medicine. The sponsors have no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The principal investigator (WTH) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
References
- 1.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045–50. doi: 10.1212/WNL.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghekar A, Li S, Lu Y, Li M, Wang MC, Albert M, et al. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013;81(20):1753–8. doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard EL, Nash F, Raiford K, Harrell LE. Recruitment of black elderly for clinical research studies of dementia: the CERAD experience. Gerontologist. 1993;33(4):561–5. doi: 10.1093/geront/33.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Hart VR, Gallagher-Thompson D, Davies HD, DiMinno M, Lessin PJ. Strategies for increasing participation of ethnic minorities in Alzheimer’s Disease Diagnostic Centers: a multifaceted approach in California. Gerontologist. 1996;36(2):259–62. doi: 10.1093/geront/36.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer’s disease in African-American and white persons. Neuroepidemiology. 2006;26(1):16–22. doi: 10.1159/000089231. [DOI] [PubMed] [Google Scholar]
- 7.Bachman DL, Stuckey M, Ebeling M, Wagner MT, Evans WJ, Hirth V, et al. Establishment of a predominantly African-American cohort for the study of Alzheimer’s disease: the South Carolina Alzheimer’s disease clinical core. Dement Geriatr Cogn Disord. 2009;27(4):329–36. doi: 10.1159/000207446. [DOI] [PubMed] [Google Scholar]
- 8.Williams MM, Scharff DP, Mathews KJ, Hoffsuemmer JS, Jackson P, Morris JC, et al. Barriers and facilitators of African American participation in Alzheimer disease biomarker research. Alzheimer Dis Assoc Disord. 2010;24(Suppl):S24–9. doi: 10.1097/WAD.0b013e3181f14a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darnell KR, McGuire C, Danner DD. African American participation in Alzheimer’s disease research that includes brain donation. Am J Alzheimers Dis Other Demen. 2011;26(6):469–76. doi: 10.1177/1533317511423020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard EL, Gwyther LP, Edmonds HL. Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: lessons learned. Alzheimer Dis Assoc Disord. 2010;24(Suppl):S19–23. doi: 10.1097/WAD.0b013e3181f12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s Disease Knowledge Scale: development and psychometric properties. Gerontologist. 2009;49(2):236–47. doi: 10.1093/geront/gnp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettens GF, Ownsworth T, Hohaus L, McKendry Y. Assessing accuracy of knowledge of cognitive effects of normal ageing and mild stage of Alzheimer’s disease. Aging Ment Health. 2014;18(3):296–303. doi: 10.1080/13607863.2013.827629. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter BD, Zoller SM, Balsis S, Otilingam PG, Gatz M. Demographic and contextual factors related to knowledge about Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2011;26(2):121–6. doi: 10.1177/1533317510394157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovner BW, Casten RJ, Harris LF. Cultural diversity and views on Alzheimer disease in older African Americans. Alzheimer Dis Assoc Disord. 2013;27(2):133–7. doi: 10.1097/WAD.0b013e3182654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson JM, Pollux PM, Mistry B, Hobson S. Beliefs about Alzheimer’s disease in Britain. Aging Ment Health. 2012;16(7):828–35. doi: 10.1080/13607863.2012.660620. [DOI] [PubMed] [Google Scholar]
- 16.Nordhus IH, Sivertsen B, Pallesen S. Knowledge about Alzheimer’s disease among Norwegian psychologists: the Alzheimer’s disease knowledge scale. Aging Ment Health. 2012;16(4):521–8. doi: 10.1080/13607863.2011.628973. [DOI] [PubMed] [Google Scholar]
- 17.Kim EJ, Jung JY. Psychometric properties of the Alzheimer’s Disease Knowledge Scale–Korean Version. J Korean Acad Nurs. 2015;45(1):107–17. doi: 10.4040/jkan.2015.45.1.107. [DOI] [PubMed] [Google Scholar]
- 18.Hughes ML, Lowe DA, Shine HE, Carpenter BD, Balsis S. Using the Alzheimer’s Association web site to improve knowledge of Alzheimer’s disease in health care providers. Am J Alzheimers Dis Other Demen. 2015;30(1):98–100. doi: 10.1177/1533317514559827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.2008–2012 American Community Survey.
- 20.Connell CM, Scott Roberts J, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(2):110–6. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percent distribution of housholds, by selected characteristics within income quintile and top 5 percent in 2011. U.B.o.L.S.a.U.C. Bureau; 2012. Editor. [Google Scholar]
- 22.Dieckmann L, Zarit SH, Zarit JM, Gatz M. The Alzheimer’s disease knowledge test. Gerontologist. 1988;28(3):402–7. doi: 10.1093/geront/28.3.402. [DOI] [PubMed] [Google Scholar]
- 23.Bowling A. Techniques of questionnaire design. In: Bowling A, S E, editors. Handbook of health research methods: invesigation, measurement and analysis. Multidisciplinary research measurement. McGraw-Hill International; Berkshire, England: pp. 394–428. 005. [Google Scholar]
- 24.Walker EA, Caban A, Schechter CB, Basch CE, Blanco E, DeWitt T, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educ. 2007;33(1):103–10. doi: 10.1177/0145721706298198. [DOI] [PubMed] [Google Scholar]
- 25.Wallston KA, Stein MJ, Smith CA. Form C of the MHLC scales: a condition-specific measure of locus of control. J Pers Assess. 1994;63(3):534–53. doi: 10.1207/s15327752jpa6303_10. [DOI] [PubMed] [Google Scholar]
- 26.Connell CM, Scott Roberts J, McLaughlin SJ. Public opinion about Alzheimer disease among blacks, hispanics, and whites: results from a national survey. Alzheimer Dis Assoc Disord. 2007;21(3):232–40. doi: 10.1097/WAD.0b013e3181461740. [DOI] [PubMed] [Google Scholar]
- 27.Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O’Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer’s disease in ethnically diverse highly educated individuals: an analysis of the NACC database. J Gerontol A Biol Sci Med Sci. 2012;67(8):890–6. doi: 10.1093/gerona/gls006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotkin J, Cox W. Best cities for minoritie, gauging the economics of opportunity. Center for Opportunity Urbanism; Orange, CA: 2015. p. 34. [Google Scholar]
- 29.Boschma J. Best and worst cities for educating blacks. National Journal. 2015 8/4/2015; Available from: http://www.nationaljournal.com/next-america/workforce/city-education-black-college-transplants-20150604.
- 30.Jefferson AL, Lambe S, Romano RR, Liu D, Islam F, Kowall N. An intervention to enhance Alzheimer’s disease clinical research participation among older African Americans. J Alzheimers Dis. 2013;36(3):597–606. doi: 10.3233/JAD-130287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruber-Baldini AL, Ye J, Anderson KE, Shulman LM. Effects of optimism/pessimism and locus of control on disability and quality of life in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(9):665–9. doi: 10.1016/j.parkreldis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Henninger DE, Whitson HE, Cohen HJ, Ariely D. Higher medical morbidity burden is associated with external locus of control. J Am Geriatr Soc. 2012;60(4):751–5. doi: 10.1111/j.1532-5415.2012.03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dysken MW, Guarino PD, Vertrees JE, Asthana S, Sano M, Llorente M, et al. Vitamin E and memantine in Alzheimer’s disease: clinical trial methods and baseline data. Alzheimers Dement. 2014;10(1):36–44. doi: 10.1016/j.jalz.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butchart J, Brook L, Hopkins V, Teeling J, Puntener U, Culliford D, et al. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology. 2015 doi: 10.1212/WNL.0000000000001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: the Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628] Nutr J. 2015;14(1):53. doi: 10.1186/s12937-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer LC, Hayes SN, Parker MW, Balls-Berry JE, Halyard MY, Pinn VW, et al. African American women’s perceptions and attitudes regarding participation in medical research: the Mayo Clinic/The Links, Incorporated partnership. J Womens Health (Larchmt) 2014;23(8):681–7. doi: 10.1089/jwh.2014.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]


