Abstract
Variability in response to methotrexate (MTX) in the treatment of Juvenile Idiopathic Arthritis (JIA) remains unpredictable and poorly understood. Based on previous studies implicating an interaction between nicotinamide phosphoribosyltransferase (NAMPT) expression and MTX therapy in inflammatory arthritis, we hypothesized that increased NAMPT expression would be associated with reduced therapeutic response to MTX in patients with JIA. A significant association was found between increased plasma concentrations of NAMPT and reduced therapeutic response in JIA patients treated with MTX. Inhibition of NAMPT in cell culture by either siRNA-based gene silencing or pharmacological inhibition with FK-866 was found to result in a 4-fold increase in the pharmacological activity of MTX. Collectively, these findings provide evidence that NAMPT inhibits the pharmacological activity of MTX and may represent a predictive biomarker of response, as well as a therapeutic target, in the treatment of JIA with MTX.
Keywords: Juvenile Idiopathic Arthritis, Biomarkers, Methotrexate, Nicotinamide Phosphoribosyltransferase
Introduction
Methotrexate (MTX) therapy continues to be a cornerstone in the treatment of Juvenile Idiopathic Arthritis (JIA). However, in the age of early and aggressive disease control, MTX therapy is mired by a delayed onset of action and a highly variable and unpredictable response profile that makes it the focus of individualized approaches to therapy (1). Understanding predictors of optimal or poor response to MTX holds the promise of more streamlined therapy in JIA. This could include more rapid initiation or avoidance of the next tier biologic therapies, and their associated costs and risks.
In Rheumatoid Arthritis (RA) the adipocytokine nicotinamide phosphoribosyltransferase (NAMPT), also commonly referred to as pre-B-cell colony-enhancing factor (PBEF) and visfatin, has been found at elevated concentrations in the plasma and synovial fluid of RA patients, has been associated with disease activity, and has been found to be predictive of treatment response (2-10). These findings have supported a role for NAMPT as both a drug target and a biomarker in RA, but the role of this adipocytokine in the JIA population is yet to be established (11, 12). Plasma NAMPT levels in a cross-sectional group of JIA patients were found by our group to be higher in patients with active disease and lower in patients actively treated with MTX therapy (13). Although these findings suggest that NAMPT is associated with disease activity and MTX response in JIA, there remains a need for evaluation of NAMPT levels in a prospective cohort of JIA patients treated with MTX to more fully understand the relationship of this adipocytokine with disease activity and drug response.
Identified and cloned based on its role in stimulating pre-B-cell colony formation, NAMPT is a ubiquitously expressed multifunctional protein that functions as both a cytosolic enzyme in the nicotinamide adenine dinucleotide (NAD) salvage pathway and as a secreted plasma protein that functions in the regulation of inflammation and metabolic activity, resulting in its designation as an adipocytokine (14-16). As a result of its role in NAD biosynthesis, NAMPT has been the target of several drug development programs and has resulted in the discovery of a number of highly potent inhibitors of its enzymatic activity, including FK-866 and GMX1778. NAMPT has been reported to stimulate CD14+ monocytes, promote the motility of leukocytes and synovial fibroblasts from RA patients, activate the transcription factors NF-kB and AP-1, induce the production of various cytokines including IL-1Ra, IL-1β, TNFα, and IL-6, and to be induced by cytokines including IL-1β and IL-6 (17-23). Despite the established pro-inflammatory role of NAMPT the mechanism through which it mediates these effects remains controversial. Early studies suggested binding of extracellular NAMPT to the insulin receptor as a basis for its activity, however, these studies have since been retracted and no specific extracellular receptor has been identified to date (24). The presence of a NAMPT receptor signaling pathway has also been supported by the induction of IL-6 by NAMPT in the presence of the inhibitor of NAMPT enzymatic activity FK-866 (25). In contrast, the role of NAMPT enzymatic activity in the pathogenesis of inflammatory arthritis has been supported by studies demonstrated the role of cellular NAD regulation in lymphocyte development and activation (14, 26). This has been supported further by studies in the collagen-induced arthritis mouse model that have found inhibition of NAMPT by either FK-866 or siRNA-based gene-silencing inhibits leukocyte activation and infiltration, cytokine production, and overall disease progression (23, 27). Further supporting the role of NAMPT enzyme activity in disease progression, depletion of NAD levels in circulating leukocytes has been associated with the efficacy of FK-866 in the collagen-induced arthritis mouse model (28).
In addition to the role of NAMPT in the pathogenesis of inflammatory arthritis, a recent study has found that inhibition of NAMPT with GMX1778, results in the depletion of cellular NAD and enhances the pharmacologic activity of the anti-folate pemetrexed (29). Similar to pemetrexed, MTX is also an antifolate, and therefore suggests that reductions in NAMPT expression may potentiate the pharmacologic activity of MTX. Although a relationship between elevated baseline NAMPT levels and increased long-term disease progression in RA patients has been demonstrated, the possibility that variation in NAMPT activity can affect the pharmacologic activity of MTX has not been evaluated (6).
This study evaluates plasma concentrations of NAMPT in a prospective cohort of JIA patients over 6-months following the initiation of MTX to determine the relationship between circulating NAMPT levels and therapeutic response. Mechanism-based studies using a cellular model to measure the activity of MTX were conducted to further evaluate the pharmacological interaction between NAMPT and MTX.
Methods
Study design and patients
Patient samples and data were collected from an ongoing single-center prospective study of JIA patients started on standardized MTX dosing (15 mg/m2/week) and followed over 6-months. Because of a national shortage in MTX for injection during this study patients were treated with either oral (n=29) or subcutaneous (n=37) MTX depending on when they were enrolled. After 3-months on therapy, non-responders to MTX monotherapy were permitted the addition of an anti-TNFα agent if deemed clinically necessary. The study was approved by the Children's Mercy Hospital institutional review board. Written informed consent/assent was obtained prior to the inclusion of each study participant and collection of patient samples.
NAMPT, IL-1α, IL-1β, IL-1Ra, IL-6, and TNFα concentrations were measured in plasma samples collected prior to the initiation of MTX therapy, and after 3- and 6-months following the initiation of MTX. Erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), active joint count (AJC), limited joint count (LJC), patient/parent global assessment of overall well-being (PT-VAS), physician global assessment of disease activity (MD-VAS), and Childhood Health Assessment Questionnaire (CHAQ) scores were determined at each visit. Clinical response was determined by the American College of Rheumatology pediatric response criteria (ACR Pedi) and the 71-joint count Juvenile Arthritis Disease Activity Score (JADAS) after 3- and 6-months on therapy.
Cell lines and reagents
A549 human lung carcinoma cells (catalogue no. CCL-185) were purchased from ATCC (Manassas, VA) and grown in DMEM (catalogue no. 11995-065) supplemented with 10% fetal bovine serum (catalogue no. 03-600-511), 100 U/mL penicillin, and 100 U/mL streptomycin (ThermoFisher Scientific, Waltham, MA). Cells were maintained at 37°C in a 5% CO2 controlled incubator and were passaged every 3 to 4 days at ∼80% confluence. Resazurin sodium salt (ThermoFisher Scientific, Waltham, MA) was re-suspended at 0.16 mg/mL in pH 7.4 PBS and passed through a 0.2 μm syringe filter. Human NAMPT specific carboxyl-terminal enzyme immunoassays (catalogue no. EK-003-80) were purchased from Phoenix Pharmaceuticals (Burlingame, CA). High performance liquid chromatography purified NAMPT siRNA (sense: 5′-CCACCCAACACAAGCAAAGUUUAUU-3′) and scrambled control siRNA (sense: 5′-CCACAACAACAAACGUUGAUCCAUU-3′) were custom synthesized (ThermoFisher Scientific, Waltham, MA). Multiplex human cytokine magnetic bead panels for the detection of IL-1α, IL-1β, IL-1Ra, IL-6, and TNFα (MILLIPLEX MAP, HCYTOMAG-60K) were purchased from EMD Millipore (Darmstadt, Germany).
NAMPT and cytokine analysis
Blood samples collected in EDTA tubes were immediately processed by centrifugation at 800×g for 10-minutes at room-temperature. Plasma samples were collected and immediately stored at -80°C prior to analysis, and were subjected to a single freeze/thaw cycle. Samples were thawed on ice and maintained at 4°C for a maximum of 48-hours prior to analysis. NAMPT concentrations were determined in duplicate by enzyme immunoassay following the manufacturer's protocol and analyzed using a BioTek Synergy HT microplate-reader (BioTek, Winooski, VT) equipped with Gen5 data analysis software. The average coefficient of variation for all samples analyzed was 8.4%. Plasma concentrations of IL-1α, IL-1β, IL-1Ra, IL-6, and TNFα were determined in duplicate by multiplex analysis using the manufacturer's protocol. Samples were analyzed on a Luminex multiplex-reader (Luminex Corporation, Austin, TX) and the resulting average coefficient of variation for the analytes were 14.7%, 18.4%, 17.5%, 16.8%, and 12.0%, respectively.
NAMPT inhibition
For each well of a six-well plate, 250 pmoles of siRNA and 5 μL of Lipofectamine 2000 (ThermoFisher Scientific, Waltham, MA) were each added to 250 μL of Opti-MEM reduced serum media (ThermoFisher Scientific, Waltham, MA) for 5-minutes at room-temperature before mixing and incubating at room-temperature for 20-minutes, and then added to 1.5 mL of antibiotic-free growth media containing 2.5×105 cells. Volumes and densities were scaled to 5×103 cells per well for 96-well plate assays. After 24-hours transfection media was replaced with normal growth media and maintained for an additional 72-hours under normal culture conditions prior to analysis. NAMPT enzymatic activity in cells was inhibited by adding 2.5 nM FK-866.
NAMPT expression and function
Western blot analysis was performed as described previously (30). Briefly, cell lysate protein was quantified using the Micro BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA). Protein (20 μg) from each sample was then subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (EMD Millipore, Billerica, MA). The membranes were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween 20 (TBS-T) at room-temperature for 1-hour and then incubated at 4°C overnight with primary antibody (Anti NAMPT antibody,1:4000). After washing three times for 10 minutes with TBS-T, the membrane was incubated with horseradish-peroxidase-linked goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Dallas, TX) at room-temperature for 1-hour. The blots were visualized with the Pierce ECL Western blot detection reagent (ThermoFisher Scientific, Waltham, MA). Total cellular NAD content was measured using a colorimetric enzyme-linked microplate assay (catalogue no. 600480) available from Cayman Chemical Company (Ann Arbor, MI). The assay was conducted in a 96-well plate format following the manufacturer's protocol.
Cellular viability
A549 cells seeded at 5×103 cells per well in optically clear black-walled 96-well plates were treated with MTX at concentrations up to 100 μM for up to 120-hours. To each well containing 100 μL of growth medium, 20 μL of 0.16 mg/mL rezasurin was added and incubated for 4-hours at 37°C and 5% CO2. The plate was protected from light and allowed to cool to room-temperature for 10 minutes prior to reading. Fluorescence signal was measured for each sample using a BioTek Cytation 3 multimode plate reader equipped with scanning excitation and emission monochromators set at an excitation and emission of 560 nm and 590 nm, respectively. Background signal was subtracted and percent viability was determined by normalizing fluorescence signal to untreated cells. Cellular protein content and cell nuclei counting were used as secondary measures of cellular viability. Protein content was quantified using the Micro BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA) and nuclei were stained with Hoechst 33342 (BD Biosciences, Franklin Lakes, NJ) and counted using Gen5 image analysis software on a BioTek Cytation 3 equipped with a DAPI filter cube and a 4× objective. The resulting log dose-response curves were fit to a 4-parameter logistic equation using SigmaPlot v12.5 (Systat Software, San Jose, CA) and the concentrations resulting in half maximal inhibition (IC50) are reported. All experiments were conducted in triplicate unless otherwise noted.
Statistical analysis
Treatment effects on measured variables in the patient samples were assessed by non-parametric testing including the Wilcoxon rank-sum test or paired analyses via the Wilcoxon signed-rank test using JMP software v11 (SAS Institute Inc, Cary, NC). Associations between continuous variables were assessed by Spearman's rank correlation analysis. Data in the cell-based studies were compared by unpaired Student's t-test analysis.
Results
Patient clinical and laboratory findings
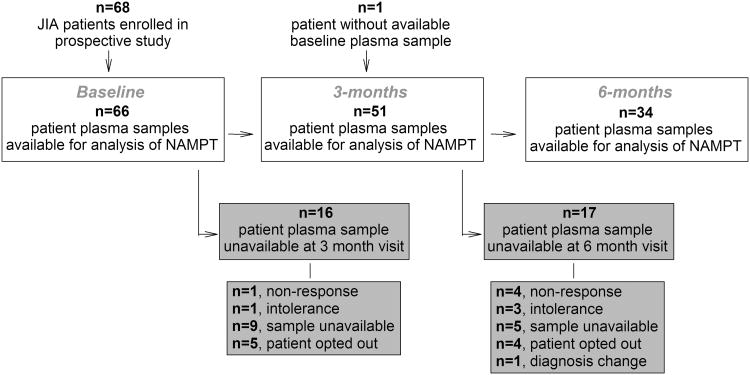
A participant flow diagram and the individual patient demographics for patients included in this study are presented in Figure 1 and Table 1, respectively. Seventy-six percent of patients with baseline samples had available samples at 3-months and 50% had available samples at 6-months with the majority of loss due to lack of sample availability due to inadequate sample volume or because the patient had not yet reached the subsequent time-point. Baseline NAMPT levels were lower among patients with unavailable samples at 3- or 6-months, as compared to those with samples for all visits (10.1 [6.8, 12.5] vs 11.8 [9.2, 18.2] ng/mL, p =0.04). However, because of the variety of reasons for lack of sample availability it is difficult to draw any conclusions based on this observation. Only 15% of the observed attrition resulted from therapeutic non-response and no difference in NAMPT levels was observed in this group compared to those that continued in the study. Consistent with the greater JIA population, the patients enrolled in this study were predominately female. Statistically significant reductions in all individual measures of disease activity were observed over the treatment period corresponding to significant reductions in composite JADAS scores following initiation of therapy and 85% of patients meeting ACR Pedi 30 response criteria by 6-months.
Figure 1.
Flow diagram of participants with plasma samples available for NAMPT analysis at their baseline, 3-month, and 6-month clinic visits. Gray boxes indicate participants for which samples at the subsequent clinic visit were unavailable for analysis.
Table 1.
Patient demographics and clinical data before the initiation of MTX (0 month), and after 3- and 6-months of therapy.
| Patient Characteristics | |||
|---|---|---|---|
|
| |||
| Visit (months) | 0 | 3 | 6 |
| Patients, n | 66 | 51 | 34 |
| Gender, female, n (%) | 48 (73) | 33 (65) | 22 (65) |
| Age, yr, median [IQR] | 11.5 [5.4, 14.4] | 10.9 [5.3, 13.8] | 10.3 [5.5, 13.9] |
| ESR, mm/h | 16 [9, 35] | 9 [7, 21]*** | 8 [7, 15]*** |
| CRP, mg/L | 0.7 [0.5, 1.8] | 0.5 [0.5, 1.3]* | 0.5 [0.5, 0.6]* |
| AJC | 5 [2, 13] | 3 [1, 7]*** | 1 [0, 5]*** |
| PT-VAS | 6 [3, 8] | 2 [0.8, 4]*** | 1 [0.5, 4]*** |
| MD-VAS | 5 [3, 7] | 2 [0, 4]*** | 1 [0, 2.5]*** |
| CHAQ | 0.5 [0.1, 1.4] | 0.12 [0, 0.66]*** | 0 [0, 0.25]*** |
| JADAS | 19 [12, 30] | 7 [4, 16.5]*** | 5 [2, 10.15]*** |
| ACR Pedi < 30, n (%) | --- | 15 (29) | 5 (15) |
| ACR Pedi > 30, n (%) | --- | 36 (71) | 29 (85) |
| ACR Pedi > 50, n (%) | --- | 27 (53) | 27 (79) |
| ACR Pedi > 70, n (%) | --- | 14 (27) | 18 (53) |
, p < 0.05;
, p< 0.01;
, p < 0.001 by paired Wilcoxon signed-rank testing.
Erythrocyte sedimentation rate (ESR); c-reactive protein (CRP); active joint count (AJC); patient/parent global assessment of overall well-being (PT-VAS); physician global assessment of disease activity (MD-VAS); Childhood Health Assessment Questionnaire (CHAQ); 71-joint count Juvenile Arthritis Disease Activity Score (JADAS); American College of Rheumatology pediatric response criteria (ACR Pedi).
Baseline NAMPT levels and disease activity
Pre-treatment plasma concentrations of NAMPT spanned a 14-fold range between 4.2 and 58.9 ng/mL and were found to be positively associated with plasma markers of inflammation including: CRP (ρ=0.27, p=0.03), IL-1α (ρ=0.40, p=0.002), IL-1β (ρ=0.27, p=0.03), IL-1Ra (ρ=0.68, p<0.0001), IL-6 (ρ=0.39, p=0.002), and TNFα (ρ=0.27, p=0.03). Baseline plasma NAMPT levels were not found to be associated with JADAS scores, or any of the other individual clinical measures of disease activity. NAMPT levels were found to be lower in females compared to males (10.3 [7.6, 10.8] vs 13.7 [10.8, 18.8] ng/mL, p =0.04), but were not found to vary significantly by disease subtype, age, weight, or body mass index. No significant differences in baseline demographics or disease activity were observed between participants with samples for all visits and those that didn't have available 3- or 6-month samples. No association between NAMPT levels and erythrocyte concentrations of MTX or its metabolites were observed.
Effect of MTX on NAMPT levels
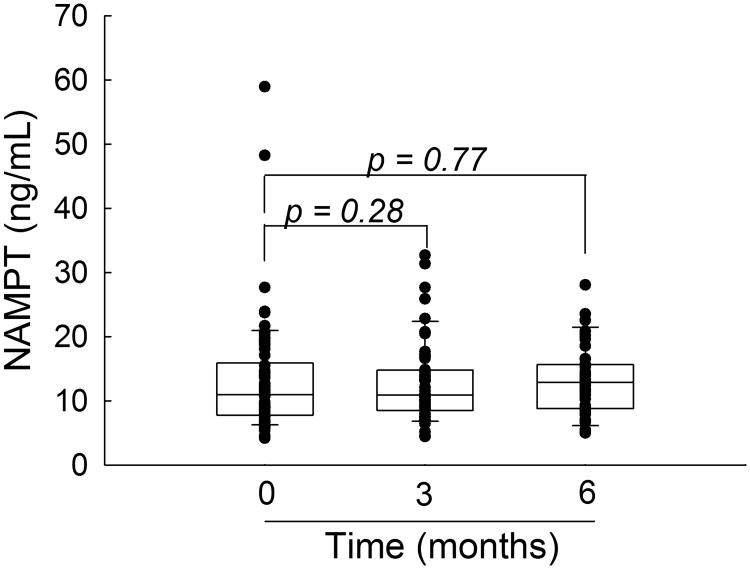
Plasma concentrations of NAMPT were measured prior to the initiation of MTX therapy and at 3- and 6-months of therapy (Figure 2). No significant change in plasma NAMPT concentrations were observed following the initiation of MTX, as determined by either paired or unpaired analysis. Changes in NAMPT concentrations from baseline over the treatment period were not found to be significantly different between responders and non-responders based on the ACR Pedi response criteria and did not correlate with changes in JADAS scores (ΔJADAS) at either 3- or 6-months.
Figure 2.
Plasma NAMPT concentrations in JIA following initiation of MTX. NAMPT concentrations were measured in plasma samples obtained from JIA patients prior to the initiation of MTX therapy and after 3- and 6-months of therapy. Data points and representative box and whisker plots are shown for each time-point. Changes in NAMPT values were evaluated by matched pair analysis using the Wilcoxon signed-rank test and the resulting p-values are provided.
NAMPT levels and therapeutic response in JIA
NAMPT levels at 3- and 6-months were assessed for their association with clinical response at these two time-points. The relationship between NAMPT levels and changes in each individual measure of disease activity and inflammation is provided in Supplemental Table 1. The primary measures of clinical response for this study were the ACR Pedi criteria and corresponding changes from baseline in JADAS (ΔJADAS). Although NAMPT levels at 3-months weren't found to be associated with ΔJADAS, lower NAMPT levels were found to be significantly associated with a greater reduction in active joint count (ΔAJC) from baseline (ρ=0.32, p=0.021). Using ACR Pedi response criteria, elevated 3-month NAMPT levels were observed in patients defined as non-responders by ACR Pedi 50 criteria compared to responders (Median [IQR]: 12.8 [9.95, 17.6] ng/mL vs. 9.28 [7.54, 14.5], p=0.035).
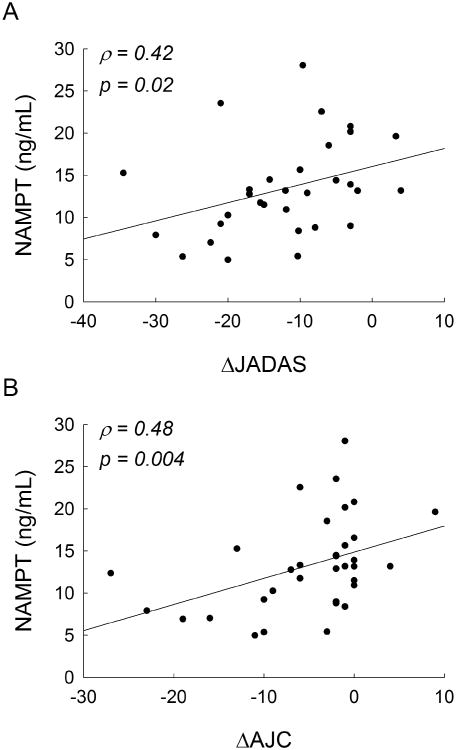
At 6-months, reductions in disease activity by ΔJADAS were found to be associated with reduced NAMPT levels measured at 6-months and were primarily driven by an association of NAMPT levels with ΔAJC (Figure 3). In line with the lack of change observed in NAMPT levels over the treatment period, it was also found that lower NAMPT levels measured at baseline were associated with improved response to therapy, as represented by a positive association with ΔAJC at 6-months (ρ=0.34, p=0.04).
Figure 3.
Association between plasma NAMPT concentrations and clinical improvement in disease activity. Linear regression plots of 6-month plasma NAMPT concentrations versus changes in (A) JADAS scores and (B) AJC in JIA patients over the 6-month treatment period. Spearman's rank correlation coefficients (ρ) and the resulting p-values are presented.
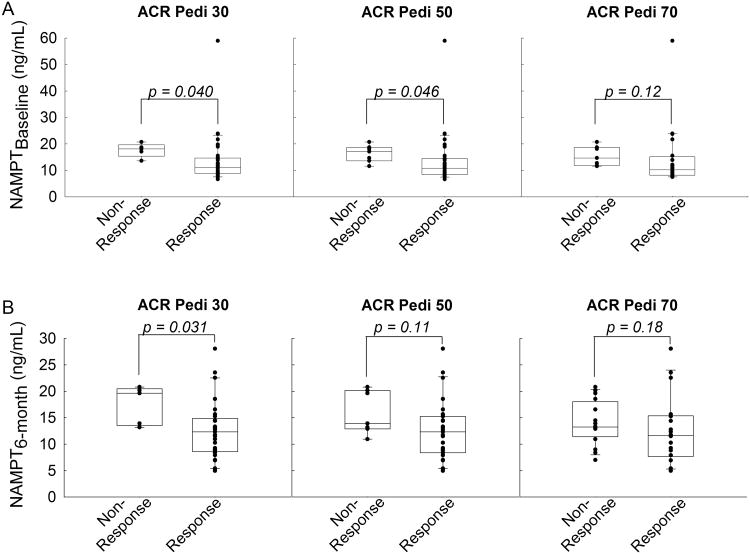
In concordance with the observed associations with ΔJADAS, elevated NAMPT concentrations were associated with non-response by ACR Pedi 30 at 6-months (Figure 4B). Similarly, elevated baseline NAMPT levels were associated with non-response by ACR Pedi 30 and ACR Pedi 50 at 6-months (Figure 4A).
Figure 4.
Relationship between plasma NAMPT concentrations and clinical improvement by ACR Pedi scoring. Plasma NAMPT levels at (A) baseline and (B) 6-months were compared between patients failing to respond to therapy (i.e. non-response) and those responding to therapy (i.e. response) based on ACR Pedi response criteria. Data points and representative box and whisker plots are shown for ACR Pedi 30, 50, and 70 responses as assessed after 6-months of therapy. NAMPT concentrations were compared between groups as indicated using the Wilcoxon rank-sum test and the resulting p-values are provided.
Reduced NAMPT expression potentiates the pharmacological activity of MTX
Although the pharmacological basis of MTX activity in JIA remains controversial, the antiproliferative activity of MTX is believed to represent a primary mechanism of therapeutic efficacy (31, 32). To investigate the role of NAMPT on the pharmacologic activity of MTX, NAMPT expression was inhibited in the A549 cell line by siRNA-based silencing of NAMPT. NAMPT levels were assessed in triplicate by western blotting and remained undetectable in the siRNA transfected cells for at least 96-hours (Figure 5A). The NAMPT antibody consistently yielded triplet bands which made verification of knockdown uncertain, therefore recombinant NAMPT (rNAMPT) was loaded on the first lane suggesting that the bottom band of the triplet represents NAMPT. NAMPT knockdown was verified by measuring cellular NAD levels and NAMPT siRNA caused an 87% reduction in cellular NAD content consistent with reduced NAMPT expression (Figure 5B). Despite the marked reduction in NAMPT and NAD levels, no change in cell viability was observed following NAMPT knockdown (Figure 5C). Nuclear counting by fluorescence microscopy using Hoechst 33342 and protein measurements by BCA analysis supported these findings and actually demonstrated an increase in cell number and protein content in cells treated with NAMPT siRNA (Supplemental Figure 1). Compared to the scrambled siRNA control, knockdown of NAMPT resulted in a ∼4-fold reduction in the concentration of MTX resulting in half-maximal inhibition of cell growth (IC50) (mean±SD: 82.8±3.3 vs. 21.6±2.4 nM, p<0.0001) (Figure 5D). NAMPT knockdown was also found to result in an increase in the growth inhibitory capacity of MTX as illustrated by the difference in cell viability at 100 μM MTX (mean±SD: 82.8±1.6% vs. 58.7±6.0%, p=0.002). Measurement of cell viability using the Hoechst 33342 nuclear staining method and the BCA protein content assay yielded a similar 4- to 5-fold reduction in IC50 values for MTX following NAMPT knockdown (Supplemental Figure 1).
Figure 5.
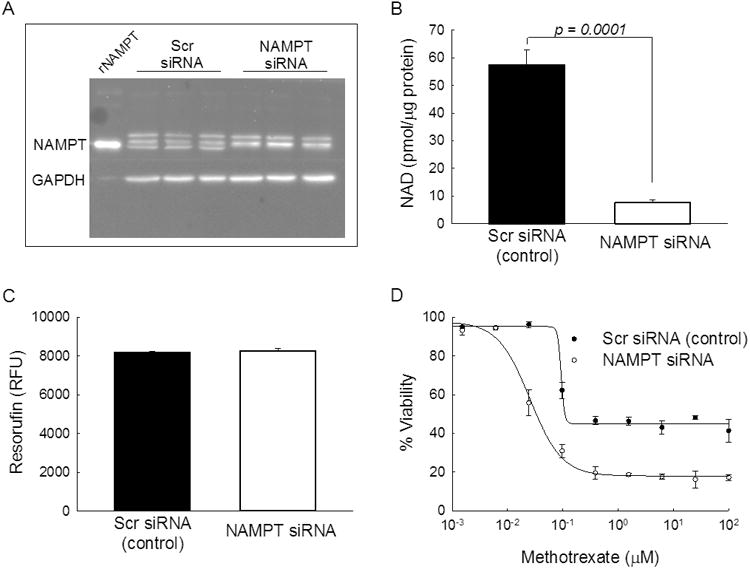
Effect of reduced cellular NAMPT expression on the pharmacological activity of MTX. A549 cells were treated with either siRNA targeting NAMPT mRNA or a scrambled control siRNA and evaluated after 96-hours for differences in (A) NAMPT expression by western blot analysis, (B) intracellular NAD levels, (C) cell viability measured as resorufin relative fluorescence units (RFU), and (D) sensitivity to the growth inhibitory effects of MTX. Results from a single experimental evaluation including three independent replicates are presented here along with the resulting mean±SD. The western blot is representative of three independent experimental evaluations and the MTX growth inhibitory response data is representative of four independent experimental evaluations.
Chemical inhibition of NAMPT potentiates the pharmacological activity of MTX
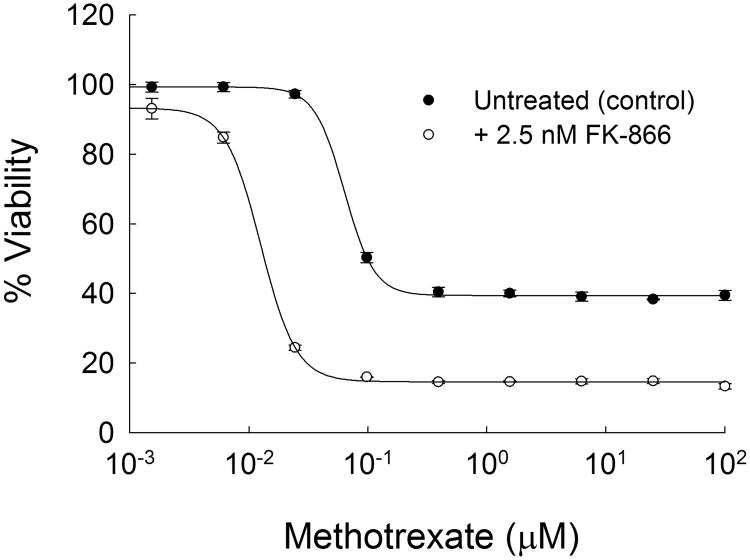
To confirm further the role of NAMPT on the pharmacological activity of MTX the competitive inhibitor of NAMPT enzymatic activity FK-866 was evaluated for its effect on MTX-mediated growth inhibition in the A549 cell line. Following the treatment procedure previously outlined for the interaction between the NAMPT inhibitor GMX1778 and pemetrexed, A549 cells were pre-treated for 48-hours with MTX prior to the addition of 2.5 nM FK-866 for an additional 72-hours (29). FK-866 at 2.5 nM was chosen as the maximum tolerated dose and resulted in less than a 10% reduction in cell viability (mean±SD: 91±4% viability). Similar to NAMPT knockdown, FK-866 treatment resulted in a greater than 4-fold reduction in the IC50 for MTX (63.4±2.7 vs. 13.5±0.9 nM, p<0.0001) (Figure 6). In agreement with the NAMPT knockdown studies it was found that FK-866 caused a significant increase in the growth inhibitory capacity of MTX as illustrated by the difference in cell viability at 100 μM MTX (86.6±0.7% vs. 60.5±1.5%, p<0.0001).
Figure 6.
Effect of chemical inhibition of NAMPT on the pharmacological activity of MTX. Cell viability was determined in A549 cells treated for 48-hours with MTX prior to the addition of 2.5 nM FK-866 or vehicle control for an additional 72-hours. Data points represent the mean±SD of viability measurements conducted in a single experiment with three independent replicates.
Discussion
These findings are the first prospective evaluation of the relationship between MTX therapy and circulating concentrations of NAMPT in JIA. Despite 85% of patients meeting the ACR Pedi 30 response criteria at 6-months, NAMPT concentrations failed to change significantly following the initiation of MTX. Consistent with the co-regulation of NAMPT and inflammatory cytokines, increased NAMPT levels at baseline were associated with increased plasma levels of IL-1α, IL-1β, IL-1Ra, IL-6, and TNFα. However, NAMPT levels were not found to be significantly associated with baseline measures of disease activity. Although these findings contradict many of the studies in RA, these findings aren't unprecedented. A study of 6-month response in RA patients similarly failed to find an association between NAMPT levels and disease activity, and failed to observe a change in NAMPT levels over the study period (33). Similarly, a study in severe RA patients treated with infliximab found that NAMPT levels were not associated with disease activity and did not change in response to therapy (34). The failure to observe any significant reduction in NAMPT over the study period does not necessarily lessen the importance of NAMPT in the pathogenesis of JIA, as MTX therapy may target the disease process downstream of NAMPT. However, it does suggest that MTX itself does not modify NAMPT expression as a basis for its pharmacological activity, as suggested by our previous findings (13). Similarly, the inability to correlate disease activity and response with changes in NAMPT levels greatly limits the potential utility of monitoring NAMPT as a reactive biomarker of response.
The observation that increased NAMPT concentrations, which appear to be stable over the treatment period, are associated with response failure suggests that NAMPT may remain a useful predictive biomarker for therapeutic response. A similar observation has been made in RA patients, where baseline plasma NAMPT levels predicted long-term disease progression (6). These findings suggest that higher pretreatment NAMPT concentrations may subsequently identify patients who are more inclined to fail MTX, which could result in earlier intervention with the next tier of biologic therapies, avoiding unnecessary delays in adequate therapy. Significant overlap was observed between responders and non-responders in our study and therefore would limit its utility as a single discriminatory biomarker of non-response, but perhaps in the future may be able to be combined with other markers of response to develop algorithms to predict drug response early in therapy.
Lastly, this data supports the role of NAMPT as a modifier of the pharmacologic activity of MTX. Inhibition of translation of NAMPT mRNA through siRNA gene-silencing and pharmacological inhibition of NAMPT enzyme activity both resulted in a similar increase in sensitivity to the growth inhibitory activity of MTX. These findings support the hypothesis that NAMPT modifies the pharmacological response to MTX through its enzymatic activity, and not through receptor signaling pathways. The previous study of pemetrexed and the NAMPT inhibitor GMX1778 found that pharmacologic inhibition of NAMPT resulted in the depletion of cellular NAD that was further exacerbated by activation of poly (ADP-ribose) polymerase (PARP) by pemetrexed resulting in depletion of the cellular NAD pool and enhanced sensitivity to pemetrexed (29). Similar to pemetrexed, MTX has also been found to activate PARP in animal models and in isolated leukocytes (35, 36). In agreement with the role of PARP in the pharmacological activity of MTX, supplementation with the NAMPT substrate and PARP inhibitor, nicotinamide, has been found to inhibit the hepatotoxic activity of MTX and promote its efficacy in a mouse model (37, 38). Additional interacting pathways for NAD metabolism and MTX have also been suggested in metabolomic studies that have found inhibition of NAMPT affects purine biosynthesis, as several steps of purine and pyrimidine biosynthesis require NAD and its intermediates (39). The fact that MTX targets enzymes in the purine and pyrimidine biosynthesis pathway as a component of its pharmacological activity further links NAMPT to the known biologic effects of MTX (40). Although the biochemical mechanism through which NAMPT affects the pharmacological activity of MTX remains unclear at this point, these finding suggest that reduced NAMPT activity is associated with an increase in the activity of MTX, and may support pharmacologic inhibition of NAMPT as a means of enhancing clinical response to MTX. To date, no in vivo studies have evaluated the combination of MTX and NAMPT inhibition. However, based on these findings the combination would be expected to result in synergism. Whether the synergism would be limited to drug efficacy, or would also result in enhanced toxicity, is difficult to assess based on this data. The targeting of lymphocytes by both NAMPT inhibitors and MTX suggests that combination would result in increased efficacy, however the basis for toxicity of these agents is less clear and may display a similar synergism (26, 31, 32).
With the paucity of biomarkers in children, resulting from many of the same problems that have historically plagued pediatric drug development (41, 42), there remains a need to identify clinical biomarkers in the pediatric population to help guide drug therapy. Therefore, despite the incomplete understanding of the biochemical basis for the observed relationship between NAMPT and MTX, these results highlight the potential role of plasma NAMPT levels as a clinical biomarker in predicting therapeutic response to MTX. Most importantly, the ability of pretreatment NAMPT levels to predict future response to MTX may fulfill a critical need in the treatment regimen of JIA. The identification of poor responders to MTX, a priori, allows clinicians to choose potentially more effective therapies at the onset of treatment, minimizing the delay in achievement of disease control and further optimizing long term outcomes in JIA.
Future studies are needed to understand more fully the mechanism through which NAMPT modifies the pharmacological activity of MTX and the role of NAMPT as a biomarker of therapeutic response to MTX in JIA. Such an understanding may present the opportunity to target NAMPT and related biochemical pathways in drug development for the treatment of JIA and further support its role as a predictive clinical biomarker in the treatment of JIA.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Response to methotrexate in the treatment of Juvenile Idiopathic Arthritis remains highly variable and continues to be unpredictable.
What question did the study address?
We hypothesized that nicotinamide phosphoribosyltransferase (NAMPT) is an important biomarker of response to methotrexate.
What this study adds to our current knowledge?
These findings demonstrate a relationship between elevated NAMPT levels and failure to respond to methotrexate that appears to result from a biochemical interaction through which NAMPT modifies the pharmacological activity of methotrexate.
How this might change clinical pharmacology or translational science?
This work may lead to 1) the use of NAMPT as a predictive biomarker of response to methotrexate, and 2) a therapeutic approach utilizing pharmacological inhibitors of NAMPT to potentiate the pharmacological activity of methotrexate.
Acknowledgments
Funding for this project was provided by Children's Mercy Kansas City, The University of Kansas, the Rheumatology Research Foundation and the National Institute of Child Health and Human Development [T32-HD069038].
The authors would like to thank Chelsey Smith and Drs. Andrew Lasky, Mark Hoeltzel, Maria Ibarra, and Cara Hoffart for their assistance in patient recruitment and sample collection for this study.
Footnotes
Authorship Contributions: R.S.F. wrote the manuscript; R.S.F., D.P.H., S.Q.Y., M.A.C., J.S.L., and M.L.B. designed the research; R.S.F., R.S., L.P., N.G., and S.I. performed the research; R.S.F. and M.L.B. analyzed the data; D.P.H. and S.Q.Y. contributed new reagents/analytical tools.
Conflict of Interest/Disclosure: The authors report no financial conflicts of interest with regard to the work presented in this manuscript.
References
- 1.Becker ML. Optimization of pediatric rheumatology therapeutics. Clinical pharmacology and therapeutics. 2012;91(4):597–606. doi: 10.1038/clpt.2011.293. [DOI] [PubMed] [Google Scholar]
- 2.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2006;65(9):1198–201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui H, Tsutsumi A, Sugihara M, Suzuki T, Iwanami K, Kohno M, et al. Visfatin (pre-B cell colony-enhancing factor) gene expression in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2008;67(4):571–2. doi: 10.1136/ard.2007.077578. [DOI] [PubMed] [Google Scholar]
- 4.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis and rheumatism. 2009;60(7):1906–14. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkady EA, Ahmed HM, Tag L, Abdou MA. Serum and synovial adiponectin, resistin, and visfatin levels in rheumatoid arthritis patients. Relation to disease activity. Z Rheumatol. 2011;70(7):602–8. doi: 10.1007/s00393-011-0834-2. [DOI] [PubMed] [Google Scholar]
- 6.Klein-Wieringa IR, van der Linden MP, Knevel R, Kwekkeboom JC, van Beelen E, Huizinga TW, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis and rheumatism. 2011;63(9):2567–74. doi: 10.1002/art.30449. [DOI] [PubMed] [Google Scholar]
- 7.Senolt L, Krystufkova O, Hulejova H, Kuklova M, Filkova M, Cerezo LA, et al. The level of serum visfatin (PBEF) is associated with total number of B cells in patients with rheumatoid arthritis and decreases following B cell depletion therapy. Cytokine. 2011;55(1):116–21. doi: 10.1016/j.cyto.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 8.El-Hini SH, Mohamed FI, Hassan AA, Ali F, Mahmoud A, Ibraheem HM. Visfatin and adiponectin as novel markers for evaluation of metabolic disturbance in recently diagnosed rheumatoid arthritis patients. Rheumatol Int. 2013;33(9):2283–9. doi: 10.1007/s00296-013-2714-3. [DOI] [PubMed] [Google Scholar]
- 9.Mirfeizi Z, Noubakht Z, Rezaie AE, Jokar MH, Sarabi ZS. Plasma levels of leptin and visfatin in rheumatoid arthritis patients; is there any relationship with joint damage? Iran J Basic Med Sci. 2014;17(9):662–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Sglunda O, Mann H, Hulejova H, Kuklova M, Pecha O, Plestilova L, et al. Decreased circulating visfatin is associated with improved disease activity in early rheumatoid arthritis: data from the PERAC cohort. PloS one. 2014;9(7):e103495. doi: 10.1371/journal.pone.0103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowell M, Evans L, Williams A. PBEF/NAMPT/visfatin: a promising drug target for treating rheumatoid arthritis? Future Med Chem. 2012;4(6):751–69. doi: 10.4155/fmc.12.34. [DOI] [PubMed] [Google Scholar]
- 12.Montecucco F, Cea M, Bauer I, Soncini D, Caffa I, Lasiglie D, et al. Nicotinamide phosphoribosyltransferase (NAMPT) inhibitors as therapeutics: rationales, controversies, clinical experience. Curr Drug Targets. 2013;14(6):637–43. doi: 10.2174/1389450111314060003. [DOI] [PubMed] [Google Scholar]
- 13.Fox EJ, Leeder JS, Ye SQ, Becker ML. Decreased expression of nicotinamide phosphoribosyltransferase in patients with juvenile idiopathic arthritis receiving methotrexate. The Journal of rheumatology. 2013;40(5):741–2. doi: 10.3899/jrheum.120639. [DOI] [PubMed] [Google Scholar]
- 14.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. European journal of immunology. 2002;32(11):3225–34. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54(5):1200–11. doi: 10.1007/s00125-010-2042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26(2):107–17. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 18.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis and rheumatism. 2006;54(7):2084–95. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 19.Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, Gay S, et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis and rheumatism. 2007;56(9):2829–39. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 20.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. Journal of immunology. 2007;178(3):1748–58. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 21.Cillero-Pastor B, Ruiz-Romero C, Carames B, Lopez-Armada MJ, Blanco FJ. Proteomic analysis by two-dimensional electrophoresis to identify the normal human chondrocyte proteome stimulated by tumor necrosis factor alpha and interleukin-1beta. Arthritis and rheumatism. 2010;62(3):802–14. doi: 10.1002/art.27265. [DOI] [PubMed] [Google Scholar]
- 22.Meier FM, Frommer KW, Peters MA, Brentano F, Lefevre S, Schroder D, et al. Visfatin/pre-B-cell colony-enhancing factor (PBEF), a proinflammatory and cell motility-changing factor in rheumatoid arthritis. The Journal of biological chemistry. 2012;287(34):28378–85. doi: 10.1074/jbc.M111.312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Presumey J, Courties G, Louis-Plence P, Escriou V, Scherman D, Pers YM, et al. Nicotinamide phosphoribosyltransferase/visfatin expression by inflammatory monocytes mediates arthritis pathogenesis. Annals of the rheumatic diseases. 2013;72(10):1717–24. doi: 10.1136/annrheumdis-2012-202403. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. The Journal of biological chemistry. 2008;283(50):34833–43. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rongvaux A, Galli M, Denanglaire S, Van Gool F, Dreze PL, Szpirer C, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. Journal of immunology. 2008;181(7):4685–95. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 27.Evans L, Williams AS, Hayes AJ, Jones SA, Nowell M. Suppression of leukocyte infiltration and cartilage degradation by selective inhibition of pre-B cell colony-enhancing factor/visfatin/nicotinamide phosphoribosyltransferase: Apo866-mediated therapy in human fibroblasts and murine collagen-induced arthritis. Arthritis and rheumatism. 2011;63(7):1866–77. doi: 10.1002/art.30338. [DOI] [PubMed] [Google Scholar]
- 28.Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PloS one. 2008;3(5):e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan M, Gravel M, Bramoulle A, Bridon G, Avizonis D, Shore GC, et al. Synergy between the NAMPT Inhibitor GMX1777(8) and Pemetrexed in Non-Small Cell Lung Cancer Cells Is Mediated by PARP Activation and Enhanced NAD Consumption. Cancer research. 2014;74(21):5948–54. doi: 10.1158/0008-5472.CAN-14-0809. [DOI] [PubMed] [Google Scholar]
- 30.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL, et al. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res. 2005;70(3):142–51. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fairbanks LD, Ruckemann K, Qiu Y, Hawrylowicz CM, Richards DF, Swaminathan R, et al. Methotrexate inhibits the first committed step of purine biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis? Biochem J. 1999;342(Pt 1):143–52. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KS, Choi HM, Ji HI, Song R, Yang HI, Lee SK, et al. Serum adipokine levels in rheumatoid arthritis patients and their contributions to the resistance to treatment. Mol Med Rep. 2014;9(1):255–60. doi: 10.3892/mmr.2013.1764. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Garcia-Unzueta MT, Berja A, Miranda-Filloy JA, de Matias JM, et al. Visfatin is not associated with inflammation or metabolic syndrome in patients with severe rheumatoid arthritis undergoing anti-TNF-alpha therapy. Clin Exp Rheumatol. 2010;28(1):56–62. [PubMed] [Google Scholar]
- 35.Dalaklioglu S, Sahin P, Ordueri EG, Celik-Ozenci C, Tasatargil A. Potential role of poly(ADP-ribose) polymerase (PARP) activation in methotrexate-induced nephrotoxicity and tubular apoptosis. Int J Toxicol. 2012;31(5):430–40. doi: 10.1177/1091581812457430. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen CH, Albertsen L, Bendtzen K, Baslund B. Methotrexate induces poly(ADP-ribose) polymerase-dependent, caspase 3-independent apoptosis in subsets of proliferating CD4+ T cells. Clinical and experimental immunology. 2007;148(2):288–95. doi: 10.1111/j.1365-2249.2007.03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroger H, Hauschild A, Ohde M, Thefeld W, Kruger D, Bache K, et al. Enhancement of the effect of methotrexate on collagen II induced arthritis in mice by nicotinamide. Inflammation. 1998;22(3):277–85. doi: 10.1023/a:1022348132132. [DOI] [PubMed] [Google Scholar]
- 38.Kroger H, Hauschild A, Ohde M, Bache K, Voigt WP, Thefeldt W, et al. Nicotinamide and methionine reduce the liver toxic effect of methotrexate. Gen Pharmacol. 1999;33(2):203–6. doi: 10.1016/s0306-3623(98)00232-8. [DOI] [PubMed] [Google Scholar]
- 39.Tolstikov V, Nikolayev A, Dong S, Zhao G, Kuo MS. Metabolomics analysis of metabolic effects of nicotinamide phosphoribosyltransferase (NAMPT) inhibition on human cancer cells. PloS one. 2014;9(12):e114019. doi: 10.1371/journal.pone.0114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funk RS, van Haandel L, Becker ML, Leeder JS. Low-dose methotrexate results in the selective accumulation of aminoimidazole carboxamide ribotide in an erythroblastoid cell line. The Journal of pharmacology and experimental therapeutics. 2013;347(1):154–63. doi: 10.1124/jpet.113.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.