Abstract
Introduction
Robust wall apposition for flow-diverter stents (FDS) may be important for endothelialization. Using a large series of experimental aneurysms treated with the Pipeline Embolization Device (PED), the objectives of this study were to 1) assess interobserver agreement for the evaluation of wall apposition on post-treatment DSA and evaluate its association with aneurysm occlusion and 2) measure the relationship between wall apposition assessed with histology and aneurysm occlusion rate after PED treatment.
Material and methods
Saccular aneurysms were created in 41 rabbits and treated with PED. DSA was performed just after the deployment of the device and at follow-up. Three investigators independently graded wall apposition on post-treatment DSA as good or poor. One histopathologist blinded to the angiographic results, graded the wall-apposition on histological samples. We examined the correlation between angiographic occlusion and wall apposition with histology and angiography.
Results
Wall apposition evaluated on histology was strongly associated with saccular aneurysm occlusion. Sensitivity and specificity of wall apposition to predict complete occlusion at follow-up were, respectively, 76.9% and 84.0% with an overall accuracy 81.6%. In this experimental study, DSA was sub-optimal to assess flow diverter apposition with moderate inter-observer agreement and low accuracy.
Conclusion
Good wall apposition is strongly associated with complete occlusion following flow-diverter therapy. In this study, DSA is suboptimal for assessing wall apposition of FDS. These findings suggest that improved tools for assessing FDS wall apposition are highly relevant.
INTRODUCTION
Flow diverter stents (FDS) are now largely accepted as standard of care in treatment of select aneurysms due to their high rates of angiographic occlusion and good clinical outcomes1-3. Because the mechanism of aneurysm occlusion following FDS treatment is likely related to stent endothelialization derived exclusively from cells in the adjacent parent artery4, it appears important to have good wall apposition to promote endothelialization5. However, to our knowledge, correlation between aneurysm occlusion and wall apposition has not previously been evaluated in FDS. Despite no evidence of this correlation, several tools are currently being evaluated to assess wall apposition of flow-diverter stents6-8.
Using a large series of elastase induced aneurysms in rabbits treated with the Pipeline Embolization Device (PED, Covidien Inc, California, USA), the objectives of this study were to 1) assess interobserver agreement for the evaluation of wall apposition on post-treatment DSA and evaluate its association with aneurysm occlusion and 2) measure the relationship between wall apposition assessed with histology and aneurysm occlusion rates after PED.
Material and Methods
In Vivo Experiments
The Institutional Animal Care and Use Committee at our institution approved animal procedures. Some of the rabbits used in this study were originally employed as part of other investigations9, 10. Elastase-induced aneurysms were created in 41 New Zealand white rabbits. Aneurysm creation procedures were performed as previously described by our study group11. Aneurysms were treated at least 3 weeks after aneurysm creation12. Two days before the treatment procedure, subjects were premedicated with aspirin (10 mg/kg PO) and clopidogrel (10 mg/kg PO); this medication regimen was continued for 1 month after embolization. The detailed procedure has been published before9, 10. All the endovascular procedures were performed with the GE Advantx DLX (Milwaukee, WI, USA) angio suite equipped with an image amplifier. Images field of view was 11 cm with a frame rate of 2 frames per second and the X -ray dose per frame was 500 µR. The spatial resolution was 1.5 lines pairs per millimeter (1.5 lp/mm).
Briefly, a 5-Fr guide catheter (Envoy; Cordis) was placed into the aortic arch and digital subtraction angiography (DSA) performed. Heparin (500 U intravenously) was administered and then a microcatheter (Marksman; Medtronic) was placed over a microguidewire (Transcend; Boston Scientific) into the subclavian artery distal to the aneurysm cavity. The wire was removed and the PED was advanced into the distal aspect of the microcatheter. The device was deployed across the neck of the aneurysm from the subclavian artery to the brachiocephalic trunk with no protrusion of the proximal landing zone in the aortic arch. The microcatheter was removed and DSA was performed through the guide catheter five minutes after deployment. No additional angioplasty was performed to improve wall apposition. No 3D acquisition has been performed.
The implanted PED was selected according to the diameter of the artery. Details regarding proximal and distal diameters of the parent artery as well as the size of the implanted PED are available in Online Table 1.
Rabbits were sacrificed at day 30 (n= 18), day 90 (n= 11), day 180 (n= 12) following the procedure. At the time of sacrifice, animals were deeply anesthetized. DSA of the aortic arch was performed to evaluate aneurysm occlusion. The animals were then euthanized with a lethal injection of pentobarbital. Aneurysm and parent artery tissue were immediately fixed in 10% neutral buffered formalin.
Data Analysis
Angiographic evaluation.
A single experienced reader, blinded to wall-apposition status (either DSA and histological evaluations), assessed angiographic aneurysm occlusion at follow-up according to a 2-point classification: complete (100 % occlusion) or incomplete occlusion.
Three investigators, blinded to histological wall apposition evaluation, independently examined selected post-processed (pixel shift) images of the post-treatment DSA to grade wall apposition status on post-treatment DSA. Wall apposition status on DSA was evaluated according to a dichotomous outcome, noted as either good or poor apposition depending on the presence or absence of contrast media visible between the stent and the parent artery (Illustrative images for good and poor wall apposition are presented in online Figures 1 and 2).
Histopathologic processing and wall apposition evaluation
One histopathologist blinded to the angiographic results did the processing and analysis for wall apposition as previously described9. After routine tissue processing, the fixed samples were embedded in paraffin. Aneurysm samples were processed at 1000 µm intervals in a sagittal orientation with use of an Isomet low-speed saw (Buehler, Lake Bluff, ILL). The metal stents were carefully removed under a dissecting microscope. The samples were then re-embedded in paraffin, sectioned at 5 to 6 µm, and stained with hematoxylineosin (H&E).
Wall apposition was evaluated according to a dichotomous outcome, with either good or poor wall apposition of the stent. The evaluation was performed at the level of the neck of the aneurysm. A good histological wall apposition meant that the stent was well apposed on the entire surface parent artery adjacent to the aneurysm ostium.
Statistical analysis
All statistical analyses were performed in R (version 3.1.1; Vienna, Austria). Kappa and ICC statistics were calculated using the irr package (version 0.84). Agreement among three readers for DSA assessment of post-treatment wall apposition was assessed using the intra-class correlation coefficient method ICC13. Cohen's kappa were also displayed for pairwise comparisons of raters. Kappa was also used to assess agreement between the reference method of histology and DSA using a consensus score agreed on by two radiologists for each method.
We calculated sensitivity, specificity and accuracy of post-treatment DSA and histologic wall apposition evaluations in predicting the occurrence of aneurysm occlusion at follow-up. Wilson-Score 95% confidence intervals are reported for each statistic. Fisher's exact test was performed to test for association between wall apposition assessed by histology at follow-up and occlusion outcome at follow-up.
RESULTS
Population and Angiographic Follow-up Outcome
Forty-one consecutively treated rabbits were included in this study. All aneurysms were saccular. The mean aneurysm size was 9.41 mm (95%CI: 8.41-10.41) in the complete occlusion group and 9.47 mm (95%CI: 8.03-10.91) in the incomplete occlusion group. There was no significant difference in aneurysms sizes across groups (t-test p value = 0.94). The length of follow-up varied from 30 days to 180 days with a mean length of follow-up of 90.0 days. Rabbits were sacrificed at day 30 (n= 18), day 90 (n= 11), day 180 (n= 12).
Follow-up DSA was available for 40 rabbits. 67.5% (n= 27/40 aneurysms) of cases had complete occlusion and 32.5 % (n=13) had incomplete occlusion. Angiographic complete occlusion rates at 30, 90 and 180 days were not statistically different with respectively 52.9% (9/17), 72.7% (8/11) and 83.3% (10/12) (Chi-square p value = .25).
Wall Apposition Evaluation on Post-Treatment DSA
Immediate post-treatment DSA was available for 41 rabbits. Wall apposition status evaluated on post-treatment DSA by the 3 independent readers demonstrated a good wall apposition rate of 61.0% (n=75/123 readings) and a poor wall apposition rate of 39.0% (n=48). The consensus DSA evaluation yielded 63.4 % (26/41) good wall apposition and 36.6% (15/41) poor wall apposition. All three readers rated similar proportions of wall apposition as poor (N=16/41, 39%). Pairwise kappa between the three readers for DSA were kappa=0.487, 0.590, and 0.385. Intra-class correlation among readers was 0.488 (95% CI 0.30-0.66), indicating moderate interobserver agreement.
Correlation Between Consensus DSA Wall Apposition and Follow-up Occlusion
Contingency table is presented in Table 1a. Sensitivity and specificity of good wall apposition evaluated on post-treatment DSA for the prediction of complete versus incomplete occlusion on follow-up DSA were, respectively, 23.1% (95% CI: 8.2 – 50.3) and 55.5 % (95% CI: 37.3 – 72.4). The overall accuracy of the wall apposition evaluated on post-treatment DSA for the prediction of complete versus incomplete occlusion at follow-up was 45.0 % (95% CI: 30.7 – 60.2).
Tables 1.
Correlations between wall apposition and follow-up DSA outcomes.
| 1a. Contingency table for wall apposition assessment on post-treatment DSA. |
| Follow-up DSA outcome | ||||
|---|---|---|---|---|
| Complete occlusion | Incomplete occlusion | Total | ||
|
Wall apposition
on post treatment DSA |
Good Wall apposition |
15 | 10 | 25 |
| Poor Wall apposition |
12 | 3 | 15 | |
| Total | 27 | 13 | 40 | |
| 1b. Contingency table for wall apposition assessment on histology. |
| Follow-up DSA outcome | ||||
|---|---|---|---|---|
| Complete occlusion | Incomplete occlusion | Total | ||
|
Wall apposition
on Histology |
Good Wall apposition |
21 | 3 | 24 |
| Poor Wall apposition |
4 | 10 | 14 | |
| Total | 25 | 13 | 38 | |
Wall Apposition Evaluation on Histology
Histological evaluation at time of follow-up DSA was available for 38 rabbits. 63.2 % of cases had good wall apposition (n=24/38 aneurysms) and 36.8 % (n=14) of cases had poor wall apposition.
Correlation Between Histological Wall Apposition Evaluation and Follow-up Occlusion
Illustrative histology images for good and poor wall apposition are presented in Figure 1. Contingency table is presented in Table 1b. Angiographic complete occlusion rates at 30, 90 and 180 days in good wall apposition aneurysms were not statistically different with respectively 77.8% (7/9), 100.0% (6/6) and 88.9% (8/9) (Chi-square p value = .44). Angiographic complete occlusion rates at 30, 90 and 180 days in poor wall apposition aneurysms were not statistically different with respectively 25.0% (2/8), 25.0% (1/4) and 50.0% (1/2) (Chi-square p value = .77).
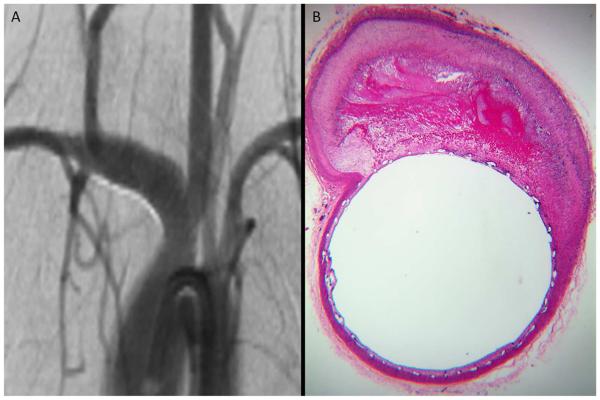
Figure 1. Histology and DSA illustrative correlation of good wall apposition associated with complete aneurysm occlusion.
Follow-up DSA objectives complete occlusion of the aneurysm sac (A). Photomicrograph (Hematoxylin-eosin stainiing, original magnification x100) at the level of the aneurysm neck shows perfect PED wall apposition with complete aneurysm pouch occlusion filled with conjunctive tissue (B).
Sensitivity and specificity of wall apposition evaluated on histology for the prediction of complete versus incomplete occlusion on follow-up DSA were respectively 76.9 % (95% CI: 49.7 – 91.8) and 84.0 % (95% CI: 65.3 – 93.6). The overall accuracy of the wall apposition evaluated on post-treatment DSA for the prediction of complete versus incomplete occlusion at follow-up was 81.6 % (95% CI: 66.6 – 90.8). Histologically-assessed wall apposition was significantly associated with occlusion at follow-up DSA (Fisher's exact test p-value <0.001, odds ratio 15.7, 95% CI 2.63-133.2).
DISCUSSION
Our study performed of a large series of experimental aneurysms demonstrated that wall apposition is a crucial determinant for saccular aneurysm occlusion after FDS treatment. Wall apposition on histology was strongly correlated with aneurysm occlusion following treatment of intracranial aneurysms with flow-diverters suggesting that, if good wall apposition is not present, adjunctive measures such as balloon inflation should be considered in some clinical settings to improve apposition so as to improve aneurysm occlusion rates.
Also, our study demonstrated that assessment of wall apposition on post-treatment DSA suffers from low interobserver agreement and is poorly predictive of final aneurysm occlusion status. We acknowledge that the DSA system used for this experimental study in rabbits is not is not optimal and that the results can not be extrapolate with DSA used in clinical angio-suite. Indeed, these findings suggest strongly that operators should consider alternative methodologies for assessing wall apposition with high-resolution contrast-enhanced cone-beam CT such as VasoCT (Philips)14, DynaCT (Siemens)15, InnovaCT (General Electrics) or with intravascular optical coherence tomography8.
This study correlates flow-diverter wall apposition with saccular aneurysms occlusion using histological evaluation. This is of high importance as wall apposition can be considered a key-factor for the occurrence of saccular aneurysms non-occlusion. One potential biological mechanism that could explain lower occlusion rates in cases of poor wall apposition is the fact that aneurysm occlusion after flow-diverter stent is not exclusively related to intra-aneurysmal thrombosis but is also driven by endothelialization of the device4. This endothelialization is relatively delayed and, we believe, derived exclusively from cells in the adjacent parent artery4. Based on this proposed mechanism, in cases of poor wall apposition, the malaposed portion of the device will fail to endothelialize due to lack of direct contact with the parent artery. This mechanism has also been discussed in the coronary intervention literature and coronary stents that lack wall apposition fail to endothelialize5,16. While our study proves that wall apposition is highly correlated with angiographic outcome, our study also found that DSA was a suboptimal tool in assessing wall apposition.
Our current results are in accordance with the clinical study published by Saake et al.6 who reported that wall apposition was difficult to assess on intra-arterial DSA in a study with 14 patients and evaluations made in consensus by two experienced neuroradiologists. In this study comparing DSA and intravenous angiographic CT (iv ACT), they did not find any significant difference between the two modalities concerning the deployment of the flow diverting stent, wall apposition of the stent in the non-aneurysmal parent vessel segments and the aneurysmal neck coverage by the stent but the reviewers preferred iv ACT for evaluation of wall apposition6. Beyond the fact that the angio-suite used in this experimental study is not optimal and differs from clinical ones, there are a number of explanations for why 2D DSA may be suboptimal in assessing wall apposition. 2D DSA cannot provide cross-sectional images thus there are limited views examining the relationship between the FDS and the vessel wall. In addition, DSA imaging acquisition is based on mask subtraction, making assessment of any contrast opacification between the subtracted device itself and the arterial wall quite difficult. Furthermore in our study, there was motion artifact due to the fact that the experimental aneurysms are not located in the brain but in the thorax and are subject to ventilation movements.
Newer tools are being currently evaluated in the setting of FDS treatment of intracranial aneurysms to assess wall apposition. Flat-panel CT with intra-arterial or intravenous opacification has a very high spatial resolution and can be done directly on the DSA table without any additional invasive procedure and low radiation6, 7, 17-22. More recently, optical coherence tomography is now being evaluated for intracranial procedures8, 23-27. Also, other endovascular techniques which are not yet used in the neuroradiology field but primarily used in cardiology, such as intravascular ultrasound24 or endoluminal optical imaging (angioscopy)28, seem promising in the assessment of wall apposition. Our study suggests that these tools could one day be preferred over DSA for the assessment of wall apposition.
In cases of poor wall apposition demonstrated on post-deployment imaging, it could be useful to improve apposition with balloon expansion. Another option is the development of flow diverter stents with higher radial force and conformability which is a key factor for better wall apposition29. However, radial force is usually low in braided devices. We surmise that new flow diverter devices with a laser-cut scaffold and a high mesh density construct on the outer diameter could give higher radial force and better wall apposition30.
Limitations
Our study is limited by its retrospective nature and the use of only selected images for the DSA readers’ assessment. In addition, it is possible that a newer generation angio-suite could provide better DSA images; 3D runs with multiplanar reconstructions might be better that 2D DSA to assess wall apposition but we did not perform these acquisitions. Another limitation to our study is the motion artifact associated with the respiratory and cardiac motion in these rabbit aneurysms. However, it is important to point out that all images underwent post-processing including pixel shifting by an experienced radiologist prior to assessment by the blinded readers. Furthermore, only one experienced reader evaluated the histological samples. However, this reader was blinded to the DSA outcomes. Regarding the choice of the appropriate device sizing was sometimes difficult because of discrepancies in the artery diameter between the proximal and distal parts but the braided stents have the ability to expand more than their labeled diameter.
We did not use previously published classification for wall apposition assessment because we mainly focused at the level of the aneurysm neck while the available classifications discussed about the device deployment in the parent artery and not specifically at the neck6. Another limitation of this study is that rabbits were sacrificed at different time-points, which can modify the outcomes after PED implantation depending of the length of follow-up, however, we did not observe any statistically significant impact of time on complete occlusion rates. Also, this study focused on wall apposition as one of the key factors influencing aneurysm occlusion after flow-diverter but some other criteria are also of high importance such as hemodynamic effects, intra-aneurysmal thrombosis, meshes density which have not been analyzed in this specific study.
CONCLUSION
This study highlights the fact that good wall apposition is key in obtaining complete occlusion of saccular aneurysms after FDS treatment. In this study, 2D DSA is suboptimal for assessing wall apposition of FDS with only moderate inter-observer agreement and low accuracy. Our inability to perform high frame rate DSAs and C-arm CT acquisitions to evaluate wall apposition must be considered when assessing the significance of these results. Our study suggests that development of new tools for the assessment of wall apposition for flow-diverter stents is needed to improve angiographic outcomes in patients treated with FDS.
Supplementary Material
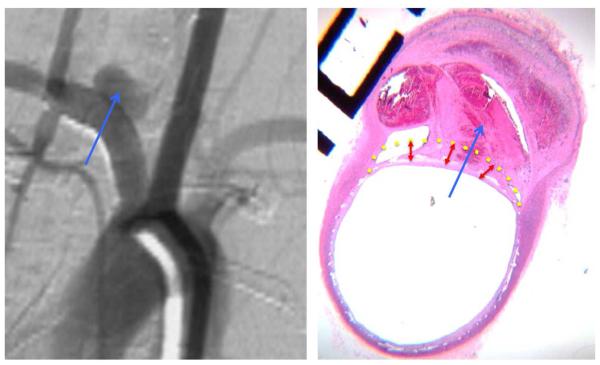
Figure 2. Histology and DSA illustrative correlation of poor wall apposition associated with incomplete aneurysm occlusion.
Follow-up DSA objectives incomplete occlusion of the aneurysm sac (blue arrow) (A). Photomicrograph at the level of the aneurysm neck (Hematoxylin-eosin dying, original magnification x100) show poor wall apposition (yellow dotted line) and filling of the aneurysm pouch with a partial thrombosis in the aneurysm sac.
Acknowledgments
FUNDING
This work was supported by research grant NS0767491 from the National Institute of Health and Medtronic. Aymeric Rouchaud was supported by a research grants from the French Society of Radiology and Therese Planiol Foundation.
Footnotes
DISCLOSURES: Juan Cebral—RELATED: Grant: NIH (research grant)*; UNRELATED: Grants/Grants Pending: NIH,* Philips,* Comments: Research grants. David Kallmes— UNRELATED: Board Membership: GE Healthcare,* Comments: Cost-effectiveness board; Consultancy: eV3/Covidien/Medtronic,* Comments: Planning and implementing clinical trials, clinical events committee, steering committee; Grants/Grants Pending: MicroVention,* Sequent,* Neurosigma,* Surmodics,* Codman,* eV3/Covidien/Medtronic,* Comments: Preclinical research and clinical trials, supply of devices; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: eV3/Covidien/Medtronic,* Comments: Presentation at FDA panel meeting. Ram Kadirvel— RELATED: Grant: NIH.
*money paid to institution
REFERENCES
- 1.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73:193–199. doi: 10.1227/01.neu.0000430297.17961.f1. discussion 199-200. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44:442–447. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 3.Wakhloo AK, Gounis MJ. Revolution in aneurysm treatment: flow diversion to cure aneurysms: a paradigm shift. Neurosurgery. 2014;61(Suppl 1):111–120. doi: 10.1227/NEU.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 4.Kadirvel R, Ding YH, Dai D, et al. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014;270:394–399. doi: 10.1148/radiol.13130796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foin N, Gutierrez-Chico JL, Nakatani S, et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 2014;7:180–189. doi: 10.1161/CIRCINTERVENTIONS.113.000931. [DOI] [PubMed] [Google Scholar]
- 6.Saake M, Struffert T, Goelitz P, et al. Angiographic CT with intravenous contrast agent application for monitoring of intracranial flow diverting stents. Neuroradiology. 2012;54:727–735. doi: 10.1007/s00234-011-0965-9. [DOI] [PubMed] [Google Scholar]
- 7.Ding D, Starke RM, Durst CR, et al. DynaCT imaging for intraprocedural evaluation of flow-diverting stent apposition during endovascular treatment of intracranial aneurysms. J Clin Neurosci. 2014;21:1981–1983. doi: 10.1016/j.jocn.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.van der Marel K, Gounis MJ, Weaver JP, et al. Grading of Regional Apposition after Flow-Diverter Treatment (GRAFT): a comparative evaluation of VasoCT and intravascular OCT. J Neurointerv Surg. 2015 doi: 10.1136/neurintsurg-2015-011843. [DOI] [PubMed] [Google Scholar]
- 9.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–2352. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 10.Kallmes DF, Ding YH, Dai D, et al. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009;30:1153–1158. doi: 10.3174/ajnr.A1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR American journal of roentgenology. 2000;174:349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara NH, Cloft HJ, Marx WF, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR American journal of neuroradiology. 2001;22:698–703. [PMC free article] [PubMed] [Google Scholar]
- 13.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 14.Kizilkilic O, Kocer N, Metaxas GE, et al. Utility of VasoCT in the treatment of intracranial aneurysm with flow-diverter stents. Journal of neurosurgery. 2012;117:45–49. doi: 10.3171/2012.4.JNS111660. [DOI] [PubMed] [Google Scholar]
- 15.Farago G, Caldiera V, Tempra G, et al. Advanced digital subtraction angiography and MR fusion imaging protocol applied to accurate placement of flow diverter device. J Neurointerv Surg. 2016;8:e5. doi: 10.1136/neurintsurg-2014-011428.rep. [DOI] [PubMed] [Google Scholar]
- 16.Attizzani GF, Capodanno D, Ohno Y, et al. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol. 2014;63:1355–1367. doi: 10.1016/j.jacc.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Aurboonyawat T, Schmidt PJ, Piotin M, et al. A study of the first-generation pipeline embolization device morphology using intraoperative angiographic computed tomography (ACT) Neuroradiology. 2011;53:23–30. doi: 10.1007/s00234-010-0709-2. [DOI] [PubMed] [Google Scholar]
- 18.Clarencon F, Piotin M, Pistocchi S, et al. Evaluation of stent visibility by flat panel detector CT in patients treated for intracranial aneurysms. Neuroradiology. 2012;54:1121–1125. doi: 10.1007/s00234-011-1002-8. [DOI] [PubMed] [Google Scholar]
- 19.Patel NV, Gounis MJ, Wakhloo AK, et al. Contrast-enhanced angiographic cone-beam CT of cerebrovascular stents: experimental optimization and clinical application. AJNR Am J Neuroradiol. 2011;32:137–144. doi: 10.3174/ajnr.A2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jou LD, Mitchell BD, Shaltoni HM, et al. Effect of structural remodeling (retraction and recoil) of the pipeline embolization device on aneurysm occlusion rate. AJNR Am J Neuroradiol. 2014;35:1772–1778. doi: 10.3174/ajnr.A3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caroff J, Mihalea C, Neki H, et al. Role of C-arm VasoCT in the use of endovascular WEB flow disruption in intracranial aneurysm treatment. AJNR Am J Neuroradiol. 2014;35:1353–1357. doi: 10.3174/ajnr.A3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poncyljusz W, Bilinski P, Safranow K, et al. The LVIS/LVIS Jr. stents in the treatment of wide-neck intracranial aneurysms: multicentre registry. J Neurointerv Surg. 2015;7:524–529. doi: 10.1136/neurintsurg-2014-011229. [DOI] [PubMed] [Google Scholar]
- 23.Hayat U, Thondapu V, Asrar Ul Haq M, et al. Optical coherence tomography to evaluate coronary stent implantation and complications. Coron Artery Dis. 2015;26(Suppl 1):e55–68. doi: 10.1097/MCA.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 24.Amoroso G, van Geuns RJ, Spaulding C, et al. Assessment of the safety and performance of the STENTYS self-expanding coronary stent in acute myocardial infarction: results from the APPOSITION I study. EuroIntervention. 2011;7:428–436. doi: 10.4244/EIJV7I4A71. [DOI] [PubMed] [Google Scholar]
- 25.Radu M, Jorgensen E, Kelbaek H, et al. Strut apposition after coronary stent implantation visualised with optical coherence tomography. EuroIntervention. 2010;6:86–93. [PubMed] [Google Scholar]
- 26.Ozaki Y, Okumura M, Ismail TF, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31:1470–1476. doi: 10.1093/eurheartj/ehq066. [DOI] [PubMed] [Google Scholar]
- 27.Sawada T, Shite J, Negi N, et al. Factors that influence measurements and accurate evaluation of stent apposition by optical coherence tomography. Assessment using a phantom model. Circ J. 2009;73:1841–1847. doi: 10.1253/circj.cj-09-0113. [DOI] [PubMed] [Google Scholar]
- 28.McVeigh PZ, Sacho R, Weersink RA, et al. High-resolution angioscopic imaging during endovascular neurosurgery. Neurosurgery. 2014;75:171–180. doi: 10.1227/NEU.0000000000000383. discussion 179-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalmar G, Hubner F, Voelker W, et al. Radial force and wall apposition of balloon-expandable vascular stents in eccentric stenoses: an in vitro evaluation in a curved vessel model. J Vasc Interv Radiol. 2002;13:499–508. doi: 10.1016/s1051-0443(07)61530-9. [DOI] [PubMed] [Google Scholar]
- 30.Mohlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol. 2015;36:1155–1161. doi: 10.3174/ajnr.A4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.