Abstract
Objective
The aims of the current research project were to investigate the efficiency of various polymers to enhance the solubility and increase the systemic absorption of piperine using hot melt extrusion technology.
Methods
Piperine 10–40% w/w and Eudragit® EPO/ Kollidon® VA 64 or Soluplus® were mixed and the resulting blends were extruded using a twin-screw extruder (Process 11, Thermo Fisher Scientific). Drug release profiles and piperine solubility studies of the extrudates were evaluated. A non-everted intestinal sac was employed for the most promising formulation, 10% w/w piperine/Soluplus®, and pure piperine to study the permeability characteristics.
Key Findings
Dissolution studies demonstrated enhancement in piperine percent release of 10% and 20% w/w piperine/Soluplus® extrudates up to 95% and 74%, respectively. The solubility of 10% and 20% piperine/Soluplus® increased more than 160- and 45-fold in water, respectively. Furthermore, permeability studies demonstrated the enhancement in piperine absorption of 10% w/w piperine/Soluplus® extrudates up to 158.9 μg/5mL compared to pure piperine at 1.3 μg/5mL within 20 minutes.
Conclusion
These results demonstrated that increasing the bioavailability of piperine may be achieved as demonstrated by findings in this study.
Keywords: Hot melt extrusion (HME), Piperine, Solubility, Permeability
Introduction
Oral drug delivery is considered as the simplest and easiest route of drug administration [1, 2]. Oral bioavailability is mainly affected by drug solubility, permeability [3-5], and first pass metabolism [6]. In fact, most of the new Active Pharmaceutical Ingredients APIs are have low water solubility with low release profiles after oral administration. The biggest challenge in the pharmaceutical industries was to enhance the solubility and the permeability of those drugs as key factors to improve their bioavailability. Various techniques have been used to improve the drug water solubility and release profile, and solid dispersions are considered to be the most successful techniques. There are two main solid dispersion manufacturing methods: the melting method, such as hot melt extrusion (HME) and solvent evaporation methods, such as spray drying [7]. HME is one of the most commonly used technique to enhance the solubility and oral bioavailability of a poorly soluble drug as a beneficial technique for solid dispersion, which involves simple dispersion of a poorly water soluble API in an inert carrier (polymer), where the drug could exist in amorphous or crystalline state. There are many advantages of using HME due to the speed and the continuous manufacturing process. Moreover, since no solvent is required, this is considered to be a green method for enhancement of the solubility and oral bioavailability of poorly soluble drugs. Depending on the polymeric carrier, HME can be also used for other purposes such as taste masking, controlling or modifying drug release, and stabilizing the active pharmaceutical ingredient.
Piperine (1-piperoylpiperidine) is a crystalline pungent alkaloid isolated from black pepper (Piper nigrum), long pepper (Piper longum), and other pepper species (family Piperaceae) [8-10]. Piperine is a poor water soluble compound with a melting point at 135°C. It has been extensively used in folk medicine in many Asian countries [9], and various studies have focused on investigating the pharmacological effects of piperine. Recently, it was reported as an anti-inflammatory, analgesic [11], anti-depressant [12], cytoprotective, anti-leukemic, and anti-oxidant agent [8, 13]. Furthermore, piperine significantly improves spatial memory and neurodegeneration in an Alzheimer's disease animal model [14]. Furthermore, piperine was reported to be a bioavailable enhancer [15]. Pattanaik et al. reported that 20 mg of oral piperine can enhance the bioavailability of phenytoin and carbamazepine by increasing their plasma dug concentration [16, 17]. Atal et al. proved that piperine is a potent inhibitor of the drug metabolism [18]. Literature reported piperine as a metabolic inhibitor that inhibits drug metabolizing enzymes, such as CYP3A4, CYP1B1, CYP1B2, and CYP2E1 [15] [19]. These results allowed researchers to incorporate piperine with an anti-cancer agent acetyl-11-keto-ß-boswellic acid to increase its bioavailability and therapeutic efficacy [20]. Furthermore, piperine modulates the permeability characteristics to increase the drug absorption through the cell membrane by increasing the vasodilation of the GIT membrane [10, 21]. Being a natural product, piperine had many advantages compared to other chemical entities, such as low cost due to easy availability of plant material and the extraction and isolation methods of piperine are easy and well known [22]. Additionally, it is safe to use.
In the current study, the main goal was to enhance the solubility and permeability of piperine in order to enhance its bioavailability and therapeutic efficacy. Three polymeric carriers Soluplus®, polyvinylpyrrolidone-co-vinylacetate 64 (Kollidon® VA 64), and Eudragit ® EPO were selected via the hot melt extrusion process to investigate the solubility and permeability of piperine in order to enhance its bioavailability. The application of HME techniques to form solid dispersions of piperine to enhance solubility coupled with ex-vivo studies to demonstrate improved permeability has not been investigated.
Materials
Piperine was purchased from Sigma-Aldrich (Milwaukee, WI 53233, USA), while Soluplus® and Kollidon® VA 64 were obtained from BASF- SE (Ludwigshafen, Germany), Eudragit ® EPO was received as a gift sample from Evonik Industries (Parsippany, NJ 07045, USA). All other chemicals and reagents used in the present study were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA).
Methods
Thermogravimetric Analysis (TGA)
TGA analysis were performed for piperine, Soluplus®, Kollidon® VA 64, and Eudragit ® EPO to evaluate their stability at the extrusion temperatures using a Perkin Elmer Pyris 1 TGA equipped with a Pyris manager software (PerkinElmer Life and Analytical Sciences, 710 Bridgeport Ave., Connecticut, USA). Approximately 10–15 mg of piperine, polymers, as well as physical mixtures were heated from 30°C to 300°C at a heating rate of 20°C/min.
Differential Scanning Calorimetry (DSC)
DSC studies were obtained using Perkin Elmer Pyris 1 DSC equipped with Pyris Manager Software (PerkinElmer Life and Analytical Sciences, 710 Bridgeport Ave., Connecticut, USA). Approximately 2–4 mg of piperine, physical mixtures or extrudates were heated from 30°C to 200°C at a heating rate of 10°C/min.
Hot Melt Extrusion (HME)
Piperine 10–40% w/w and Eudragit® EPO, Kollidon® VA 64, or Soluplus® (Table 1) were mixed using a V-shell blender (Patrtreson-Kelley Twin Shell Dry Blender) for 10 minutes. The resulting physical mixture blends were extruded using a twin-screw extruder (Process 11 Twin Screw Extruder, ThermoFisher Scientific) at the screw speed of 150 rpm, at a temperature range of 100– 130°C. All extrudates were milled and sieved through an ASTM #35 mesh to obtain a uniform particle size.
Table 1.
Pipeline formulation composition of HME
| Formulation | Piperine (PIP) (%) | Eudragit ® PEO (%) | Soluplus ® (%) | Kollidon® VA 64 (%) |
|---|---|---|---|---|
| P1 | 10 | 90 | - | - |
| P2 | 20 | 80 | - | - |
| P3 | 40 | 60 | - | - |
| P4 | 10 | - | 90 | - |
| P5 | 20 | - | 80 | - |
| P6 | 40 | - | 60 | - |
| P7 | 10 | - | - | 90 |
| P8 | 20 | - | - | 80 |
| P9 | 40 | - | - | 60 |
Scanning Electron Microscopy (SEM)
The morphology and physical state of the extrudates were evaluated using SEM analysis. Samples were mounted on adhesive carbon pads placed on aluminum and were sputter coated with gold using a Hummer® 6.2 sputtering system (Anatech LTD, Springfield, VA) in a high vacuum evaporator. A JEOL JSM-5600 scanning electron microscope operating (SEM) at an accelerating voltage of 10kV was used for imaging.
Fourier transforms infrared spectroscopy (FTIR)
FTIR spectra of piperine, polymeric carriers, physical mixtures, as well as extrudates were performed using Agilent Cary 630 FTIR spectrometer equipped with a DTGS detector. The same (2–4 mg) was placed directly on ATR, and the scanning range was 400–4000 cm−1 and the resolution was 1 cm−1.
High Performance Liquid Chromatography (HPLC)
HPLC analyses were performed on all samples to ascertain the piperine content using a Waters HPLC equipped with Empower software to analyze the data. HPLC consisted of a Waters e2695 separation Module and Waters 2489 UV/Visible detector, (Waters Technologies Corporation, 34 Maple St., Milford, MA 0157). The column used was Phenomenex Luna C18 (5 μ, 250 × 4.6 mm). The mobile phase consisted of a mixture of 0.1% ortho phosphoric acid and acetonitrile (45:55 v/v) with a flow rate of 1.2 mL/min and 20 μL injection volume. The column temperature was 35°C (±2). The UV detector wavelength for piperine detection was set at λmax 262 nm [23].
Solubility test
The solubility was determined at pH 1.2, 5 (water), and 6.8 for formulations P4–P9 as well as pure piperine. Samples equivalent to approximately 100 mg of piperine based on the drug load of each formulation were shaken in 20-mL scintillation vials containing 10 mL tested pH solution. The samples were shaken at 80 rpm and at 25°C using a Thermo Scientific Precision Reciprocal Shaking Bath. A sample volume of 1 mL was collected at 4, 10, 20, and 24 h. The samples were filtered and analyzed by HPLC, and 1 mL of the same pH solution was added back to the scintillation vials to maintain the volume.
In-vitro Drug Release
A sample equivalent to 40 mg piperine of each extrudate as well as pure piperine were filled in gelatin capsules, and the in vitro drug release profiles were run using a USP type II dissolution apparatus. The used dissolution medium was 900 mL of 0.1 N HCl maintained at 37°C. A sample volume of 2 mL was taken at 10, 20, 30, 45, 60, 90, and 120 min and was filtered and analyzed using HPLC; then, 2 mL of fresh dissolution medium was added back to the dissolution vessel at each time point to maintain the volume.
Statistical analysis
Statistical analysis was performed to compare the effects of different polymers and drug loading on piperine drug release utilizing the Kruskal-Wallis test, a non-parametric multiple comparison test followed by the Dunn's test to identify the individual differences between the formulations using SPSS software (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.) . Values of P < 0.05 were considered significant.
Ex-vivo permeability model (non-everted intestinal sac)
The permeability studies were performed for the most promising formulation (P4) and pure piperine was used as the control. Non-everted intestinal sacs of male Sprague-Dawley rats, weighing approximately 200–250 g, were provided from Charles River (Wilmington MA 01887, USA). Sacs of 4–5 cm in length were prepared. Each sac was filled with 0.5 mL of incubated Krebs-Ringer bicarbonate phosphate buffer, pH 7.4 with piperine 5 mg/mL. Each non-everted sac was placed in a 25-mL beaker containing of 5 mL Krebs-Ringer bicarbonate phosphate buffer, pH 7.4. A sample volume of 1 mL was taken from the solution outside the sac at 0, 20, 50, 80, and 120 min. The samples were filtered and analyzed for the content using HPLC. Fresh Krebs-Ringer buffer (1 mL) was added back to the beaker at each time point to maintain the volume. Cumulative dissolution profiles were calculated.
Results and discussion
Pre-formulation Studies
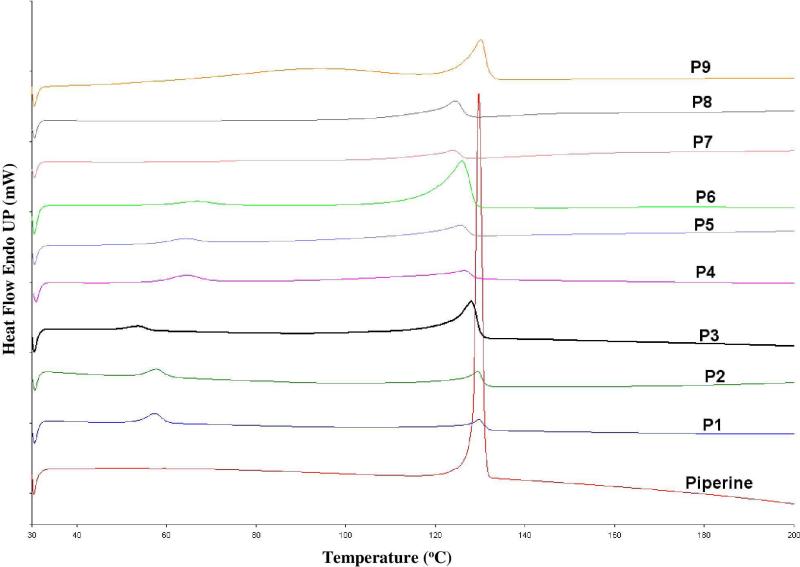
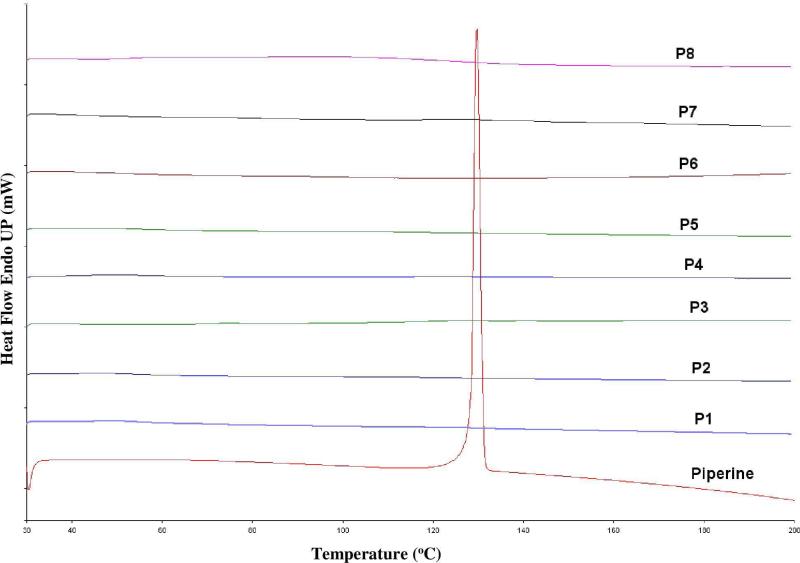
Under all utilized processing temperatures, TGA data showed no decrease in sample weight, which indicated that all formulations in the study were stable under all applied extrusion temperatures. DSC data showed the endothermic crystalline melting peak at 135°C for pure piperine and all the physical mixture samples (Figure 1). Alternatively, all the extrudates showed absence of crystalline melting peaks in DSC data (Figure 2).
Figure 1.
DSC thermogram of 10%, 20%, and 40% PIP/ Eudragit ® PEO, PIP/ Soluplus® and PIP/ Kollidon® VA 64 physical mixtures.
Figure 2.
DSC thermogram of 10%, 20%, and 40% of PIP/ Eudragit ® PEO, PIP/ Soluplus® and PIP/ Kollidon® VA 64 extrudates.
Hot Melt Extrusion (HME)
HME process was carried out using Process 11 Twin Screw Extruder, from Thermo Fisher Scientific. The Thermo Fisher standard screw configuration was used in this study, which consists of four conveying zones and three mixing zones (Figure 3). The HME processing conditions such as extrusion temperature, screw speed, and torque values are shown in Table 2. The resulting extrudates were transparent, greenish yellow and brittle, except for P3 (40% piperine/ Eudragit® EPO), which was opaque. This observation can be explained by the fact that 40% of the drug load exceeded the Eudragit ® EPO carrier capacity allowing the extrudate to mimic the appearance of the piperine only. All extrudates were milled using a coffee grinder and sieved through an ASTM #35 mesh to obtain a uniform particle size.
Figure 3.
SEM of extrudates: a) 10% w/w piperine/Soluplus®, b) 20% w/w piperine/Soluplus®, c) 40% w/w piperine/Soluplus®, d) 20% piperine/Eudragit ® EPO, e) 20% piperine/Kollidon ® VA64.
Table 2.
Processing parameters for hot melt extrusion of PIP/ Eudragit ® PEO, PIP/ Soluplus® and PIP/ Kollidon® VA 64 formulations
| Formulation | Processing Temperature (°C) | Screw Speed (rpm) | Torque (Nm) | Extrudate Image |
|---|---|---|---|---|
| P1 | 110 | 150 | 6-7 |
|
| P2 | 110 | 150 | 4-5 |
|
| P3 | 110 | 150 | 3 |
|
| P4 | 120 | 150 | 7-8 |
|
| P5 | 120 | 150 | 4-5 |
|
| P6 | 120 | 150 | 3-4 |
|
| P7 | 130 | 150 | 7-8 |
|
| P8 | 130 | 150 | 3-4 |
|
| P9 | 130 | 150 | 3-4 |
|
Scanning electron microscopy (SEM)
SEM images showed absence of crystals in 10% w/w piperine/Soluplus® indicating that piperine was dispersed in the Soluplus® polymer carrier as an amorphous form. This may help to enhance the solubility and bioavailability of this formulation. However, crystals were evident in all other formulations with different ratios (Figure 3). These results are not consistent with the DSC results that showed absence of the piperine crystalline peak in the resulting extrudates. This observation be explained by the fact that all polymers used in this study, i.e., Eudragit® EPO, Kollidon® VA 64, and Soluplus® have a Tg of 45°C, 101°C, and 70°C, respectively, which is lower than the piperine melting point. Due to this, the polymers used will soften before the melting temperature of piperine allowing the piperine to solubilize in the polymer matrix and prevent the melting peak appearance.
Fourier transforms infrared spectroscopy (FTIR)
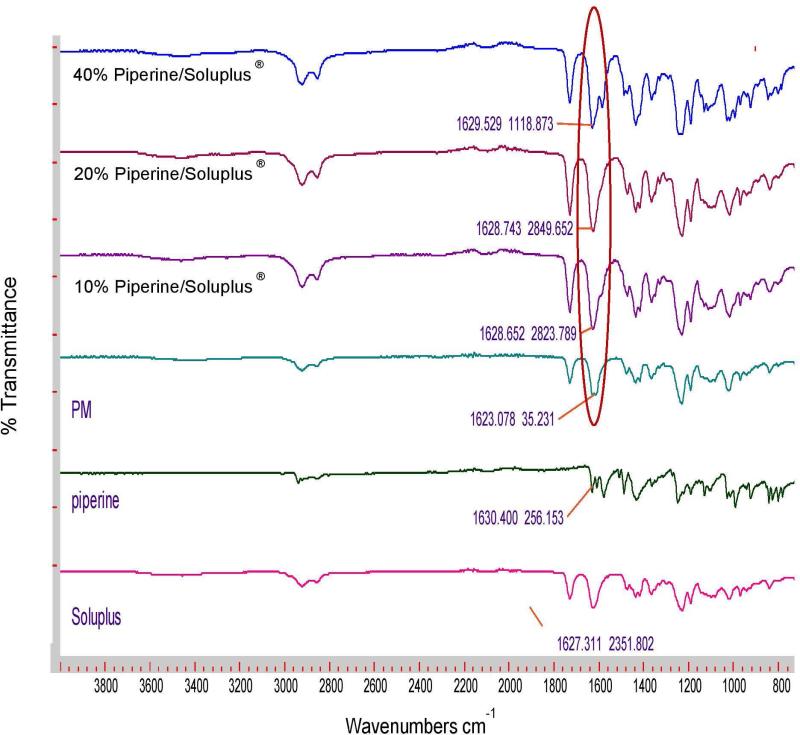
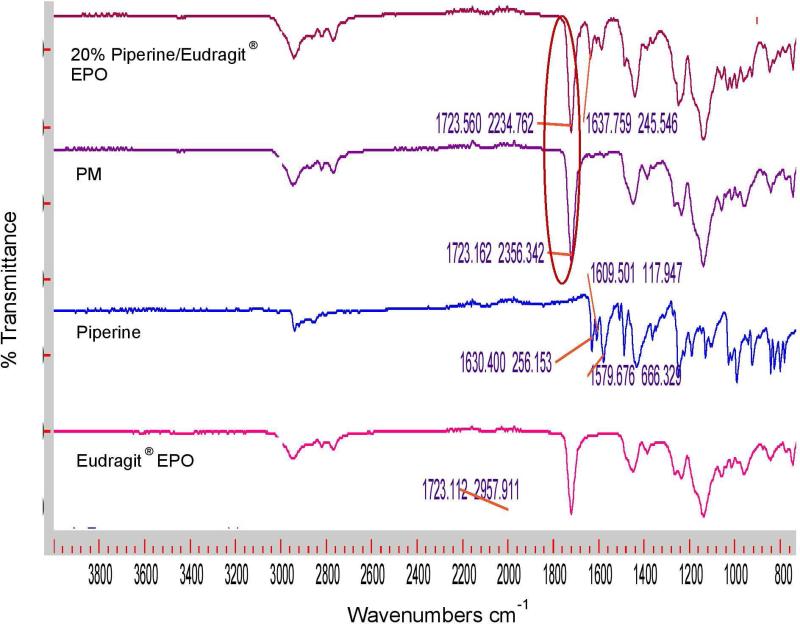
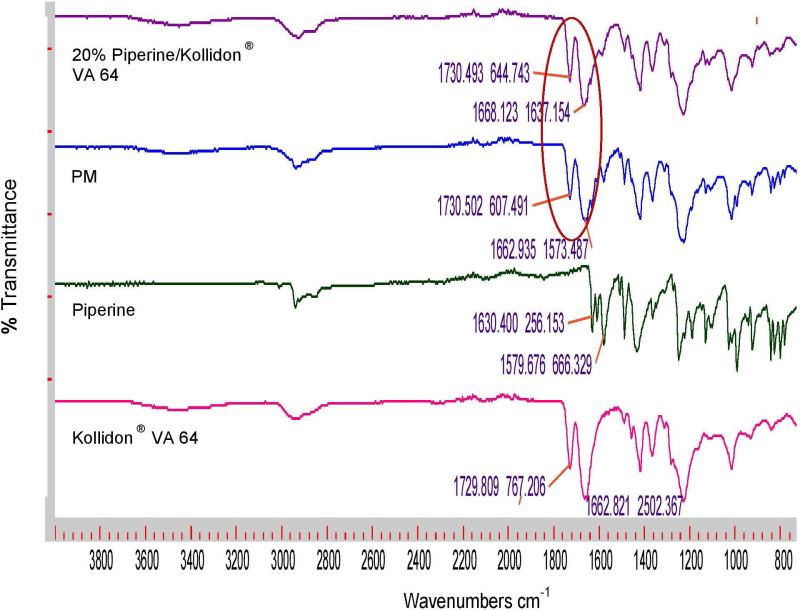
FTIR analysis was performed to evaluate any chemical or physical interaction between piperine and the different polymeric carriers. Figure 4a shows the FTIR spectra of Soluplus and their formulations; the physical mixture displays a sharp peak at 1,623 cm−1, and this peak shifted to approximately 1,629 cm−1 for all Soluplus formulations, indicating the presence of hydrogen bonding between piperine and Soluplus®. In contrast, there were no observed shifts in Eudragit® EPO, and Kollidon® VA 64 formulations (Figures 4b and c). The formation of hydrogen bonding will increase the stability of the Soluplus solid dispersion formulations and prevent recrystallization of piperine [7, 24, 25].
Figure 4a.
FTIR spectra of Soluplus®, piperine, physical mixture, 10% piperine /Soluplus®, 20% piperine /Soluplus®, and 40% piperine /Soluplus®.
Figure 4b.
FTIR spectra of Eudragit ® EPO, piperine, 20% piperine/Eudragit ® EPO physical mixture and extrudates.
Figure 4c.
FTIR spectra of Kollidon ® VA 64, piperine, 20% piperine/Kollidon® VA 64 physical mixture and extrudates.
Solubility Evaluation
Solubility study was performed for formulations P4, P5, P6, P7, P8, and P9 using three different solvents pH (1.2, 5 [water], and 6.8) to evaluate whether piperine solubility is pH dependent or not. The pH did not have any effect on the solubility of piperine as there were slight changes in the solubility values at pH 1.2 and 6.8 (Table 3). Furthermore, the solubility of piperine in formulations P4 P5, P6, P7, P8, and P9 increased more than 160, 45, 25, 20, 5, and 3 fold, respectively, compared to the water solubility of pure piperine (Table 3). This significant enhancement in the water solubility of piperine in P4 was due to the dispersion of piperine in its amorphous state which was supported by SEM. Amorphous solid dispersion play a significant role in increasing the solubility and dissolution rate of poorly water soluble APIs [26-29]. In general, amorphous API is more soluble than crystalline ones, because of its higher effective surface area than the crystalline form [30-32]. However, the main problem associated with amorphous API is its instability, which can led to recrystallization during storage as well as during dissolution [33]. In contrast, amorphous solid dispersions are stable as polymeric carriers prevent API recrystallization [34].
Table 3.
Solubility of pipeline and pipeline formulation in water, pH 1.2 and 6.8
| Formulation | Solubility in water (mg/L) | Solubility in pH 1.2 (mg/L) | Solubility in pH 6.8 (mg/L) |
|---|---|---|---|
| Piperine | 1 | 0.9 | 0.9 |
| P4 | 163 | 117 | 117 |
| P5 | 47 | 39 | 45 |
| P6 | 27 | 21 | 27 |
| P7 | 20 | 18 | 17 |
| P8 | 6 | 6 | 6 |
| P9 | 3 | 3 | 3 |
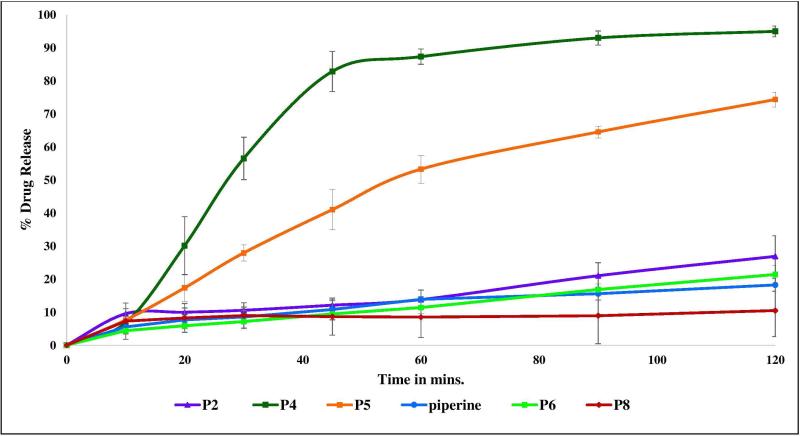
In-vitro Release Profiles
Dissolution studies demonstrated improvement in piperine drug release of 10% and 20% w/w piperine/Soluplus® extrudates up to 95% and 74%, respectively (Figure 5). However, no effect on piperine drug release profiles for other formulations was noted due to the remaining crystalline piperine. These results confirmed that the dispersion of piperine in the amorphous state plays a big role in enhancing the solubility [35] and drug release. Additionally, it can be concluded that the maximum drug loading capacity of piperine in Soluplus to form stabilized amorphous solid dispersion is approximately 10%. Soluplus® Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer is a relatively new polymer designed mainly for use in solid dispersion preparation APIs [36]. It acts as a polymeric stabilizer and solubilizing agent to improve the solubility and bioavailability of poor water soluble APIs [37]. It is a good polymeric carrier candidate to prepare amorphous solid dispersions of many poor water soluble APIs such as clotrimazole, carbamazepine, griseofulvine, and itraconazole by improving their dissolution profiles as well as their intestinal absorption and bioavailability [37]. In the recent study, Soluplus® was successfully used to prepare 10% piperine amorphous solid dispersion with a significant increase (P < 0.05) in the piperine release profile.
Figure 5.
In-vitro release profiles of 10% w/w piperine/Soluplus®, 20% w/w piperine/Soluplus®, 40% w/w piperine/Soluplus®, 20% piperine/Eudragit ® EPO, 20% piperine/Kollidon ® VA64 and pure piperine.
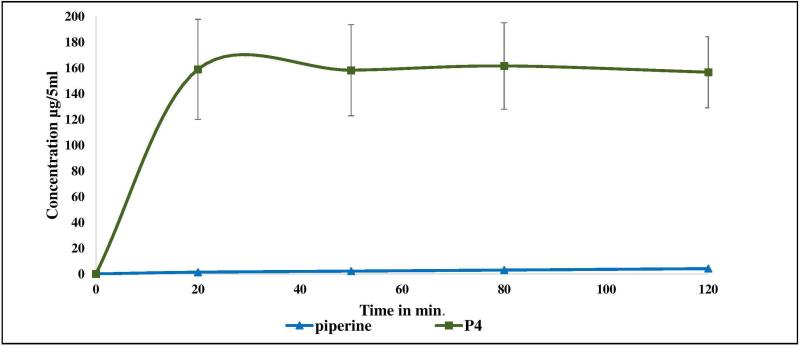
Ex-vivo permeability model (non-everted intestinal sac)
There are many in-vivo, ex-vivo, and in-vitro assays used to evaluate the intestinal drug permeability [38]. These assays include perfusion, diffusion chambers, gut sac, cultured cell, and artificial membrane [39]. The results of a research investigation showed that there was a good correlation between rat and human oral absorption. There are two types of rat intestinal sac models that are used to evaluate the drug permeability: everted and non-everted sacs. Some studies have been performed to compare the permeability values of some APIs through both everted and non-everted sacs and no significant difference was observed in the permeability results. In this study, the non-everted rat intestinal sac model was used to study piperine absorption. The permeability study results demonstrated the enhancement in piperine absorption of 10% w/w piperine/Soluplus® up to 158.9 μg/5mL compared to 1.4 μg/5mL in the case of pure piperine within 20 min (Figure 6) as a direct result of the solubility improvement (over 112-fold increase in absorption).
Figure 6.
Piperine absorption characteristics using jejunum non-everted sac under normal physiological conditions (Krebs Ringer bicarbonate phosphate buffer, pH 7.4).
4.6. Conclusion
HME was successfully used to enhance the solubility of the psychoactive natural product piperine. The drug release profiles of 10% and 20% w/w piperine/Soluplus® hot melt extrudates were significantly enhanced due to piperine in its amorphous state. In addition, the formation of hydrogen bonds between piperine and Soluplus® may inhibit drug recrystallization and assist in the maintenance of the drug in its amorphous form thereby enabling good formulation stability. Furthermore, a significant improvement in piperine permeability was confirmed using a noneverted rat intestinal sac. These results demonstrate that an increase in the absorption and bioavailability of piperine are possibilities.
Acknowledgments and Funding
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors also thank the Pii Center for Pharmaceutical Technology for contributions to in this project.
Footnotes
Conflict of Interest
The authors report no conflicts of interest to disclose.
References
- 1.Youn YS, et al. Improved intestinal delivery of salmon calcitonin by Lys 18-amine specific PEGylation: Stability, permeability, pharmacokinetic behavior and in vivo hypocalcemic efficacy. Journal of controlled release. 2006;114(3):334–342. doi: 10.1016/j.jconrel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Guggi D, Krauland AH, Bernkop-Schnürch A. Systemic peptide delivery via the stomach: in vivo evaluation of an oral dosage form for salmon calcitonin. Journal of controlled release. 2003;92(1):125–135. doi: 10.1016/s0168-3659(03)00299-2. [DOI] [PubMed] [Google Scholar]
- 3.Pade V, Stavchansky S. Link between drug absorption solubility and permeability measurements in Caco-2 cells. Journal of pharmaceutical sciences. 1998;87(12):1604–1607. doi: 10.1021/js980111k. [DOI] [PubMed] [Google Scholar]
- 4.Hörter D, Dressman J. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Advanced drug delivery reviews. 2001;46(1):75–87. doi: 10.1016/s0169-409x(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 5.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. Journal of Clinical Pharmacology. 2002;42(6):620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, et al. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. International journal of pharmaceutics. 2004;285(1):135–146. doi: 10.1016/j.ijpharm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug discovery today. 2007;12(23):1068–1075. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Chonpathompikunlert P, Wattanathorn J, Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer's disease. Food and Chemical Toxicology. 2010;48(3):798–802. doi: 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Wattanathorn J, et al. Piperine, the potential functional food for mood and cognitive disorders. Food and Chemical Toxicology. 2008;46(9):3106–3110. doi: 10.1016/j.fct.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9(3):224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, et al. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacological Research. 2000;41(6):657–662. doi: 10.1006/phrs.1999.0640. [DOI] [PubMed] [Google Scholar]
- 12.KONTUSH A, SCHEKATOLINA S. Vitamin E in neurodegenerative disorders: Alzheimer's disease. Annals of the New York Academy of Sciences. 2004;1031(1):249–262. doi: 10.1196/annals.1331.025. [DOI] [PubMed] [Google Scholar]
- 13.Selvendiran K, et al. Cytoprotective effect of piperine against benzo [a] pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Fitoterapia. 2003;74(1):109–115. doi: 10.1016/s0367-326x(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 14.Borre YE, et al. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology. 2014;79:738–749. doi: 10.1016/j.neuropharm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Kesarwani K, Gupta R. Bioavailability enhancers of herbal origin: An overview. Asian Pacific journal of tropical biomedicine. 2013;3(4):253–266. doi: 10.1016/S2221-1691(13)60060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattanaik S, et al. Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytotherapy Research. 2006;20(8):683–686. doi: 10.1002/ptr.1937. [DOI] [PubMed] [Google Scholar]
- 17.Pattanaik S, et al. Pharmacokinetic interaction of single dose of piperine with steady-state carbamazepine in epilepsy patients. Phytotherapy Research. 2009;23(9):1281–1286. doi: 10.1002/ptr.2676. [DOI] [PubMed] [Google Scholar]
- 18.Atal C, Dubey R, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Journal of Pharmacology and Experimental Therapeutics. 1985;232(1):258–262. [PubMed] [Google Scholar]
- 19.Majeed M, Badmaev V, Rajendran R. Use of piperine as a bioavailability enhancer. Google Patents. 1999 [Google Scholar]
- 20.Brough C, et al. Pharmaceutical formulations of acetyl-11-keto-b-boswellic acid, diindolylmethane, and curcumin for pharmaceutical applications. Google Patents. 2012 [Google Scholar]
- 21.Khajuria A, Zutshi U, Bedi K. Permeability characteristics of piperine on oral absorption--an active alkaloid from peppers and a bioavailability enhancer. Indian journal of experimental biology. 1998;36(1):46–50. [PubMed] [Google Scholar]
- 22.Ribeiro TS, et al. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorganic & medicinal chemistry letters. 2004;14(13):3555–3558. doi: 10.1016/j.bmcl.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Moorthi C, et al. Application of validated RP–HPLC–PDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. Journal of Pharmacy Research. 2013;7(3):224–229. [Google Scholar]
- 24.Vasanthavada M, et al. Phase behavior of amorphous molecular dispersions II: Role of hydrogen bonding in solid solubility and phase separation kinetics. Pharmaceutical research. 2005;22(3):440–448. doi: 10.1007/s11095-004-1882-y. [DOI] [PubMed] [Google Scholar]
- 25.Schachter DM, Xiong J, Tirol GC. Solid state NMR perspective of drug–polymer solid solutions: a model system based on poly (ethylene oxide). International journal of pharmaceutics. 2004;281(1):89–101. doi: 10.1016/j.ijpharm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Van den Mooter G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discovery Today: Technologies. 2012;9(2):e79–e85. doi: 10.1016/j.ddtec.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Blagden N, et al. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Advanced drug delivery reviews. 2007;59(7):617–630. doi: 10.1016/j.addr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Lakshman JP, et al. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Molecular pharmaceutics. 2008;5(6):994–1002. doi: 10.1021/mp8001073. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy M, et al. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Molecular pharmaceutics. 2008;5(6):981–993. doi: 10.1021/mp800061r. [DOI] [PubMed] [Google Scholar]
- 30.Pignatello R, et al. Preparation, characterisation and photosensitivity studies of solid dispersions of diflunisal and Eudragit RS100® and RL100®. International journal of pharmaceutics. 2001;218(1):27–42. doi: 10.1016/s0378-5173(01)00597-x. [DOI] [PubMed] [Google Scholar]
- 31.Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. International journal of pharmaceutics. 2002;231(2):131–144. doi: 10.1016/s0378-5173(01)00891-2. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata Y, et al. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. International journal of pharmaceutics. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Alonzo DE, et al. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharmaceutical research. 2010;27(4):608–618. doi: 10.1007/s11095-009-0021-1. [DOI] [PubMed] [Google Scholar]
- 34.Overhoff KA, et al. Solid dispersions of itraconazole and enteric polymers made by ultra-rapid freezing. International journal of pharmaceutics. 2007;336(1):122–132. doi: 10.1016/j.ijpharm.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 35.Shah S, et al. Melt extrusion with poorly soluble drugs. International journal of pharmaceutics. 2013;453(1):233–252. doi: 10.1016/j.ijpharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Shamma RN, Basha M. Soluplus®: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technology. 2013;237:406–414. [Google Scholar]
- 37.Hardung H, Djuric D, Ali S. Combining HME & solubilization: Soluplus®—the solid solution. Drug Deliv Technol. 2010;10(3):20–7. [Google Scholar]
- 38.Ruan L-P, et al. Prediction of human absorption of natural compounds by the noneverted rat intestinal sac model. European journal of medicinal chemistry. 2006;41(5):605–610. doi: 10.1016/j.ejmech.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Volpe DA. Application of method suitability for drug permeability classification. The AAPS journal. 2010;12(4):670–678. doi: 10.1208/s12248-010-9227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]