Abstract
Degenerative brain changes in Alzheimer’s disease may occur in reverse order of normal brain development based on the retrogenesis model and prior results. The aim of this study was to test whether evidence of reverse myelination was observed in mild cognitive impairment (MCI) using a novel, data-driven analytic approach based on lifespan developmental data. Whole-brain high-resolution diffusion tensor imaging scans were obtained for 31 subjects with MCI and 79 demographically matched healthy older adults. Comparisons across corpus callosum (CC) regions of interest (ROIs) showed decreased fractional anisotropy (FA) in the body, but not the genu or splenium; early-, middle- and late-myelinating ROIs restricted to the CC revealed decreased FA in late-, but not early- or middle-myelinating ROIs. Voxelwise group differences were found via tract-based spatial statistics revealing areas of lower FA in MCI, but whole-brain differences were equally distributed across early-, middle- and late-myelinating regions. Overall, results within the CC support the retrogenesis model, although caution is needed when generalizing these results beyond the CC.
Keywords: Diffusion Tensor Imaging, Magnetic Resonance Imaging, Mild Cognitive Impairment, Corpus Callosum, Aging, Alzheimer’s disease
1. Introduction
White matter changes are well-documented in Alzheimer’s dementia (AD) (Brun and Englund, 1986; Salat et al., 2010; Stricker et al., 2009) and its prodromal stages, including Mild Cognitive Impairment (MCI) (Delano-Wood et al., 2010; Fellgiebel et al., 2005; Parente et al., 2008; Stricker et al., 2013; Wang et al., 2009). However, the pattern and underlying mechanism of these changes remain unclear. One reason for this uncertainty is that few diffusion tensor imaging (DTI) studies have tested a priori hypotheses based on a specified neuropathologic mechanism. The retrogenesis model of AD provides a testable model and posits that white matter degeneration reflects myelin breakdown that develops in a pattern that is the reverse of myelogenesis (Bartzokis, 2009; Reisberg et al., 1999). White matter pathways with large diameter fibers that myelinate first in development, such as primary motor fibers, are the last to be affected by AD. In contrast, white matter pathways with small diameter fibers that myelinate much later in normal development, such as neocortical association and allocortical fibers, are the first to be affected by the AD degenerative process (Bartzokis, 2004). Recent studies have demonstrated support for the retrogenesis model in Alzheimer’s dementia patients (Alves et al., 2012; Benitez et al., 2014; Stricker et al., 2009), although support for this model is less clear in MCI (Benitez et al., 2014; Fieremans et al., 2013).
Assumptions about the order of myelination throughout development varies across study methods used and contributes to difficulty in interpreting results within the context of the retrogenesis model (e.g. volumetric imaging, diffusion imaging, and histological procedures may provide differing results). Consistent with the pattern of gray matter (GM) cortical development (Giedd et al., 1999; Gogtay and Thompson, 2010; Pfefferbaum et al., 1994), pathological studies of myelination of human infants suggest a pattern of white matter development that is proximal to distal, projection to association, and occipital to frontal (Kinney et al., 1988; Yakovlev and Lecours, 1967). More recent studies have attempted to examine lifespan white matter changes in vivo utilizing magnetic resonance imaging (MRI) methodology, including white matter volumetric study, diffusion parameters, and tractography. Indeed, there is evidence that association tracts continue to develop into the twenties, followed by projection fibers into the thirties (Lebel and Beaulieu, 2011; Lebel et al., 2012). Further, in a cross-sectional study Westlye and colleagues (2010) found that peaks in tract-based spatial statistics (TBSS) fractional anisotropy (FA), mean diffusivity (MD) and radial diffusivity (RD) occurred on average between 29 and 36 years, whereas white matter volume segmentation noted growth into the sixth decade. In normal aging, white matter pathways undergo a non-linear, hierarchical maturation pattern, such that there is a rapid acceleration of development (increases in FA and decreases in MD) during early life, a mid-life plateau, and a decline in later adulthood; however, there are regional differences in the course of these 3-phase models. Regions with fronto-temporal connections typically develop at a slower rate than regions with less extensive connections (Hasan et al., 2007; Lebel et al., 2008; Westlye et al., 2010). Those with protracted development, including portions of the corpus callosum, have been shown to demonstrate rapid development in early childhood, a later plateau stage, as well as accelerated decline in tract integrity with age (Lebel et al., 2008; Sridharan et al., 2014; Sullivan and Pfefferbaum, 2006; Teipel et al., 2014; Walhovd et al., 2011; Westlye et al., 2010). However, there are methodological considerations differentiating these studies, including MRI method (volumetric, DTI, and/or fiber tracking), regional vs. whole brain analysis, and participant inclusion. Overall, it appears that cortico-cortical association pathways followed by commissural pathways represent the latest-myelinating regions in the brain.
The corpus callosum has frequently been targeted for investigating changes in early- versus late-myelinating regions in studies of aging and AD (Bartzokis et al., 2003; Bartzokis et al., 2007; Delano-Wood et al., 2008; Di Paola et al., 2010a; Di Paola et al., 2010b; Fieremans et al., 2013; Hanyu et al., 1999; Head et al., 2004). It is often assumed that the genu is later-myelinating relative to the splenium due to the general tendency of frontal regions to myelinate later than posterior regions (Kinney et al., 1988) and the predominance of fibers connecting prefrontal regions in the genu, versus temporoparietal fibers in the splenium (Lebel et al., 2010; Preti et al., 2012). Decreased white matter integrity within the corpus callosum has been previously reported in patients with AD relative to healthy controls (Alves et al., 2012; Duan et al., 2006; Hanyu et al., 1999; Naggara et al., 2006; Parente et al., 2008; Preti et al., 2012; Takahashi et al., 2002; Teipel, 2007; Teipel et al., 2012) and MCI (Alves et al., 2012; Delano-Wood et al., 2008; Di Paola et al., 2010a; Fellgiebel et al., 2004; Liu et al., 2013; Scrascia et al., 2014; Ukmar et al., 2008; Wang et al., 2009; Wang et al., 2014; Xie et al., 2006). However, inconsistencies exist in the literature base regarding the location of abnormalities within the corpus callosum, when changes emerge across the disease course and the underlying mechanisms driving the changes. In particular, posterior callosal abnormalities (e.g., splenium) have been hypothesized to reflect Wallerian degeneration attributable to cortical AD neuropathology (Alves et al., 2012; Bozzali et al., 2002; Di Paola et al., 2010a; Liu et al., 2013; Wang et al., 2013; Wang et al., 2014), whereas degeneration of more anterior callosal regions (e.g., genu) have been suggested to reflect retrogenesis of those areas (Alves et al., 2012; Di Paola et al., 2010a; Liu et al., 2013; Wang et al., 2014; Xie et al., 2006). DTI studies of MCI and AD using ROI methods have frequently omitted the body of the CC (Bozzali et al., 2002; Bozzao et al., 2001; Delano-Wood et al., 2008; Duan et al., 2006; Fellgiebel et al., 2004; Head et al., 2004; Naggara et al., 2006; Parente et al., 2008; Stahl et al., 2007; Takahashi et al., 2002; Ukmar et al., 2008; Wang et al., 2009); however, those studies that have included it or used a whole-brain voxelwise analysis approach have often demonstrated decreased white matter integrity in the body of the CC in MCI and AD (Alves et al., 2012; Di Paola et al., 2010a; Liu et al., 2013; Preti et al., 2012; Xie et al., 2006).
Interestingly, some assumptions within the literature about the developmental order within the CC were recently challenged by data presented by Lebel and colleagues (2010), whose results suggest that the body of the CC should not be omitted from studies of MCI and AD. The authors divided the callosum into 7 separate regions, creating boundaries that are functionally as well as anatomically distinct. While their lifetime white matter development findings were consistent with prior studies demonstrating an inverted-U curve of increased FA and decreased MD throughout adolescence and early adulthood, followed by a plateau and reverse growth in later adulthood, prior fit models were unable to assess relative timing of the 7 regions across the lifetime (Hasan et al., 2007). Their results suggested an “outer-to-inner” pattern of decreasing white matter integrity with age within the CC and indicate that examination of the entire callosum, including the body, is necessary to account for potential differences in neurodevelopmental timing.
Given the variation in assumptions about the order of myelination, we aimed to take a data-driven approach to testing the retrogenesis model, with a focus on the corpus callosum. We defined myelination order according to previously acquired data across a large normative sample spanning ages 8–85 (Westlye et al., 2010), and used a priori, empirically-defined ROIs to test the retrogenesis model within the corpus callosum and across the whole-brain. We predicted that FA would be lower in patients with MCI relative to healthy older adults in late-myelinating regions. We further expected lower FA in the body of the CC in patients with MCI relative to healthy older adults based on recent data suggesting this region to be late-myelinating relative to the genu and splenium (Lebel et al., 2010).
2. Methods
2.1 Participants
One hundred-ten participants underwent neuropsychological testing and brain MRI. Participants were recruited from two overlapping studies conducted at VA Boston Healthcare System. Twenty-eight participants were selected (based on their agreement to undergo structural MRI) from a larger sample recruited from the community through the Harvard Cooperative Program on Aging (HCPA) Claude Pepper Older American Independence Center (OAIC). These participants responded to a HCPA newsletter asking for healthy community-dwelling older African Americans. Eighty-two participants were part of the Understanding Cerebrovascular and Alzheimer’s Risk in the Elderly (UCARE) program, recruited through the Boston University Alzheimer’s Disease Center (BUADC) based on the criteria of being neurologically healthy and having a first-degree family relative with AD. Data were analyzed on a subset of individuals presented previously (Stricker et al., 2013). Participants were excluded for the following: history of head trauma of “mild” severity or greater (Holm et al., 2005; within our sample loss of consciousness did not exceed 15 minutes), more than one head injury, any neurological disorder including dementia (i.e., Parkinson’s disease, AD, vascular dementia), severe psychiatric illness, or brain surgery. All participants were literate with at least a 9th grade education. The VA Boston Healthcare System’s institutional review board approved the study according to the Helsinki Declaration, and informed consent was obtained from each participant.
2.2 MCI Criteria
Retrospective diagnosis of MCI was made by applying the “comprehensive” criteria (Jak et al., 2009) based on neuropsychological test scores across four cognitive domains including memory, attention/processing speed, language and executive functions, as previously described (Stricker et al., 2013). To contribute to MCI classification, at least two performances within a cognitive domain fell one standard deviation (SD) or more below published normative expectations for that domain. All participants scored ≥ 24 (Caucasians) or ≥ 23 (African Americans; Pedraza et al., 2012) on the MMSE. Participants were classified as amnestic MCI (aMCI) if memory was impaired (either alone or with additional domains impaired) and as nonamnestic MCI (naMCI) if domain(s) other than memory were impaired. Participants were classified as healthy controls (HC) if performance on no more than one measure within a cognitive domain fell more than 1 SD below normative data. Using these criteria, 31 participants met criteria for MCI (14 aMCI, 17 naMCI), and 79 participants were classified as HC. Self-report measures included the Geriatric Depression Scale (GDS; (Yesavage et al., 1982) and the Lawton and Brody instrumental activities of daily living (IADL) questionnaire (Lawton and Brody, 1969).
2.3 Neuroimaging Protocol
Five participants were scanned using a Siemens 1.5 Tesla Sonata system, with the following parameters: DTI: repetition time (TR)=9000 ms echo time (TE)=68 ms, 60 slices total, acquisition matrix=128 × 128 (field of view; FOV=256 × 256 mm), slice thickness=2 mm (for 2 mm isotropic voxels) with 0 mm gap, with a b value=700 s/mm2, 10 T2 and 60 diffusion weighted images, and one image, the T2-weighted “low b” image with a b-value=0 s/mm2 as an anatomical reference volume. The remaining 105 participants were scanned on the upgraded Siemens 1.5 Avanto System, with slightly different parameters: DTI: repetition time (TR)=7200 ms echo time (TE)=77 ms, 60 slices total, acquisition matrix=128 × 128 (field of view; FOV=256 × 256 mm), slice thickness=2 mm (for 2 mm isotropic voxels) with 0 mm gap, with a b value=700 s/mm2, 10 T2 and 60 diffusion weighted images, and one image, the T2-weighted “low b” image with a b-value=0 s/mm2 as an anatomical reference volume.
2.4 Image Processing
Diffusion data went through a multistep processing pipeline involving tools from the FreeSurfer image analysis suite and FSL (http://www.fmrib.ox.ac.uk.fsl/), specifically Dtifit from FMRIB’s Diffusion Toolbox (Behrens et al., 2003) and TBSS (Tract-Based Spatial Statistics, (Smith et al., 2006), part of FSL (Smith et al., 2004). See our previous work (Leritz et al., 2010; Salat et al., 2010) for additional details. FA, a measure of the diffusion displacement probability of water within white matter, was used as the primary metric of white matter integrity for this study. Lower FA is generally taken to indicate lower white matter integrity.
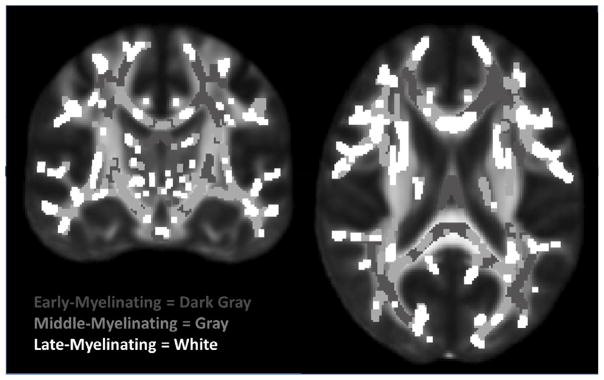
Regions of interest (ROIs) restricted to the TBSS skeleton for early-, middle-, and late-myelinating regions were created based on available data. Order of myelination of different white matter regions was empirically determined based on data presented in Westlye et al. (2010) wherein age trajectories of FA was determined from 430 healthy subjects (age range 8–85). Summary data was provided in the form of a TBSS skeleton map with voxel values corresponding to age of non-parametric locally weighted polynomial regression (LOESS; (Cleveland and Devlin, 1988) estimated peak FA (see Westlye et al., 2010 for details). This was divided into 3 ROI myelination tertiles (see Figure 1) defined as early-myelinating (age FA peak <25.5), middle-myelinating (age FA peak 25.5–29.08) and late-myelinating (age FA peak >29.08).
Figure 1.
Early-, Middle-, and Late-Myelinating ROIs. ROIs dilated for visualization purposes only.
ROIs limited to the TBSS skeleton for the genu, body and splenium of the corpus callosum (CC) were created using the Johns Hopkins University (JHU) white matter labels, available as part of the FSL suite. In addition, the genu, body and splenium ROIs were added together to create one larger CC mask, and then the above described early-, middle and late-myelinating ROIs were applied to that mask. This yielded early-, middle- and late-myelinating ROIs restricted to the corpus callosum (subsequently referred to as CC-early, CC-middle and CC-late). Average FA was derived for each white matter ROI and was extracted from voxels limited to the TBSS skeleton to reduce the influence of partial volume contamination.
2.5 Statistical Analyses
Independent samples t-tests were used to examine group differences across CC ROIs. Voxelwise statistics were performed across the TBSS skeleton to test for whole-brain differences in FA across MCI and HC groups. As the primary goal was to evaluate the proportion of voxels significant across the myelination tertile ROIs and not report on the exact localization of differences found, the voxelwise analysis did not control for multiple comparisons. A minimum cluster size of 10 was required and a mask of all significant voxels was saved. Masks for the early-, middle- and late-myelinating ROIs were then applied to the significant clusters mask to determine what percentage of the total of significant voxels from the voxelwise analyses fell within early-, middle- and late-myelinating regions. Chi-square analyses were used to compare frequencies of significant voxels across the 3 tertile ROIs. A significance level of p < .05 was used for all analyses.
3. Results
3. 1 Participants
The MCI and HC groups were comparable on age (t = −0.56, p = 0.58), education (t = 0.17, p=0.87), sex (χ2 = 0.04, p = 0.85), ethnicity (χ2 = 3.16, p = 0.08), Lawton and Brody IADL score (t = 1.83, p = 0.07), GDS (t = −0.93, p = 0.36), and MMSE (t = 1.77, p = 0.08). Due to targeted recruitment, a large percentage (92%) had a self-reported positive family history of AD, and this percentage was comparable across MCI and HC groups (χ2=0.05, p=.82). See Table 1.
Table 1.
Demographics and FA by group.
| HC (n = 79) | MCI (n = 31) | |
|---|---|---|
| Age | 67.81 (9.02) | 68.94 (10.64) |
| Education | 14.94 (2.83) | 14.84 (2.46) |
| Sex: n male/female | 29/50 | 12/19 |
| Ethnicity: n Caucasian/AA | 55/24 | 16/15 |
| MMSE | 28.07 (1.65) | 27.40 (1.96) |
| Lawton-Brody IADL | 26.58 (0.96) | 26.16 (1.37) |
| GDS | 4.03 (5.59) | 5.13 (5.64) |
| FA Genu of CC | 0.682 (0.049) | 0.680 (0.041) |
| FA Body of CC* | 0.659 (0.042) | 0.635 (0.044) |
| FA Splenium of CC | 0.760 (0.041) | 0.753 (0.034) |
| FA CC Early | 0.662 (0.040) | 0.654 (0.035) |
| FA CC Middle | 0.695 (0.043) | 0.680 (0.040) |
| FA CC Late* | 0.691 (0.042) | 0.666 (0.046) |
Note. HC = normal controls; MCI = Mild Cognitive Impairment; AA = African American; MMSE = Mini Mental Status Examination; FA = fractional anisotropy; CC = corpus callosum.
p < .05
3.2 Corpus Callosum
The MCI group demonstrated lower FA than the HC group in the body of the CC (t = 2.69, p = .008); see Table 1. There were no significant group differences in the genu (t = 0.27, p = 0.79) or splenium (t = 0.83, p = 0.41). To determine the relative order of myelination of each CC ROI, we applied the myelination tertile ROI map to the JHU CC ROIs. The genu was comprised of predominantly early-myelinating voxels (58.5%) [40.0% middle- and 1.5% late-myelinating]. The body was comprised of predominantly late-myelinating voxels (47.8%) [37.2% middle-and 15.1% early-myelinating]. The splenium was comprised of predominantly middle- (50.5%) and early-myelinating (47.8%) voxels [1.8% late-myelinating]. Average FA extracted from the entire corpus callosum for early-, middle-, and late-myelinating tertiles, respectively, showed no significant group differences in the CC-early (t = 0.90, p = 0.37) or CC-middle (t = 1.66, p = 0.10) ROIs, but the MCI group showed decreased FA in the CC-late ROI (t = 2.70, p = 0.008) relative to the HC group.
3.3 Whole-brain Voxelwise Results
Whole-brain voxelwise statistics demonstrated numerous areas of decreased FA in the MCI group relative to the HC group (approximately 15% of all voxels were significant). There were no significant findings for the other contrast (MCI > HC). Chi-square analyses revealed no differences in the frequency of significant voxels across the early-, middle- and late-myelinating ROIs (χ2 = 0.91, p = 0.64). These results indicate that group differences are equally likely regardless of order of myelination.
4. Discussion
The current findings revealed decreased FA in late-myelinating regions within the CC and decreased FA in the body of the CC (comprised predominantly of late-myelinating WM) in individuals with MCI relative to healthy older adults. These results suggest that the pattern of changes in white matter integrity in MCI within the CC support the retrogenesis model when late-myelinating regions are empirically defined in an independent dataset.
In contrast to previous research which considered the genu as a late-myelinating region given its connections to prefrontal white matter, findings from Lebel et al. (2010) and the data presented here both demonstrate a general trend for outer regions (e.g., genu and splenium) of the CC to myelinate earlier than inner regions (e.g., body). Our finding of decreased white matter integrity in the body of the CC in MCI in this study, combined with previous evidence that this region is later-myelinating relative to the genu and splenium, suggests that the body is an important region in white matter neurodevelopment and worth assessment in future studies. Our results are consistent with other findings showing decreased white matter integrity in this CC region in MCI and AD (Alves et al., 2012; Di Paola et al., 2010a; Liu et al., 2013; Preti et al., 2012; Xie et al., 2006). The genu and splenium, which did not reveal significant findings in ROI analyses, may be composed of predominantly early- and middle-myelinating fibers. Previous studies have shown varying results within these regions, with some revealing decreased white matter integrity in the anterior CC in patients with MCI versus healthy older adults (Alves et al., 2012; Di Paola et al., 2010a; Liu et al., 2013; Scrascia et al., 2014; Stahl et al., 2007; Wang et al., 2009; Wang et al., 2014; Xie et al., 2006), while others have revealed no anterior CC abnormalities in MCI (Delano-Wood et al., 2008; Ukmar et al., 2008) or in patients with AD (Duan et al., 2006; Naggara et al., 2006). Decreased white matter integrity in the splenium is reported to be a fairly consistent finding across studies of AD (see Chua et al., 2008 for review); thus, it is somewhat surprising that no differences across MCI and HC groups were found in this ROI in our study. Although the splenium has been frequently implicated in DTI studies of AD patients, this finding has been less consistent in MCI, with some studies noting lower white matter integrity (Delano-Wood et al., 2008; Parente et al., 2008; Ukmar et al., 2008), and others reporting null results in the posterior CC (Alves et al., 2012; Di Paola et al., 2010a; Stahl et al., 2007); this inconsistency in the literature may help to explain our results and suggests a need for additional examinations. Further, some studies have also failed to show any differences in white matter integrity in ROIs of the genu and splenium in MCI or AD relative to healthy controls (Bozzao et al., 2001; Fellgiebel et al., 2004; Stahl et al., 2007), consistent with our results.
In contrast to our CC findings, results of the whole-brain voxelwise analysis did not support the retrogenesis model. Decreased white matter integrity in MCI was equally likely across early-, middle- and late-myelinating regions. It is possible that interhemispheric fibers may be more likely to reflect developmentally-based order of myelination because of properties inherent to the CC. For example, the CC is composed predominantly of bidirectional interhemispheric connections conjoined with few crossing fibers and a known, clearly delineated pattern of increasing anisotropy from anterior to posterior (Aboitiz et al., 1992; Hofer and Frahm, 2006). Any alterations to CC white matter integrity, including loss of myelination or reduced spatial organization of white matter, may be more easily detected in the CC relative to smaller white matter pathways that contain more crossing fibers and have less well defined anisotropy profiles. It may also be that other properties of the CC, such as higher signal-to-noise ratio and higher reliability given its greater homogeneity of fibers, may also help explain the discrepancy between results within the CC and those of the whole-brain analysis.
This study has a number of limitations. First, it will be important for future research to replicate these results. Because follow-up data are unavailable and MCI can reflect a variety of etiologies, it is possible that the results presented herein represent normal variability in white matter morphology that is indicative of normative cognitive strengths and weakness, rather than an Alzheimer’s disease trajectory. Reproducing these results in an MCI cohort that later converts to Alzheimer’s dementia would help verify that results represent a pattern of change expected early in the Alzheimer’s pathological process. Second, changes in CC white matter have been shown to correlate with the distribution of Wallerian degeneration at cortical regions following ischemic damage (de Lacoste et al., 1985). It is possible then that the findings reported here supporting retrogenesis in the CC reflect a larger, brain-wide pattern of pathogenesis that follows the retrogenesis model that were not detectable in the whole-brain voxel-based white matter analysis. Future studies could use cortical thickness analyses to investigate this hypothesis. Similarly, although decreased FA has been associated with decreased myelin in mouse models (Harsan et al., 2006), decreased diffusion anisotropy is due to changes in myelin as well as other factors, such as axonal structure and homogeneity of the direction of fiber pathways within a voxel (Madler et al., 2008; Song et al., 2002). Thus, the myelination tertile ROIs cannot be assumed to only reflect increased myelination with age as FA does not represent a direct measure of myelination, and is thus not likely to capture all relevant myelin-related neurodevelopmental or neurodegenerative variability. Third, due to small sample sizes and an effort to limit the number of analyses, we chose not to analyze aMCI and naMCI subgroups separately given evidence that naMCI frequently represents early Alzheimer’s disease (Fischer et al., 2007; Rountree et al., 2007; Schneider et al., 2009). However, this may make comparisons with other MCI studies more difficult, as samples including only aMCI are more common. Fourth, there may be disparities between the sample used to generate the myelination tertile ROIs (Westlye et al., 2010) and the sample under investigation in this study. The participants from which the myelination tertile ROIs were derived were of above average cognitive functioning and are not necessarily representative of the general population or of the developmental trajectory of the sample used here. In contrast, the neurocognitive performance of participants in this sample was widely distributed and included participants with MCI. Additionally, cohort effects inherent in the cross-sectional design used by Westlye and colleagues may contribute to the current results in that group-wise differences in early development (e.g. nutrition, education) could potentially alter white matter developmental trajectory. Longitudinal studies could help clarify the role of environmental and genetic factors on white matter development and degeneration over the lifespan. Finally, the diffusion data for both the Westlye study and the current study were derived from the center of each pathway based on the processing methodology employed (TBSS). Thin myelinated fibers closer to the cortex or towards the periphery of white matter pathways may be more vulnerable to changes with age or neurodegeneration than the centers of white matter pathways and deeper structures (Bartzokis et al., 2004; Marner et al., 2003; Sandell and Peters, 2001). Therefore, the center-derived DTI methodology used here may predispose findings to be less sensitive to changes in these lateral regions than in regions such as the CC. Alternate methods of projecting anisotropy maps may allow future studies to investigate age-related white matter changes more sensitively in these more thinly myelinated regions. Lastly, considering other imaging indices of white matter integrity and myelination in conjunction with DTI metrics may provide a complementary view of the age-trajectories of white matter across the human lifespan.
In conclusion, these findings support retrogenesis as a mechanism underlying white matter integrity changes within the corpus callosum among individuals with MCI. The historical lack of emphasis upon understanding development of brain white matter tracts in the study of callosal changes in AD may explain the lack of consistency that has existed in the literature to date across anterior, posterior and middle regions of the corpus callosum. The current methodology based on empirical data rather than longstanding assumptions about order of myelination provides a novel perspective on interpretation of CC findings that will contribute to improved understanding regarding the presence and pattern of regional callosal involvement in MCI.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, VISN 1 Career Development Award to Nikki Stricker. This research was also conducted while Nikki Stricker was a Gilbert Foundation/AFAR Research Grant recipient. This work was also supported by the Medical Research Service VA Merit Review Awards to William Milberg and Regina McGlinchey, the National Institute of Neurologic Disorders and Stroke (grant numbers K23NS062148); the National Institute of Nursing Research (grant number R01NR010827), the National Institute on Aging (grant numbers P60AG08812, P01AG004390); the Research Council of Norway (204966/F20), and by the National Institute on Drug Abuse (2T32DA015036).
Footnotes
The authors report no conflicts of interest.
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain research. 1992;598(1–2):143–53. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Alves GS, O’Dwyer L, Jurcoane A, Oertel-Knochel V, Knochel C, Prvulovic D, Sudo F, Alves CE, Valente L, Moreira D, Fusser F, Karakaya T, Pantel J, Engelhardt E, Laks J. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PloS one. 2012;7(12):e52859. doi: 10.1371/journal.pone.0052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiology of aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of neurology. 2003;60(3):393–8. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Tingus K, Huang D, Mendez MF, Edwards N, Mintz J. Apolipoprotein E affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biol Psychiatry. 2007;62(12):1380–7. doi: 10.1016/j.biopsych.2007.03.024. S0006-3223(07)00318-6 [pii] [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: novel therapeutic targets for preventing cognitive decline and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2004;6(6 Suppl):S53–9. doi: 10.3233/jad-2004-6s604. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benitez A, Fieremans E, Jensen JH, Falangola MF, Tabesh A, Ferris SH, Helpern JA. White matter tract integrity metrics reflect the vulnerability of late-myelinating tracts in Alzheimer’s disease. NeuroImage Clinical. 2014;4:64–71. doi: 10.1016/j.nicl.2013.11.001. S2213-1582(13)00149-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72(6):742–6. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzao A, Floris R, Baviera ME, Apruzzese A, Simonetti G. Diffusion and perfusion MR imaging in cases of Alzheimer’s disease: correlations with cortical atrophy and lesion load. AJNR American journal of neuroradiology. 2001;22(6):1030–6. [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19(3):253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21(1):83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local ditting. Journal of the American Statistical Association. 1988;83:596–610. [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. Journal of neuropathology and experimental neurology. 1985;44(6):578–91. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment: differential influence of lesion type on neuropsychological functioning. Stroke; a journal of cerebral circulation. 2008b;39(3):794–9. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, Wierenga CE, Delis DC, Theilmann RJ, Salmon DP. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiology of aging. 2010;31(10):1721–31. doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini AR, Sancesario G, Passafiume D, Caltagirone C, Spalletta G. When, where, and how the corpus callosum changes in MCI and AD: a multimodal MRI study. Neurology. 2010a;74(14):1136–42. doi: 10.1212/WNL.0b013e3181d7d8cb. 74/14/1136 [pii] [DOI] [PubMed] [Google Scholar]
- Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer’s disease and mild cognitive impairment using different MRI techniques: a review. Journal of Alzheimer’s disease : JAD. 2010b;20(1):67–95. doi: 10.3233/JAD-2010-1370. R06Q23414651653H [pii] [DOI] [PubMed] [Google Scholar]
- Duan JH, Wang HQ, Xu J, Lin X, Chen SQ, Kang Z, Yao ZB. White matter damage of patients with Alzheimer’s disease correlated with the decreased cognitive function. Surg Radiol Anat. 2006;28(2):150–6. doi: 10.1007/s00276-006-0111-2. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiology of aging. 2005;26(8):1193–8. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004;18(1):101–8. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- Fieremans E, Benitez A, Jensen JH, Falangola MF, Tabesh A, Deardorff RL, Spampinato MV, Babb JS, Novikov DS, Ferris SH, Helpern JA. Novel white matter tract integrity metrics sensitive to Alzheimer disease progression. AJNR American journal of neuroradiology. 2013;34(11):2105–12. doi: 10.3174/ajnr.A3553. ajnr.A3553 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288–91. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and cognition. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Asano T, Sakurai H, Imon Y, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted and magnetization transfer imaging of the corpus callosum in Alzheimer’s disease. J Neurol Sci. 1999;167(1):37–44. doi: 10.1016/s0022-510x(99)00135-5. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. Journal of neuroscience research. 2006;83(3):392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, Ewing-Cobbs L, Fletcher JM. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. NeuroImage. 2007;34(4):1497–505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral cortex. 2004;14(4):410–23. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited-Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holm L, Cassidy JD, Carroll LJ, Borg J. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2005;37(3):137–41. doi: 10.1080/16501970510027321. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368–75. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of neuropathology and experimental neurology. 1988;47(3):217–34. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(30):10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. NeuroImage. 2010;52(1):20–31. doi: 10.1016/j.neuroimage.2010.03.072. S1053-8119(10)00359-9 [pii] [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, Rudolph JL, Schnyer DM, Barber CE, Lipsitz LA, McGlinchey RE. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010;24(2):199–208. doi: 10.1037/a0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yin C, Xia S, Jia L, Guo Y, Zhao Z, Li X, Han Y, Jia J. White matter changes in patients with amnestic mild cognitive impairment detected by diffusion tensor imaging. PloS one. 2013;8(3):e59440. doi: 10.1371/journal.pone.0059440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magnetic resonance imaging. 2008;26(7):874–88. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of comparative neurology. 2003;462(2):144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res. 2006;146(3):243–9. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Parente DB, Gasparetto EL, da Cruz LC, Jr, Domingues RC, Baptista AC, Carvalho AC, Domingues RC. Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer’s disease. AJR Am J Roentgenol. 2008;190(5):1369–74. doi: 10.2214/AJR.07.2617. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Clark JH, O’Bryant SE, Smith GE, Ivnik RJ, Graff-Radford NR, Willis FB, Petersen RC, Lucas JA. Diagnostic validity of age and education corrections for the Mini-Mental State Examination in older African Americans. Journal of the American Geriatrics Society. 2012;60(2):328–31. doi: 10.1111/j.1532-5415.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of neurology. 1994;51(9):874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Preti MG, Baglio F, Lagana MM, Griffanti L, Nemni R, Clerici M, Bozzali M, Baselli G. Assessing corpus callosum changes in Alzheimer’s disease: comparison between tract-based spatial statistics and atlas-based tractography. PloS one. 2012;7(4):e35856. doi: 10.1371/journal.pone.0035856. PONE-D-11-15104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Franssen EH, Hasan SM, Monteiro I, Boksay I, Souren LE, Kenowsky S, Auer SR, Elahi S, Kluger A. Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer’s and other dementing processes. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl 3):28–36. doi: 10.1007/pl00014170. [DOI] [PubMed] [Google Scholar]
- Rountree SD, Waring SC, Chan WC, Lupo PJ, Darby EJ, Doody RS. Importance of subtle amnestic and nonamnestic deficits in mild cognitive impairment: prognosis and conversion to dementia. Dementia and geriatric cognitive disorders. 2007;24(6):476–82. doi: 10.1159/000110800. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. The Journal of comparative neurology. 2001;429(4):541–53. doi: 10.1002/1096-9861(20010122)429:4<541::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of neurology. 2009;66(2):200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrascia F, Curcio G, Ursini F, Trotta L, Quintiliani L, Migliore S, Altamura C, Pitocco F, Altavilla R, Melgari JM, Quattrocchi CC, Vernieri F. Relationship among diffusion tensor imaging, EEG activity, and cognitive status in mild cognitive impairment and Alzheimer’s disease patients. Journal of Alzheimer’s disease : JAD. 2014;38(4):939–50. doi: 10.3233/JAD-130788. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sridharan A, Bendlin BB, Gallagher CL, Oh JM, Willette AA, Alexander AL, Kemnitz JW, Colman RJ, Weindruch RH, Johnson SC. Effect of age and calorie restriction on corpus callosal integrity in rhesus macaques: a fiber tractography study. Neuroscience letters. 2014;569:38–42. doi: 10.1016/j.neulet.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243(2):483–92. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- Stricker NH, Salat DH, Foley JM, Zink TA, Kellison IL, McFarland CP, Grande LJ, McGlinchey RE, Milberg WP, Leritz EC. Decreased white matter integrity in neuropsychologically defined mild cognitive impairment is independent of cortical thinning. Journal of the International Neuropsychological Society : JINS. 2013;19(8):925–37. doi: 10.1017/S1355617713000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. NeuroImage. 2009;45(1):10–6. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30(6):749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neuroscience letters. 2002;332(1):45–8. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Lerche M, Kilimann I, O’Brien K, Grothe M, Meyer P, Li X, Sanger P, Hauenstein K. Decline of fiber tract integrity over the adult age range: a diffusion spectrum imaging study. Journal of magnetic resonance imaging : JMRI. 2014;40(2):348–59. doi: 10.1002/jmri.24420. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde ALW, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. NeuroImage. 2007;34:985–95. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde AL, Fellgiebel A, Filippi M, Hampel H, Kloppel S, Hauenstein K, Ewers M group E.s. Anatomical MRI and DTI in the diagnosis of Alzheimer’s disease: a European multicenter study. Journal of Alzheimer’s disease : JAD. 2012;31(Suppl 3):S33–47. doi: 10.3233/JAD-2012-112118. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer’s disease and in patients with mild cognitive impairment by using diffusion tensor imaging. La Radiologia medica. 2008;113(6):915–22. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiology of aging. 2011;32(5):916–32. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Lv PY, Wang HB, Li ZL, Li N, Sun ZY, Zhao BH, Huang Y. Diffusion tensor imaging measures of normal appearing white matter in patients who are aging, or have amnestic mild cognitive impairment, or Alzheimer’s disease. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2013;20(8):1089–94. doi: 10.1016/j.jocn.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. AJNR American journal of neuroradiology. 2009;30(5):893–9. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PN, Chou KH, Chang NJ, Lin KN, Chen WT, Lan GY, Lin CP, Lirng JF. Callosal degeneration topographically correlated with cognitive function in amnestic mild cognitive impairment and Alzheimer’s disease dementia. Human brain mapping. 2014;35(4):1529–43. doi: 10.1002/hbm.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral cortex. 2010;20(9):2055–68. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66(12):1845–9. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. F. A. Davis Company; Philadelphia: 1967. pp. 3–70. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]