Abstract
Following massive small bowel resection, the remnant bowel compensates by a process termed adaptation. Adaptation is characterized by villus elongation and crypt deepening which increases the capacity for absorption and digestion per unit length. The mechanisms/mediators of this important response are multiple. The purpose of this review is to highlight major basic contributions in elucidating a more comprehensive understanding of this process.
Intestinal adaptation is an important response to massive small bowel resection (SBR) and represents a mitogenic signal to the intestine culminating in a compensatory expansion in mucosal digestive and absorptive surface area per unit length. Clinically, adaptation is heralded by the gradual tolerance of enteral nutrition that could not be tolerated at earlier time points. A complete adaptation response allows for tolerance of all nutrition to be absorbed from the gut, without the need for supplemental parenteral feeding. The expression of several immediate-early genes within the remnant bowel has been recorded to be elevated within hours of intestinal resection1, 2. Similarly, in a murine model of SBR, alterations in wet weight as well as DNA and protein content in the remnant bowel are elevated as soon as 24 hours, but prior to the initiation of enteral feeding3.
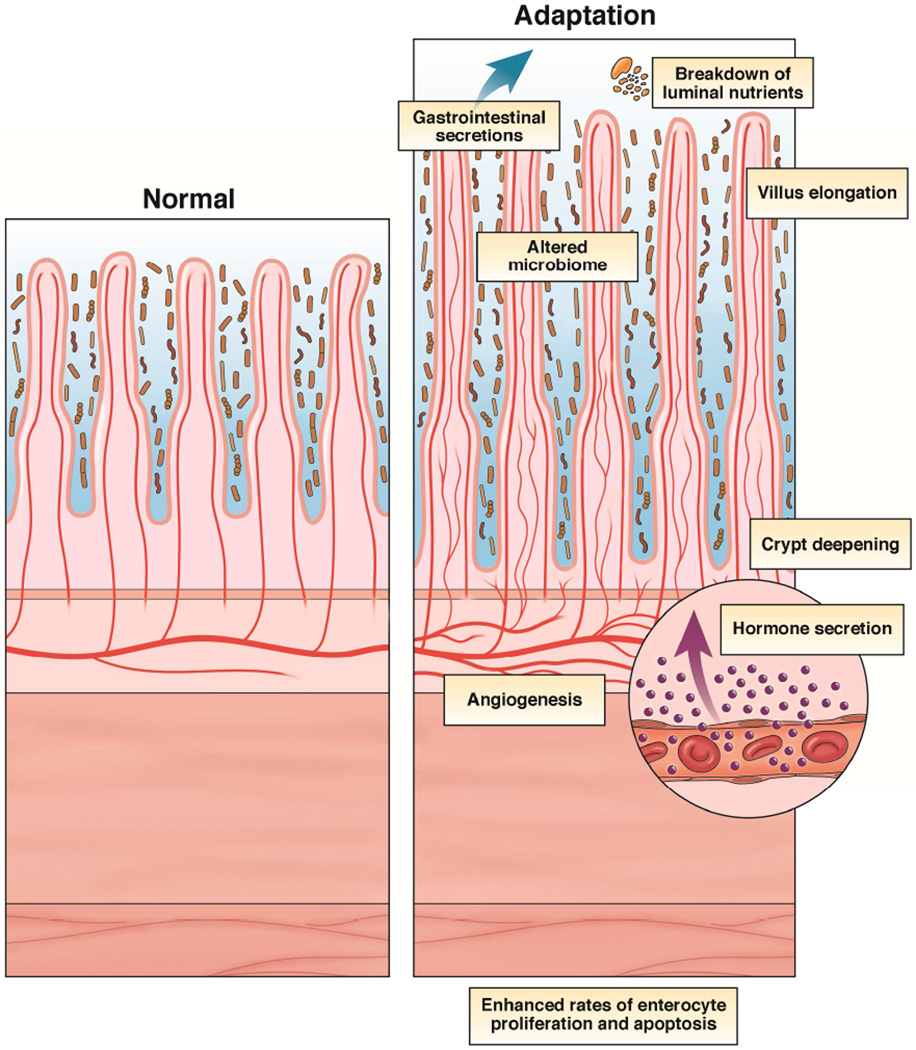
Adaptation is structurally characterized by taller villi and deeper crypts, as well as enhanced rates of enterocyte proliferation and apoptosis. While these features are a renowned characteristic of adaptation in animal models of massive SBR, similar structural alterations have not consistently been described in humans. In one study, the intestine was evaluated in a uniform population of infants with neonatal necrotizing enterocolitis who required bowel resection4. Comparing villus height and crypt depth at the normal margin of tissue at the time of resection with the time of ostomy takedown revealed significant increases in both parameters. In another report, a 70%– 75% increase in villus height was documented in the small intestine of 13 patients at 2 years following jejuno-ileal bypass5. In addition, significant increases in crypt depth and cell number/crypt in the colon of 12 patients with jejuno-colonic anastomosis compared with healthy controls was identified at a mean of 9.8 years following resection6. Unfortunately, the histological status of the small intestine was not evaluated in that study. In contrast, other studies have failed to demonstrate changes in rates of enterocyte proliferation, crypt depth, or villus height in the small intestine of patients with short gut syndrome compared with controls7–9. All of the above mentioned human studies are comprised of small patient numbers, variable lengths of resected intestine, assorted amounts of enteral feeding, and analysis at single time points after SBR. Despite these limitations, animal models for studying resection-induced adaptation continue to provide important mechanistic insights.
Mechanisms of adaptation
The mechanisms and mediators of intestinal adaptation are multi-factorial and include intraluminal nutrients, gastrointestinal secretions, as well as hormones10, 11 (Figure 1). In general, most research has focused on various growth factors and how they affect rates of enterocyte proliferation as the primary driver of resection-induced mucosal growth. It should be considered, however that enhanced rates of enterocyte proliferation may actually occur secondary to growth of subepithelial structures.
Figure 1.
Factors which play a role in resection-induced intestinal adaptation.
Intraluminal Nutrients
Enteral nutrients appear to stimulate intestinal adaptation via several mechanisms including direct contact with epithelial cells as well as stimulated secretion of trophic gastrointestinal hormones and pancreaticobiliary secretions12. The contributions of luminal nutrients to the adaptive response of the intestine is underscored by the observations that gut mucosal atrophy is associated with starvation and is reversed by refeeding. Further, surgical transposition of a segment of the ileum into the more proximal intestinal stream results in structural and functional “jejunalization” of the transposed ileum13, 14. Not only is the presence of luminal nutrition important for adaptation, but so is the composition of the nutrition. Luminal administration of non-nutrient substrates has little effect on adaptation. More complex nutrients requiring more metabolic energy to absorb and digest have been suggested to induce the greatest adaptation response, presumably by virtue of an increased functional workload of the enterocyte. Enteral fats appear to be the most trophic of the macronutrients in inducing adaptation15. More specifically, longer-chain and more polyunsaturated fats as present in fish oil may provide an even greater adaptive stimulus16–18.
Gastrointestinal Secretions
Multiple experimental observations contribute to the notion that endogenous gastrointestinal secretions are important for adaptation. Experimental models whereby the ampulla of Vater is surgically transposed of to areas more distal in the gastrointestinal tract induces in villus hyperplasia beyond the transposed segment19, 20. Bile alone has been demonstrated to stimulate intestinal RNA and DNA content when directly delivered to the mid-small bowel, but the effect seems to be more profound when combined with the pancreatic secretions20. In other studies, pancreatic secretions seem to be more trophic to the intestinal mucosa when compared with bile20. Further evidence that pancreaticobiliary secretions are important for post-resection adaptation is the observation that somatostatin, an agent which dramatically diminishes the output of endogenous gastrointestinal secretions, is also associated with an inhibited adaptation response21.
Humoral Factors
A surgical model of vascular parabiosis in which two rats share a common circulation has provided one of the most compelling studies endorsing the contributions of hormones to resection-induced adaptation22. In that report, intestinal resection in one animal was associated with adaptive changes in the intestine of the other unoperated animal. Multiple endogenous humoral factors that have been suggested to play a role in intestinal adaptation and include growth hormone, insulin-like growth factor, glucagon-like peptide-2, epidermal growth factor (EGF), leptin, thyroxine, and corticosteroids, to name but a few23. Many of these factors have either been found to be elevated in the serum of patients who have undergone SBR, or exogenous administration of these agents following SBR has resulted in enhanced parameters of adaptation.
Growth Hormone
Growth hormone (GH) is a 191 amino acid, single-chain protein produced in the anterior pituitary gland. This growth factor is known to be a major regulator of postnatal growth in mammals as well as playing an important role in the regulation of lipid and carbohydrate metabolism24, 25. Because GH has been shown to induce growth and proliferation in many different tissues and cell lines, its role in the setting of short gut syndrome has been studied extensively. The receptor for GH has been found throughout the intestine—in cells of the muscularis propria, submucosa, muscularis mucosa, lamina propria, and intestinal epithelium26. Because of its widespread distribution for this receptor in the gut, GH has been proposed to directly stimulate intestinal growth. In addition to directly stimulating growth of the intestinal layers, GH is a major stimulus for the production of insulin-like growth factor – 1 (IGF-1), another intestinotrophic hormone, whose role in intestinal adaptation will be discussed in later.
In animal studies, exogenous GH has resulted in significantly increased small bowel length, mucosal height, jejunal villus height, and/or glutamine and leucine transport in animals that had undergone intestinal resection27–29. In contrast, other reports have failed to demonstrate an effect of GH on postresection mucosal growth30, 31. GH appears to be more effective in combination with glutamine, an enterocyte-preferred fuel. Several, but not all animal studies evaluating GH plus glutamine have demonstrated improvements in structural measures of intestinal adaptation32–35.
In a clinical trial, Byrne and colleagues first demonstrated clinical benefit by administering GH and glutamine in ten patients with short bowel syndrome who had been on long term parenteral nutrition36. This study inspired multiple subsequent clinical trials with mixed results. In a 2010 Cochrane Review, Wales et al analyzed 5 clinical trials of GH with or without glutamine and suggested a positive effect of GH on weight gain and energy absorption37. In the majority of trials, the effects were short-lived an returned to baseline shortly after cessation of therapy. The evidence to recommend this therapy was therefore inconclusive and the clinical utility of this treatment was questioned. Somatropin is a recombinant form of human GH and has been recently shown to enhance fat free mass through the stimulation of protein synthesis and and decrease proteolysis in response to feeding38. In that study, improvements in de novo synthesis and intestinal absorption increase glutamine availability over the physiologic range, suggesting that beneficial effects of GH may not require supplemental glutamine.
Insulin-like-growth hormone-1 (IGF-1)
IGF-1 is a hormone produced chiefly in the liver and to a lesser degree in the gastrointestinal tract and has commanded much attention as an enterotrophic hormone. Like GH, IGF-1 has been shown to enhance rates of enterocyte proliferation after SBR39. These observations, along with the localization of IGF-1 production, its receptor, and regulatory binding proteins in the intestine, make IGF-1 an attractive target for modulating adaptation responses40, 41.
It has been considered that IGF-1 is the mediator of the effects attributed to GH32, 42 Both functional and structural parameters of adaptation have been shown to be amplified by IGF-1. Vanderhoof et al found an increase in the activity of the ileal digestive enzymes sucrase, maltase, and leucine aminopeptidase when IGF-1 was given after SBR43. In rats with SBS, IGF-1 treatment allowed the rats to be weaned from parenteral nutrition43. In that study, IGF-1 – treated short bowel rats were also found to have greater body weights and increased lean body mass.
In addition to the effects of IGF-1 on enterocytes, our laboratory has revealed a possible effect of IGF-1 on the smooth muscle of the intestine. We performed SBR procedures on transgenic mice who overexpress IGF-1 specifically in smooth muscle cells44. We found that these mice increased the length of their remnant intestine far more (approximately 2-fold) than nontransgenic control mice that also underwent SBR. Of note, these transgenic mice did not exhibit the normal adaptive response of increasing villus height and crypt depth in the early phases of adaptation. The intestinal lengthening response preceded villus growth, which was noted at later postoperative time points. These experiments suggest that the IGF-1-stimulated muscular lengthening might be an important trigger for enhanced villus and crypt growth. As such, it is plausible to consider that enterocyte proliferation occurs secondary to growth of the underlying mesenchyme, as opposed to being the primary stimulus for villus lengthening.
In contrast with the positive findings above, adaptation responses appear to be preserved following SBR in both IGF-1-null mice as well as in a strain of mice in whom IGF-1 receptor expression was disrupted specifically in enterocytes45. These findings have several implications. First, they suggest that enterocytes are not a major cell compartment for IGF-1 receptor signaling during adaptation. Thus, the beneficial effects of exogenous IGF-1 may involve IGF-1 receptors in other cells within the bowel wall. This notion would be supported by the magnified intestinal lengthening demonstrated after SBR in mesenchymal IGF-1 transgenic mice as described above44. In addition, these findings would offer the possibility that other ligands (such as insulin or IGF-2) for the IGF receptor may be able to compensate for the lack of IGF-1 expression. Despite the significant preclinical work that has been done, no human clinical trials with IGF-1 have been reported.
Glucagon-like peptide – 2 (GLP-2)
GLP-2 is an enterotrophic hormone and a member of the pituitary adenylate cyclase activating peptide glucagon superfamily. GLP-2 is synthesized in enteroendocrine L cells of the distal ileum and proximal colon46, 47. Within this 33 amino acid protein, the second amino acid in the sequence is alanine, which makes the hormone sensitive to degradation by the exopeptidase dipeptidyl peptidase-448, 49. Substitution of glycine for alanine at position 2 makes a synthetic analog of GLP-2 (Teduglutide) that is resistant to enzymatic degradation and significantly extends its half-life50.
GLP 2 exerts its effects through the GLP-2 receptor, which has been identified on intestinal enteroendocrine cells, enteric neurons, and subepithelial myofibroblasts51–53. Secretion of GLP-2 by intestinal L cells is driven by both direct stimulation of nutrients in the distal bowel and vagally mediated pathways, which are activated by the presence of nutrients in the proximal bowel54. Ingestion of nutrients, particularly long-chain fatty acids, plays a major role in GLP-2 secretion55. In patients with short bowel syndrome, the presence of a colon in continuity with the small intestine is important for nutrient-stimulated increases in GLP-256, 57. This finding may help explain why presence of the colon reduces the likelihood that a patient with short bowel syndrome will require parenteral nutrition.
The intestinal effects of GLP-2 have been studied both in animal models and in human clinical trials. When given to rodents, GLP-2 stimulates intestinal mucosal growth58, 59. In addition to elongated intestinal villi and crypts, GLP-2 administration augments rates of crypt cell proliferation and attenuates rates of apoptosis. Other effects mediated by GLP-2 include reduced gastric motility, inhibited gastric acid secretion, and increased mesenteric blood flow60–62. GLP-2 also acts on the enteric nervous system, which may play a key role in its ability to stimulate mucosal growth. After administration of GLP-2, cellular changes have been detected in enteric neurons before affecting the intestinal crypts, suggesting that many of the effects of GLP-2 may be mediated by the enteric nervous system52. Along this line, a potential role for the enteric nervous system was suggested by studies of Ret-heterozygous mutant mice who displayed enhanced adaptation responses to SBR63.
In several studies of adult patients with short bowel syndrome treated with teduglutide showed increases in villus height and decreases in parenteral nutrition and fluid requirements64–66. Teduglutide is now approved for clinical use in parenteral nutrition-dependent adults with short bowel syndrome67.
Epidermal Growth Factor
Human EGF is a 53 amino acid protein found in platelets, macrophages, urine, saliva, breast milk, and plasma. EGF is a member of a family of ligands sharing a common EGF receptor (EGFR), which also includes transforming growth factor, heparin-binding EGF-like growth factor, amphiregulin, epiregulin, epigen, betacellulin, and neuregulins 1–468. This growth factor has been shown to induce growth in the epithelia of multiple tissues to include skin, lung, tracheal, corneal, and gastrointestinal tract69.
An important role for EGF as a mediator of adaptation was initially suggested by a study in which EGF was administered to rats after SBR and demonstrated significant increases in weight gain as well as other parameters of adaptation70. Through several subsequent experimental paradigms, enhanced resection-induced adaptation responses have been verified following stimulation of the EGF receptor either by exogenous EGF71, 72, in EGF transgenic mice73 or administration of another EGF receptor ligand (transforming growth factor-α)74. Alternatively, inhibiting EGF receptor signaling by removing the submandibular glands – a major source of endogenous EGF in the mouse75, performing SBR procedures in waved-2 mice with diminished EGF receptor activity76, or administration of a pharmacologic EGF receptor inhibitor77 all resulted in attenuated adaptation responses.
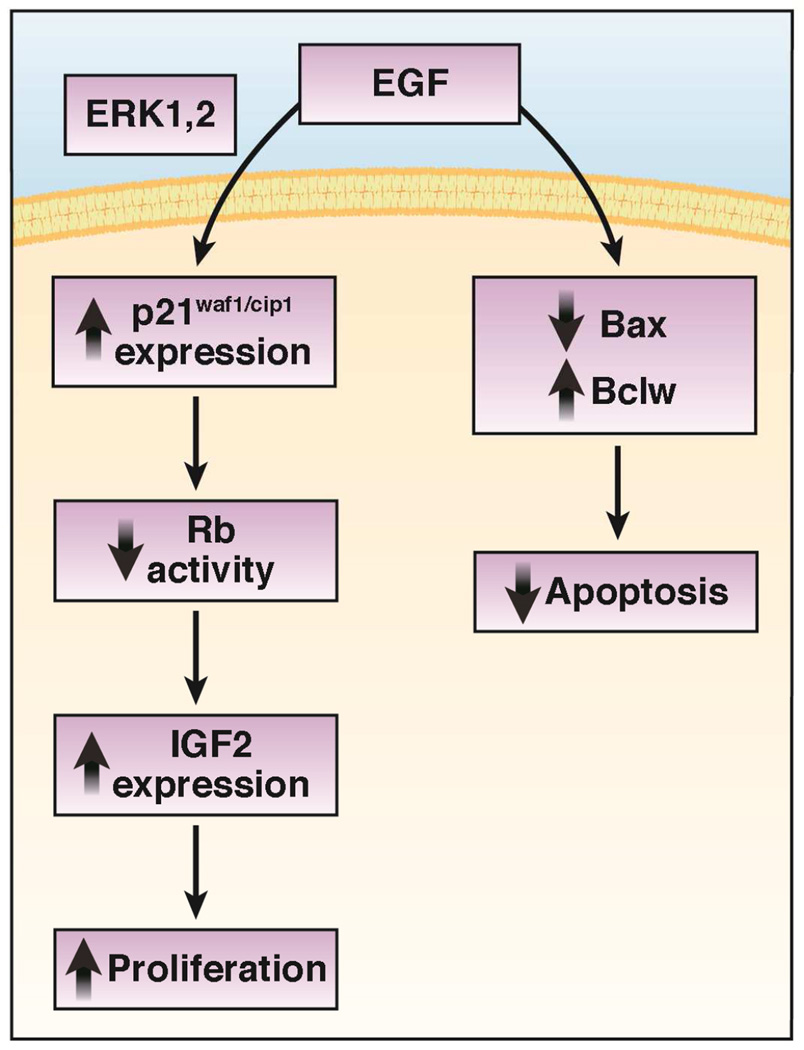
Since the intestinal mucosa is a very dynamic organ containing some of the most rapidly proliferating cells in the body, the relationship between rates of cell production and cell death must be precise. Any imbalance may result in either intestinal mucosal atrophy or neoplasia. In studies focused on mechanisms for EGF receptor regulation of proliferation revealed that expression of the cell cycle inhibitor p21waf1/cip1 (p21) was increased and paradoxically required for EGF-directed proliferation of enterocytes in vitro78. In this study, a critical region of the p21 promoter was found to be activated by EGF receptor stimulation. This promoter activity required activated extracellular signal-regulated kinase (ERK) 1/2 and contained a putative binding site for the transcription factor Sp1. The requirement for this cell cycle regulatory protein was verified in earlier experiments in which p21-null mice demonstrated no induction of enterocyte proliferation after the stimulus of SBR79.
In seeking potential mechanisms for how p21 regulates adaptation, we initially expanded upon the observation that p21 affects stem cell populations within bone marrow80 We therefore sought to determine the effect of p21 deficiency on intestinal stem cells. In these studies, we were unable to demonstrate differences in the expression of several stem cell markers or numbers of crypt-base columnar cells in p21-null versus control mice81. However, we did identify increased expression of another cell cycle inhibitor Retinoblastoma protein (Rb) within the crypt cells of the p21-deficient mice82. The significance of Rb expression was established by genetically inactivating a single Rb allele in the p21-null animals, which restored enterocyte proliferation and adaptation responses. In another study, rates of enterocyte proliferation and villus growth were magnified when Rb expression was completely disrupted within the intestinal epithelium of unoperated mice83.
Independent of p21, EGF receptor stimulation has been demonstrated to directly inactivate Rb by phosphorylation in cultured enterocytes84. One simple explanation for how Rb deficiency results in enterocyte proliferation is the fact that the activity of this cell cycle inhibitor is attenuated. Alternatively, enhanced IGF-2 expression has been shown to be associated with Rb-deficiency in enterocytes85. In this study, genetic disruption of IGF-2 expression in intestinal Rb-deficient mice prevented the mucosal hyperplasia associated with Rb deficiency. Since acute disruption of intestinal Rb expression after intestinal resection results in amplified adaptation responses86, future experiments focused upon illuminating this previously unrecognized role for Rb as a critical player in the molecular mechanism of resection-induced enterocyte proliferation and adaptation appear justified.
Similar to proliferation, rates of enterocyte apoptosis are also elevated following SBR87–90. Since rates of enterocyte production must be perfectly matched by rates of enterocyte loss, these findings made biological sense. It appears that the proaptotic Bcl-2 family member Bax is a major mediator of resection-induced enterocyte apoptosis. Bax expression is elevated in the intestine after SBR88, 91, and coincides with reduced expression of an anti-apoptotic Bcl-2 family member Bcl-w91. Indeed, the proliferative crypt compartment is the site for the greatest changes in Bax and Bcl-w expression92. When intestinal resections were performed in Bax-null mice, the expected increase in enterocyte apoptosis did not occur, despite normal induction of enterocyte proliferation93. In the setting of intestinal resection, apoptotic and adaptive responses are preserved in both tumor necrosis factor receptor 1-null and Fas-null mice94. These results suggest that the mechanism for increased enterocyte apoptosis following massive SBR does not appear to involve the extrinsic, death receptor-mediated pathway. Further, the apoptosis response to SBR is not a simple passive response to increased rates of enterocyte proliferation.
The ultimate utility of focusing on both the rates of proliferation and apoptosis is that future growth factor and/or pharmacologic therapy targeted to stimulate proliferation while at the same time inhibit apoptosis may result in an even greater expanded mucosal surface area than either intervention alone. The benefits of this dual therapeutic approach was suggested by co-administration of EGF (to inhibit apoptosis and stimulate proliferation) and a pharmacologic apoptosis (pan-caspase) inhibitor after SBR resulting in greater mucosal growth95. Explicit understanding of these mechanisms will be necessary to optimize this novel therapy. A summary of key signaling events which have been established to be involved with EGF-directed enhanced enterocyte proliferation and attenuated rates of apoptosis is presented in Figure 2.
Figure 2.
Key signaling events that have been established to play a role in mechanisms for how EGF amplifies resection-induced intestinal adaptation.
Angiogenesis
We have previously identified a significant induction of capillary growth within adapting intestinal villi96 (40). It is presently unclear whether this angiogenic response occurs as a result of stimulated enterocyte production or whether this angiogenic response is a primary signal to induce enterocyte proliferation. Photoacoustic microscopy applied to live mice immediately following intestinal resection revealed that both blood flow and arterial oxygen saturation were reduced and oxygen extraction was elevated within the remnant intestine97. This immediate response was associated with elevated expression of hypoxia-inducing factor-1α98. It is therefore possible that the immediate response to SBR results in a hypoxic milieu which may initiate a series of hypoxia-regulated genes capable of signaling for enterocyte proliferation. This notion is supported by a study reporting rescued adaptation responses in the intestine of vascular endothelial growth factor deficient mice after SBR99.
One proangiogenic chemokine is CXCL-5 and has been demonstrated to be elevated in the adapting intestine after SBR96. The expression of endothelial CXCL-5 appears to be modulated by EGF100. Indeed, genetic disruption of CXCL-5 expression prevents adaptive angiogenesis following SBR101. In this study, it was surprising that villus growth occurred despite the lack of an angiogenic response. In a subsequent series of experiments, CXCL-5 null mice were found to have impaired intestinal lipid absorption after SBR102. It is therefore plausible to conclude that the angiogenic response to intestinal resection is more important for functional, rather than structural adaptation.
Gut Microbiome
Small bowel bacterial overgrowth (SBBO) and catheter-related bloodstream infections are two of the most common complications in patients with intestinal failure and directly impact morbidity and mortality103, 104. SBBO generally results from the development of dilated loops of intestine with impaired peristalsis. This anatomic alteration sets the stage for stasis, disruption of the enteric flora, secretory diarrhea, malabsorption, gut mucosal inflammation, D-lactic acid production, and bacterial translocation into either the portal circulation or mesenteric lymph nodes. Despite these well-known events, data supporting the occurrence of bacterial translocation and microbiological features of SBBO in humans is both limited and indirect. Prior studies have utilized employ cultures of duodenal aspirate, absorption of various sugar markers as a surrogate for intestinal permeability, or hydrogen and/or 14C-D-xylose breath testing. Reliance on culturable organisms alone is restricted by the fact that less than 50% of bacterial species in the gut cannot be cultured.
Recent advances in high-throughput sequencing of the 16S ribosomal (rRNA) gene of luminal gut bacteria have established a significant association between the intestinal microbiome and various intestinal epithelial and metabolic responses to a wide spectrum of diseases and conditions. Using 16S sequencing, massive SBR has been shown in several animal models (mouse, piglet) to be associated with significant alterations in the gut microbiome105–107.
There have been limited pediatric clinical studies involving the gut microbiome in the context of short bowel syndrome. In one report, the gut microbiota in 11 children with short bowel syndrome was studied and found a reduced bacterial diversity associated with an increased relative abundance of Proteobacteria 108. A confounding variable of this study was that the majority of patients on parenteral nutrition (PN) were receiving antibiotics at the time of stool sampling. Another six patients had already weaned from PN. In another study of 23 children with intestinal failure, there was also a reduced bacterial diversity associated with an increased relative abundance of Proteobacteria in patients that required PN while an overabundance of Lactobacilli in patients that had already weaned from PN 109. In the PN patients, Proteobacteria was associated with a greater degree of liver injury. These data offer the possibility that the gut microbiome may be a major contributor in the pathogenesis of cholestasis and hepatic injury in patients with intestinal failure.
Mayeur et al. studied 16 patients with short gut syndrome and revealed a marked dysbiosis in fecal microbiota, with a predominance of Lactobacillus/Leuconostoc group, while Clostridium and Bacteroides were under-represented 110. The presence of fecal lactate (56% of patients) was used to define a Lactate-accumulator group (LA), while absence of fecal lactate (44% of patients) defined a Non lactate-accumulator group (NLA). The LA group has lower serum HC03-levels and were at risk of D-encephalopathic reactions. Furthermore, all patients of the NLA group and those accumulating preferentially L isoform in the LA group had never developed D-acidosis. The D/L fecal lactate ratio may therefore be a relevant index to predict risk for D-lactate encephalopathy. There has been a recent case report of a child with D-lactic acidosis and short bowel syndrome successfully managed by fecal transplantation 111
Through metagenomic and biochemical analysis, the intestinal microbiota of genetically obese mice have been shown to have an increased capacity for energy harvest from the diet 112. In that study, transfer of stool into the gastrointestinal tract of germ free mice resulted in a significant increase in body fat. Stool from obese mice display a proportional increase in Fermicutes phyla (which includes the genus Lactobacilli) in their intestinal lumen. It is therefore plausible to investigate whether the altered intestinal microbiota in patients with intestinal failure adapts to provide greater energy harvest for the host.
The paucity of published data regarding direct interrogation of the microbiota in the setting of intestinal failure represents a significant gap in our understanding of this important morbidity. These data will direct a more informed scientific rationale for current therapeutic interventions such as antibiotic administration, prebiotics, probiotics, operative reduction in small bowel caliber, or even future interventions such as microbiota manipulation via fecal transplantation.
Summary
Adaptation is critical for survival following massive intestinal loss. In children with intestinal failure, roughly half will have a complete adaptation response and wean completely from parenteral nutrition113. Within the other half of patients, and equal proportion will either die or require a small bowel transplant. Current 5-year graft survival rates following intestinal transplantation are roughly 50%114 with significant patient morbidity associated with significant immunosuppression. Basic research designed to elucidate specific mechanisms for resection-induced adaptation responses are therefore critical for the future design of more targeted, innovative therapies to enhance this important response.
Acknowledgments
Grant Support – National Institutes of Health NIDDK – DK059288, DK104698, March of Dimes, and the St. Louis Children’s Hospital Foundation – Children’s Surgical Sciences Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures – Dr. Warner serves as a consultant for Shire Human Genetic Therapies, Inc.
Reference List
- 1.Rubin DC, Swietlicki EA, Wang JL, et al. Regulation of PC4/TIS7 expression in adapting remnant intestine after resection. Am J Physiol. 1998;275:G506–G513. doi: 10.1152/ajpgi.1998.275.3.G506. [DOI] [PubMed] [Google Scholar]
- 2.Sacks AI, Warwick GJ, Barnard JA. Early proliferative events following intestinal resection in the rat. Journal of Pediatric Gastroenterology & Nutrition. 1995;21:158–164. doi: 10.1097/00005176-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small bowel resection in the mouse. Journal of the American College of Surgeons. 1996;183:441–449. [PubMed] [Google Scholar]
- 4.McDuffie LA, Bucher BT, Erwin CR, et al. Intestinal adaptation after small bowel resection in human infants. J Pediatr. Surg. 2011;46:1045–1051. doi: 10.1016/j.jpedsurg.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doldi SB. Intestinal adaptation following jejuno-ileal bypass. Clin Nutr. 1991;10:138–145. doi: 10.1016/0261-5614(91)90049-i. [DOI] [PubMed] [Google Scholar]
- 6.Joly F, Mayeur C, Bruneau A, et al. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie. 2010;92:753–761. doi: 10.1016/j.biochi.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe SJ, Haymond MW, Bennet WM, et al. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology. 1994;107:379–388. doi: 10.1016/0016-5085(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 8.Porus RL. Epithelial Hyperplasia Following Massive Small Bowel Resection in Man. Gastroenterology. 1965;48:753–757. [PubMed] [Google Scholar]
- 9.Ziegler TR, Fernandez-Estivariz C, Gu LH, et al. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am. J. Clin. Nutr. 2002;75:922–930. doi: 10.1093/ajcn/75.5.922. [DOI] [PubMed] [Google Scholar]
- 10.Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr. 2014;38:23S–31S. doi: 10.1177/0148607114525210. [DOI] [PubMed] [Google Scholar]
- 11.Sangild PT, Ney DM, Sigalet DL, et al. Animal models of gastrointestinal and liver diseases. Animal models of infant short bowel syndrome: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1147–G1168. doi: 10.1152/ajpgi.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. New Engl J Med. 1978;298:1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- 13.Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clinical Science. 1967;32:139–149. [PubMed] [Google Scholar]
- 14.Altmann GG, Leblond CP. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. American Journal of Anatomy. 1970;127:15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- 15.Booth IW. Enteral nutrition as primary therapy in short bowel syndrome. [Review] Gut. 1994;35:S69–S72. doi: 10.1136/gut.35.1_suppl.s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr. Gastroenterol. Nutr. 1999;28:41–45. doi: 10.1097/00005176-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Vanderhoof JA, Park JH, Herrington MK, et al. Effects of dietary menhaden oil on mucosal adaptation after small bowel resection in rats. Gastroenterology. 1994;106:94–99. doi: 10.1016/s0016-5085(94)94589-6. [DOI] [PubMed] [Google Scholar]
- 18.Choi PM, Sun RC, Guo J, et al. High-fat diet enhances villus growth during the adaptation response to massive proximal small bowel resection. J. Gastrointest. Surg. 2014;18:286–294. doi: 10.1007/s11605-013-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson RC, Bauer FL, Ross JS, et al. Contributions of bile and pancreatic juice to cell proliferation in ileal mucosa. Surgery. 1978;83:570–576. [PubMed] [Google Scholar]
- 20.Altmann GG. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. American Journal of Anatomy. 1971;132:167–177. doi: 10.1002/aja.1001320204. [DOI] [PubMed] [Google Scholar]
- 21.Bass BL, Fischer BA, Richardson C, et al. Somatostatin analogue treatment inhibits post-resectional adaptation of the small bowel in rats. American Journal of Surgery. 1991;161:107–111. doi: 10.1016/0002-9610(91)90369-o. discussion 111-2. [DOI] [PubMed] [Google Scholar]
- 22.Williamson RC, Buchholtz TW, Malt RA. Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology. 1978;75:249–254. [PubMed] [Google Scholar]
- 23.McMellen ME, Wakeman D, Longshore SW, et al. Growth factors: possible roles for clinical management of the short bowel syndrome. Semin. Pediatr. Surg. 2010;19:35–43. doi: 10.1053/j.sempedsurg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71(Suppl 2):36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 25.Lichanska AM, Waters MJ. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet. 2008;24:41–47. doi: 10.1016/j.tig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Delehaye-Zervas MC, Mertani H, Martini JF, et al. Expression of the growth hormone receptor gene in human digestive tissue. J Clin Endocrinol Metab. 1994;78:1473–1480. doi: 10.1210/jcem.78.6.8200952. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou PH, Canarelli JP, Leroy C, et al. Stimulation by recombinant human growth hormone of growth and development of remaining bowel after subtotal ileojejunectomy in rats. J Pediatr Gastroenterol Nutr. 1994;18:446–452. doi: 10.1097/00005176-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Shulman DI, Hu CS, Duckett G, et al. Effects of short-term growth hormone therapy in rats undergoing 75% small intestinal resection. Journal of Pediatric Gastroenterology & Nutrition. 1992;14:3–11. doi: 10.1097/00005176-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Iannoli P, Miller JH, Ryan CK, et al. Epidermal growth factor and human growth hormone accelerate adaptation after massive enterectomy in an additive, nutrient-dependent, and site-specific fashion. Surgery. 1997;122:721–728. doi: 10.1016/s0039-6060(97)90079-9. discussion. [DOI] [PubMed] [Google Scholar]
- 30.Washizawa N, Gu LH, Gu L, et al. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2004;28:399–409. doi: 10.1177/0148607104028006399. [DOI] [PubMed] [Google Scholar]
- 31.Ljungmann K, Grofte T, Kissmeyer-Nielsen P, et al. GH decreases hepatic amino acid degradation after small bowel resection in rats without enhancing bowel adaptation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G700–G706. doi: 10.1152/ajpgi.2000.279.4.G700. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Wu ZH, Xie JX, et al. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin. Nutr. 2001;20:159–166. doi: 10.1054/clnu.2000.0379. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Li YX, Li N, et al. Glutamine enhances the gut-trophic effect of growth hormone in rat after massive small bowel resection. J Surg Res. 2001;99:47–52. doi: 10.1006/jsre.2001.6108. [DOI] [PubMed] [Google Scholar]
- 34.Spadoni JM, Aguilar-Nascimento JE, Silva MH, et al. Effects of the combined use of glutamine and growth hormone in the intestinal adaptation after massive resection of the small bowel in rats. Acta Cir Bras. 2005;20:382–389. doi: 10.1590/s0102-86502005000500008. [DOI] [PubMed] [Google Scholar]
- 35.Vanderhoof JA, Kollman KA, Griffin S, et al. Growth hormone and glutamine do not stimulate intestinal adaptation following massive small bowel resection in the rat. J Pediatr Gastroenterol Nutr. 1997;25:327–331. doi: 10.1097/00005176-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Byrne TA, Persinger RL, Young LS, et al. A new treatment for patients with short-bowel syndrome. Growth hormone, glutamine, and a modified diet. Annals of Surgery. 1995;222:243–254. doi: 10.1097/00000658-199509000-00003. discussion 254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wales PW, Nasr A, de Silva N, et al. Human growth hormone and glutamine for patients with short bowel syndrome. Cochrane Database Syst Rev. 2010:CD006321. doi: 10.1002/14651858.CD006321.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Seguy D, Darmaun D, Duhamel A, et al. Growth hormone enhances fat-free mass and glutamine availability in patients with short-bowel syndrome: an ancillary double-blind, randomized crossover study. Am J Clin Nutr. 2014;100:850–858. doi: 10.3945/ajcn.113.071845. [DOI] [PubMed] [Google Scholar]
- 39.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am. J. Physiol Regul. Integr. Comp Physiol. 2003 doi: 10.1152/ajpregu.00014.2003. [DOI] [PubMed] [Google Scholar]
- 40.Ohneda K, Ulshen MH, Fuller CR, et al. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology. 1997;112:444–454. doi: 10.1053/gast.1997.v112.pm9024298. [DOI] [PubMed] [Google Scholar]
- 41.Winesett DE, Ulshen MH, Hoyt EC, et al. Regulation and localization of the insulin-like growth factor system in small bowel during altered nutrient status. American Journal of Physiology. 1995;268:G631–G640. doi: 10.1152/ajpgi.1995.268.4.G631. [DOI] [PubMed] [Google Scholar]
- 42.Lund PK. Molecular basis of intestinal adaptation: the role of the insulin-like growth factor system. Ann. N. Y. Acad. Sci. 1998;859:18–36. doi: 10.1111/j.1749-6632.1998.tb11108.x. [DOI] [PubMed] [Google Scholar]
- 43.Vanderhoof JA, McCusker RH, Clark R, et al. Truncated and native insulinlike growth factor I enhance mucosal adaptation after jejunoileal resection. Gastroenterology. 1992;102:1949–1956. doi: 10.1016/0016-5085(92)90318-s. [DOI] [PubMed] [Google Scholar]
- 44.Knott AW, Juno RJ, Jarboe MD, et al. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am. J. Physiol Gastrointest. Liver Physiol. 2004;287:G562–G570. doi: 10.1152/ajpgi.00438.2003. [DOI] [PubMed] [Google Scholar]
- 45.Sun RC, Choi PM, Guo J, et al. Insulin-like growth factor 2 and its enterocyte receptor are not required for adaptation in response to massive small bowel resection. J Pediatr Surg. 2014;49:966–970. doi: 10.1016/j.jpedsurg.2014.01.035. discussion 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayo KE, Miller LJ, Bataille D, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 47.Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–544. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- 48.Munroe DG, Gupta AK, Kooshesh F, et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavares W, Drucker DJ, Brubaker PL. Enzymatic- and renal-dependent catabolism of the intestinotropic hormone glucagon-like peptide-2 in rats. Am J Physiol Endocrinol Metab. 2000;278:E134–E139. doi: 10.1152/ajpendo.2000.278.1.E134. [DOI] [PubMed] [Google Scholar]
- 50.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yusta B, Huang L, Munroe D, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 52.Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12497–12502. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orskov C, Hartmann B, Poulsen SS, et al. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Roberge JN, Brubaker PL. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology. 1991;128:3169–3174. doi: 10.1210/endo-128-6-3169. [DOI] [PubMed] [Google Scholar]
- 55.Xiao Q, Boushey RP, Drucker DJ, et al. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999;117:99–105. doi: 10.1016/s0016-5085(99)70555-x. [DOI] [PubMed] [Google Scholar]
- 56.Jeppesen PB, Hartmann B, Thulesen J, et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut. 2000;47:370–376. doi: 10.1136/gut.47.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure [see comments] Gut. 1999;45:559–563. doi: 10.1136/gut.45.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai CH, Hill M, Asa SL, et al. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am. J. Physiol. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 59.Drucker DJ, Erlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wojdemann M, Wettergren A, Hartmann B, et al. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33:828–832. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]
- 61.Wojdemann M, Wettergren A, Hartmann B, et al. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab. 1999;84:2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- 62.Bremholm L, Hornum M, Henriksen BM, et al. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol. 2009;44:314–319. doi: 10.1080/00365520802538195. [DOI] [PubMed] [Google Scholar]
- 63.Hitch MC, Leinicke JA, Wakeman D, et al. Ret heterozygous mice have enhanced intestinal adaptation after massive small bowel resection. Am. J. Physiol Gastrointest. Liver Physiol. 2012;302:G1143–G1150. doi: 10.1152/ajpgi.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tappenden KA, Edelman J, Joelsson B. Teduglutide enhances structural adaptation of the small intestinal mucosa in patients with short bowel syndrome. J Clin Gastroenterol. 2013;47:602–607. doi: 10.1097/MCG.0b013e3182828f57. [DOI] [PubMed] [Google Scholar]
- 65.O'Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11:815–823. e1–3. doi: 10.1016/j.cgh.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 66.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481. e3. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Seidner DL, Schwartz LK, Winkler MF, et al. Increased intestinal absorption in the era of teduglutide and its impact on management strategies in patients with short bowel syndrome-associated intestinal failure. JPEN J Parenter Enteral Nutr. 2013;37:201–211. doi: 10.1177/0148607112472906. [DOI] [PubMed] [Google Scholar]
- 68.Dreux AC, Lamb DJ, Modjtahedi H, et al. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53. doi: 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 69.Yates RA, Nanney LB, Gates RE, et al. Epidermal growth factor and related growth factors. [Review] International Journal of Dermatology. 1991;30:687–694. doi: 10.1111/j.1365-4362.1991.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 70.Chaet MS, Arya G, Ziegler MM, et al. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. Journal of Pediatric Surgery. 1994;29:1035–1039. doi: 10.1016/0022-3468(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 71.Shin CE, Helmrath MA, Falcone RJ, et al. Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route, and timing of administration. Journal of Surgical Research. 1998;77:11–16. doi: 10.1006/jsre.1998.5336. [DOI] [PubMed] [Google Scholar]
- 72.Thompson JS. Epidermal growth factor and the short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 1999;23:S113–S116. doi: 10.1177/014860719902300528. [DOI] [PubMed] [Google Scholar]
- 73.Erwin CR, Helmrath MA, Shin CE, et al. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am. J. Physiol. 1999;277:G533–G540. doi: 10.1152/ajpgi.1999.277.3.G533. [DOI] [PubMed] [Google Scholar]
- 74.Falcone RA, Jr, Stern LE, Kemp CJ, et al. Intestinal adaptation occurs independent of transforming growth factor- alpha. J. Pediatr. Surg. 2000;35:365–370. doi: 10.1016/s0022-3468(00)90042-3. [DOI] [PubMed] [Google Scholar]
- 75.Helmrath MA, Shin CE, Fox JW, et al. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery. 1998;124:848–854. [PubMed] [Google Scholar]
- 76.Helmrath MA, Erwin CR, Warner BW. A defective EGF receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res. 1997;69:76–80. doi: 10.1006/jsre.1997.5033. [DOI] [PubMed] [Google Scholar]
- 77.O'Brien DP, Nelson LA, Williams JL, et al. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J. Surg. Res. 2002;105:25–30. doi: 10.1006/jsre.2002.6440. [DOI] [PubMed] [Google Scholar]
- 78.Sheng G, Bernabe KQ, Guo J, et al. Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology. 2006;131:153–164. doi: 10.1053/j.gastro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Stern LE, Falcone RA, Jr, Kemp CJ, et al. p21 (WAF1/CIP1) is required for the mitogenic response to intestinal resection. J. Surg. Res. 2000;90:45–50. doi: 10.1006/jsre.2000.5834. [DOI] [PubMed] [Google Scholar]
- 80.Mantel C, Luo Z, Canfield J, et al. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 81.Longshore SW, Nair R, Perrone EE, et al. p21(waf1/cip1) deficiency does not perturb the intestinal crypt stem cell population after massive small bowel resection. J. Pediatr. Surg. 2009;44:1065–1071. doi: 10.1016/j.jpedsurg.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leinicke JA, Longshore S, Wakeman D, et al. Regulation of retinoblastoma protein (Rb) by p21 Is critical for adaptation to massive small bowel resection. J Gastrointest Surg. 2012;16:148–155. doi: 10.1007/s11605-011-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo J, Longshore S, Nair R, et al. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J. Biol. Chem. 2009;284:134–140. doi: 10.1074/jbc.M806133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo J, Sheng G, Warner BW. Epidermal growth factor-induced rapid retinoblastoma phosphorylation at Ser780 and Ser795 is mediated by ERK1/2 in small intestine epithelial cells. J. Biol. Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- 85.Choi P, Guo J, Erwin CR, et al. IGF-2 mediates intestinal mucosal hyperplasia in retinoblastoma protein (Rb)-deficient mice. J. Pediatr. Surg. 2013;48:1340–1347. doi: 10.1016/j.jpedsurg.2013.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi PM, Sun RC, Sommovilla J, et al. IGF-2 is necessary for retinoblastoma-mediated enhanced adaptation after small-bowel resection. J Gastrointest Surg. 2014;18:1887–1893. doi: 10.1007/s11605-014-2586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helmrath MA, Erwin CR, Shin CE, et al. Enterocyte apoptosis is increased following small bowel resection. J Gastrointest Surg. 1998;2:44–49. doi: 10.1016/s1091-255x(98)80102-9. [DOI] [PubMed] [Google Scholar]
- 88.Tang Y, Swartz-Basile DA, Swietlicki EA, et al. Bax is required for resection-induced changes in apoptosis, proliferation, and members of the extrinsic cell death pathways. Gastroenterology. 2004;126:220–230. doi: 10.1053/j.gastro.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 89.Thompson JS, Barent B. Effects of Intestinal Resection on Enterocyte Apoptosis. J. Gastrointest. Surg. 1999;3:672–677. doi: 10.1016/s1091-255x(99)80092-4. [DOI] [PubMed] [Google Scholar]
- 90.Welters CF, Piersma FE, Hockenbery DM, et al. The role of apoptosis during intestinal adaptation after small bowel resection [In Process Citation] J. Pediatr. Surg. 2000;35:20–24. doi: 10.1016/s0022-3468(00)80006-8. [DOI] [PubMed] [Google Scholar]
- 91.Stern LE, Falcone RA, Jr, Kemp CJ, et al. Effect of Massive Small Bowel Resection on the Bax/Bcl-w Ratio and Enterocyte Apoptosis. J. Gastrointest. Surg. 2000;4:93–100. doi: 10.1016/s1091-255x(00)80038-4. [DOI] [PubMed] [Google Scholar]
- 92.Bernal NP, Stehr W, Coyle R, et al. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology. 2006;130:412–423. doi: 10.1053/j.gastro.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Stern LE, Huang F, Kemp CJ, et al. Bax is required for increased enterocyte apoptosis after massive small bowel resection. Surgery. 2000;128:165–170. doi: 10.1067/msy.2000.107370. [DOI] [PubMed] [Google Scholar]
- 94.Knott AW, O'Brien DP, Juno RJ, et al. Enterocyte apoptosis after enterectomy in mice is activated independent of the extrinsic death receptor pathway. Am. J. Physiol Gastrointest. Liver Physiol. 2003;285:G404–G413. doi: 10.1152/ajpgi.00096.2003. [DOI] [PubMed] [Google Scholar]
- 95.Bernal NP, Stehr W, Profitt S, et al. Combined pharmacotherapy that increases proliferation and decreases apoptosis optimally enhances intestinal adaptation. J Pediatr Surg. 2006;41:719–724. doi: 10.1016/j.jpedsurg.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Martin CA, Perrone EE, Longshore SW, et al. Intestinal resection induces angiogenesis within adapting intestinal villi. J. Pediatr. Surg. 2009;44:1077–1082. doi: 10.1016/j.jpedsurg.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowland KJ, Yao J, Wang L, et al. Immediate alterations in intestinal oxygen saturation and blood flow after massive small bowel resection as measured by photoacoustic microscopy. J. Pediatr. Surg. 2012;47:1143–1149. doi: 10.1016/j.jpedsurg.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowland KJ, Yao J, Wang L, et al. Up-regulation of hypoxia-inducible factor 1 alpha and hemodynamic responses following massive small bowel resection. J. Pediatr. Surg. 2013;48:1330–1339. doi: 10.1016/j.jpedsurg.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parvadia JK, Keswani SG, Vaikunth S, et al. Role of VEGF in small bowel adaptation after resection: the adaptive response is angiogenesis dependent. Am. J. Physiol Gastrointest. Liver Physiol. 2007;293:G591–G598. doi: 10.1152/ajpgi.00572.2006. [DOI] [PubMed] [Google Scholar]
- 100.McMellen ME, Wakeman D, Erwin CR, et al. Epidermal growth factor receptor signaling modulates chemokine (CXC) ligand 5 expression and is associated with villus angiogenesis after small bowel resection. Surgery. 2010;148:364–370. doi: 10.1016/j.surg.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rowland KJ, Diaz-Miron J, Guo J, et al. CXCL5 is required for angiogenesis, but not structural adaptation after small bowel resection. J Pediatr Surg. 2014;49:976–980. doi: 10.1016/j.jpedsurg.2014.01.034. discussion 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diaz-Miron J, Sun R, Choi P, et al. The effect of impaired angiogenesis on intestinal function following massive small bowel resection. J Pediatr Surg. 2015;50:948–953. doi: 10.1016/j.jpedsurg.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cole CR, Ziegler TR. Small bowel bacterial overgrowth: a negative factor in gut adaptation in pediatric SBS. Curr. Gastroenterol. Rep. 2007;9:456–462. doi: 10.1007/s11894-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 104.Cole CR, Frem JC, Schmotzer B, et al. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J. Pediatr. 2010;156:941–947. 947. doi: 10.1016/j.jpeds.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sommovilla J, Zhou Y, Sun RC, et al. Small Bowel Resection Induces Long-Term Changes in the Enteric Microbiota of Mice. J. Gastrointest. Surg. 2014 doi: 10.1007/s11605-014-2631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Devine AA, Gonzalez A, Speck KE, et al. Impact of ileocecal resection and concomitant antibiotics on the microbiome of the murine jejunum and colon. PLoS. ONE. 2013;8:e73140. doi: 10.1371/journal.pone.0073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lapthorne S, Pereira-Fantini PM, Fouhy F, et al. Gut microbial diversity is reduced and is associated with colonic inflammation in a piglet model of short bowel syndrome. Gut Microbes. 2013;4:212–221. doi: 10.4161/gmic.24372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engstrand Lilja H, Wefer H, Nystrom N, et al. Intestinal dysbiosis in children with short bowel syndrome is associated with impaired outcome. Microbiome. 2015;3:18. doi: 10.1186/s40168-015-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Korpela K, Mutanen A, Salonen A, et al. Intestinal Microbiota Signatures Associated With Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115584388. [DOI] [PubMed] [Google Scholar]
- 110.Mayeur C, Gratadoux JJ, Bridonneau C, et al. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS. ONE. 2013;8:e54335. doi: 10.1371/journal.pone.0054335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davidovics ZH, Vance K, Etienne N, et al. Fecal Transplantation Successfully Treats Recurrent D-Lactic Acidosis in a Child With Short Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115619931. [DOI] [PubMed] [Google Scholar]
- 112.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 113.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J. Pediatr. 2012;161:723–728. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mercer DF, Iverson AK, Culwell KA. Nutrition and small bowel transplantation. Nutr Clin Pract. 2014;29:615–620. doi: 10.1177/0884533614539354. [DOI] [PubMed] [Google Scholar]