Abstract
Reward mediates the acquisition and long-term retention of procedural skills in humans. Yet, learning under rewarded conditions is highly variable across individuals and the mechanisms that determine interindividual variability in rewarded learning are not known. We postulated that baseline functional connectivity in a large-scale frontostriatal-limbic network could predict subsequent interindividual variability in rewarded learning. Resting-state functional MRI was acquired in two groups of subjects (n = 30) who then trained on a visuomotor procedural learning task with or without reward feedback. We then tested whether baseline functional connectivity within the frontostriatal-limbic network predicted memory strength measured immediately, 24 h and 1 month after training in both groups. We found that connectivity in the frontostriatal-limbic network predicted interindividual variability in the rewarded but not in the unrewarded learning group. Prediction was strongest for long-term memory. Similar links between connectivity and reward-based memory were absent in two control networks, a fronto-parieto-temporal language network and the dorsal attention network. The results indicate that baseline functional connectivity within the frontostriatal-limbic network successfully predicts long-term retention of rewarded learning.
Keywords: reward learning, brain connectivity, learning, memory, resting state functional magnetic resonance imaging, ventral striatum
INTRODUCTION
Humans vary in their capacity to acquire and retain memories [Forstmann et al., 2010; Zimerman et al., 2013], and one fundamental determinant for this variability is the degree to which reward facilitates learning [Buitrago et al., 2004; Ericsson et al., 1993; Forstmann et al., 2010; Zimerman et al., 2013]. Previous studies focused on the contribution of specific brain regions to reward-based memory including the ventral striatum [Wächter et al., 2009], midbrain, and hippocampus [Adcock et al., 2006; Kuhl et al., 2010; Wittmann et al., 2005]. However, reward-related brain activity is present beyond this circuitry and throughout the human brain [Vickery et al., 2011], mainly in structures along the mesocortical and mesolimbic dopaminergic pathways [Liu et al., 2011; Sescousse et al., 2013].
While there has been increasing interest in the role of reward in procedural and declarative learning [Adcock et al., 2006; McGinty et al., 2013; Wittmann et al., 2005], a systems-level mechanistic understanding of the way by which reward modulates learning across individuals remains missing. This gap in knowledge is of translational relevance as deficits in rewarded learning have been well documented in neuropsychiatric disorders such as depression [Kumar et al., 2008; Vrieze et al., 2012], schizophrenia [Gold et al., 2008; Strauss et al., 2011] and addiction [Hyman et al., 2006; Volkow et al., 2009] and in neurological conditions such as Parkinson’s disease [Bodi et al., 2009; Peterson et al., 2009] and Stroke [Rochatet al., 2013].
To address this question, we assessed functional interactions within a large-scale network of brain areas implicated in reward processing using resting-state functional magnetic resonance imaging (fMRI) [Biswal et al., 1995; Friston et al., 1994]. Resting-state fMRI provides valuable information on intrinsic interactions among local and distributed brain networks [Albert et al., 2009; Birn, 2007; Vahdat et al., 2011; van den Heuvel and Hulshoff Pol, 2010; Van Dijk et al., 2010]. We, thus, evaluated the extent to which baseline functional connectivity in a frontostriatal-limbic functional network [Di Martino et al., 2008] predicted memory in two groups of healthy subjects (n = 30) half of whom then trained on a visuomotor procedural learning task with and the other half without reward feedback. Since the neural substrates of immediate and more long-term procedural memory differ [Dayan and Cohen, 2011; Doyon and Benali, 2005], and these forms of memory appear to be differentially affected by reward [Abe et al., 2011; Dayan and Cohen, 2011; Wächter et al., 2009] links between baseline network connectivity and memory measured at different time intervals after training were examined.
MATERIALS AND METHODS
Participants
Thirty-Six right-handed, healthy volunteers (20 males; mean age 24.3 ± 3.1 years) gave written informed consent approved by the Combined Neuroscience Institutional Review Board, National Institutes of Health. Inclusion criteria were normal physical and neurological examinations, no MRI contraindications and no use of psychoactive medication. We also required a minimum of 6 h sleep prior to every testing session. Data from six subjects (four from the rewarded and two from the unrewarded group, see below) were later excluded due to excessive head movements with predefined criteria of translation or rotation movements above 1.5 mm during fMRI acquisition. Therefore, our final pool of participants included 30 volunteers (16 males, mean age 24.2 ± 2.7 years).
Procedural Learning Task
Subjects were assigned to one of two groups: a rewarded (15 subjects; eight males, mean age 24 ± 2.4 years) or an unrewarded (15 subjects; eight males; mean age 24.5 ± 2.9 years) group, both of whom trained on a modified version of a previously published visuomotor learning task used for studying procedural learning and memory [Dayan et al., 2014; Reis et al., 2009; Schambra et al., 2011]. Sitting in front of a laptop computer (Dell, Latitude E5510, screen size 15.6″) placed at comfortable viewing height and distance, subjects were asked to move a cursor through a numbered sequence of five targets (four gates and one thick line, Fig. 1A) horizontally arranged on the screen. Movement of the cursor was controlled by pinching a force-transducer with the distal phalanx of the thumb and the proximal interphalangeal joint of the index of the right dominant hand (Fig. 1B). When pressure was applied to the transducer, the cursor moved to the right, and by releasing pressure, the cursor moved back to the left. Subjects had to accurately move the cursor through the following sequence: Target 1, return, Target 2, return, Target 3, return, Target 4, return, and Target 5. In both groups, auditory feedback (a “beep” sound) was given whenever a target was reached successfully, that is, the cursor was moved in between the lines of a gate (or on the line of Target 5) for a minimum of 0.2 sec. A trial was successful if it was completed within 8 sec without overshooting beyond the boundaries of any of the targets. A GO-Signal, which was displayed as a bright green circle below the target zones, indicated the beginning of each trial. The GO-signal was switched off when a trial was completed or the subject overshot the target boundaries. Disappearance of the Go-signal signaled the subject to wait for the next trial. The sequence of targets was the same for all subjects and did not change over the course of the experiment. Subjects were instructed to be as accurate as possible while completing the sequence within the allocated time (8 sec). During training, the rewarded group received visual feedback, indicating: “You Win: $0.6,” after the completion of each successful trial. A “Total Winnings” display was shown beneath this section, accumulating winnings throughout the entire training session. If a trial was unsuccessful, subjects received no reward feedback (“You Win: $0”). Thus, in our training paradigm accuracy was rewarded, rather than a combination of accuracy and speed [Dayan et al., 2014; Reis et al., 2009; Schambra et al., 2011]. As a control, the unrewarded group trained on the same task in the absence of reward feedback at the end of each trial. Both groups received identical real time visual depiction of cursor movements and heard auditory tones indicating a successfully reached target and a completed trial. Unbeknownst to the subjects, both groups ultimately received an identical compensation for their participation in the study after the third session, when the experiment was completed.
Figure 1.
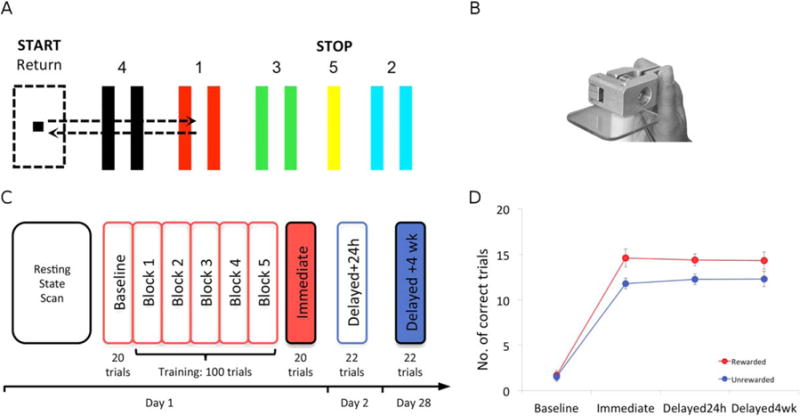
Behavioral training on a visuomotor task. A: Subjects had to move a cursor through a sequence of five targets horizontally arranged on a computer screen (1-Return, 2-Return, 3-Return, 4-Return, 5). B: Pinching a force-transducer with the right hand moved the cursor to the right (i.e., toward a target) whereas releasing pressure moved it back to the left (toward the start position). C: Experimental setup of the 3-session experiment. Day one began with an 8 min resting state scan. Subjects then trained on the visuomotor task outside of the scanner for 30 min. Tests of procedural memory were administered immediately (Immediate), one day (Delayed24h) and 4 weeks (Delayed4wk) after training. D: While at baseline performance between these two groups was comparable, differences between the groups emerged after training at the Immediate, Delayed24h and Delayed4wk tests.
Experimental Setup
Session 1 began with an 8-min resting-state fMRI scan (Fig. 1C). Subjects were instructed to lay still inside the scanner, close their eyes, and avoid falling asleep. Foam inserts were used to limit head movements. After scanning, baseline performance was measured with 20 trials of the visuomotor learning task. Subsequently, subjects trained on the visuomotor learning task (5 blocks of 20 trials each, approximately lasting 30 min) with or without reward feedback. After training, memory was measured [Dayan and Cohen, 2011] immediately after (Immediate), the following day (Delayed24h, ± 110 min) and a month later (Delayed4wk, ± 0.6 days). Testing on the Delayed24h and Delayed4wk consisted of 22 trials, where the first two trials were discarded to exclude the warm-up effect [Ajemian et al., 2010; Schmidt and Lee, 2011].
Baseline and test measurements (Immediate, Delayed24h, Delayed4wk) were administered in the absence of reward feedback in both groups. Two subjects in the unrewarded group dropped out prior to the third testing session and, thus, data in the Delayed4wk was obtained from n = 13 in this group.
Imaging Setup
Scanning was performed on a 3.0-T GE Signa HDx scanner using an 8-channel coil. T2*-weighted images were acquired using a gradient echo echoplanar imaging sequence (201 volumes; 38 ascending axial slices, 4 mm thickness, 2.400 ms repetition time, 35 ms echo time, 90° flip angle, 24 × 24 cm2 field of view, 64 × 64 matrix size). In addition, high-resolution 3D magnetization prepared rapid gradient echo images (1 × 1 × 1 mm) were acquired from each subject to allow for volume-based statistical analysis and for visualization purposes.
fMRI Preprocessing
Data were preprocessed using Brainvoyager QX. Anatomical images were aligned to an ACPC plane and then transformed to Talairach space [Talairach and Tournoux, 1988]. The first two functional volumes were discarded to insure the experimental data were acquired after the scanner reached steady-state magnetization. The remaining 199 volumes were used for analysis. Preprocessing of functional data included slice scan time correction, head motion correction, spatial smoothing with a Gaussian filter of 4 mm full width half maximum (FWHM) and temporal filtering using low-pass frequency filter of 0.01 Hz, and high-pass Gaussian-FWHM filter of 4 sec, resulting in frequencies between 0.01 and 0.1 Hz and reducing low-frequency drifts and high-frequency noise [Meindl et al., 2010; Wang et al., 2006]. Each subject’s functional images were then coregistered to the corresponding 3-D anatomical image to produce a 4-D volume time course. Every coregistration was checked manually and adjusted if needed.
Network Identification
Seed-based functional connectivity analysis was performed with the data from all 30 subjects (i.e., collapsed across groups) to identify a network of areas showing functional connectivity with the right nucleus accumbens (NAcc), a subset of the ventral striatum crucial in reward processing. Further analysis compared the results with those obtained with a left NAcc seed. Because of its small size and variable localization, NAcc was identified anatomically for every subject individually using a published procedure based on anatomical landmarks [Neto et al., 2008]. A spherical volume of interest (VOI; 123 voxels, voxel size 1 × 1 × 1 mm) was placed within each subject’s localized NAcc. The average Talairach coordinates of the NAcc VOIs across the 30 subjects were 8/7/−4 (± 0.86/1.1/0.95), which is in agreement with a recent meta-analysis of reward-processing studies and with a previous study of ventral striatum functional connectivity [Di Martino et al., 2008; Liu et al., 2011]. Average time series were then extracted from each NAcc VOI and served as predictors in a multisubject general linear model. Heart rate and respiration data from every subject were used as nuisance covariates to account for the influence of these slowly fluctuating signals on the BOLD contrast. Statistical activation maps were corrected for multiple comparisons using a false discovery rate (FDR) correction at P < 0.01. VOIs with identical shape and size (spherical, 512 voxels, voxel size 1 × 1 × 1 mm) were placed at the center of each cluster of activity, of activity that showed connectivity with the NAcc. Beta-weights from these VOIs were then extracted and averaged to compute a subject specific index of network connectivity.
Two control networks, the dorsal attention network [Buckner et al., 2013; Fox et al., 2006; Thomas Yeo et al., 2011] and a fronto-parieto-temporal language network [Tomasi and Volkow, 2012] were defined following the same procedure as described earlier but using the right intraparietal sulcus (rIPS) and left inferior frontal gyrus (lIFG), respectively, as seeds. Seed coordinates were identical in each subject, and were 27/−58/49 for rIPS [Fox et al., 2006] and −49/25/18 for lIFG [Tomasi and Volkow, 2012]. In the dorsal attention network, where voxels showed highly significant correlation with the rIPS seed, a higher minimal statistical threshold was used (t = 12, compared to t = 7) to allow for the identification of distinct nodes within the network. These networks were chosen because we expected them to have little overlap with the reward circuits in the frontostriatal-limbic pathway. Single region beta-weights were extracted and connectivity indices were again computed for each subject by averaging beta-weights across all nodes within each of the networks.
Data Analysis
Accuracy (number of correct trials in a block) served as the behavioral outcome measure. Group performance was analyzed using a two-way repeated-measures analysis of variance, where “Group” (rewarded, unrewarded) served as the between-subjects factor and “Testing Session” (all testing sessions) as the within-subject factor. Pearson’s correlations were used to examine links between memory scores and network connectivity within subjects. Between-group comparisons of correlations between network connectivity and memory were performed with a Fisher r-to-z transformation [Fisher, 1915]. Statistical analyses were performed with SPSS and MATLAB. Robust correlation tests were performed using the “Robust Correlation Toolbox” in MATLAB [Pernet et al., 2012]. Significance levels for all statistical tests were set at P < 0.05 and were corrected for multiple comparisons.
RESULTS
Baseline performance was comparable in the rewarded and unrewarded groups (1.7 ± 0.5 and 1.5 ± 0.5 correct trials, respectively). There was a significant effect of Group (F1,26=4.28, P < 0.05, Fig. 1D) in the absence of a Group × Testing session interaction (F3,78 = 1.48, ns). Thus, both groups’ performance in the task improved immediately post training (P < 0.01) reaching 14.6 ±1 and 11.8 ± 0.6 correct trials in the rewarded and unrewarded groups, respectively. Memory then remained relatively stable at 24 h (14.4 ± 0.7 and 12.3 ± 0.6 correct trials in the rewarded and unrewarded groups, respectively) and 4 weeks (14.3 ± 1 and 12.3 ± 0.8 correct trials, respectively) testing times.
We then evaluated the relationship between baseline connectivity and memory. A seed-based functional connectivity analysis focusing on the brain regions showing functional connectivity with the right NAcc [Cauda et al., 2011; Di Martino et al., 2008] identified a frontal-cingulate-striato-limbic network (Fig. 2A and Table I, Methods). Further analysis averaged the beta-weights (regression coefficients) across all nodes in the network allowing computation of an index of network connectivity for each individual subject. Average connectivity in this network was comparable across the rewarded and unrewarded groups (t28 = 1.88, ns).
Figure 2.
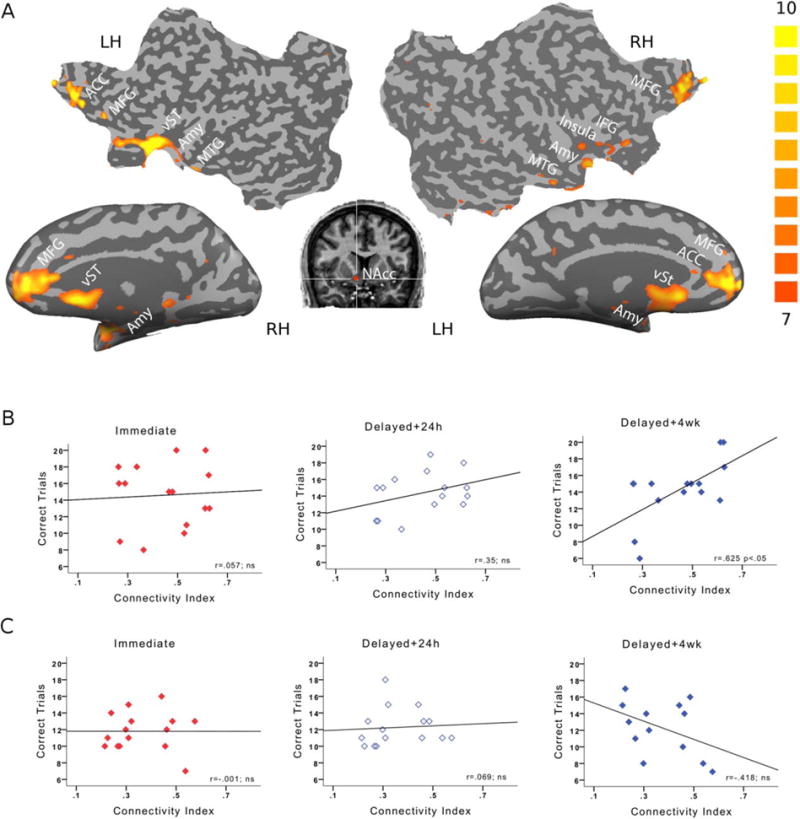
Frontostriatal-limbic network. A: Seed-based connectivity analysis with NAcc revealed a network composed of frontal, striatal, and limbic regions, visualized here on inflated and flattened brains (FDR-corrected at P < 0.01). B: Correlations of average network connectivity with memory at Immediate, Delayed24h and Delayed4wk testing points in the rewarded group. Only long-term memory (Delayed4wk) showed significant correlations with average network connectivity. C: Correlations of average network connectivity with memory in the unrewarded group. No significant correlations were found.
TABLE I.
Components of the frontostriatal-limbic network
| l/r | Region | BA | X | Y | Z |
|---|---|---|---|---|---|
| r | Middle Temporal Gyrus | 21 | 49 | 5 | −24 |
| r | Parahippocampal Gyrus | 21 | 40 | 7 | −32 |
| r | Insula | 36 | 7 | −6 | |
| r | Amygdala (ventral) | 33 | −2 | −21 | |
| r | Uncus | 20 | 31 | −4 | −34 |
| r | Cingulate Gyrus | 32 | 3 | 22 | 28 |
| r | Amygdala (dorsal) | 26 | −5 | −12 | |
| r | Medial Frontal Gyrus | 10 | 13 | 52 | 15 |
| r | Medial Pallidum | 10 | −2 | −10 | |
| r | Thalamus | 7 | −30 | −4 | |
| r | Midbrain | 4 | −11 | −11 | |
| r | Cerebellum (Culmen) | 5 | −54 | −15 | |
| l | Anterior Cingulate | 24 | −2 | 24 | 1 |
| l | Cerebellum (Culmen) | 1 | −55 | 5 | |
| l | Medial Frontal Gyrus | 9 | −5 | 47 | 23 |
| l | Medial Pallidum | −17 | −15 | −6 | |
| l | Amygdala | −34 | −4 | −12 | |
| l | Middle Temporal Gyrus | 21 | −36 | −4 | −24 |
Talairach coordinates of the center of each cluster within the frontostriatal-limbic network. Every volume of interest was of identical shape and size (spherical; 512 voxels).
In the rewarded group, network connectivity correlated with memory at Delayed4wk (r = 0.625, P < 0.01, Bonferroni corrected for multiple comparisons; Fig. 2B; see also Supporting Information Fig. 1) but not at the Immediate or Delayed24h testing times (r = 0.057; ns and r = 0.35; ns, respectively; Fig. 2B). Significant correlations at Delayed4wk were not restricted to the network connectivity index but were also evident in pairwise correlations with multiple single regions within the network (Supporting Information Fig. 2A). In the unrewarded group, network connectivity did not correlate with memory at any testing time (Immediate: r = −0.001; ns, Delayed24h: r = 0.069; ns, Delayed4wk: r = −0.418; ns, Fig. 2C and Supporting Information Fig. 2B). Correlations between baseline connectivity and memory were significantly different between groups at Delayed4wk (z = 2.75; P < 0.01) but not at the Immediate and Delayed24h tests (z = 0.14, ns and z = 0.73, ns, respectively).
Additional analyses were carried out to confirm the reliability of the significant correlation between network connectivity and learning scores at Delayed4wk in the rewarded group. First, the correlation between network connectivity and scores at Delayed4wk was preserved after a leave-one-out cross validation procedure (with r-values ranging from 0.563 to 0.694, all significant at P < 0.02). We next tested the correlation using two robust correlation procedures, where outliers are down weighted or removed and accounted for in significance testing [Pernet et al., 2012]. The first procedure, the percentage-bend correlation [Wilcox, 1994], is robust against the influence of marginal outliers and estimates linear associations without computing a Pearson’s correlation coefficient [Pernet et al., 2012]. The second procedure, skipped-correlations [Wilcox, 2004], directly reflects Pearson’s r and protects against the influence of bivariate outliers. The correlation between network connectivity and scores at Delayed4wk in the rewarded group was significant using both techniques (Bend correlation: r = 0.63, P < 0.02; skipped correlation: r = 0.62, P < 0.05).
The literature points to the involvement of both right and left NAcc in reward processing [Liu et al., 2011], with some evidence for right lateralization [Cauda et al., 2011; Di Martino et al., 2008; Knutson et al., 2001]. While, for brevity, our focus here was on right NAcc, comparable results were obtained also when the network was defined using a left NAcc seed (Supporting Information Fig. 3). A significant correlation was found between network connectivity defined with left NAcc and learning scores at Delayed4wk for the rewarded (r = 0.552, P < 0.02) but not for the unrewarded group (r = −0.425; ns). These correlations were significantly different (z = 2.51, P < 0.02). Moreover, average network connectivity between the networks based on right and left NAcc did not differ (t = 1.675, P = 0.105) and showed a strong correlation across subjects (r = 0.678; P < 0.01).
To evaluate the topographic specificity of the findings, we examined two control networks. The first network, referred to as the language network [Tomasi and Volkow, 2012], is composed of frontal parietal and temporal regions, all showing functional connectivity with left IFG (FDR-corrected at P < 0.01, Fig. 3A and Supporting Information Table 1). The second control network, known as the dorsal attention network [Buckner et al., 2013; Fox et al., 2006] is primarily composed of frontal and parietal regions, that show functional connectivity with the right IPS (FDR-corrected at P < 0.01, Fig. 3B and Supporting Information Table 2). Baseline connectivity within neither the language network (Fig. 3C), nor the dorsal attention network (Fig. 3D) correlated with any of the memory measures in the rewarded group (all P values > 0.091). Similarly, connectivity in neither of the networks correlated significantly with any of the memory measures in the unrewarded group (all P values > 0.089; see Supporting Information Fig. 4A,B). Consistently, no significant differences between the correlations obtained for the rewarded and unrewarded groups were found (all z tests < 1.2, all ns).
Figure 3.
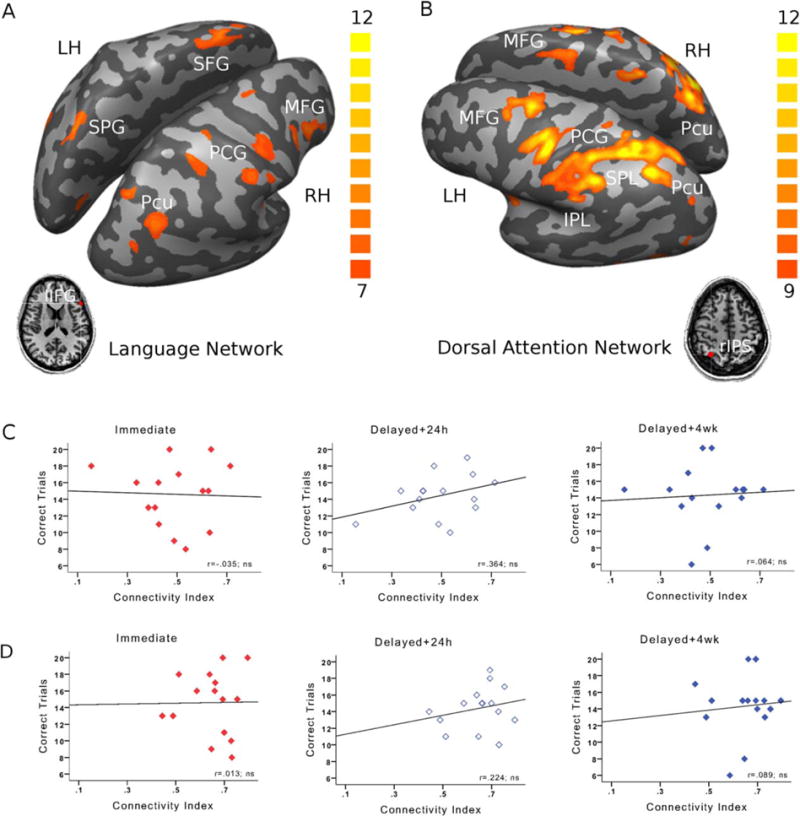
Specificity of the memory-connectivity links to the frontostriatal-limbic network. A: The fronto-parieto-temporal language network, composed of regions showing functional connectivity with left IFG (FDR-corrected at P < 0.01) was used as a first control network. B: The dorsal attention network, composed of regions showing functional connectivity with right IPS (FDR-corrected at P < 0.01) was used as a second control network. C: In the rewarded group, no significant correlations were obtained between any of the memory measures and connectivity in the fronto-parieto-temporal language network or D: the dorsal attention network. Similar results were obtained for the unrewarded group (Supporting Information Fig. 4 A,B).
DISCUSSION
The results demonstrate that baseline functional connectivity within a frontostriatal-limbic network predicts the formation of long-term procedural memory acquired with rewarded feedback. The specificity of the frontostriatal-limbic network for predicting reward-based long-term memory was established by training a second group of subjects who performed the same learning task in the absence of reward feedback. There were significantly weaker correlations between network connectivity and procedural memory in this group. Similar links between connectivity and reward-based memory were absent in two control networks, confirming the topographic specificity of the findings.
The frontostriatal-limbic network studied here is composed of regions implicated in reward–related computations. First, the NAcc, part of the ventral striatum, is engaged in reward anticipation [Knutson et al., 2001; Schott et al., 2008], encoding of reward prediction errors [Abler et al., 2006; Rodriguez et al., 2006] and evaluation of reward [Liu et al., 2007]. In the context of reward-based learning, it has been proposed that the striatum integrates dopaminergic reward signals with sensory cues via corticostriatal and thalamo-striatal afferents to modulate the generation of motor commands [Hosp et al., 2011; Wickens et al., 2003]. This effect is mediated by direct and indirect output pathways to the motor cortex (M1) [Wickens et al., 2003], including a projection from the ventral tegmental area (VTA) to M1 [Hosp et al., 2011]. Moreover, it was recently reported that activity within a local neuronal network in the rat’s NAcc is strongly predictive of performance of a locomotor task [McGinty et al., 2013]. Other regions within the frontostriatal-limbic network have also been implicated in reward-related functions. Activity in ventromedial prefrontal cortex (vmPFC), supports the formation of memory under predictable reward [Bialleck et al., 2011]. The orbitofrontal cortex (OFC) is engaged in encoding of reward value [Elliott et al., 2003] and in processing motivational relevant variables [Elliott et al., 2000; Fellows and Farah, 2005]. The anterior cingulate cortex is essential for integrating reward and motor responses [Williams et al., 2004], while other structures including the thalamus, amygdala, and vmPFC interact during associative memory encoding acquired under reward [Gaffan and Murray, 1990]. Additionally, medial temporal lobe interactions with the prefrontal cortex (PFC) are required for transitioning recently acquired memories into long-term memories [Frankland and Bontempi, 2005; Simons and Spiers, 2003]. Similarly, the amygdala is not only activated by rewarding stimuli [Canli et al., 2002] but also couples with the striatum during learning [Popescu et al., 2009] and interacts with the medial temporal lobe to coordinate memory formation [Dolcos et al., 2004]. Anatomically, direct projections from VTA to the hippocampus contribute to reward-based formation of adaptive memory, as do disinhibitory projections from NAcc and globus pallidum and excitatory projections from the PFC to VTA [Shohamy and Adcock, 2010]. The amygdala, PFC and NAcc also receive direct dopaminergic projections from VTA sending disinhibiting projections back to VTA [Düzel et al., 2009]. Altogether, these findings support our current focus on the frontostriatal-limbic network and fit in well with its role in predicting interindividual variability in rewarded learning.
Interestingly, connectivity in the frontostriatal-limbic network predicted procedural memory 4 weeks after but not immediately post-training. These preliminary findings raise the hypothesis that this network may play a more prominent role in consolidation of rewarded learning over time, taking place after the end of training [Dayan and Cohen, 2011]. Consistent with our findings, reward has been shown to specifically facilitate long-term procedural memory [Abe et al., 2011; Dayan et al., 2014], plausibly through engaging similar circuitries as those included in the frontostriatal-limbic network. While our results cannot specifically provide evidence as to whether different networks are involved in the formation of memory immediately, one day or several weeks after training, the strengthening of correlations over time imply that the same network could be engaged in memory formation, across all temporal windows, dynamically reshaping through systems consolidation, a process known to last between weeks to months [Dudai, 2004]. Additional work is needed to confirm this suggestion.
The results are consistent with the view that reward processing is distributed throughout the human brain [Liu et al., 2011; Vickery et al., 2011]. Integrative processing within this large-scale network is likely rooted in anatomical connections between core network nodes [Haber and Knutson, 2010]. For instance, projections from limbic areas [Friedman et al., 2002; Fudge et al., 2002] and the OFC reach the ventral striatum, and similarly from the ventral striatum to pallidum and midbrain [Haber et al., 1995]. Human studies demonstrated diffusion MRI-based structural connectivity between the striatum, ventromedial frontal cortex and limbic regions such as the uncus and the amygdala [Johansen-Berg et al., 2008; Lehéricy et al., 2004]. Links between functional connectivity as detected at rest and structural network architecture have been widely reported [Greicius et al., 2009; Tomassini et al., 2011], and may potentially provide the neuroanatomical basis for the results reported here. Namely, stronger functional connectivity within the frontostriatal-limbic network may facilitate long-term memory retention by maximizing the efficiency of information processing in both local and distant network interactions [Sepulcre et al., 2010], conceivably through an underlying variation in structural connectivity [Fields, 2011].
Procedural learning is crucial for rehabilitation after focal brain lesions such as stroke [Dimyan and Cohen, 2011]. Stroke often severely disrupts motor function, requiring patients to relearn basic procedural skills. Various rehabilitative interventions for stroke utilize reinforcement schemes [Krakauer, 2006; Pulvermüller et al., 2001], and reward can modulate specific functional abnormalities after stroke [Malhotra et al., 2013]. However, stroke patients, particularly those with lesions in the basal ganglia, thalamus, insula, and PFC show deficits in their sensitivity to reward [Rochat et al., 2013], necessitating tools that could potentially differentiate among patients who are more and less likely to respond to reward. Thus, it is conceivable that functional connectivity within frontostriatal-limbic circuits may help in predicting interindividual variability in the success of reinforcement-based interventions after brain lesions.
CONCLUSIONS
In summary, we report that baseline task-free functional connectivity in a frontostriatal-limbic network predicts formation of long-term memories acquired under rewarded training. Baseline variation in intrinsic task-free brain connectivity may thus underlie the interindividual differences that characterize reward learning.
Supplementary Material
Acknowledgments
We would like to thank G. Dold and G. Melvin for their help with setting up the experiment, A. Harris for help with data coding and N. Censor, SL Liew, and J. Gonzalez-Castillo for helpful comments.
Contract grant sponsor(s): Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health and by the Mentoring Program for Excellent Students of the University Medical Center Hamburg-Eppendorf (to JMH); Scholarship from Claussen-Simon Stiftung (to JMH).
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: The authors declare no competing financial interests.
References
- Abe M, Schambra H, Wassermann EM, Luckenbaugh D, Schweighofer N, Cohen LG. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol. 2011;21:557–562. doi: 10.1016/j.cub.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Ajemian R, D’Ausilio A, Moorman H, Bizzi E. Why professional athletes need a prolonged period of warm-up and other peculiarities of human motor learning. J Mot Behav. 2010;42:381–388. doi: 10.1080/00222895.2010.528262. [DOI] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialleck KA, Schaal H-P, Kranz TA, Fell J, Elger CE, Axmacher N. Ventromedial prefrontal cortex activation is associated with memory formation for predictable rewards. PLoS ONE. 2011;6:e16695. doi: 10.1371/journal.pone.0016695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM. The behavioral significance of spontaneous fluctuations in brain activity. Neuron. 2007;56:8–9. doi: 10.1016/j.neuron.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibo G, Takats A, Bereczki D, Gluck MA. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav Brain Res. 2004;155:249–256. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Averbeck BB, Richmond BJ, Cohen LG. Stochastic reinforcement benefits skill acquisition. Learn Mem. 2014;21:140–142. doi: 10.1101/lm.032417.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JFW. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100:363–406. [Google Scholar]
- Fellows LK, Farah MJ. Dissociable elements of human foresight: A role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005;43:1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Fields RD. Imaging learning: the search for a memory trace. Neuroscientist. 2011;17:185–196. doi: 10.1177/1073858410383696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers E-J, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA. 2010;107:15916–15920. doi: 10.1073/pnas.1004932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: Combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. J Neurosci. 1990;10:3479–3493. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: A deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele P-F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim D-S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra PA, Soto D, Li K, Russell C. Reward modulates spatial neglect. J Neurol Neurosurg Psychiatr. 2013;84:366–369. doi: 10.1136/jnnp-2012-303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–922. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl T, Teipel S, Elmouden R, Mueller S, Koch W, Dietrich O, Coates U, Reiser M, Glaser C. Test-retest reproducibility of the default-mode network in healthy individuals. Hum Brain Mapp. 2010;31:237–246. doi: 10.1002/hbm.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 2008;11:13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Wilcox R, Rousselet GA. Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Front Psychol. 2012;3:606. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, Elliott C, Song DD, Makeig S, Sejnowski TJ, Poizner H. Probabilistic reversal learning is impaired in Parkinson’s disease. Neuroscience. 2009;163:1092–1101. doi: 10.1016/j.neuroscience.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12:801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub E. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat L, Van der Linden M, Renaud O, Epiney J-B, Michel P, Sztajzel R, Spierer L, Annoni J-M. Poor reward sensitivity and apathy after stroke: implication of basal ganglia. Neurology. 2013;81:1674–1680. doi: 10.1212/01.wnl.0000435290.49598.1d. [DOI] [PubMed] [Google Scholar]
- Rodriguez PF, Aron AR, Poldrack RA. Ventral–striatal/nucleus–accumbens sensitivity to prediction errors during classification learning. Hum Brain Mapp. 2006;27:306–313. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra HM, Abe M, Luckenbaugh DA, Reis J, Krakauer JW, Cohen LG. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol. 2011;106:652–661. doi: 10.1152/jn.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning. 5th. Human Kinetics Publishers; Champaign, IL: 2011. [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze H-J, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BTT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol. 2010;6:e1000808. doi: 10.1371/journal.pcbi.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci (Regul Ed) 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci. 2011;31:16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery TJ, Chun MM, Lee D. Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron. 2011;72:166–177. doi: 10.1016/j.neuron.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T. Reduced Reward Learning Predicts Outcome in Major Depressive Disorder. Biol Psychiatry. 2012;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächter T, Lungu OV, Liu T, Willingham DT, Ashe J. Differential effect of reward and punishment on procedural learning. J Neurosci. 2009;29:436–443. doi: 10.1523/JNEUROSCI.4132-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wilcox R. Inferences Based on a Skipped Correlation Coefficient. J Appl Statistics. 2004;31:131–143. [Google Scholar]
- Wilcox RR. The percentage bend correlation coefficient. Psychometrika. 1994;59:601–616. [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73:10–15. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


