Abstract
Background
Intimate partner violence (IPV) is associated with higher HIV incidence, reduced condom use, and poor adherence to antiretroviral therapy and other medications. IPV may also affect adherence to pre-exposure prophylaxis (PrEP).
Methods
We analyzed data from 1785 HIV-uninfected women enrolled in a clinical trial of PrEP among African HIV-serodiscordant couples. Experience of verbal, physical, or economic IPV was assessed at monthly visits by face-to-face interviews. Low PrEP adherence was defined as clinic-based pill count coverage <80% or plasma tenofovir levels <40 ng/mL. The association between IPV and low adherence was analyzed using generalized estimating equations, adjusting for potential confounders. In-depth interview transcripts were examined to explain how IPV could impact adherence.
Results
16% of women reported IPV during a median of 34.8 months of follow-up (IQR 27.0 - 35.0). Overall, 7% of visits had pill count coverage <80% and 32% had plasma tenofovir <40 ng/mL. Women reporting IPV in the past 3 months had increased risk of low adherence by pill count (adjusted RR 1.49, 95% CI 1.17-1.89) and by plasma tenofovir (adjusted RR 1.51, 95% CI 1.06-2.15). Verbal, economic, and physical IPV were all associated with low adherence. However, the impact of IPV diminished and was not statistically significant 3 months after the reported exposure. In qualitative interviews, women identified several ways in which IPV affected adherence, including stress and forgetting, leaving home without pills, and partners throwing pills away.
Conclusion
Women who reported recent IPV in the Partners PrEP Study were at increased risk of low PrEP adherence. Strategies to mitigate PrEP non-adherence in the context of IPV should be evaluated.
Keywords: Intimate partner violence, pre-exposure prophylaxis, adherence, HIV prevention
Introduction
Randomized trials have demonstrated that oral antiretroviral pre-exposure prophylaxis (PrEP) is effective for HIV prevention in several populations, including heterosexual men and women1,2, men who have sex with men3–5, and injection drug users.6 Based on these data, the World Health Organization (WHO) recommends PrEP as part of a comprehensive HIV prevention package for people at substantial risk of HIV infection.7 Several PrEP demonstration projects are testing strategies to maximize population impact and cost-effectiveness.8,9
One population eligible for PrEP targeting is sexually active women in sub-Saharan Africa.10 In this region, women have considerably higher incidence of HIV than men, particularly at young ages.11 Intimate partner violence (IPV) is also common, with lifetime prevalence estimates ranging from 36-71%.12 IPV is associated with an increased risk of HIV infection,13–20 with two prospective studies showing that HIV incidence is approximately 50% higher among women who have experienced IPV than women with no IPV history.21,22 In the study by Kouyoumdjian et al., the effect size was similar for physical, sexual, and verbal IPV; increased with IPV frequency and severity; and persisted for more than one year after the last violent episode.22 In the context of violent relationships, individual-level biomedical interventions such as PrEP may be more effective for HIV prevention than behavioral interventions requiring cooperation of both partners.22,23 However, for PrEP to prevent HIV infection, consistently high adherence is necessary during periods of potential exposure.24–26 Women who experience IPV have lower adherence to several medication regimens, including antiretroviral therapy for HIV treatment and methadone treatment for drug addiction 27–30; IPV may also be a barrier to PrEP adherence.31
With programs or demonstration projects beginning to offer PrEP to women, including women who experience IPV, it is important to understand whether adherence levels will be high enough for PrEP to be effective. If IPV is associated with low PrEP adherence, additional, targeted adherence support may be required for IPV survivors. We conducted a prospective cohort study to examine whether recent and/or past exposure to IPV is associated with low PrEP adherence among HIV-uninfected women participating in a clinical trial of PrEP.
Methods
Study Population
The population for this analysis was all HIV-uninfected women participating in the Partners PrEP Study, a phase 3, randomized, double-blind, placebo-controlled clinical trial that demonstrated the efficacy of daily oral PrEP among HIV-uninfected members of HIV serodiscordant couples. The design, procedures, and outcomes of the trial are described elsewhere.1,32 Briefly, from 2008-2012, 4747 couples were randomized and followed at 9 research sites in Kenya and Uganda. HIV-uninfected partners were randomly assigned to once-daily tenofovir disoproxil fumarate (TDF), emtricitabine (FTC)/TDF, or placebo, and followed monthly for 12-48 months. All couples received a package of HIV prevention services, including risk-reduction counseling, couples' counseling, and condoms. The study protocol was approved by the University of Washington Human Subjects Review Committee and ethics review committees at each of the study sites. All participants provided written informed consent in English or their local language.
Data Collection
Experience of IPV was assessed monthly by asking whether the participant had been verbally, physically, or economically abused by her partner since the last study visit. Participants were asked in the context of a risk-reduction counseling session, in local languages, and in a manner considered culturally appropriate for each study site.33 Although the wording of the question varied and was context specific, all interviewers were experienced in couples' counseling and were trained through multiple role-plays to assess and document IPV on case report forms according to standard protocols. If the participant reported IPV, the type (verbal, physical, or economic), frequency, and consequences (e.g., relationship breakup, income loss) were documented on a structured questionnaire.
PrEP adherence was measured by clinic-based pill counts and plasma tenofovir concentrations. For all HIV-uninfected participants, pill counts were conducted on returned, unused medication tablets each month at the study clinic. Plasma samples were collected and stored at visit months 1, 3, and quarterly thereafter, plus at any visit where a participant tested positive for HIV. Plasma tenofovir concentrations were measured in a subset of participants only, using ultra-performance liquid chromatography-mass spectrometry assay methods.34 Of the 1297 plasma tenofovir measurements, 606 (47%) were from 113 randomly sampled women, and 691 (53%) were from 302 women purposively selected for other secondary analyses.32,34–37
Demographic characteristics were collected separately from the participant and her partner at enrollment, including age, income, education, weekly alcohol intake, marital status, relationship duration, and how long the couple had known they were serodiscordant. Data on sexual behavior, including coital frequency, condom use, and outside partnerships, were collected by interviewer-administered questionnaires at monthly intervals for the participant and quarterly intervals for her partner. We relied on participant reports for dyad-level data such as relationship duration and coital frequency, and on partner reports for his individual-level data, including demographic characteristics and outside partnerships. Monthly HIV testing and annual STI testing were conducted using methods described previously.38
Data Analysis
At each study visit, women were categorized as having no IPV reported to date in the study, IPV reported in the past 3 months, or IPV reported in the study and >3 months ago. This approach enabled us to distinguish between an acute effect of recent IPV on PrEP adherence, compared to a more long-lasting effect.
Pill count coverage was defined as the percentage of days between study visits when a pill was available to be taken, calculated as (number of pills dispensed – number of pills counted) ÷ number of days between study visits. Coverage was dichotomized into high (≥ 80%) or low (<80%), consistent with other HIV prevention studies39,40 and with recent pharmacodynamic modeling suggesting that 6 of 7 doses per week of oral FTC/TDF PrEP may be required to protect female genital tissue from HIV infection.41 Although clinic-based pill counts are an imperfect measure of adherence, they were strongly correlated with other objective measures of adherence in the Partners PrEP Study.30,31 Visits were excluded if the participant was not taking study drug for a protocol-defined reason, such as pregnancy, breastfeeding, seroconversion, or toxicity concerns. Missed visits were included, because one possible consequence of IPV could be failure to attend clinic visits, and adherence was set to zero when the number of days since the last visit exceeded the number of pills dispensed. About 2% (1400/59,806) of eligible follow-up visits were missed.
Plasma tenofovir adherence was dichotomized at 40 ng/mL. This concentration is based on the lower 95% confidence interval 24 hours after dose for directly observed daily dosing at steady state, but is also consistent with a single dose taken in the last 24 hours.42–45 We selected this threshold because it is more sensitive to occasional missed doses than a threshold of detectable vs. non-detectable, and because data suggest that near-daily dosing may be necessary to achieve adequate vaginal concentrations of activated intracellular metabolites of tenofovir diphosphate and FTC triphosphate required for effective protection from HIV.46,47
We evaluated the associations between IPV and each PrEP adherence measure using univariate and multivariable (adjusted) generalized estimating equation (GEE) Poisson models with an exchangeable correlation matrix and robust standard errors, to account for repeated measures for each participant.48,49 Multivariable models adjusted a priori for age, study site, and time in study. We also evaluated the following covariates as potential confounders and retained them in the model if they resulted in meaningful changes (>10%) to the estimated risk ratios: baseline covariates of partnership duration, years in the known HIV serodiscordant partnership, age difference with HIV-infected partner, income, education, alcohol intake; and time-varying covariates of HIV-infected partner's report of outside sexual partners, and participant's reports of outside sexual partners and any sex with their HIV-infected partner. Because changes in the participant's sexual behavior could be either a cause or a consequence of IPV, the last two time-varying covariates were lagged by 3 months to ensure that they preceded both the exposure and the outcome. Because the amount of missing data was small (<5% of visits), we conducted complete case analyses. Risk estimates did not change under different assumptions about the values of missing data. In sensitivity analyses, we excluded participants with pill count coverage >103%, indicating that fewer pills were returned than would be expected based on the number of days since the last visit. Previous studies suggest that coverage above that threshold may indicate lower adherence.34,50 To maximize statistical power, we included measurements from both randomly sampled and purposefully sampled participants in our plasma tenofovir analysis. We also restricted the analysis to randomly sampled participants to assess the potential for selection bias.
Additional analyses examined the effects of type of IPV (physical, verbal, or economic) and frequency of physical and verbal IPV on PrEP adherence measured by pill count coverage. The comparison group for each of these analyses was women who reported no IPV to date in the study. We used a Cox proportional hazards model to determine whether IPV was associated with higher HIV incidence in this cohort. The adjusted hazard ratios (aHRs) controlled for study arm and did not change with adjustment for age, marital/cohabiting status, number of children, any sex or unprotected sex in past month, male partner viral load and circumcision status, HSV-2 status at enrollment, or DMPA use.
Qualitative data
As part of an ancillary adherence sub-study, in-depth qualitative interviews were conducted with 88 HIV-uninfected participants (40 female and 48 male) at a single study site in Uganda. Methods and other findings from these interviews are described elsewhere.51,52 Participants were purposively sampled based on adherence levels, as estimated from unannounced home-based pill counts conducted as part of the sub-study procedures. The study enrolled all 58 participants at the site whose adherence dropped below 80% at some point during the sub-study (“low adherers”), and a sample of 30 participants with 100% adherence throughout the sub-study (“perfect adherers”). Women in the qualitative sample were older than in the overall study (median age 36 years, IQR 29.5-40.5), had fewer years of schooling (median 3, IQR 0-5), and were more likely to earn an income (97.5%). They were similar to the overall sample on marital status, relationship duration, and number of children. The interview addressed participants' experiences of taking PrEP, accounts of missed doses and lapses in adherence, and strategies for sustaining adherence. Interviews were conducted a minimum of three months and median of 21 months after enrollment (IQR 16-24 months). Data were analyzed using an inductive process to understand social influences that appeared to impact adherence. For this analysis, we reviewed the seven transcripts that contained references to IPV, including four low adherers and three perfect adherers. Relevant content was organized to reveal patterns in the data. The ancillary adherence sub-study was approved by the Partners Health Care Human Research Committee, the University of Washington Human Subjects Review Committee, and the Uganda National Council on Science and Technology. Separate written consent was obtained from all participants for this sub-study.
Results
Participant characteristics
Characteristics of the 1785 HIV-uninfected female participants are shown in Table 1. Mean age was 33.2 years, participants had completed an average of 5.6 years of school, and 69.6% had earned an income in the past 3 months. The vast majority of participants were married (99.2%), with a mean relationship duration of 12.9 years, and had mutually-disclosed HIV serodiscordant status for a mean of 1.4 years.
Table 1. Participant characteristics at baseline and during follow-up.
| Enrollment (Mean (SD) or N(%)) | N | Total (N=1785) | Any IPV in study (N=288) | No IPV in study (N=1497) | P^ |
|---|---|---|---|---|---|
| Demographic and Relationship Characteristics | |||||
| Age (years) | 1785 | 33.2 (7.5) | 32.6 (7.2) | 33.4 (7.6) | 0.08 |
| Partner age (years) | 1785 | 39.2 (8.1) | 38.2 (8.2) | 39.4 (8.0) | 0.03 |
| Age difference (participant age - partner age) | 1785 | 6.0 (6.0) | 5.7 (6.3) | 6.0 (5.9) | 0.4 |
| Ugandan (vs. Kenyan) | 1785 | 1203 (67.4%) | 216 (75.0%) | 987 (65.9%) | 0.003 |
| Years of school | 1785 | 5.6 (3.8) | 5.4 (3.7) | 5.6 (3.8) | 0.4 |
| Any income | 1785 | 1242 (69.6%) | 255 (78.1%) | 1017 (67.9%) | 0.001 |
| Married | 1785 | 1770 (99.2%) | 286 (99.3%) | 1484 (99.1%) | 0.8 |
| Partnership duration (years) | 1711 | 12.9 (8.3) | 12.2 (7.7) | 13.0 (8.4) | 0.1 |
| Years known discordant | 1781 | 1.4 (1.7) | 1.6 (1.6) | 1.3 (1.7) | 0.02 |
| Number of children | 1785 | 3.9 (2.2) | 3.8 (2.2) | 3.9 (2.2) | 0.6 |
| Behavioral Characteristics | |||||
| Number of drinks per week | 1785 | 0.2 (0.9) | 0.3 (0.8) | 0.2 (0.9) | 0.3 |
| Number of sex acts with study partner, past month | 1785 | 5.5 (5.0) | 6.0 (6.2) | 5.4 (4.7) | 0.1 |
| Unprotected sex with study partner, past month | 1785 | 406 (22.8%) | 69 (24.0%) | 337 (22.5%) | 0.6 |
| Sex with outside partner, past month | 1785 | 8 (0.5%) | 0 (0.0%) | 8 (0.5%) | 0.2 |
| Male partner reports outside partner, past month | 1785 | 264 (14.8%) | 44 (15.3%) | 220 (14.7%) | 0.8 |
| Any STI diagnosis* | 1636 | 221 (13.5%) | 32 (12.2%) | 189 (13.8%) | 0.5 |
|
| |||||
| Follow-Up (per person) | N | Total (N=1785) | Any IPV in study (N=288) | No IPV in study (N=1497) | |
|
| |||||
| Number of visits | 1785 | 33.7 (9.4) | 35.7 (8.4) | 33.3 (9.6) | <0.001 |
| Duration of follow-up (months) | 1785 | 31.2 (8.3) | 33.0 (7.4) | 30.9 (9.5) | <0.001 |
| Number of visits reporting IPV: | 288 | ||||
| 1 | -- | 198 (68.8%) | -- | -- | |
| 2 | -- | 57 (19.8%) | -- | -- | |
| 3 | -- | 19 (6.6%) | -- | -- | |
| 4 or more | -- | 14 (4.9%) | -- | -- | |
| Ever pregnant | 1785 | 397 (22.2%) | 57 (19.8%) | 340 (22.7%) | 0.3 |
| Any STI diagnosis* | 1781 | 258 (14.5%) | 51 (17.7%) | 207 (13.9%) | 0.09 |
| HIV seroconversion | 1781 | 57 (3.2%) | 9 (3.1%) | 48 (3.2%) | 0.9 |
|
| |||||
| Follow-Up (per visit) | N | Total (N = 58,406 visits) | Any IPV† (N = 419 visits) | No IPV (N = 57,987 visits) | |
|
| |||||
| Number of sex acts with study partner, past month | 57,439 | 3.7 (4.1) | 3.5 (5.3) | 3.7 (4.1) | 0.4 |
| Any sex with study partner, past month | 57,439 | 46,538 (81.0%) | 288 (68.7%) | 46,250 (81.1%) | <0.001 |
| Number of unprotected sex acts, past month | 57,439 | 0.5 (1.9) | 1.0 (3.7) | 0.4 (1.8) | 0.001 |
| Any unprotected sex with study partner, past month | 57,439 | 7,111 (12.4%) | 94 (22.4%) | 7,017 (12.3%) | <0.001 |
| Outside sexual partner, past month | 58,384 | 1,329 (2.3%) | 13 (3.1%) | 1,316 (2.3%) | 0.3 |
| Male partner reports outside partner, past month | 55,310 | 8,069 (14.8%) | 79 (19.8%) | 7,990 (14.8%) | 0.005 |
Any positive test result for chlamydia, gonorrhea, syphilis, or trichomonas. At enrollment, 149 women were missing 1 or more STI tests at enrollment and had no positive tests
IPV was reported at 437 total visits, of which 18 were enrollment visits and 419 were follow-up visits.
P values are based on t-tests with unequal variance for continuous variables and Pearson's chi-squared tests for categorical variables
Prevalence and correlates of IPV
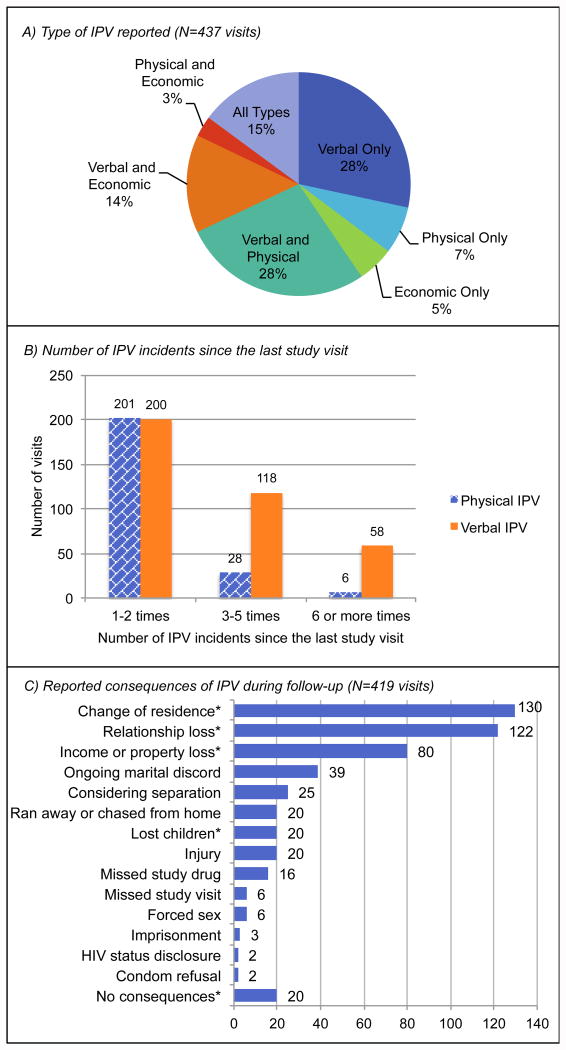
Over the course of the study, 288 women (16.1%) reported IPV at 437 visits (0.7% of 60,191 total visits). Of these women, 198 (68.8%) reported IPV at 1 study visit, 19.8% at 2 visits, 6.6% at 3 visits, and 4.9% at ≥4 visits. Most women reported multiple types of IPV (Figure 1A). Verbal IPV was the most common, reported at 376 visits, followed by physical IPV (235 visits) and economic IPV (212 visits). At 53% of visits with verbal IPV, women reported 1-2 incidents since the last monthly study visit. Three to 5 incidents were reported at 31% of visits, and ≥6 incidents at 15%. At the majority of visits with physical IPV (86%), women reported 1-2 incidents since the last visit, with 13% reporting 3-5 incidents and 3% reporting ≥6 incidents (Figure 1B). The most common consequences of IPV were change of residence, relationship loss, and income/property loss. Missed doses of study drug and missed study visits were also reported, though these were not predetermined response categories and were mentioned infrequently (Figure 1C).
Figure 1.
Descriptive statistics for IPV exposure during the study. A) Type of IPV reported at each study visit in which any IPV was reported. B) Number of IPV episodes reported since the last study visit. Data were not collected on frequency of economic IPV. C) Reported consequences of IPV. Consequences marked with an asterisk (*) were explicitly listed as response options, while those without the asterisk were described by participants in the open-ended “other” category. Note: participants were only asked to describe IPV consequences during follow-up visits. Data are missing for the IPV episodes reported at enrollment
Women who reported IPV were similar to women who reported no IPV on most demographic, relationship, and behavioral characteristics (Table 1). Baseline characteristics associated with subsequent reporting of IPV included having younger partners (mean 38.2 versus 39.4 years), having mutually-disclosed HIV serodiscordant status for slightly longer (1.6 versus 1.3 years), and reporting any income (78.1% versus 67.9%). Seventy-five percent of women who reported IPV were from Uganda (versus Kenya), compared to 65.9% of women who did not report IPV in the study. At visits with IPV, women were less likely to report sexual activity with their study partners than at visits with no IPV (68.7% versus 81.1%), more likely to report unprotected sex (22.4% versus 12.3%), and more likely to have partners who reported an outside sexual partner (19.8% versus 14.8%).
Adherence to PrEP and association with IPV
Pill count coverage was high among most women, regardless of reported IPV (mean 95.3%, standard deviation [SD] 19.8%, Table 2); the proportion of visits with pill count coverage <80% was 7.0%. Among visits with plasma tenofovir measurements, 32.0% had concentrations <40 ng/mL.
Table 2. Summary of adherence by measure and IPV status.
| Adherence Measure | |||||
|---|---|---|---|---|---|
|
| |||||
| Pill count coverage | Plasma tenofovir levels | ||||
|
| |||||
| Number of Visits | Mean (SD) | <80% N (%) | Number of Visits | <40 ng/mL N (%) | |
| IPV in study, ≤ 3 months ago | 1,100 | 95.5% (18.7%) | 88 (8.0%) | 38 | 16 (42.1%) |
| IPV in study, >3 months ago | 5,471 | 94.8% (21.5%) | 433 (7.9%) | 142 | 40 (35.2%) |
| No IPV to date in study | 43,562 | 95.5% (19.6%) | 2,962 (6.9%) | 1117 | 349 (31.2%) |
| Total | 50,165 | 95.3% (19.8%) | 3,510 (7.0%) | 1297 | 415 (32.0%) |
Table 3 presents crude and adjusted risk ratios (aRRs) for the association of IPV with PrEP adherence. After adjusting for age, study site, time on study, and male partner reports of outside sex partners, women were 50% more likely to have low PrEP adherence at visits with IPV in the past 3 months, compared to visits with no IPV to date in the study. This association was consistent regardless of whether adherence was measured by pill count (aRR 1.49, 95% confidence interval [CI] 1.17-1.89, p=0.001) or by plasma tenofovir (aRR 1.51, 95% CI 1.06-2.15, p=0.02). Adherence at visits >3 months after reported IPV was similar to adherence at visits with no IPV to date in the study (aRR for pill count: 1.08, 95% CI 0.86-1.36, p=0.5, aRR for plasma tenofovir: 0.95, 95% CI 0.73-1.24, p=0.7). There was no evidence of effect modification by time on study, or by country for recent IPV; for IPV occurring in the study > 3 months ago, lower adherence persisted in Kenya but not in Uganda (p-interaction =0.02) in the pill count analysis but not in the plasma tenofovir analysis.
Table 3. Effect of IPV exposure on each PrEP adherence outcome: univariate and multivariable results.
| Pill count coverage <80% | Tenofovir <40 ng/mL | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk ratio (95% CI) | p | Adjusted* risk ratio (95% CI) | p | Risk ratio (95% CI) | p | Adjusted* risk ratio (95% CI) | p | |
| IPV in study, ≤ 3 months ago | 1.28 (1.03–1.59) | 0.03 | 1.49 (1.17–1.89) | 0.001 | 1.41 (1.01–1.99) | 0.05 | 1.51 (1.06–2.15) | 0.02 |
| IPV in study, >3 months ago | 1.27 (1.05–1.54) | 0.02 | 1.08 (0.86–1.36) | 0.5 | 1.15 (0.91–1.45) | 0.3 | 0.95 (0.73–1.24) | 0.7 |
| No IPV to date in study | 1.00 | -- | 1.00 | -- | 1.00 | -- | 1.00 | -- |
Adjusted for age (years), study site, time on study (days), and whether male partner reports outside sex partner
The association between IPV in the past 3 months and low adherence was similar in women who reported sex in the past month (aRR: 1.53, 95% CI 1.11-2.10, p=0.009) and women reported no sex in the past month (aRR 1.44, 95% CI 1.04-1.99, p=0.03). Risk estimates did not substantially change when pill count analyses excluded participants with coverage >103%, when tenofovir analyses were restricted to the randomly sampled cohorts, or when adjusting for other potential confounding factors listed in the Methods.
When different types of IPV were considered separately, effect sizes for pill count coverage were similar for recent (past 3 month) verbal IPV (aRR 1.65, 95% CI 1.17-2.33, p=0.005) and recent economic IPV (aRR 1.48, 95% CI 1.14-1.92, p=0.003). The effect of recent physical IPV was not statistically significant (aRR = 1.27, 95% CI 0.89-1.82, p=0.2). However, the frequency of IPV since the last study visit was higher for verbal IPV (mean 4.1 episodes, SD 6.9) than for physical IPV (mean 1.7 episodes, SD 2.1), and the risk of low adherence increased significantly with increasing frequency of recent physical IPV (aRR = 1.09 for each additional episode of IPV within the reporting period, 95% CI 1.04-1.14, p<0.001) and recent verbal IPV (aRR = 1.02 for each additional episode, 95% CI 1.02-1.03, p<0.001). Type and frequency of IPV reported in the study and >3 months ago were not associated with adherence.
IPV and HIV incidence
There were 48 HIV seroconversions among women with no IPV to date in the study, 9 among women with IPV during the study and >3 months ago, and 2 among women with IPV in the past 3 months, resulting in HIV incidence rates of 1.2, 1.3, and 2.2 per 100 person-years, respectively. The associations between IPV and HIV incidence were not statistically significant (aHR for IPV in the past 3 months: 1.54, 95% CI 0.37-6.51, p=0.6; aHR for IPV > 3 months ago: 1.26, 95% CI 0.55-2.90, p=0.6).
Participant reports on IPV and PrEP use
Although the in-depth interviews did not specifically ask about IPV, seven women raised the topic when describing adherence challenges and strategies. Three patterns of how IPV interfered with adherence were evident in the transcripts (Table 4). Some women explained how violence and discord in the home made it difficult to remember to take the pills (Panel 4A). Others described running away during violent episodes, either because they feared for their safety or because they were chased away by their partners; they did not take their pills with them when leaving the house, so these episodes could result in missed doses (Panel 4B). In two cases, women's partners threatened to take or throw away their pills, either as a form of punishment or because they blamed relationship discord on the pills themselves (Panel 4C). Some women also described ways to surmount these challenges, and maintained high adherence despite experiences of IPV. Two women reported sending their children to retrieve their pills after they had run away from the house, and another was able to replace pills her husband had thrown away by explaining her situation to the study staff (Panel 4D).
Table 4. Excerpts from qualitative interviews on how IPV impacts PrEP adherence.
| Pattern | Example |
|---|---|
| A) Stress | Now when you don't have peace or you have slept outside, can't the day end when you are embroiled in quarrels and forget about the drugs? (participant QLA028) |
| If the family is not fine and there is no co-operation between you and your husband, that eventually affects the way one swallows his/her medicine. But if the home is okay and there is peace, even the children will be allowed to remind you. (participant QLA055) | |
| B) Leaving home without study drug | Of course I go without drugs. Now if we fight and I run away, can I go with the drugs? Or I just run for safety and look for refuge somewhere? (participant QLA 028) |
| The whole of December and November, I was in serious problems; I could not remember to swallow medicine… he would chase me out of the house and I would not get a chance to take my medicine with me. He would chase me and I spend the nights in the middle of nowhere; sometimes in church or in the bush…it was hard for me to remember to swallow medicine. Our relationship was not good; it was a very difficult moment in our relationship. (participant QLA 055) | |
| We fought, my husband and I, so I ran away to my parents' home. In the process, I left the bottles behind. (participant QPA 002) | |
| It's hard for me to remember each and every day I missed. But one common reason that has led me to miss my medicine on several occasions is..; My husband likes taking alcohol. And when he drinks, he becomes violent. There are times when he chase me and I run out of the house. Sometimes, that happens before I swallow the medicine and you find that I don't have a chance to go back. (participant QLA 053) | |
| C) Partner throws away or threatens to take study drugs | …there is some problem that happened where we quarreled at home and he threw away my drugs …He was telling me that; “let me throw away these pills and we will remain the same because it seems they are the ones making you behave like that. He had taken some alcohol which was forcing him to behave like that. (participant QPA 020) |
| He was saying that since I refused to use condoms, he would also swallow my drugs… We quarreled over it, he chased me and I slept in the kitchen, from there I never looked back, I went back to my parents…(participant QPA 016) | |
| HD) Resilience | When my child brought me what to put on in the morning, he also carried my drug bottles along. The man was busy staging a roadblock carrying a panga that I should not dare step in the house looking for clothes, while the children bypassed him and entered the bedroom where they picked clothes and drugs and brought them to me. So ever since I started taking the drugs, I have never stopped or missed taking them. (participant QPA 016) |
| It is my son who sneaked the bottles out of the house and brought them to me. My husband had actually locked everything in the bedroom. So I told my son to devise all means possible to get for me my pill bottles. (participant QPA 002) | |
| … he threw away my drugs, but I gathered them again and when the study staff came to visit us, I explained to them. They told me to come to the clinic the following day and get more drug. (participant QPA 020) |
Discussion
Overall, adherence to PrEP was high among women in the Partners PrEP Study, regardless of IPV history. However, women who reported IPV in the past 3 months had an increased risk of low PrEP adherence. The association did not persist for more than 3 months after the violence occurred, suggesting that among this group of women, the effects of IPV on adherence were acute and time-limited. Qualitative findings suggested several pathways through which IPV may cause short-term adherence lapses, including stress, being forced to leave the home, or a partner trying to take pills away from the participant; some women also developed strategies to maintain high adherence during IPV episodes.
This is the first study to examine the association between IPV and PrEP adherence. IPV was associated with lower condom and diaphragm use in one prospective study53 and with lower ART uptake, self-reported adherence, and viral suppression in a meta-analysis.27 Other studies have described the importance of partner support and disclosure of product use for good adherence in PrEP and microbicide trials52,54–59, and the role of violence as a barrier to disclosure.57,59 Since couples enrolled in the Partners PrEP Study together, our findings suggest that IPV impacts adherence even when women are using PrEP with their partner's knowledge and consent. It will be important for PrEP demonstration projects targeting high-risk women to collect data on this risk factor going forward.
At 16%, the period prevalence of IPV during this study was similar to that reported in another study of HIV serodiscordant couples using the same instrument33, but lower than national Demographic and Health Survey estimates of spousal violence in the past 12 months: 41% in Kenya and 45% in Uganda.60,61 Our study population consisted of women in stable, long-term relationships who were willing to be tested for HIV with their partners and enroll in a couples-based prevention study. Women with these characteristics may be less likely to experience IPV than women in the general population or those participating in other PrEP trials. Several qualitative studies have noted the predominance of violence in the lives of PrEP and microbicide study participants in some geographies, such as for some women in South Africa where the majority of VOICE and FEM-PrEP participants resided.62,63 Our estimates of the impact of IPV on PrEP adherence may be based on more moderate or infrequent IPV, and the effect may be stronger or more persistent in other populations. However, women reporting IPV in our study population reported higher risk sexual behavior, such as unprotected sex and having a partner who reported outside partners, relationship loss, change of residence, and property loss, suggesting that they experienced meaningful consequences of IPV.
Strengths of this study include a large sample size, prospective study design, and integration of quantitative and qualitative methods. Our findings were robust to different measures of PrEP adherence, adjustment for multiple potential confounding factors, and several sensitivity analyses to address misclassification, missing data, and selection bias. The association between recent IPV and PrEP adherence persisted when we restricted the analysis to women reporting sexual activity with their study partners, suggesting that IPV increases the risk of low adherence during periods in which women are at risk for HIV acquisition. Although the study was not powered to test for an association between IPV and HIV incidence, the hazard ratio point estimates are consistent with previous estimates of a 50% increase in HIV incidence associated with IPV.21,22,33
An important limitation to our study is that classification of IPV was based on self-report and may be under-reported. Our measurement tool did not ask about sexual IPV, violence severity, history of IPV before enrollment, or specific violent acts such as hitting, slapping, or threatening. Women may not have disclosed IPV if they did not consider specific acts to be abusive or if they did not feel comfortable discussing IPV with the study staff. In addition, IPV was assessed in the context of a counseling session rather than with a standardized question. This approach may have increased disclosure of IPV, but IPV assessment may have differed between sites. We cannot distinguish whether differences in reported IPV and its effects represent true differences in the rate of IPV by site or country, differences in participant willingness to report IPV, or differences in IPV ascertainment by study interviewers. If the degree of under-reporting is the same among women with low versus high PrEP adherence, this would likely underestimate the risk of low adherence associated with IPV. Although the proportion of visits with low adherence was higher when measured by plasma tenofovir levels than by pill-count coverage, the risk estimates for IPV and PrEP adherence were consistent between adherence measures, increasing our confidence in the results. Although the qualitative interviews were conducted at only one study site, a review of the narrative descriptions of IPV episodes and consequences on study case report forms suggest that the mechanisms described were relevant to other sites in Uganda and to Kenya as well.
In sub-Saharan Africa, targeting PrEP to high-risk women may be a cost-efficient approach to reducing HIV incidence64–66, but high adherence levels are required during periods of risk. Demonstration projects are ongoing to evaluate the feasibility of this approach and to identify strategies for implementation.67–69 Given the high prevalence of IPV in this region, and its impact on HIV risk, IPV should be considered when identifying high-risk women. In our cohort, the vast majority of women were able to take PrEP consistently, regardless of IPV history, but IPV in the short-term was associated with lower adherence in some subjects. Efforts to target PrEP towards women with IPV should recognize the risk of low adherence, and interventions should be evaluated to promote PrEP adherence in the context of violence. A potential intervention could integrate lessons from successful PrEP adherence programs70 and from interventions to improve ART adherence among HIV infected women with histories of abuse71, and could include motivational interviewing and problem-solving approaches to help women identify ways that IPV impacts their adherence and develop approaches to prevent violence or avoid lapses in adherence associated with IPV.70,71 Individual or group counseling approaches should be evaluated; group counseling may involve a social support network that could increase self-efficacy for adherence.72 Some women in our study reported strategies to maintain adherence in the face of IPV, and lessons from these examples of resilience could help in developing successful interventions. Such interventions could increase the prevention benefit of PrEP by promoting effective use in a population at high risk of HIV.
Acknowledgments
The authors thank the couples who participated in this study and the teams at the study sites and at the University of Washington for work on data collection and management.
Partners PrEP Study Team: University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam R. Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly R. Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi. Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (CLS) of the Wits Health Consortium (University of the Witwatersrand, Johannesburg, South Africa).
Source of funding: This work has been supported by research grants from the Bill & Melinda Gates Foundation (OPP47674), the US National Institutes of Health (R01 MH095507, T32 AI007140, F31 MH107258), and the ARCS© Foundation Seattle Chapter Endowment Fund. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Meetings at which parts of the data were presented: These findings were presented in part at the 2015 Conference on Retroviruses and Opportunistic Infections (CROI); February 23-26, Seattle, WA USA
Conflicts of interest: We have no conflicts of interest to declare.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack S, Dunn D. Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA: 2015. Pragmatic open-label randomised trial of pre-exposure prophylaxis: the PROUD study. Abstract 22LB. [Google Scholar]
- 5.Molina J, Capitant C, Charreau I, et al. Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2015. On demand PrEP with oral TDF-FTC in MSM: Results of the ANRS Ipergay trial. Abstract 23LB. [Google Scholar]
- 6.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: 2015. [PubMed] [Google Scholar]
- 8.World Health Organization. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men, and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- 9.AVAC. [June 18, 2015];Ongoing and Planned PrEP Demonstration and Implementation Studies. 2015 Apr; Available at: http://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and-implementation-studies.
- 10.World Health Organization. WHO Technical Update on Pre-Exposure Prophylaxis (PrEP) Geneva, Switzerland: 2015. [Google Scholar]
- 11.UNAIDS. The GAP Report. Geneva, Switzerland: 2014. [Google Scholar]
- 12.Garcia-Moreno C, Jansen H, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: findings from the WHO multi-country study on women's health and domestic violence. Lancet. 2006;368(9543):1260–69. doi: 10.1016/S0140-6736(06)69523-8. [DOI] [PubMed] [Google Scholar]
- 13.He H, McCoy H, Stevens SJ, Stark MJ. Violence and HIV sexual risk behaviors among female sex partners of male drug users. Women Health. 1998;27:161–175. doi: 10.1300/J013v27n01_10. [DOI] [PubMed] [Google Scholar]
- 14.Kalichman SC, Williams EA, Cherry C, Belcher L, Nachimson D. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Women's Heal. 1998;7:371–378. doi: 10.1089/jwh.1998.7.371. [DOI] [PubMed] [Google Scholar]
- 15.van der Straten A, King R, Grinstead O, Vittinghoff E, Serufilira A, Allen S. Sexual coercion, physical violence, and HIV infection among women in steady relationships in Kigali, Rwanda. AIDS Behav. 1998;2(1):61–73. [Google Scholar]
- 16.Zierler S, Witbeck B, Mayer K. Sexual violence against women living with or at risk for HIV infection. Am J Prev Med. 1996;12:304–310. [PubMed] [Google Scholar]
- 17.Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health. 1997;87(6):6–8. doi: 10.2105/ajph.87.6.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriola KRJ, Louden T, Doldren MA, Fortenberry RM. A meta-analysis of the relationship of child sexual abuse to HIV risk behavior among women. Child Abuse Negl. 2005;29(6):725–746. doi: 10.1016/j.chiabu.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Manfrin-Ledet L, Porche DJ. The state of science: violence and HIV infection in women. J Assoc Nurses AIDS Care. 2003;14(6):56–68. doi: 10.1177/1055329003252056. [DOI] [PubMed] [Google Scholar]
- 20.Stockman JK, Lucea MB, Campbell JC. Forced sexual initiation, sexual intimate partner violence and HIV risk in women: a global review of the literature. AIDS Behav. 2013;17(3):832–47. doi: 10.1007/s10461-012-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet. 2010;376(9734):41–8. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 22.Kouyoumdjian FG, Calzavara LM, Bondy SJ, et al. Intimate partner violence is associated with incident HIV infection in women in Rakai, Uganda. AIDS. 2013;27(8):1331–1338. doi: 10.1097/QAD.0b013e32835fd851. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JC, Campbell JC, Farley JE. Interventions to address HIV and intimate partner violence in sub-Saharan Africa: a review of the literature. J Assoc Nurses AIDS Care. 2013;24(4):383–90. doi: 10.1016/j.jana.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med. 2013;64:219–32. doi: 10.1146/annurev-med-050911-163701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis. AIDS. 2015;29(22):1277–1285. doi: 10.1097/QAD.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatcher AM, Smout EM, Turan JM, Christofides N, Stoeckl H. Intimate partner violence and engagement in HIV care and treatment among women. AIDS. 2015;29(16):2183–94. doi: 10.1097/QAD.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 28.Mugavero M, Ostermann J, Whetten K, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 29.Lopez EJ, Jones DL, Villar-Loubet OM, Arheart KL, Weiss SM. Violence, coping, and consistent medication adherence in HIV-positive couples. AIDS Educ Prev. 2010;22(1):61–8. doi: 10.1521/aeap.2010.22.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hien DA, Nunes E, Levin FR, Fraser D. Posttraumatic stress disorder and short-term outcome in early methadone treatment. J Subst Abuse Treat. 2000;19(1):31–7. doi: 10.1016/s0740-5472(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 31.Koenig LJ, Lyles C, Smith DK. Adherence to antiretroviral medications for HIV pre-exposure prophylaxis: lessons learned from trials and treatment studies. Am J Prev Med. 2013;44(1 Suppl 2):S91–8. doi: 10.1016/j.amepre.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 32.Baeten JM, Donnell D, Mugo NR, et al. Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2014;14(11):1055–1064. doi: 10.1016/S1473-3099(14)70937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Were E, Curran K, Delany-Moretlwe S, et al. A prospective study of frequency and correlates of intimate partner violence among African heterosexual HIV serodiscordant couples. AIDS. 2011;25(16):2009–18. doi: 10.1097/QAD.0b013e32834b005d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000172. Epublished 2014 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celum C, Morrow RA, Donnell D, et al. Daily oral tenofovir and emtricitabine–tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1–uninfected men and women. Ann Intern Med. 2014;161:11. doi: 10.7326/M13-2471. [DOI] [PubMed] [Google Scholar]
- 36.Pattacini L, Murnane PM, Baeten JM, et al. Antiretroviral pre-exposure prophylaxis does not enhance immune responses to HIV in exposed but uninfected persons. J Infect Dis. 2014:1–10. doi: 10.1093/infdis/jiu815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews LT, Heffron R, Mugo NR, et al. High medication adherence during periconception periods among HIV-1-uninfected women participating in a clinical trial of antiretroviral pre-exposure prophylaxis. 2014;67(1):91–97. doi: 10.1097/QAI.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (80- ) 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cottrell ML, Yang KH, Prince HMA, et al. A translational pharmacology approach to predicting HIV pre-exposure prophylaxis outcomes in men and women using tenofovir disoproxil fumarate ± emtricitabine. J Infect Dis. 2016 Feb 24;:1–29. doi: 10.1093/infdis/jiw077. Epublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol. 2007;47:751–759. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- 43.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakuda TN, Schöller-Gyüre M, De Smedt G, et al. Assessment of the steady-state pharmacokinetic interaction between etravirine administered as two different formulations and tenofovir disoproxil fumarate in healthy volunteers. HIV Med. 2009;10:173–181. doi: 10.1111/j.1468-1293.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 45.Kiser JJ, Fletcher CV, Flynn PM, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008;52(2):631–637. doi: 10.1128/AAC.00761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottrell ML, Yang KH, Prince HMA, et al. HIV Research for Prevention. Cape Town, South Africa: 2014. Predicting effective Truvada® PrEP dosing strategies with a novel PK-PD model incorporating tissue active metabolites and endogenous nucleotides (EN) Abstract OA22.06 LB. [Google Scholar]
- 47.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155(3):515–518. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 49.Yelland LN, Salter AB, Ryan P. Performance of the modified poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–992. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 50.Donnell DJ, Baeten JM, Hong T, et al. Correlation between pill counts and biologic effects in an HIV-1 prevention clinical trial: implications for measuring adherence. AIDS Behav. 2013;17(2):632–9. doi: 10.1007/s10461-012-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware NC, Pisarski EE, Haberer JE, et al. Lay social resources for support of adherence to antiretroviral prophylaxis for HIV prevention among serodiscordant couples in sub-Saharan Africa: a qualitative study. AIDS Behav. 2014 doi: 10.1007/s10461-014-0899-4. Epublished 01 October 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59(5):463–8. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kacanek D, Bostrom A, Montgomery ET, et al. Intimate partner violence and condom and diaphragm nonadherence among women in an HIV prevention trial in southern Africa. J Acquir Immune Defic Syndr. 2013;64(4):400–8. doi: 10.1097/QAI.0b013e3182a6b0be. [DOI] [PubMed] [Google Scholar]
- 54.Van Der Straten A, Stadler J, Montgomery E, et al. Women's experiences with oral and vaginal pre-exposure prophylaxis: The VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corneli A, Perry B, Agot K, Ahmed K, Malamatsho F, Van Damme L. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PLoS One. 2015;10(4):e0125458. doi: 10.1371/journal.pone.0125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansoor LE, Abdool Karim Q, Yende-Zuma N, et al. Adherence in the CAPRISA 004 tenofovir gel microbicide trial. AIDS Behav. 2014;18(5):811–9. doi: 10.1007/s10461-014-0751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery CM, Lees S, Stadler J, et al. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care. 2008;20(6):733–740. doi: 10.1080/09540120701693974. [DOI] [PubMed] [Google Scholar]
- 58.Montgomery ET, van der Straten A, Stadler J, et al. Male partner influence on women's HIV prevention trial participation and use of pre-exposure prophylaxis: the importance of “understanding”. AIDS Behav. 2015;19(5):784–793. doi: 10.1007/s10461-014-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanham M, Wilcher R, Montgomery ET, et al. Engaging male partners in women's microbicide use: evidence from clinical trials and implications for future research and microbicide introduction. J Int AIDS Soc. 2014;17(3 Suppl 2):19159. doi: 10.7448/IAS.17.3.19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uganda Bureau of Statistics (UBOS) and ICF International Inc. 2011 Uganda Demographic and Health Survey. Kampala, Uganda: UBOS and Calverton; Maryland: ICF International Inc; 2012. [Google Scholar]
- 61.Kenya National Bureau of Statistics (KNBS), ICF Macro. Kenya Demographic and Health Survey 2008-09. Calverton, Maryland, USA: 2010. [Google Scholar]
- 62.Stadler J, Delany-Moretlwe S, Palanee T, Rees H. Hidden harms: Women's narratives of intimate partner violence in a microbicide trial, South Africa. Soc Sci Med. 2014;110:49–55. doi: 10.1016/j.socscimed.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann MA, Montgomery ET, Stadle J, Laborde N, van der Straten A. VOICE-C participant narratives of rape: What they mean for female-initiated HIV prevention products. AIDS Res Hum Retroviruses. 2014;30(S1):A86. [Google Scholar]
- 64.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The Cost and Impact of Scaling Up Pre-exposure Prophylaxis for HIV Prevention: A Systematic Review of Cost-Effectiveness Modelling Studies. PLoS Med. 2013;10(3) doi: 10.1371/journal.pmed.1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walensky RP, Park J-E, Wood R, et al. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women. Clin Infect Dis. 2012;54(10):1504–13. doi: 10.1093/cid/cis225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pretorius C, Stover J, Bollinger L, Bacaër N, Williams B. evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. [June 19, 2015];Sustainable Healthcenter Implementation PrEP Pilot Study (SHIPP) clinicaltrials.gov.: NCT02074891. Available at: https://clinicaltrials.gov/ct2/show/NCT02074891.
- 68.Gender-Specific Combination HIV Prevention for Youth in High Burden Settings (MP3-Youth) doi: 10.2196/resprot.5833. clinicaltrials.gov.: NCT01571128. Available at: https://clinicaltrials.gov/ct2/show/NCT01571128. [DOI] [PMC free article] [PubMed]
- 69.LVCT Health. [June 19, 2015];Demonstrating effective delivery of daily oral HIV pre-exposure prophylaxis (PrEP) as part of an HIV combination prevention intervention among young women at high HIV risk, female sex workers and men who have sex with men in Kenya (PrEP Demonstration Proj. 2015 Available at: http://www.lvcthealth.org/images/pdf/oral_prep.pdf.
- 70.Psaros C, Haberer JE, Katabira E, et al. An intervention to support HIV preexposure prophylaxis adherence in HIV-serodiscordant couples in Uganda. J Acquir Immune Defic Syndr. 2014;66(5):522–9. doi: 10.1097/QAI.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyatt GE, Longshore D, Chin D, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–62. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 72.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–96. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]