Abstract
Background
High rates of 23S rDNA mutations implicated in macrolide resistance have been identified in Treponema pallidum (T.p.) samples from syphilis patients in many countries. Nonetheless, some clinicians have been reluctant to abandon azithromycin as a treatment for syphilis, citing the lack of a causal association between these mutations and clinical evidence of drug resistance. While azithromycin resistance has been demonstrated in vivo for the historical Street 14 strain, no recent T.p. isolates have been tested. We used the well-established rabbit model of syphilis to determine the in vivo efficacy of azithromycin against 23S rDNA mutant strains collected in 2004–5 from patients with syphilis in Seattle, WA.
Methods
Groups of 9 rabbits were each infected with a strain containing 23S rDNA mutation A2058G (strains UW074B, UW189B, UW391B) or A2059G (strains UW228B, UW254B, and UW330B), or with one wild type strain (Chicago, Bal 3, and Mexico A). After documentation of infection, 3 animals per strain were treated with azithromycin; 3 were treated with benzathine penicillin G; and 3 served as untreated control groups. Treatment efficacy was documented by darkfield microscopic evidence of T. pallidum, serological response, and rabbit infectivity test.
Results
Azithromycin uniformly failed to cure rabbits infected with strains harboring either 23S rDNA mutation, although benzathine penicillin G was effective. Infections caused by wild type strains were successfully treated by either azithromycin or benzathine penicillin G.
Conclusions
A macrolide resistant phenotype was demonstrated for all strains harboring a 23S rDNA mutation, demonstrating that either A2058G or A2059G mutation confers in vivo drug resistance.
Keywords: syphilis, drug resistance, treatment
INTRODUCTION
Benzathine penicillin G is an inexpensive and effective treatment for syphilis, and there is no confirmed clinical or laboratory evidence of penicillin resistance in Treponema pallidum (T.p.). This treatment, however, requires painful intramuscular injections, and clinicians have long sought a single dose oral regimen for syphilis. Azithromycin, a long-acting oral ketolide antibiotic, is effective against the standard laboratory strain of T.p. (Nichols) in the rabbit model1 and several early studies confirmed the efficacy of azithromycin for syphilis treatment in non-pregnant adults.2–4
When syphilis rates in the US began to increase in 2000, single dose azithromycin began to be used more widely to treat syphilis. This regimen was particularly attractive for partner-delivered treatment of sexual contacts. In 2002, however, azithromycin treatment failures were identified in San Francisco, and samples from these individuals contained the 23S A2058G mutation.5 Samples from San Francisco, Dublin, and Seattle documented a high prevalence of the A2058G mutation in circulating strains of T.p., with an increasing frequency of the mutation over time.6–9 Harboring a strain with this mutation was associated with past macrolide exposure.10 A second 23S rDNA mutation, A2059G, was identified in a secondary syphilis patient who failed therapy with the macrolide antibiotic spiramycin, and the mutation was subsequently identified in 18% of samples from patients with syphilis in the Czech Republic.11
The development of a rapid, isolation-independent molecular method to test patient samples led to widespread reports of varying prevalence rates of these mutations. For example, up to 97% of samples tested in Shanghai, China, have the A2058G mutation,12 whereas this mutation has been identified in very low frequency in Taiwan.13,14 The A2059G mutation has been seen in multiple sites, although in lower frequency.9,15 The rise in syphilis rates, particularly among men who have sex with men (MSM), has resulted in a corresponding observation of 23S rDNA mutations in patient samples in London,16 Dublin,8 South Africa,17 US,18 Seattle,9 and China.19
Despite the reported association of the A2058G and A2059G 23S rDNA mutations with treatment failure after treatment with macrolides,20 there has been controversy about the clinical relevance of the mutations.21 In a paper describing a large multi-site equivalence study of azithromycin vs. benzathine penicillin G for treatment of early syphilis, the authors suggest that the question of whether 23S mutations contribute to syphilis treatment failure among individuals treated with azithromycin remains unresolved.21
The rabbit model of syphilis offers a definitive means to examine the correlation between 23S rDNA mutations and efficacy of azithromycin therapy. Our ongoing syphilis research programs have included isolation of T. pallidum strains from patient samples (blood, cerebrospinal fluid, chancre exudate) for many years, and these strains have been tested for 23S rDNA mutations.9,10 We used six of these strains, containing either the A2058G or A2059G mutation, to examine in vivo susceptibility of T. pallidum to azithromycin in the rabbit model.
Materials and Methods
Strains
Six T.p. strains isolated from blood of patients with untreated syphilis were selected to represent the A2058G and A2059G 23S mutations and, when possible, to represent different molecular strain types within groups (Table 1).9,10 Wild type, historical strains were obtained originally from Paul Hardy and Ellen Nell at Johns Hopkins University. These strains had not been tested previously for susceptibility in vivo to azithromycin. All strains were stored as glycerol stocks in liquid nitrogen prior to propagation in rabbits as previously described.22
TABLE 1.
Strains used in this study
| Group | Strain | Molecular Strain Type | Source* |
|---|---|---|---|
| Wild Type | Bal 3 | 12a/c | Baltimore, unknown |
| Chicago29 | 14a/a | Chicago, 1951 | |
| Mexico A29 | 14d/e | Mexico, 1953 | |
| A2058G | UW074B | 14d/g | Seattle, 2004 |
| UW189B | 13d/d | Seattle, 2004 | |
| UW391B | 14d/f | Seattle, 2005 | |
| A2059G | UW228B | 15d/f | Seattle, 2004 |
| UW254B | 15d/f | Seattle, 2004 | |
| UW330B | 15d/f | Seattle, 2005 |
Location, year
Molecular demonstration of 23S rDNA mutation
23S rDNA mutations were confirmed using the published restriction fragment length polymorphism (RFLP) methods previously described.6,11 Briefly, an initial 23S rDNA polymerase chain reaction (PCR) product specific to T.p. genomic DNA served as template for a nested PCR. For restriction enzyme digestion, the A2058G mutation was detected with MboII (New England Biolabs, Ipswich, MA) and the A2059G mutation with BsaI (NEB). Wild type amplicons were not cut by either enzyme.
Infections
Outbred adult male New Zealand white rabbits (2.5 – 3.5 kg) were obtained from R & R Rabbitry, Stanwood, WA. Initially, each rabbit had negative serological results by fluorescent treponemal antibody-absorption (FTA-ABS) and Venereal Disease Research Laboratory (VDRL, Becton, Dickinson and Company, Sparks, MD) tests. Rabbits were fed antibiotic-free food and water and housed at 16 – 18°C. These studies were approved by the University of Washington Institutional Animal Care & Use Committee, and care was provided in full accordance with the Guide for the Care and Use of Laboratory Animals.
Groups of 9 rabbits were infected intradermally (ID) with one of the three wild type strains or with one of two Seattle isolates that contained A2059G mutations. Intradermal infection is preferable to intratesticular infection because the skin lesions can be readily aspirated for darkfield microscopic examination for the presence of viable T.p.to determine how quickly the antibiotic treatment results in bacterial clearance (see Table 2). However, ID infection with the other four Seattle strains (3 containing the A2058G mutation and one strain with the A2059 mutation) does not reproducibly induce robust skin lesions; therefore, groups of 9 rabbits were infected intratesticularly with each of these strains.
TABLE 2.
Darkfield detection of T.p. in intradermally infected rabbits following treatment
| Strain | Benzathine Penicillin G | Azithromycin | Untreated |
|---|---|---|---|
| Wild Type | |||
| Chicago | 0/3* | 0/3 | 3/3 |
| Mexico A | 0/3 | 0/3 | 3/3 |
| Bal 3 | 0/3 | 0/3 | 3/3 |
| A2059G | |||
| UW228B | 0/3 | 3/3 | 3/3 |
| UW330B | 0/3 | 3/3 | 3/3 |
Number of rabbits with DF-detectable T.p. in lesion aspirates by day10/number of rabbits per group.
Intradermal Infections (ID)
Wild Type (Chicago, Bal 3, Mexico A); A2059G mutation (UW228B, UW330B): Rabbit backs were clipped free of fur, and each rabbit was infected ID at 10 sites with a 0.1mL volume of 107 T.p./mL (106 T.p. per site). Lesions were examined daily for size and progression for 10 days after initiation of treatment. Once lesions were evident, aspirates were taken for darkfield (DF) microscopic examination prior to treatment and every 2 days after initiation of treatment; all rabbits in the treatment studies had treponemes detectable by DF prior to initiation of treatment. After treatment, rabbits whose lesion aspirates became and remained DF-negative on three consecutive time points were declared DF-negative and were no longer examined by this method. The microscopist was blind to the treatment status and infecting strain of the animals from which the samples were collected.
Intratesticular Infections (IT)
A2058G mutation (UW074, UW189, UW391); A2059G mutation (UW254B): To match the intradermal infection experiments, the same total number of bacteria (107) was delivered into one testicle per rabbit. Clinical evidence of orchitis (monitored twice weekly) or darkfield evidence of T.p. in a testicular aspirate was considered to be proof of active infection prior to initiation of treatment.
Treatment
For each strain tested, there were three treatment groups with 3 rabbits per group: control (no treatment), azithromycin, benzathine penicillin G. The timing of initiation of treatment was dependent upon clinical evidence of infection (DF+ aspirate or orchitis), thus the timing of treatment initiation varied among strains. Azithromycin-treated animals received one 45mg capsule PO daily (15 mg/kg), roughly equivalent (w/w) to 1 g/day in humans, for 10 days and consistent with our previous study.1 Benzathine penicillin G (BPG)-treated animals received a single IM injection (200,000 units; equivalent w/w to 4.8 million units for humans).1
Serological testing
Blood was collected from all rabbits every two weeks post infection for 8–14 weeks for quantitative VDRL testing. Serological assays for all time points were performed on the same day to minimize test-to-test variability; the technologist was blinded to the treatment status of the animals from which the samples were collected. Mean log2 titers +/− SE were calculated for each treatment group at each time point: nonreactive= −1.0; weakly reactive= −0.5; (reactive at a titer of 1:1) R1=0 and R2=1, etc.
Rabbit Infectivity Test
Upon completion of serological monitoring, all rabbits were euthanized for rabbit infectivity testing (RIT) as described previously.23 Popliteal lymph nodes from each rabbit were minced in normal rabbit serum, pooled by group, and each pool was injected IT into a naïve rabbit for detection of persistent infection in the donors. Development of either DF+ orchitis or VDRL and FTA-ABS seroconversion in the recipient rabbit during a 3 month observation period was considered to be evidence of transferred infection. Observation was discontinued in a recipient rabbit when either of these criteria was met. When recipient rabbits failed to develop evidence of T.p. infection by the end of the observation period, the donors were assumed to be cured by antibiotic treatment.
RESULTS
Three criteria were used to determine susceptibility of a T.p. strain to antibiotic treatment: disappearance of T.p. by DF microscopic examination of lesion aspirates; four-fold reduction in VDRL titer or reversion to nonreactive following treatment; and failure of tissues to transfer active infection to recipient rabbits. Table 2 summarizes the darkfield microscopic evidence of treponemal infection following completion of treatment in rabbits infected intradermally. All rabbits treated with BPG had no detectable T.p. in lesion aspirates by day 2 after treatment initiation and remained DF-negative for two subsequent examinations. After treatment with azithromycin, rabbits infected with wild type strains had no detectable T.p. in lesion aspirates by day 4 after initiation of treatment and remained DF-negative for two subsequent examinations. In contrast, all rabbits infected ID with the two strains containing the A2059G mutation and treated with azithromycin had detectable T.p. in lesion aspirates throughout the 10-day observation period. All untreated intradermally-infected rabbits had detectable T.p. in lesion aspirates throughout the 10-day observation period.
In untreated individuals with syphilis, serum VDRL or rapid plasma reagin (RPR) titers increase through the secondary stage of disease. After treatment, a 4-fold reduction in titer is an indication of the efficacy of treatment. A similar picture is seen in the rabbit model of syphilis, regardless of the route of infection. Figure 1 shows the mean+/− SE log2 titers of serum VDRL antibody over the course of infection and treatment for a representative strain in each 23S rDNA mutation group. Serological evidence of treatment success was seen following BPG treatment for all strains, and following azithromycin treatment of wild type strains. Rabbits infected with strains containing either 23S rDNA mutation failed to show a significant reduction in titer post azithromycin, following the same pattern of increasing titer as seen in untreated animals infected with that same strain.
FIGURE 1.
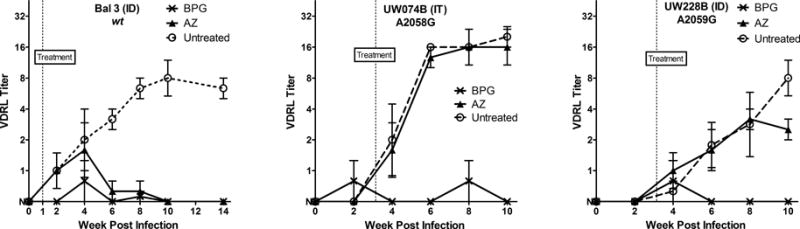
Mean (+/− SE) serum VDRL titers in untreated- (open circle), BPG- (x), and azithromycin- (triangle) treated rabbits. The data represent 3 rabbits per treatment group, and mean serum VDRL titers are shown through 10–14 weeks post-infection, and at least six weeks following initiation of treatment. One representative strain is shown for each 23S group: wild type, A2058G, and A2059G, as noted. For calculating mean +/− SE, titers were converted to log2 with nonreactive= −1.0; weakly reactive= −0.5; R1= 0, and R2=1, etc. Antilogs of mean +/−SE of titers are shown in this figure with nonreactive set at y=0.
The gold standard test for persistent T.p. is the highly sensitive RIT.23 As shown in Table 3, tissue from BPG treatment groups for all strains failed to transfer infection to recipient rabbits during the 3-month observation period, indicating effective treatment by BPG for all strains. Conversely, all rabbits receiving tissues from untreated animals for all strains developed either DF+ orchitis or seroconversion (VDRL and FTA-ABS). Azithromycin treatment of rabbits infected with any of the 3 wild type strains was effective (RIT-negative), but tissues from animals infected with any of the 6 strains containing A2058G or A2059G 23S rDNA mutations remained infectious for the recipient animals, indicating functional resistance to azithromycin in treated animals.
TABLE 3.
Summary of Results of Rabbit Infectivity Test
| Strain | Benzathine penicillin G | Azithromycin | Untreated |
|---|---|---|---|
| Wild Type | |||
| Chicago | N* | N | DF+ (1mo) |
| Mexico A | N | N | R16 (2mo) |
| Bal 3 | N | N | R8 (2mo) |
| A2058G | |||
| UW189B | N | R8 (2mo) | R16 (2mo) |
| UW391B | N | R8 (2mo) | R8 (1mo) |
| UW074B | N | R16 (3mo) | R16 (1mo) |
| A2059G | |||
| UW228B | N | R2 (1mo) | R4 (1mo) |
| UW254B | N** | R16 (7wk) | R64 (7wk) |
| UW330B | N | R16 (1mo) | DF+ (1 mo) |
N = Nonreactive serum VDRL at three months post-transfer, R = Reactive serum VDRL with reciprocal titer (time post-transfer at which seroconversion was first detected), DF+ = Darkfield positive aspirate from testicular aspirate; no titer available.
Euthanized at 7wk due to unrelated illness.
DISCUSSION
We undertook this study to address uncertainty in the scientific and medical communities regarding the clinical relevance of 23S rDNA mutations in T. pallidum. In other bacteria, the A2058G and A2059G 23S rDNA mutations confer functional resistance to macrolide antibiotics, yet concern has been raised in the syphilis literature about whether the presence of these mutations in T.p. conveys a macrolide resistant phenotype.21
The Street 14 strain, isolated in 1977, is resistant to macrolide antibiotics in vivo, but demonstration of resistance in more recently isolated strains lends particular relevance to the management of today’s patients. In many areas, the prevalence of strains containing a 23S rDNA resistance mutation is greater than 80–85%.9,12,24,25 Surveillance for these mutations can provide practical information as illustrated by a recent report from Taiwan, where azithromycin was able to be used for syphilis treatment during a recent BPG shortage because of a known low frequency of strains carrying mutations.26 In the US and Western Europe, the strains containing 23S rDNA mutations are reported in the MSM population consistent with the epidemiology of syphilis in these regions, although studies of separate demographic groups have not been reported. The persistence of the mutations in the population over many years suggests that the A2058G and A2059G mutations in T. p. may not confer a significant fitness disadvantage. If true, then these resistant strains may continue to circulate widely in the future, even in the absence of antibiotic pressure.
Our results clearly link in vivo azithromycin resistance to 23S rDNA mutations in 6 recently isolated strains. To avoid the possibility that we were testing separate isolates of the same circulating strain, we endeavored to select strains with different molecular types when possible. Although three strains with A2058G mutations and different molecular type were available, all of our isolates containing A2059G were of the same type. They were isolated over the course of 12-month period, so they may or may not represent a single strain. Nonetheless, all three isolates were clearly resistant to azithromycin. To ensure rigor in our investigations, we used multiple methods for demonstrating persistence of infection after treatment: darkfield microscopic examination of material aspirated from skin lesions or infected testes, the course of serum VDRL titers before and following treatment, and the RIT, which is the gold standard measure of the presence of infectious T.p. in a treated person or experimental animal.
Penicillin remains the preferred treatment for syphilis. Benzathine penicillin G is the form of penicillin that provides treponemicidal levels of penicillin in blood for multiple weeks following a single administration. This long duration of drug levels is considered necessary because T.p. divides very slowly (30–33 hours),27 and the drug must be present during cell wall synthesis to be effective. A recent study by Tipple et al. showed that, following BPG treatment of three primary and secondary syphilis patients, blood was clear of T.p. DNA by PCR after a mean of 32 hours, and chancre exudate from the single patient with primary syphilis was cleared of DNA by 56 hours.28 Consistent with the data in humans, rabbits treated with BPG have no T.p. detectable in lesion exudate by DF microscopy ~1 day post-BPG.6 The PCR assay used in the Tipple study could be more sensitive than darkfield microscopy or may have detected residual nucleic acids from dead organisms. Strains with proven penicillin resistance have not been isolated from patients, and clinical failures are uncommon following BPG treatment.
Despite the efficacy of penicillin, the availability of an effective, oral single dose treatment for syphilis would be welcomed by patients and clinicians. It was hoped that azithromycin would be that drug, but the high frequency of 23S rDNA mutations, particularly in light of our findings, makes azithromycin inappropriate in most geographical regions. The rapid dissemination of T.p. strains containing these mutations over many countries and multiple continents has essentially removed azithromycin and other macrolides from the repertoire of drugs that can be used to treat syphilis. Continued surveillance for 23S mutations is important for tracking the movement of resistant strains and for identifying the development of new mutations.
Acknowledgments
Source of Funding: Research reported in this publication was supported by the National Institutes of Health under award numbers R03AI094122, R01AI42143, R01AI63940 (SAL) and R01NS034235 (CMM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BPG
benzathine penicillin G
- DF
darkfield microscopy
- FTA-ABS
fluorescent treponemal antibody-absorption
- PO
per os: by mouth
- RIT
rabbit infectivity test
- T.p.
Treponema pallidum
- VDRL
Venereal Disease Research Laboratory
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Lukehart SA, Fohn MJ, Baker-Zander SA. Efficacy of azithromycin for therapy of active syphilis in the rabbit model. J Antimicrob Chemother. 1990 Jan;25(Suppl A):91–9. doi: 10.1093/jac/25.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- 2.Verdon MS, Handsfield HH, Johnson RB. Pilot study of azithromycin for treatment of primary and secondary syphilis. Clin Infect Dis Off Publ Infect Dis Soc Am. 1994 Sep;19(3):486–8. doi: 10.1093/clinids/19.3.486. [DOI] [PubMed] [Google Scholar]
- 3.Hook EW, 3rd, Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann Intern Med. 1999 Sep 21;131(6):434–7. doi: 10.7326/0003-4819-131-6-199909210-00007. [DOI] [PubMed] [Google Scholar]
- 4.Rekart ML, Patrick DM, Chakraborty B, et al. Targeted mass treatment for syphilis with oral azithromycin. Lancet Lond Engl. 2003 Jan 25;361(9354):313–4. doi: 10.1016/s0140-6736(03)12335-5. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Brief report: azithromycin treatment failures in syphilis infections–San Francisco, California, 2002–2003. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. Mar, pp. 197–8. Morbidity and Mortality Weekly Report. [Google Scholar]
- 6.Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004 Jul 8;351(2):154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell SJ, Engelman J, Kent CK, et al. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin Infect Dis. 2006 Feb 1;42(3):337–45. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 8.Muldoon E, Mulcahy F. Syphilis Resurgence in Dublin, Ireland. Int J STD AIDS. 2011;22:493–7. doi: 10.1258/ijsa.2011.010438. [DOI] [PubMed] [Google Scholar]
- 9.Grimes M, Sahi SK, Godornes BC, et al. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex Transm Dis. 2012 Dec;39(12):954–8. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra CM, Colina AP, Godornes C, et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J Infect Dis. 2006 Dec 15;194(12):1771–3. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 11.Matejkova P, Flasarova M, Zakoucka H, et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol. 2009 Jun;58(Pt 6):832–6. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 12.Martin IE, Gu W, Yang Y, Tsang RS. Macrolide reistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49:515–21. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Chang S-Y, Lee N-Y, et al. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J Clin Microbiol. 2012 Jul;50(7):2299–304. doi: 10.1128/JCM.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme K, Behets F, Ravelomanana N, et al. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex Transm Dis. 2009 Dec;36(12):775–6. doi: 10.1097/OLQ.0b013e3181bd11dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grillova L, Petrosova H, Mikalova L, et al. Molecular Typing of Treponema pallidum in the Czech Republic during 2011 to 2013: Increased Prevalence of Identified Genotypes and of Isolates with Macrolide Resistance. J Clin Microbiol. 2014 Oct 1;52(10):3693–700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect. 2011 Oct;87(6):486–8. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 17.Müller EE, Paz-Bailey G, Lewis DA. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex Transm Infect. 2012 Oct;88(6):470–4. doi: 10.1136/sextrans-2011-050322. [DOI] [PubMed] [Google Scholar]
- 18.A2058G Prevalence Workgroup. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012 Oct;39(10):794–8. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 19.Chen X-S, Yin Y-P, Wei W-H, et al. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin Microbiol Infect. 2013 Oct;19(10):975–9. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 20.Katz KA, Klausner JD. Azithromycin resistance in Treponema pallidum. Curr Opin Infect Dis. 2008 Feb;21(1):83–91. doi: 10.1097/QCO.0b013e3282f44772. [DOI] [PubMed] [Google Scholar]
- 21.Hook EW, 3rd, Behets F, Van Damme K, et al. A Phase III Equivalence Trial of Azithromycin versus Benzathine Penicillin for Treatment of Early Syphilis. J Infect Dis. 2010;201:1729–35. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 22.Lukehart SA, Marra CM. Isolation and laboratory maintenance of. Treponema pallidum Curr Protoc Microbiol. 2007 Nov; doi: 10.1002/9780471729259.mc12a01s7. Chapter 12:Unit 12A 1. [DOI] [PubMed] [Google Scholar]
- 23.Turner TB, Hardy PH, Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969;45(3):183–96. doi: 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P, Li K, Lu H, Qian Y, Gu X, Gong W, et al. Azithromycin treatment failure among primary and secondary syphilis patients in Shanghai. Sex Transm Dis. 2010 Nov;37(11):726–9. doi: 10.1097/OLQ.0b013e3181e2c753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read P, Jeoffreys N, Tagg K, Guy RJ, Gilbert GL, Donovan B. Azithromycin-Resistant Syphilis-Causing Strains in Sydney, Australia: Prevalence and Risk Factors. J Clin Microbiol. 2014 Aug 1;52(8):2776–81. doi: 10.1128/JCM.00301-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C-J, Tang H-J, Chang S-Y, et al. Comparison of serological responses to single-dose azithromycin (2 g) versus benzathine penicillin G in the treatment of early syphilis in HIV-infected patients in an area of low prevalence of macrolide-resistant Treponema pallidum infection. J Antimicrob Chemother. 2016 Mar;71(3):775–82. doi: 10.1093/jac/dkv379. [DOI] [PubMed] [Google Scholar]
- 27.Cumberland MC, Turner TB. The rate of multiplication of Treponema pallidum in normal and immune rabbits. Am J Syph Gonorrhea Vener Dis. 1949 May;33(3):201–12. [PubMed] [Google Scholar]
- 28.Tipple C, Jones R, McClure M, Taylor G. Rapid Treponema pallidum clearance from blood and ulcer samples following single dose benzathine penicillin treatment of early syphilis. PLoS Negl Trop Dis. 2015 Feb;9(2):e0003492. doi: 10.1371/journal.pntd.0003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner TB, Hollander DH. Biology of the Treponematoses. Geneva: World Health Organization; 1957. [PubMed] [Google Scholar]


