Abstract
One of the most common drug dependencies occurring with alcoholism is cocaine dependence. This combination is particularly worrisome because of the increased risk of cardiovascular events associated with their co-abuse. Though it's well known that ethanol increases the cardiovascular effects of cocaine by inhibiting cocaine clearance and the formation of cocaethylene, it has also been postulated that ethanol enhances cocaine's cardiovascular effects independent of the two latter mechanisms. This study investigated the cardiovascular pharmacodynamics of the cocaine-ethanol interaction to determine if ethanol directly enhanced the cardiovascular effects of cocaine. Dogs (n=6) were administered 3mg/kg of IV cocaine alone and in combination with 1g/kg of IV ethanol on separate study days. Blood pressure, heart rate, and ECG were monitored continuously and blood samples collected periodically after drug administration. Concentration-time data were fitted to a two-compartment model, and concentration-effect data fitted to a simple Emax model using WinNonlin. Pharmacokinetic and pharmacodynamic parameters were compared between the two treatment phases by a paired t-test. The administration of ethanol before cocaine resulted in a decrease in cocaine clearance, but there were no differences in any of the other pharmacokinetic or pharmacodynamic parameter values between the cocaine alone and cocaine+ethanol phases. As has been demonstrated in previous animal and human studies, the clearance of cocaine was decreased by prior administration of ethanol. However, ethanol did not change the concentration-effect relationship of the cardiovascular response to cocaine administration. It is concluded from this study that ethanol does not directly enhance the cardiovascular effects of cocaine.
Introduction
It is well accepted that the co-abuse of ethanol with cocaine causes an increase in cardiovascular effects and toxicity when compared to cocaine alone (Farre et al. 1993; McCance-Katz et al. 1998; Mehta et al. 2002; Schechter and Meehan 1995). Various investigators have speculated that the increase in cardiovascular effects when cocaine and ethanol are co-administered is secondary to the formation of the active metabolite, cocaethylene, due to the inhibition of cocaine's metabolism by ethanol, and a result of a pharmacodynamic interaction between cocaine and ethanol.(Farre et al. 1997; Henning and Wilson 1996; McCance-Katz et al. 1993; Pan and Hedaya 1999; Perez-Reyes and Jeffcoat 1992; Schechter and Meehan 1995)
The combined administration of cocaine and ethanol results in the formation of cocaethylene, an active metabolite formed by transesterification between cocaine and ethanol. Cocaethylene's pharmacologic activity has been studied in animals and found to be similar to cocaine's, but cocaethylene has been reported to be more toxic than cocaine with a lower LD50 in rats than cocaine (Hearn et al. 1991). It clearly has the potential to contribute to the increased cardiovascular effects when cocaine and ethanol are coadministered. Ethanol in addition to resulting in the formation of cocaethylene also inhibits the clearance of cocaine from the body. The decreased clearance causes a greater accumulation of cocaine plasma concentrations with repeated administrations such as during a cocaine binge and an increase in the duration of activity due to the inhibition of cocaine's metabolism.
Thus, there is evidence from previous studies that the formation of cocaethylene and the inhibition of cocaine's metabolism are contributing factors to the increased cardiovascular effects seen after the co-administration of cocaine and ethanol, but there is not any evidence available to ascertain the potential pharmacodynamic interaction between cocaine and ethanol of which some investigations have speculated. The present study was designed to determine if ethanol administration prior to giving cocaine enhanced the effects on the cardiovascular system compared to cocaine given alone. The dog was deemed a good model for the study of a pharmacodynamic interaction between cocaine and ethanol because our previous experience using the cocaine and ethanol doses given in this study did not produce quantifiable plasma concentrations of cocaethylene (Parker et al. 1998). Thus, the combined effects of cocaine and ethanol on the cardiovascular system could be evaluated without the being confounded by the formation of the active metabolite, cocaethylene.
Methods
Animal Model
This protocol was approved by the Animal Care and Use Committee of the University of Tennessee, Memphis. Six, adult, conditioned, mongrel dogs weighting between 17.2 and 20.6 kg underwent a one-week training program to acclimate them to standing in a nylon sling. Following this training period, each dog underwent a surgical procedure under general anesthesia for placement of an arterial indwelling silicone catheter with a subcutaneous access port (V-A-P Access Port, model 6PV, Access Technologies, Skokie, IL). The dogs were given post operative antibiotics to prevent infection and allowed to convalesce for seven days following surgery. Catheter patency was maintained by flushing daily with heparinized saline (250 units/ml).
Experimental Protocol
On each study day, the dog was put in the sling and an intravenous catheter placed in a foreleg vein for drug administration. Blood pressure was monitored continuously by connecting a Gold Stantham P23D6 pressure transducer and continuously monitored using an Electronics of Medicine VR-16 multi-channel recorder (Pleasantville, NY) via the arterial access port. Dogs received a 3 mg/kg dose of cocaine as the hydrochloride salt delivered by an infusion pump over approximately five minutes. On study days when both ethanol and cocaine were given, 1g/kg of ethanol was given immediately prior to the cocaine infusion as a 40-minute infusion of an ethanol-saline solution. Each dog received cocaine alone and ethanol prior to cocaine on separate study days in this repeated measures design.
Arterial blood samples (4 ml) were collected for the determination of cocaine, benzoylecgonine, and cocaethylene plasma concentrations at 0, 3, 5, 10, 20, 35, 65, 125, 185, and 425 minutes after the start of the cocaine infusion. Samples were collected into Vacutainer® tubes containing 30 mg of sodium fluoride, gently mixed and put on ice immediately. Within thirty minutes of sample collection, plasma was separated by centrifugation for 10 minutes at 2000 rpm and stored on ice until transferred to an −70°C freezer until analysis. Plasma cocaine and cocaethylene concentrations were determined by an HPLC method developed in our laboratory as previously described (Williams et al. 1996).
Data Analysis
The following general equation was fit to the cocaine plasma concentrations using WinNonlin (version 4.1),
where Ci is the ith coefficient, λi is the ith exponent, n equals the number of coefficient and exponent pairs, t equals the time after the start of the infusion, T is equal to the infusion time, and t-T equals the post infusion time (when t ≤ T, t=T). Clearance (Cl) was calculated by:
and volume of distribution at steady state (Vss) was calculated by:
and half-life (t½) was calculated by:
where λz equals the elimination rate constant of the terminal slope.
The concentration-effect relationship was evaluated by fitting a simple Emax model to the concentration-effect plot from zero to 65 minutes using the following model equation and a weight of 1.0 (no weight):
where Eo is the effect at baseline, Emax is the maximum effect, EC50 is the plasma concentration that achieves one-half the maximum effect, and C is the measured plasma concentration.
The maximum observed effect for heart rate, systolic and diastolic blood pressure, and QRS duration expressed as the percent increase over baseline, and pharmacokinetic and pharmacodynamic model parameter estimates were compared between the cocaine 3 mg/kg and cocaine 3 mg/kg + ethanol 1g/kg treatment phases using a paired t-test.
Results
The model of best fit to the cocaine plasma concentration-time data was a two compartment model using a weight factor of 1/y^ (the reciprocal of the predicted concentration). The estimates of the pharmacokinetic parameters are given in Table 1 along with the peak concentrations achieved (Cmax), the maximum effect of each measured cardiovascular parameter as a percent relative to baseline, and the mean ± standard deviation for the parameters of the simple Emax fits, Eo (baseline effect), EC50 (cocaine plasma concentration at one-half the Emax), and the Emax (the maximum possible effect). There was a significant decrease in the Cl of cocaine when ethanol administration preceded the cocaine infusion (1.24 versus 0.79 L/min, p<0.05). The peak effects are similar though there appears to be a slightly greater effect of the cocaine-ethanol combination on blood pressure at the latter time points as seen in Figure 1. However, no significant differences in the blood pressure effect of cocaine alone and after ethanol administration occurred at any time point. The maximum effect was not altered by the administration of 1 g/kg of ethanol prior to cocaine administration with no differences in the percent increases in heart rate, systolic blood pressure, diastolic blood pressure, or QRS duration between the two treatment phases (see Table 1). The parameter estimates of the simple Emax model also did not differ between treatment phases with essentially superimposable predicted concentration-effect relationships for heart rate, systolic and diastolic blood pressure (see Figure 2).
Table 1. Pharmacokinetic and Pharmacodynamic Parameter Estimates.
Estimates ± standard deviation from pharmacokinetic analysis, the maximum percent increase over baseline for heart rate, systolic and diastolic blood pressure, and QRS duration, and the estimates for Eo, EC50, and Emax from six dogs given cocaine alone and cocaine + ethanol. Only the Cl was found to be significantly different between the cocaine alone and cocaine + ethanol treatment phases.
| Cocaine | Cocaine+Ethanol | |||||
|---|---|---|---|---|---|---|
| Pharmacokinetics | ||||||
| k (min−1) | 0.016±0.0040 | 0.0119±0.0041 | ||||
| Vss (L/kg) | 2.5±0.61 | 3.1±0.97 | ||||
| Cl (L/min) | 1.24±0.123 | 0.79±0.283† | ||||
| Cmax (ng/ml) | 2838±497 | 2804±1016 | ||||
| Maximum Effect over Baseline | ||||||
| Heart Rate | 49±31% | 53±28% | ||||
| Systolic BP | 46±13% | 49±28% | ||||
| Diastolic BP | 88±44% | 79±37 | ||||
| QRS | 18±10% | 24±10% | ||||
| Simple Emax | Eo | EC50 | Emax | Eo | EC50 | Emax |
| Heart Rate (beats/min) | 93±16 | 4482±5330 | 188±62 | 96±15 | 1477±1239 | 164±47 |
| Systolic BP (mmHg) | 152±22 | 276±161 | 224±19 | 148±23 | 565±404 | 233±35 |
| Diastolic BP (mmHg) | 94±15 | 619±164 | 166±13 | 94±10 | 403±183 | 153±9 |
| QRS (msec) | 76±7 | 11797±6014 | 128±32 | 61±8 | 599±7549 | 94±17 |
Figure 1.
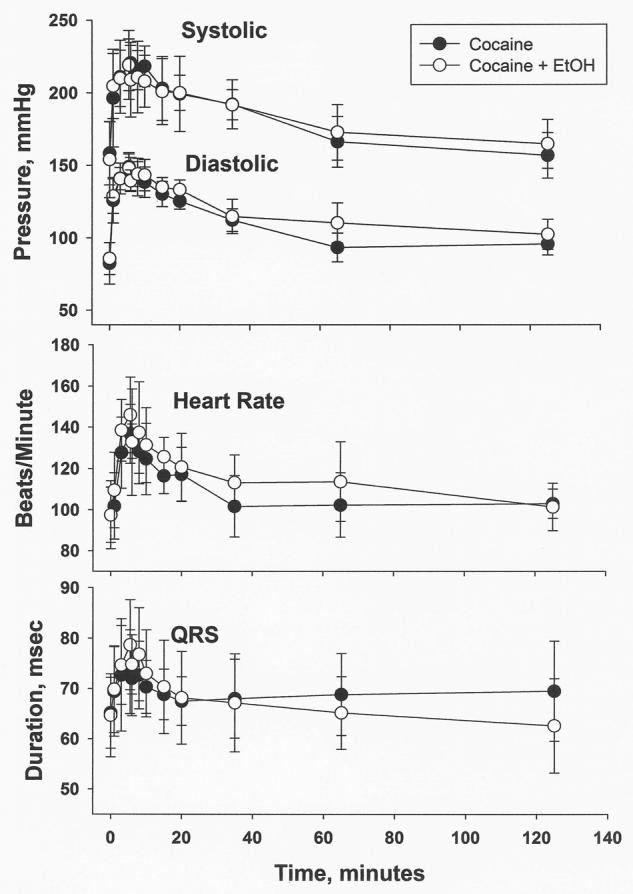
Plots of blood pressure, heart rate, and QRS duration (mean±standard deviation, n=6) versus time: from 0 to 125 minutes post-infusion of 3 mg/kg of cocaine given alone and after a 3 mg/kg cocaine infusion preceded by a 1 g/kg ethanol infusion.
Figure 2.
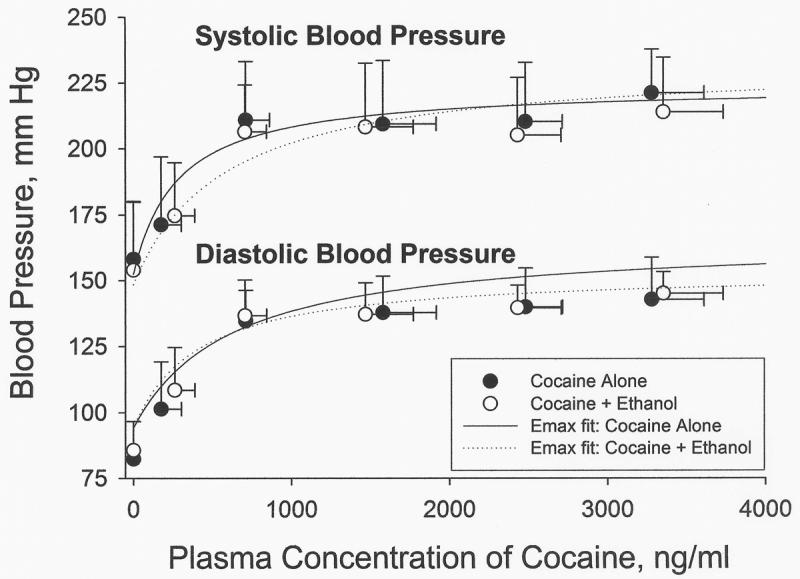
Concentration-Effect plots of blood pressure. Each data point represents the mean concentration-effect with the vertical error bar indicating the standard deviation of the measured effect and the horizontal error bar representing the standard deviation in the cocaine plasma concentration. The solid and broken lines are derived from the simple Emax model equation using the mean (n=6) values of Eo, EC50, and Emax for cocaine alone and cocaine + ethanol. The virtually superimposable Emax fits indicate that ethanol does not alter cocaine's concentration-effect relationship.
Discussion
The interaction between cocaine and ethanol results in increased morbidity and mortality due primarily to cardiovascular toxicity (Henning et al. 1994; McCance-Katz et al. 1998; Pennings et al. 2002; Rose 1994). Three mechanisms have been proposed as potentially contributing to the enhanced cardiovascular toxicity when cocaine and ethanol are co-administered; the inhibition of cocaine elimination by ethanol, the formation of the active metabolite, cocaethylene, in the presence of ethanol, and a pharmacodynamic interaction between cocaine and ethanol (Henning and Wilson 1996; Henning et al. 1994; McCance-Katz et al. 1998; Wilson et al. 2001). The metabolism of cocaine to inactive metabolites by carboxylesterases is inhibited by ethanol causing a decrease in cocaine clearance (Farre et al. 1993; McCance-Katz et al. 1998; Perez-Reyes and Jeffcoat 1992) and increased exposure to cocaine. Cocaine is an extensively metabolized drug with only 2% of an intravenous dose recovered from the urine as unchanged drug (Ambre et al. 1988). The predominant pathway for cocaine's elimination is by enzymatic hydrolysis with the formation of the inactive metabolites benzoylecgonine and ecgonine methyl ester that undergo renal elimination (Ambre 1985; Ambre et al. 1984). In most individuals studied, the greatest proportion of the administered dose is eliminated as benzoylecgonine followed by ecgonine methyl ester (Ambre et al. 1988; Cone et al. 1998; Kolbrich et al. 2006).
Ingestion of ethanol prior to cocaine administration decreases the clearance of cocaine and reduces the formation of benzoylecgonine. The presence of ethanol also results in the formation of the active metabolite, cocaethylene, by transesterification with ethanol (Bourland et al. 1998). Cocaethylene is reported to have similar pharmacological activity on the cardiovascular system and in the central nervous system as cocaine. Direct comparisons of the cardiovascular effects of cocaine and cocaethylene report it is equipotent or slightly less potent than cocaine on heart rate, diastolic blood pressure, and systolic blood pressure (Hart et al. 2000; Schindler et al. 2001), and it is a more potent sodium channel blocker in the myocardium than cocaine (Xu et al. 1994). Both, the formation of cocaethylene and the inhibition of cocaine elimination contribute to an increase in the cardiovascular effects when ethanol is co-ingested with cocaine. A third potential mechanism is direct synergistic activity between cocaine and ethanol on the cardiovascular system (Farre et al. 1993; Henning et al. 1994; McCance-Katz et al. 1998). It is hypothesized that while ethanol has minimal cardiovascular effects when given alone that it might enhance the cardiovascular effects of cocaine by a mechanism separate from the inhibition of cocaine elimination or formation of cocaethylene.
In the present study, the pharmacokinetic and pharmacodynamic interaction between cocaine and ethanol was studied in the dog. This allowed an evaluation of the cocaine-ethanol interaction without the confounding factor of cocaethylene formation as no quantifiable concentrations of cocaethylene occurred (lower limit of quantification = 25 ng/ml). In a previous study conducted in our laboratory of cocaethylene's pharmacodynamics in the dog, a 1 mg/kg dose of cocaethylene producing only mild cardiovascular effects resulted a mean peak concentration (n=6) of 1381 ng/ml (Parker et al. 1998). This previous data demonstrated that a cocaethylene concentration of 25 ng/ml is a subphysiologic concentration relative to the cardiovascular effects measured in this study.
There was no evidence of synergistic or additive cardiovascular effects of cocaine-ethanol administration as the concentration-effect relationship was the same when cocaine was given alone and when it was given after the administration of 1g/kg of ethanol. These results suggest that a pharmacodynamic interaction between cocaine and ethanol does not contribute to the increased cardiovascular effects that occur with the co-administration of cocaine and ethanol.
The co-ingestion of cocaine and ethanol has been studied in both animals and humans. The inhibition of cocaine's conversion to benzoylecgonine with a significant decrease in cocaine's clearance ranging from 10 – 25% has been a consistent finding in several species studied (Hart et al. 2000; McCance-Katz et al. 1998; Pan and Hedaya 1999; Parker et al. 1998). However, there has been inconsistency in the reported alteration in peak cocaine concentrations and pharmacological effects with the co-administration of cocaine and ethanol. Some studies report an increase in peak cocaine concentrations or effects (Cami et al. 1998; Farre et al. 1997; Farre et al. 1993; Foltin and Fischman 1989; McCance-Katz et al. 1998; Perez-Reyes and Jeffcoat 1992) while other studies report no significant changes (Henning et al. 1994; Laizure et al. 2003; Parker et al. 1996; Uszenski et al. 1992). The differing results can be reconciled by taking into account the route of cocaine administration used in the studies. Cocaine behaves pharmacokinetically as a high-extraction, hepatically eliminated drug undergoing significant first-pass metabolism, which will result in a significant difference in cocaine disposition depending on the route of administration. Studies administering cocaine orally, by insufflation (snorting), or intraperitoneally that are subject to first-pass metabolism will demonstrate increased cocaine peak concentrations and effects in the presence of ethanol, while administration by routes not subject to first-pass metabolism i.e., inhalation (smoking) or intravenously, will demonstrate no change in the peak cocaine concentration or effect.
The other contributing factor to enhanced cardiovascular effects of the cocaine-ethanol combination is the formation of cocaethylene. After a single dose of intravenous cocaine in humans approximately 17% of the administered dose is ultimately converted to cocaethylene (Harris et al. 2003). This relatively low rate of formation is consistent with the low cocaethylene concentrations found in patients admitted to emergency rooms exhibiting signs of cocaine toxicity and forensic studies in which the reported cocaethylene concentrations ranged from 0 to 249 ng/ml (Bailey 1993; Blaho et al. 2000; Brookoff et al. 1996; Harris et al. 2003). The corresponding cocaine concentrations ranged from 26 to 1455 ng/ml. This data, though limited, does not support the contention that the slower elimination of cocaethylene will lead to increased exposure to cocaethylene compared to cocaine with repeated cocaine dosing in the presence of ethanol. This presumption has often been stated based on the longer half-life of cocaethylene. However, the maximum plasma concentrations achieved after repeated dosing is determined by the fraction of cocaine converted to cocaethylene and the clearance of the drug not its volume or half-life. In humans cocaethylene is reported to have a larger volume of distribution than cocaethylene, 2.74 versus 1.94 L/Kg, which will result in a longer half-life. The reported mean clearance values differed by only about 20% (Hart et al. 2000). Thus, the accumulation of cocaethylene after the repeated administration of cocaine in the presence of ethanol does not appear to result in high concentrations of cocaethylene due to the relatively small fraction of the cocaine dose converted to cocaethylene and its rapid clearance. This may explain why cocaethylene levels in patients admitted to emergency rooms with cocaine toxicity after cocaine and ethanol ingestion have demonstrated relatively low cocaethylene concentrations even though cocaethylene has a significantly longer half-life than cocaine.
The co-abuse of ethanol with cocaine results in an increase in the cardiovascular effects of cocaine on the cardiovascular system by the inhibition of cocaine hydrolysis and the formation of the active metabolite, cocaethylene. In this study of cocaine and ethanol co-administration in the dog, there was no evidence of a pharmacodynamic interaction contributing to the enhanced cardiovascular effects reported when cocaine and ethanol are co-administered.
Acknowledgments
This study was supported by a National Institutes of Health grant from the National Heart, Lund and Blood Institute, R15HL54311
Abbreviations
- Emax
The maximum possible response
- EC50
The plasma concentration that produces 50% of the maximum possible response
- Eo
The effect at baseline prior to agonist administration
- Cmax
The peak plasma drug concentration
- Cl
The clearance of drug from the body
- k
The terminal elimination rate constant for a drug
- Vss
The volume of distribution at steady state for a drug
References
- Ambre J. The urinary excretion of cocaine and metabolites in humans: a kinetic analysis of published data. J Anal Toxicol. 1985;9:241–5. doi: 10.1093/jat/9.6.241. [DOI] [PubMed] [Google Scholar]
- Ambre J, Fischman M, Ruo TI. Urinary excretion of ecgonine methyl ester, a major metabolite of cocaine in humans. J Anal Toxicol. 1984;8:23–5. doi: 10.1093/jat/8.1.23. [DOI] [PubMed] [Google Scholar]
- Ambre J, Ruo TI, Nelson J, Belknap S. Urinary excretion of cocaine, benzoylecgonine, and ecgonine methyl ester in humans. J Anal Toxicol. 1988;12:301–6. doi: 10.1093/jat/12.6.301. [DOI] [PubMed] [Google Scholar]
- Bailey DN. Plasma cocaethylene concentrations in patients treated in the emergency room or trauma unit. Am J Clin Pathol. 1993;99:123–7. doi: 10.1093/ajcp/99.2.123. [DOI] [PubMed] [Google Scholar]
- Blaho K, Logan B, Winbery S, Park L, Schwilke E. Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. Am J Emerg Med. 2000;18:593–8. doi: 10.1053/ajem.2000.9282. [DOI] [PubMed] [Google Scholar]
- Bourland JA, Martin DK, Mayersohn M. In vitro transesterification of cocaethylene (ethylcocaine) in the presence of ethanol. esterase-mediated ethyl ester exchange esterase-mediated ethyl ester exchange. Drug Metab Dispos. 1998;26:203–6. [PubMed] [Google Scholar]
- Brookoff D, Rotondo MF, Shaw LM, Campbell EA, Fields L. Coacaethylene levels in patients who test positive for cocaine. Ann Emerg Med. 1996;27:316–20. doi: 10.1016/s0196-0644(96)70266-4. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Gonzalez ML, Segura J, de la Torre R. Cocaine metabolism in humans after use of alcohol. Clinical and research implications. Recent Dev Alcohol. 1998;14:437–55. doi: 10.1007/0-306-47148-5_22. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Tsadik A, Oyler J, Darwin WD. Cocaine metabolism and urinary excretion after different routes of administration. Ther Drug Monit. 1998;20:556–60. doi: 10.1097/00007691-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Gonzalez ML, Teran MT, Roset PN, Menoyo E, Cami J. Cocaine and alcohol interactions in humans: neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther. 1997;283:164–76. [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Cami J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–73. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of the combination of cocaine and marijuana on the task-elicited physiological response. NIDA Res Monogr. 1989;95:359–60. [PubMed] [Google Scholar]
- Harris DS, Everhart ET, Mendelson J, Jones RT. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug Alcohol Depend. 2003;72:169–82. doi: 10.1016/s0376-8716(03)00200-x. [DOI] [PubMed] [Google Scholar]
- Hart CL, Jatlow P, Sevarino KA, McCance-Katz EF. Comparison of intravenous cocaethylene and cocaine in humans. Psychopharmacology (Berl) 2000;149:153–62. doi: 10.1007/s002139900363. [DOI] [PubMed] [Google Scholar]
- Hearn WL, Rose S, Wagner J, Ciarleglio A, Mash DC. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacol Biochem Behav. 1991;39:531–3. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- Henning RJ, Wilson LD. Cocaethylene is as cardiotoxic as cocaine but is less toxic than cocaine plus ethanol. Life Sci. 1996;59:615–27. doi: 10.1016/0024-3205(96)00227-5. [DOI] [PubMed] [Google Scholar]
- Henning RJ, Wilson LD, Glauser JM. Cocaine plus ethanol is more cardiotoxic than cocaine or ethanol alone. Crit Care Med. 1994;22:1896–906. [PubMed] [Google Scholar]
- Kolbrich EA, Barnes AJ, Gorelick DA, Boyd SJ, Cone EJ, Huestis MA. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J Anal Toxicol. 2006;30:501–10. doi: 10.1093/jat/30.8.501. [DOI] [PubMed] [Google Scholar]
- Laizure SC, Mandrell T, Gades NM, Parker RB. Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases. Drug Metab Dispos. 2003;31:16–20. doi: 10.1124/dmd.31.1.16. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biol Psychiatry. 1998;44:250–9. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- Mehta MC, Jain AC, Billie M. Effects of cocaine and alcohol alone and in combination on cardiovascular performance in dogs. Am J Med Sci. 2002;324:76–83. doi: 10.1097/00000441-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Hedaya MA. Cocaine and alcohol interactions in the rat: effect on cocaine pharmacokinetics and pharmacodynamics. J Pharm Sci. 1999;88:459–67. doi: 10.1021/js980282p. [DOI] [PubMed] [Google Scholar]
- Parker RB, Laizure SC, Williams CL, Mandrell TD, Lima JJ. Evaluation of dose-dependent pharmacokinetics of cocaethylene and cocaine in conscious dogs. Life Sci. 1998;62:333–42. doi: 10.1016/s0024-3205(97)01115-6. [DOI] [PubMed] [Google Scholar]
- Parker RB, Williams CL, Laizure SC, Mandrell TD, LaBranche GS, Lima JJ. Effects of ethanol and cocaethylene on cocaine pharmacokinetics in conscious dogs. Drug Metab Dispos. 1996;24:850–3. [PubMed] [Google Scholar]
- Pennings EJ, Leccese AP, Wolff FA. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97:773–83. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992;51:553–63. doi: 10.1016/0024-3205(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Rose JS. Cocaethylene: a current understanding of the active metabolite of cocaine and ethanol. Am J Emerg Med. 1994;12:489–90. doi: 10.1016/0735-6757(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM. The lethal effects of ethanol and cocaine and their combination in mice: implications for cocaethylene formation. Pharmacol Biochem Behav. 1995;52:245–8. doi: 10.1016/0091-3057(95)00098-h. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Zheng JW, Goldberg SR. Effects of cocaine and cocaine metabolites on cardiovascular function in squirrel monkeys. Eur J Pharmacol. 2001;431:53–9. doi: 10.1016/s0014-2999(01)01406-6. [DOI] [PubMed] [Google Scholar]
- Uszenski RT, Gillis RA, Schaer GL, Analouei AR, Kuhn FE. Additive myocardial depressant effects of cocaine and ethanol. Am Heart J. 1992;124:1276–83. doi: 10.1016/0002-8703(92)90412-o. [DOI] [PubMed] [Google Scholar]
- Williams CL, Laizure SC, Parker RB, Lima JJ. Quantitation of cocaine and cocaethylene in canine serum by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;681:271–6. doi: 10.1016/0378-4347(95)00536-6. [DOI] [PubMed] [Google Scholar]
- Wilson LD, Jeromin J, Garvey L, Dorbandt A. Cocaine, ethanol, and cocaethylene cardiotoxity in an animal model of cocaine and ethanol abuse. Acad Emerg Med. 2001;8:211–22. doi: 10.1111/j.1553-2712.2001.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Crumb WJ, Jr., Clarkson CW. Cocaethylene, a metabolite of cocaine and ethanol, is a potent blocker of cardiac sodium channels. J Pharmacol Exp Ther. 1994;271:319–25. [PubMed] [Google Scholar]


