Abstract
Cancer immunotherapy is one the most effective approaches for treating patients with tumors, as it bolsters the generation and persistence of memory T cells. In preclinical work, it has been reported that adoptively transferred CD4+ and CD8+ lymphocytes that secrete IL-17A (i.e., Th17 and Tc17 cells) regress tumors to a greater extent than IFN-γ+Th1 or Tc1 cells in vivo. Herein, we review the mechanisms underlying how infused Th17 and Tc17 cells regress established malignancies in clinically relevant mouse models of cancer. We also discuss how unique signaling cues—such as co-stimulatory molecules (ICOS and 41BB), cytokines (IL-12 and IL-23) or pharmaceutical reagents (Akt inhibitors, etc.)—can be exploited to bolster the therapeutic potential of IL-17+ lymphocytes with an emphasis on using this knowledge to improve next-generation clinical trials for patients with cancer.
Keywords: Th17, Tc17, Cancer, Immunotherapy, ACT
Introduction
After decades of skepticism that the immune system could be harnessed to kill tumors, new approaches for effectively treating cancer patients have emerged promising in the clinic. For example, patients with melanoma experienced robust antitumor responses after treatment with a combination of antibodies that block co-inhibitory molecules PD-1 and CTLA-4 on exhausted T cells [1]. Advances in gene therapy also show that the patient's T cells can be engineered with a tumor antigen-specific receptor [2–4]. Adoptive transfer of these engineered T cells into patients with acute lymphocytic leukemia mediated treatment outcomes of record efficacy, resulting in complete remission in ~90 % of children and adult patients [5, 6]. Novel clinical trials also reveal compelling evidence that mutation-reactive CD4+ T cells are capable of mediating tumor regression in a patient with epithelial cancer [7]. Consequently, now even the most cynical folks believe that the body's own immune system can be harnessed to kill tumors. These clinical examples are just some of the reasons why the journal Science Magazine selected cancer immunotherapy as the 2013 “Breakthrough of the Year” [8].
While there has been success in cancer immunotherapy in the past few years, there exists room for improvement. Not all individuals benefit from these approaches. For instance, adoptive cell therapy (ACT)-based clinical trials do not consistently mediate cures in patients with solid tumors, marred by the use of exhausted T cells [9–11]. A lack of means of developing durable T cell potency has hampered advances in the field. Herein, we highlight recent efforts to generate memory T cells, as these cells are persistent and mount rapid recall responses to tumors. We first review basic properties of memory CD4+ and CD8+ T cells in tumor immunity. We then focus on an emerging memory CD4+ T cell subset with stem cell properties that secretes IL-17A, called Th17 cells. From a clinical perspective, Th17 cells’ potential for longevity and self-renewal present hope for mediating prolonged patient responses against tumor recurrence. We discuss ways to manipulate Th17 as well as IL-17-producing CD8+ T cells, termed Tc17 cells, via co-stimulation, cytokines and pharmaceutics to potentiate treatment outcome in patients.
Memory CD8+ versus CD4+ T cells in cancer immunotherapy
Cytotoxic CD8+ T cells have long been regarded as the ideal cell for ACT, due to their ability to lyse tumors directly. However, as CD8+ T cells are expanded ex vivo to large numbers prior to infusion into patients, they become progressively more differentiated and less effective in vivo. Even though these effector memory CD8+ T cells (denoted by their high CD44 and low CD62L expression) are potent initially, they do not persist and tumors relapse [12]. Stem and central memory CD8 lymphocytes, which are antigen experienced, yet less differentiated, than effector memory CD8+ T cells, have emerged more efficacious and persistent in clinically relevant mouse models and in human clinical trials, as discussed elsewhere [13–16]. Although CD8+ T cells are important in mounting immunity to tumors, it has been shown in clinical trials that they are not always effective when infused alone into patients with melanoma. One reason for this poor outcome by CD8+ T cells is that the tumor finds ways to hide from the T cells. Specifically, it is well appreciated that CD8+ T cells recognize tumor endogenous antigens in the context of MHC class I, which are downregulated due to genetic instability and heterogeneity of tumor cells. This phenomenon impairs CD8+ T cell-mediated recognition of tumors [17]. Thus, although CD8+ T cells are the “frontline” defenders against the transformed cell, it seems they are not always able to protect the host for tumor relapse. Some evidence suggests that CD4+ T cells (along with CD8+ T cells) are promising, as they are able to coordinate with and sustain the rest of the immune system to attack the tumor. Helper CD4+ T cells recognize MHC class II on DCs, and tumors are inherently equipped to engage the entire immune system to fight against tumors long term, thus rendering them appealing for next-generation clinical trials.
There has been increased enthusiasm for the use of personalized CD4+ T cells for the adoptive immunotherapy of cancer, due to their promise in a recent clinical trial. In this trial, Tran and co-workers used whole-exome sequencing-based approach to reveal that tumor-infiltrating lymphocytes from a patient with metastatic cholangiocarcinoma contained CD4+ Th1 cells recognize a mutation in erbb2 interacting protein expressed by the cancer. After the transfer of TIL containing about 25 % mutation-specific Th1 cells, the patient achieved prolonged disease stabilization. After tumor progression, the patient was retreated with ~95 % of their mutation-reactive Th1 cells. The patient again experienced tumor regression, underscoring that a CD4+ T cell response against a mutated tumor antigen can mediate regression of a metastatic epithelial cancer [7, 18]. One classic way that helper CD4+ T cells contribute to anticancer immunity is by producing effector cytokines. In preclinical models, it has been shown that the cytokines produced by CD4+ T cells can mediate the recruitment of cytotoxic CD8+ T cells, as well as attracting neutrophils and NK cells to the tumor [17]. Further evidence shows that CD4+ T cells can directly lyse tumors upon transfer into the host [17, 19]. Collectively, these data uncover that CD4+ T cells have unrealized potential to kill tumors, perhaps by circumventing some limitations that render CD8+ T cells ineffective long term.
T helper subsets in tumor immunity
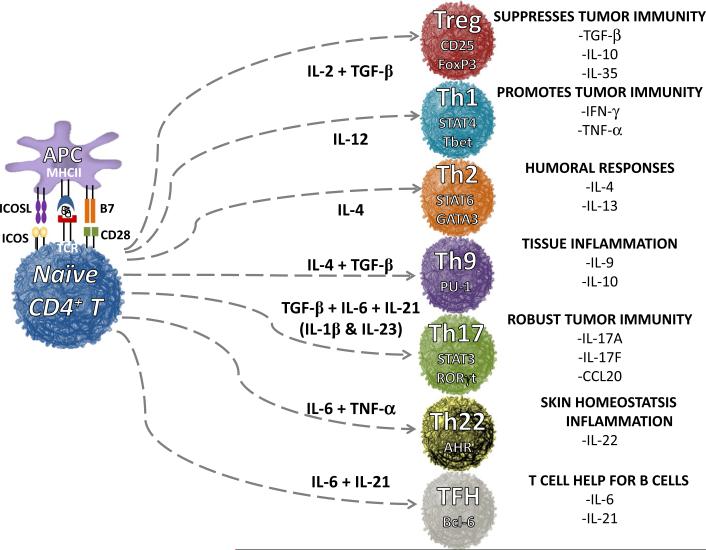
Naïve CD4+ T cells differentiate into distinct subsets and elicit either immune rejection or suppression of tumors [20]. Depending on the signaling cues that naïve CD4+ lymphocytes receive during activation, they can induce distinct transcriptional factors that impact their fate [21]. Depending on the cytokines and co-stimulatory/co-inhibitory cues received, CD4+ T cells differentiate into one of several subsets, including Th1, Th2, Th9, Th17, Th22, TFH and regulatory T cells (Treg) [22–24], as depicted in Fig. 1.
Fig. 1.
Differentiation of helper T cell subsets from naïve CD4+ T cells mediated by cytokines and co-signals. Th1, Th2, Th17, Th9, Th22, follicular T helper cells (TFH) and regulatory T cells (Treg) are induced via various cytokines and co-stimulatory molecules produced by antigen-presenting cells, such as dendritic cells and macrophages. These distinct subsets regulate immune response to foreign, self and tumor antigens. FoxP3 forkhead box P3, STAT4 signal transducer and activator of transcription protein 4, T-bet T-box transcription factor, STAT6 signal transducer and activator of transcription protein 6, GATA3 transacting T cell-specific transcription factor, PU-1 (ETS—domain transcription factor), STAT3 signal transducer and activator of transcription protein 3, RORγt retinoic acid-related orphan receptor gamma, AHR aryl hydrocarbon receptor and Bcl-6 B cell lymphoma 6 protein
Nearly 30 years ago, two helper CD4 subsets were defined: Th1 cells that secrete interferon gamma (IFN-γ) promote cell-mediated immunity, while Th2 cells that produce interleukin 4 (IL-4) support humoral immunity [25]. Both subsets augment, while Treg cells dampen antitumor CD8+ T cell activity [25–29]. Twenty years after the identification of Th1 and Th2 subsets, a third and distinct helper subset classified by its ability to secrete IL-17A, called Th17 cells, was discovered [30] and has shed tremendous light on the role of Th17 biology in autoimmunity and in tumor immunity [31]. At least in the context of murine cells, CD8+ T cells have been found to skew to a Tc1, Tc2 or Tc17 phenotype as well [32, 33].
Th17 cells contribute to autoimmunity, while their role in tumor immunity remains partly elucidated [34]. Some reports show that Th17 cells eradicate tumors, while others reveal that they promote tumor growth [35]. Recent work has provided insight into the conflicting roles of Th17 functions in tumor immunity and has shown that Th17 fate is regulated by many factors, including the stimuli to which they are exposed to during activation, including TCR signal strength, intestinal bacterial antigens, co-stimulation/co-inhibition and cytokines [36–39] as well as the tumor microenvironment and the context of the therapeutic intervention [31, 40]. Understanding the cues that control Th17 responses in the tumor is important for advancing the field. Herein, we discuss the role of Th17 and Tc17 in ACT, which have shown potential in murine and humanized ACT mouse models of cancer.
Th17 cell function, phenotype and plasticity
Naïve CD4+ T cells undergo differentiation into specific subsets via distinct cytokines. How particular cytokines influence their differentiation toward various subsets is in Fig. 1 and described elsewhere [20, 23]. Briefly, CD4+ T cells differentiate into a Th1 phenotype via IL-12 and into a Th17 phenotype via TGF-β and IL-6 [41]. IL-23 maintains Th17 proliferation and function long term [42]. The Kuchroo laboratory reported that IL-23-induced TGF-β3 promotes pathogenic Th17 cells in autoimmunity, as indicated by their gene expression of Tbx21, RORC, ICOS, IL-23R, IL-7R and more [43]. How driving Th17 cells toward a regulatory versus pathogenic profile impacts their ability to kill tumors is under investigation, and the level and amount of certain cytokines have clearly been shown to impact their ability to survive, function and regress tumors in ACT models [38, 44, 45], as discussed later in this review.
Traditionally, Th17 cells have been characterized by their capacity to secrete IL-17A, IL-17F, IL-22 and CCL20 [46–48]. Master transcription factors retinoic acid-related orphan receptor (ROR)γt, RORα and interferon regulatory factor 4 (IRF4) [49–53] control Th17 cell development and function. IL-23 supports Th17 generation and was found to induce the expression of runt-related transcription factor 1 and 3 (RUNX1 and RUNX3) [54]. RUNX1 and RUNX3 promote Th17 differentiation by enhancing RORγt expression [55]. Th17 cells can be detected in humans, as they express heightened levels of various extracellular proteins on their cell surface. These markers include chemokine receptor 6 (CCR6), the inducible co-stimulator (ICOS), IL-23 receptor (IL-23R), CD146 (MCAM) and ectoenzyme CD26. These Th17 markers have not only helped investigators discern them from other subsets [56–59], but helped investigators determine their phenotype and role in various diseases. Human Th17 cells are mainly effector memory lymphocytes with a small population of central memory cells. Human Th17 cells express high CD45RO, low CD45RA and low/intermediate amounts of CCR7 and CD62L [60].
Not only do Th17 cells possess a differentiated phenotype, but they can convert into a Th1-like lineage over time—denoted by their ability to switch from mainly IL-17A producers to IFN-γ producers and/or IFN-γ/IL-17A double producers over time. This process is known as plasticity [61]. Th17 plasticity can hamper an investigator's ability to discriminate Th17 from Th1 cells. Alas, the surface marker lectin-like receptor CD161 can be used to distinguish these subsets [62]. Th17 cell precursors can be detected by the expression of CD161 on T cells from cord blood, and these cells secrete IL-17A when activated in the presence of TGF-β and IL-23. Conversely, when Th17 cells are exposed to IL-12, they convert into a hybrid T cell phenotype that co-produces IFN-γ and IL-17A and expresses RORγt, T-bet and CD161. IL-12 not only induces T-bet but also represses histone markers in the RORγt locus [63]. T-bet then interacts with RUNX1 or RUNX3 to disrupt their interaction with RORγt [54]. In the presence of IL-12, RUNX1 binds to the IFN-γ promoter. T-bet and RUNX1 activation is needed for the maximum secretion of IFN-γ by Th17 cells. In the presence of Th17-promoting cytokines and at low RUNX1 levels, the Th17 phenotype is retained and mainly secretes IL-17A. Depending on the level of RUNX1 and inflammatory cytokines, the formation of the RUNX1/T-bet complex in Th17 cells leads to the generation of IFN-γ+IL-17A+ T cell independent of RORγt expression [54]. Continued exposure of Th17 cells to IL-12 forces them to a Th1 phenotype that no longer secretes IL-17A, and these cells, coined “non-classic Th1 cells,” are different from classic Th1 cells by their RUNX1/3 and CD161 expression. Although it is clear that there exists a significant difference in the function, transcription and phenotype of non-classic and classic Th1 cells, it remains elusive how these distinct cells regulate tumor immunity. It is likely that investigators will define their distinct roles in cancer immunotherapy in the near future.
The distribution of Th17 cells in the body
Th17 cells rarely reside in the peripheral blood of cancer patients, but are slightly more abundant in the tumor [64–67]. Th17 cells impair immune surveillance and promote tumor growth in mice and humans [68, 69]. The protumorigenic properties of Th17 cells are not discussed in this review, but can be found elsewhere [20]. In other instances, Th17 cells mediate potent antitumor responses in mice with tumors to a greater extent than Th1 cells (often thought to be the ideal subset for tumor immunity) [70, 71]. Those intriguing studies involved an ACT approach, which comprised of ex vivo expanding Th17 cells to large numbers (these Th17 cells expressed a T cell receptor (TCR) or chimeric antigen receptor (CAR) that recognizes tumor antigens, such as tyrosinase and mesothelin) [70]. This approach suggests that ACT trials utilizing Th17 cells would be promising in human patients. Improving the persistence and antitumor activity of Th17 cells and their influence on other immune cells via various cues is discussed below.
Factors and pharmaceutics that regulate antitumor Th17 cell properties
Many factors regulate Th17 cells in the tumor. These factors include: (1) the source of the Th17 cells (arising naturally via tumor growth or transferred following ex vivo manipulation); (2) the functional phenotype of the cells driven by particular cytokines (effector or regulatory); and/or (3) exposure to distinct therapeutic interventions (such as chemotherapy, ACT or checkpoint modulators) [20, 37, 72–74]. Defining how Th17 cells regulate immune responses in the context of these factors, as well as how these factors impact patient survival, is of profound interest to the field. Curiously, Th17 cells possess either regulatory or inflammatory properties depending on the stimuli they encounter [75, 76]. This property may explain why Th17 cells mediate antitumor activity in some experimental regimens but foster tumor growth in other situations.
One explanation for the provocative nature of Th17 cells in tumor immunity could be that different tumor types promote Th17 cells with distinct fates. Indeed, the high number of Th17 cells that infiltrate tumors in patients with colon cancer correlates with poor prognosis [77]. Conversely, improved survival is associated with more Th17 cells in ovarian cancer patients [78–82]. These contrasting findings make it difficult to ascribe if Th17 cells are good or bad in cancer progression. Yet, there is still more to consider: how tumors regulate downstream signaling pathways in Th17 cells might also impact their survival in vivo. Indeed, natural versus induced Th17 cells differentially regulate Akt and mTOR pathways [83]. The role of Akt is particularly relevant given recent developments that pharmacologic inhibition of the serine/threonine Akt pathway augments antiviral memory CD8+ T cells in vivo [84]. In the context of adoptive immunotherapy, pharmacologic inhibition of Akt was found to enable the expansion of tumor-infiltrating lymphocytes (TILs) with the transcriptional, metabolic and functional properties characteristic of memory T cells. Akt inhibition enhanced the persistence and antitumor activity of TIL after infusion into lymphodepleted mice with melanoma [69]. Although it is clear that pharmacologic Akt inhibition enhances the persistence of antitumor CD8+ T cells, it remains unexplored how these pathways influence Th17 cells in tumor immunity in mice and humans. On that same note, it remains unexplored how other commonly used pharmaceutics, such as histone deacetylase (HDAC) inhibitors (found to modulate IL-6-dependent CD4+ T cell polarization [85]), will regulate the disease pathogenesis of distinct malignancies. We predict that these questions will be resolved soon and manifest discoveries that allow the field to create new ways to generate potent cellular products.
Memory CD8+ T cell and Th17 cell responses to tumors
Large numbers of naturally arising tumor-infiltrating lymphocytes (TILs) or TCR-engineered T lymphocytes have been generated to treat patients with melanoma at a few distinct institutes around the world. Aside from melanoma, and until recently, it has been challenging to generate T cells against other malignancies that recognize and kill tumors. Alas, engineering T cells to express antigen receptors (e.g., TCRs or CARs) against a particular malignancy has broadened the utility of ACT to treat patients beyond melanoma. Using this approach, translational T cell therapy groups have now treated patients with synovial cell sarcoma, cervical cancer, chronic/acute lymphocytic leukemia, multiple myeloma as well as epithelial carcinomas [5, 86–89]. Besides hematological cancers, most ACT-based clinical trials have not reached their full potential, marred by the use of short-lived lymphocytes [11]. Consequently, there is a need to generate clinical-grade T cell product with durable memory responses to immunosuppressive tumors. In the past few years, it has become clear that infused Th17 cells display persistence and the ability to drive rapid responses to aggressive malignancies in vivo [90]. Although transferred Th17 cells have not been used in the clinic, preclinical data imply that they might be ideal for cellular therapy. Our understanding of memory has been informed from studies of CD8+ T cells in infectious disease and cancer models. Thus, below, we review memory CD8+ T cell subsets (e.g., naïve, stem, central and effector) in tumor immunity and then reflect on these discoveries with CD8+ T cells with the objective of improving the antitumor properties of memory CD4+ T cells in cancer immunotherapy [91, 92].
Upon antigen recognition, T cells undergo clonal expansion followed by a contraction phase and the development of memory [93]. CD8+ T cells can acquire central memory qualities upon in vitro culture with IL-15; these cells possess potent antitumor activity in vivo compared to effector memory cells [94, 95]. Memory CD8+ T cells with stem cell memory properties, generated in vitro, destroy tumor even more effectively than central memory cells [13]. The prominent feature of these CD8+ T cells seems to be that the younger their phenotype, the more potent their potential antitumor response [96, 97]. The ability of CD4+ T cell subsets to persist with young phenotypes and consequently better protect the host for cancer is less understood.
Th17 cells possess a high proliferative potential upon antigenic re-encounter in vivo compared to their Th1 counterparts [90]. The finding that Th17 persists in vivo was unexpected given that they express extracellular markers of an effector memory phenotype in vitro (e.g., low CD62L and CCR7 and high CD44 levels). Yet, Th17 cells masqueraded as terminally differentiated effectors in vitro. However, once infused, Th17 cells unregulated CD62L and CCR7 in vivo, phenotypic of a less differentiated cell. Lef1 and Tcf7 (genes in the Wnt/β-catenin pathway) were expressed on Th17 cells to a greater extent than Th1 cells [90]. Th17 cells also converted into Th1-like progeny in vivo and possessed a self-renewing capacity. Dual functions were needed for Th17 cell-mediated tumor destruction because cells deficient in IFN-γ or IL-17A had impaired antitumor activity in vivo. Thus, in vivo Th17 cells are durable, polyfunctional and possess robust recall responses to cancers, all hallmarks of a CD4+ T lymphocyte with stemness.
Likewise, human Th17 cells display durable memory [60]. When transferred into xenograft mice, Th17 cells mediated antitumor immunity and persisted in vivo. These cells expressed antiapoptotic genes and were resistant to activation-induced cell death [98]. The molecular pathways associated with memory Th17 cells are attractive targets to augment cancer therapies. Investigators are devising clever ways to generate durable T cells to promote curative responses in patients. It is likely that human Th17 cells will be promising in the clinic, and they induce cytotoxic CD8+ T cells to traffic to and ablate tumor growth, as discussed below.
Do Th17 cells need CD8+ cells? The importance of team building...
Th17 cells activate CD8+ T cells in mice with melanoma, which improves the antitumor effect [71]. Th17 cells promote dendritic cell recruitment, thereby sponsoring the generation of cytotoxic CD8+ T cells in the tumor. Th17 cells secrete CCL20, which mediates CD8+ T cell trafficking to melanoma. Mice deficient in CCR6 (the receptor for CCL20) did not respond to Th17 therapy, suggesting Th17 cells bolster CD8+ T cell activation via a CCL20/CCR6 homing mechanisms. Collectively, this work suggests that Th17 cells orchestrate other immunological partners, such as CD8+ T cells, to de-bulk cancer.
Other reports caution against the simple idea that Th17 cells synergize with CD8+ T cells to kill tumors. CD4+ T cells have been reported to kill tumors directly [99]. These contrasting results highlight the need for follow-up studies on the role of Th17 with other immune cells in the tumor. Th17 cells—depending on context—mediate tumor regression in mice. Interactions between Th17 and CD8+ T cells may have certain consequences on treatment outcome; however, another question, with implications for treatment efficacy, concerns the proportion of Th17 and Treg cells on each other and on tumor regression.
The Treg/Th17 paradigm in tumor immunity
Treg cells suppress tumor immunity. In contrast to Treg cells, Th17 cells potentiate antitumor immune responses in ACT. Yet, there is a close relationship between Treg and Th17 cells due to their common need for cytokine IL-2 [100–102]. IL-2 signaling exerts opposing effects on Th17 and Treg cells in the tumor [67]. IL-2 has been reported to augment Th17 cell generation, which dampens Treg function in the tumor. These findings suggest that infused Th17 cells might reduce the Treg numbers in the host; abrogation of Treg suppression (via host preconditioning with chemotherapy and/or radiotherapy) offers one explanation for why the therapeutic outcome in Th17 treated mice is effective [37, 90]. Alternatively, it is possible that Treg cells require IL-2 for their in vivo maintenance and outcompete other subsets for this cytokine via a high-affinity IL-2 receptor alpha. Thus, it is conceivable that Treg cells impair Th17 persistence by depriving them of IL-2. Instead, given that IL-2 impairs Th17 differentiation, Treg cells may paradoxically support Th17 engraftment by acting as a IL-2 sink [103]. Consequently, host Treg depletion would impair Th17 longevity in vivo. Experiments that deplete Treg in mice would shed light on Th17–Treg paradigm in tumor immunity.
Exploiting antitumor Th17 and Tc17 cells in the clinic
Clinical trials with ACT primarily use bulk T cells, derived from TILs or gene-engineered lymphocytes to treat cancer patients. In these trials, T cells are expanded with IL-2 and a CD3 agonist (i.e., OKT3) or are expanded with magnetic beads coated with CD3 and CD28 agonists [104, 105]. Th17 cells have not been used in the clinic, but gene therapy now permits the opportunity to redirect these cells to treat a broad range of malignancies [2, 106–110]. This approach could avoid the use of exhausted T cells [11, 12]. Such therapies would exploit cytokines and co-stimulatory molecules reported to potentiate antitumor Th17 cell quality. Below, we discuss the emerging roles of host preconditioning, pro-inflammatory cytokines IL-12 and IL-23, and co-stimulation in regulating the antitumor activity of Th17 and Tc17 cells in vivo.
ICOS versus CD28 co-stimulation on Th17 cells
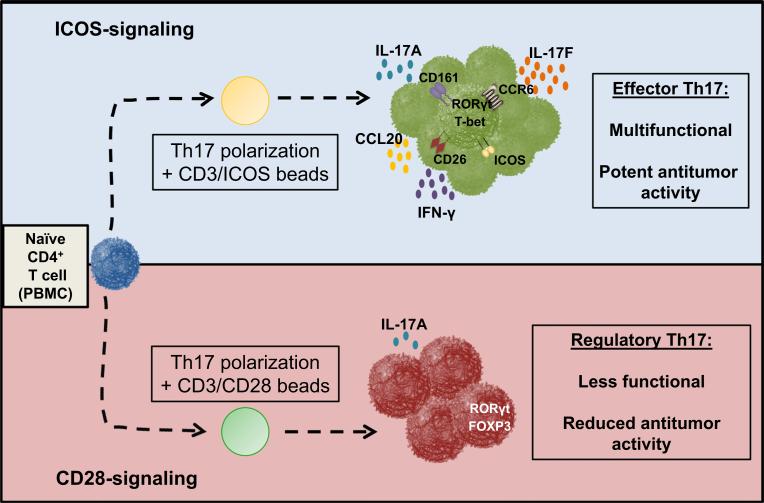
Th17 cells require two signals to be activated: The first signal is antigen specific (“signal 1”) and involves TCR-dependent recognition of peptide presented by MHC II on antigen-presenting cells and the second signal is co-stimulation (“signal 2”) provided by molecules in the B7-CD28/TNF receptor superfamily [111, 112]. Immunologists often use CD28 to study Th17 cell biology. Yet, CD28 signaling was found to suppress the generation of human Th17 cells [37]. Conversely, ICOS was critical for their generation. ICOS induced RORγt and T-bet expression in Th17 cells, leading to their robust capacity to secrete IL-17A and IFN-γ compared to CD28-stimulated Th17 cells, as shown in Fig. 2. Importantly, ICOS promoted the robust antitumor activity of CAR positive Th17 cells after transfer into mice bearing established human tumors superior to infusion of those expanded with CD28. In tumor models with murine Th17 cells, IFN-γ and IL-17A were essential for Th17-mediated regression of tumor, as antibody blockade of IFN-γ or IL-17A abrogated their effectiveness [70, 90]. More work is needed to determine the role of IL-17A, IFN-γ and other cytokines produced by ICOS-activated human Th17 cells in the regression tumors in vivo, and this information can be used to design T cell-based clinical trials for cancer.
Fig. 2.
Respective roles of CD28 and ICOS in human Th17 cell function and antitumor activity. The nature of the co-stimulatory molecule signaling determines the functional fate of human Th17 cells. ICOS co-stimulation enhances proliferation and expansion of inflammatory Th17 cells that secrete IFN-γ, IL-17A and IL-17F, while CD28 co-stimulation yields smaller numbers of Th17 cells that appear to be restrained in terms of what molecules they secrete, including less IFN-γ, IL-17A and IL-17F. Importantly, ICOS-stimulated Th17 cells kill human tumors to a greater extent than those stimulated with CD28
ICOStomizing Th17/Tc17 cells with CARs
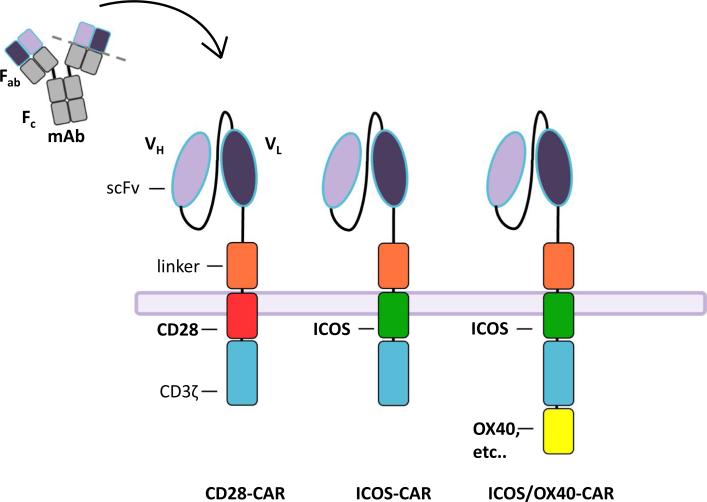
Ligating Th17 cells with an ICOS agonist ex vivo enhanced their capacity to kill tumors in vivo. Thus, the question arises: Does including ICOS signaling into a CAR construct improve the antitumor activity of T cell in vivo? The idea of including ICOS [113, 114] in CARs is interesting as most constructs use CD28 or 41BB intracellular domains in third-generation CARs containing the CD3ζ chain (Fig. 3). Comparative analysis of the three different constructs of CAR containing (with CD3ζ) CD28, ICOS or 4-1BB revealed, as expected, that only ICOS signaling enhanced IL-17A, IL-17F, IL-22 and IFN-γ production by T cells when cultured with antigen-positive cancers. This work further supports the original finding that ICOS generates Th1/Th17 cells [37]. ICOS-CAR Th17-polarized cells did not regress human tumors to a greater extent than Th17 cells engineered with CD28 or 4-1BB-CAR. Yet, ICOS-CAR Th17 cells persisted better than CD28-CAR or 4-1BB-CAR Th17 cells. These data might suggest that CD3/ICOS stimulation with beads used at the beginning of the culture is sufficient to generate durable human antitumor Th17 cells (regardless of the CAR/co-signal used to engineer them). This interpretation is possible as cord blood Th17 cells stimulated with ICOS were found to retain their original functional characteristics when first stimulated with a CD28. Regardless, it is interesting that continued ICOS signaling imparted by the ICOS-CAR increased Th17 persistence, a feature associated with favorable outcome in cancer patients.
Fig. 3.
Exploiting ICOS and OX40 in CAR Th17/Tc17 cells redirected to recognize and kill tumors. Gene transfer is used to redirect lymphocytes to express chimeric antigen receptors that target tumor antigens in an MHC-independent manner. CARs are fusion proteins composed of an extracellular portion derived from an antibody (ScFv), a linker that dimerizes with intracellular signaling domains derived from T cell signaling proteins. CAR constructs used in the clinic contain CD3ζ as well as a co-stimulatory endodomains (e.g., CD28 or 4-1BB). CARs to generate Th17 cells consist of an ICOS co-stimulatory endodomains linked to CD3ζ. An OX40 co-stimulatory domain could possibly be added to enhance antitumor immunity, as OX40/OX40 ligand signaling favors effector T cells over Treg cells
To bypass programming with many cytokines (such as TGF-β, IL-6, IL-21, IL-23, anti-IL-2, anti-IFN-γ and anti-IL-4, see Fig. 1), it might be rational to sort out bona fide Th17 cells via extracellular markers (e.g., CCR6, CD26) from the peripheral blood of patients rather than cytokine programming them. Indeed, Th17 cells can comprise ~8–20 % of the CD4+ T cell population. Sorted Th17 cells could then be engineered with an ICOS-CAR to maintain their characteristics long term. This sorting approach could be attractive given that T cell cultures from cancer patients might be biased mainly to a Treg or Th1 phenotype resistant to skewing to a Th17 phenotype compared to lymphocytes obtained from a healthy donor.
Gene array data revealed that ICOS-based CARs induced higher expression of IL-1 receptor (IL-1R) and NCS1 (neuronal calcium sensor-1) on Th17 cells than those engineered with CD28- or 41BB-based CARs [115]. This finding is interesting given that the IL-1 receptor (IL-1R)/Toll-like receptor (TLR) superfamily plays an essential role in the regulation of inflammatory responses and was found to support Th17 differentiation and commitment [116]. How ICOS signaling induces this receptor and how IL-1β and/or TLR agonists regulate Th17 cells in lymphodepleted host, where these microbial signals are exacerbated [117], will be important to investigate. Likewise, it is compelling that NCS-1 is expressed in ICOS-CAR Th17 cells, as calcium signaling is essential for induction of nuclear orphan receptor pathways to drive Th17 differentiation [118]. Thus, pharmaceutical reagents that modulate the calcium channel may be considered for the development of therapeutic agents to regulate cancer immunotherapy [119].
Finally, it is worth contemplating the finding that ICOS-CAR supports the generation of human CD4+ Th17 cells to a greater degree than human CD8+ Tc17 cells, while 41BB-CAR supported CD8+ Tc17 cells over CD4+ Th17 cells. These new data might imply that different co-signaling domains in CARs differentially regulate the fate of human CD4+ and CD8+ T cells in clinical trials, a consequence which remains unclear in the patient's treatment outcome. However, this interpretation should be critically evaluated, particularly given that these CAR/co-stimulation experiments were conducted in NSG mice, where it is hard to clearly interpret the interplay between human lymphocytes with other immune cells (which are murine cells) in vivo.
ICOS co-stimulation augments antitumor Tc17 cells
Recently, ICOS was reported to augment the antitumor activity of IL-17A+CD8+ T cells in the pmel-1 CD8+ melanoma syngeneic model. In contrast to the work in NSG models, ICOS does not merely augment IL-17A and IFN-γ production and expansion of Th17 cells but also bolsters CD8+ Tc17 cells as well [120]. Using mice deficient in ICOS or the ICOS ligand, ICOS was found to support Tc17 cell responses to tumors. In vivo, blockade of the ICOS–ICOS ligand pathway impaired the capacity of Tc17 cells to kill melanoma in mice. On the other hand, activating Tc17 cells in vitro with an ICOS agonist augmented their capacity to mount immunity to tumors in vivo. Interestingly, host lymphodepletion increased ICOS ligand on activated dendritic cells, which promoted the in vivo expansion of antitumor cytotoxic ICOShiTc17 cells. Microbial translocation induced ICOS ligand expression on DCs and likely acts to bolster type 17 CD8+ and CD4+ T cells. ICOS signaling also increased IL-2Rα, IL-7Rα and IL-23R expression on Tc17 but not Tc1 cells and enhanced Tc17 in vivo engraftment. Moreover, ICOS−/−Tc17 cells expressed less CXCR3 and more suppressive ectoenzyme CD39 on their cell surface than WT Tc17 cells [120], which may have compromised their capacity to traffic to and activate in the tumor. This work has implications for the design of T cell-based cancer therapies, as it shows for the first time that ICOS signaling improves Tc17 persistence and function via inducing cytokine receptors and other molecules known to help them survive or traffic to the tumor.
Host lymphodepletion to trigger Tc17 plasticity via activated APCs and IL-12 signaling
ICOS signaling is not the only cue that augments Tc17 plasticity. Host preconditioning also drives Tc17 plasticity, thereby enhancing their ability to ablate tumors [45]. Specifically, Bowers et al. found that dendritic cells from irradiated mice drove Tc17 plasticity via IL-12 signaling. IL-12 increased in the serum of mice exposed to escalating doses of lymphodepletion. Compared to Tc1 cells, Tc17 cells regressed melanoma in myeloablated mice (9 Gy TBI with stem cell support) to a greater extent than in lymphoreplete (no TBI) or non-myeloablated (5 Gy TBI) mice. Moreover, Tc17 cells mediated cures in myeloablated mice. Tc17 cells converted from mainly IL-17A producers into IL-17A+IFN-γ+ double producers after transfer, and these cells converted more rapidly in myeloablated mice than in lymphoreplete animals. Lymphodepletion triggered the innate immune system and increased co-stimulatory molecules on DCs, including the ICOS ligand. Also, irradiated DCs secreted pro-inflammatory cytokines IL-12 and IL-23 to a greater extent than monocytes. Interestingly, only IL-12 secreted by TBI activated host DCs appeared to augment the antitumor activity of donor Tc17 cells, as blocking endogenous IL-12 reduced their therapeutic efficacy in myeloablated mice. Finally, priming Tc17 cells in vitro with exogenous IL-12 enhanced their functional plasticity and capacity to regress melanoma in vivo [45]. These data show that chemotherapy/TBI bolsters antitumor Th17 activity via IL-12 signaling.
Along with IL-12, it has been reported that the dose of TGF-β, IL-1β, IL-23 and IL-2 used to program CD4+ T cells to a Th17 phenotype can distinctly regulate their functional fate and capacity to regress tumors [38, 44, 45]. These findings underscore that small changes to cytokines, co-stimulation and pharmaceutical reagents in the Th17 culture can have unforeseen consequences in shaping memory responses to tumors. Understanding how to exploit these cues to augment antitumor Th17 cells is important and is being actively investigated in the field.
Conclusions
Although there has been success in the field of cancer immunotherapy, there exists the need for improvement. Not all patients respond to immunotherapy or some patients experience short-lived responses. In this review, we highlighted recent efforts to generate memory T cells that persist and mount potent antitumor immunity in vivo. Central and stem memory CD8+ T cells have shown promise for enhancing tumor immunity and can be generated using drugs that manipulate the Wnt/β-catenin or PI3 kinase pathway [15, 69, 121, 122]. Th17 cells have also been reported to eradicate large tumors [70], yet it remains unexplored how pharmaceutically manipulating Th17 cells with PI3 kinase or glycolytic inhibitors will impact their fate. From a clinical standpoint, the potential for durable memory Th17 cells presents excitement in the field. Th17 and Tc17 cell products can be generated via various cues, including distinct co-stimulation and cytokines. All of these manipulations appear to generate cell products that persist in the host. Although Th17/Tc17 cells have been defined to possess stemness properties due to the expression of genes in the Wnt/β-catenin signal pathway, this quality has not been tested formally via serial transfer experiments to see if only one cell can repopulate itself and give rise to other T cell progeny. Using serial transfer of one cell to confidently declare stemness was first executed in an infectious disease model, where the investigators elegantly showed that CD8+ lymphocytes that express high levels of CD62L on their cell surface have stemness—as the transfer of single CD62L+ but not CD62L− T cell could repopulate and protect another host from infection long term [123]. Given that Th17 cells express nominal CD62L, it is logical to argue that Th17 cells, in contrast to central memory CD8+ T cells, do not have stemness. However, this conclusion is questionable given that Th17 cells persist in vivo better than Th1 cells (which express more CD62L). Thus, it will be important to perform serial transfer experiments with one (or more) Th17 versus Th1 cells to address the potential of CD4+ subsets to reconstitute and protect the host from tumor challenge. Indeed, more knowledge of the mechanisms regulating the antitumor responses in Th17 and Tc17 cells via these distinct signals is warranted to create safe yet potent vaccine and cellular therapies for patients with cancer.
Acknowledgments
We thank the scientific and clinical team and the patients at the Medical University of South Carolina for help and guidance in the development of new cancer immunotherapies.
Funding This work was supported in part by National Cancer Institute Grant 5R01CA175061, South Carolina Clinical & Translational Research Grant UL1 TR000062, American Cancer Society-Internal Research Grant 016623-004, Medical University of South Carolina start-up funds to Chrystal Paulos, and Jeane B. Kempner Foundation Grant and American Cancer Society Postdoctoral Fellowship (122704-PF-13-084-01-LIB) Grant support for Michelle Nelson. This work was also supported in part by Ruth L. Kirschstein National Research Service Award, National Cancer Institute, National Institutes of Health Fellowships awarded to Stefanie Bailey (F31 CA192787-01) and to Jacob Bowers (F30 CA200272-01).
Abbreviations
- ACT
Adoptive cell therapy
- CAR
Chimeric antigen receptor
- ICOS
Inducible co-stimulator
- IRF4
Interferon regulatory factor 4
- ROR
Retinoic acid-related orphan receptor
- RUNX
Runt-related transcription factor
- Tc17
IL-17-producing CD8 cytotoxic T cell
- TCR
T cell receptor
- Th17
IL-17-producing CD4 helper T cell
Footnotes
Compliance with ethical standards
Conflict of interest Chrystal Paulos has a patent for the expansion of Tc17 and Th17 cells using the inducible co-stimulator agonist and inducible co-stimulator ligand-expressing artificial antigen-presenting cells and beads. All other authors declare no financial or commercial conflict of interest.
References
- 1.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. doi:10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3:95ra73. 2011 doi: 10.1126/scitranslmed.3002842. doi:10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. doi:10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson MH, Paulos CM. Novel immunotherapies for hematologic malignancies. Immunol Rev. 2015;263:90–105. doi: 10.1111/imr.12245. doi:10.1111/imr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. doi:10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. doi:10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran E, Turcotte S, Gros A, et al. Cancer immuno-therapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. doi:10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. doi:10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. doi:10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: Which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. doi:10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulos CM, Suhoski MM, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. doi:10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. doi:10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. doi:10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattinoni L. Memory T cells officially join the stem cell club. Immunity. 2014;41:7–9. doi: 10.1016/j.immuni.2014.07.003. doi:10.1016/j.immuni.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. doi:10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. doi:10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. doi:10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. doi:10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. doi:10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. doi:10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+) T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. doi:10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. doi:10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. doi:10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. doi:10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. doi:10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. doi:10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. doi:10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antony PA, Restifo NP. CD4+ CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamoto K, Kosaka A, Tsuji T, et al. Critical role of the Th1/Tc1 circuit for the generation of tumor-specific CTL during tumor eradication in vivo by Th1-cell therapy. Cancer Sci. 2003;94:924–928. doi: 10.1111/j.1349-7006.2003.tb01377.x. doi:10.1111/j.1349-7006.2003.tb01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. doi:10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. doi:10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. doi:10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrikant PA, Rao R, Li Q, Kesterson J, Eppolito C, Mischo A, Singhal P. Regulating functional cell fates in CD8 T cells. Immunol Res. 2010;46:12–22. doi: 10.1007/s12026-009-8130-9. doi:10.1007/s12026-009-8130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris TJ, Grosso JF, Yen HR, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. doi:10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. doi:10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purvis HA, Stoop JN, Mann J, et al. Low-strength T-cell activation promotes Th17 responses. Blood. 2010;116:4829–4837. doi: 10.1182/blood-2010-03-272153. doi:10.1182/blood-2010-03-272153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med 2:55ra78. 2010 doi: 10.1126/scitranslmed.3000448. doi:10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S, Thyagarajan K, Kesarwani P, et al. Reducing CD73 expression by IL1β-Programmed Th17 cells improves immunotherapeutic control of tumors. Cancer Res. 2014;74:6048–6059. doi: 10.1158/0008-5472.CAN-14-1450. doi:10.1158/0008-5472.CAN-14-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Torchinsky MB, Gobert M, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. doi:10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viaud S, Saccheri F, Mignot G, et al. The intestinal micro-biota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. doi:10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. doi:10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. doi:10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. doi:10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. doi:10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Bowers JS, Nelson MH, Kundimi S, Bailey SR, Huff LW, Schwartz KM, Cole DJ, Rubinstein MP, Paulos CM. Dendritic cells in irradiated mice trigger the functional plasticity and antitumor activity of adoptively transferred Tc17 cells via IL12 signaling. Clin Cancer Res. 2015;21:2546–2557. doi: 10.1158/1078-0432.CCR-14-2294. doi:10.1158/1078-0432.CCR-14-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. doi:10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. doi:10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 48.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. doi:10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. doi:10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. doi:10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. doi:10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. doi:10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 53.Ciofani M, Madar A, Galan C, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. doi:10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Godec J, Ben-Aissa K, et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity. 2014;40:355–366. doi: 10.1016/j.immuni.2014.01.002. doi:10.1016/j.immuni.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. doi:10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. doi:10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17—producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. doi:10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 58.Bengsch B, Seigel B, Flecken T, Wolanski J, Blum HE, Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J Immunol. 2012;188:5438–5447. doi: 10.4049/jimmunol.1103801. doi:10.4049/jimmunol.1103801. [DOI] [PubMed] [Google Scholar]
- 59.Kamiyama T, Watanabe H, Iijima M, Miyazaki A, Iwamoto S. Coexpression of CCR6 and CD146 (MCAM) is a marker of effector memory T-helper 17 cells. J Dermatol. 2012;39:838–842. doi: 10.1111/j.1346-8138.2012.01544.x. doi:10.1111/j.1346-8138.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 60.Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. doi:10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. doi:10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cosmi L, De Palma R, Santarlasci V, et al. Human inter-leukin 17—producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. doi:10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. doi:10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. doi:10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Obermajer N, Wong JL, Edwards RP, et al. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J Exp Med. 2013;210:1433–1445. doi: 10.1084/jem.20121277. doi:10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: A TGF-β-dependent immunosuppressive activity? Trends Mol Med. 2012;18:742–749. doi: 10.1016/j.molmed.2012.09.007. doi:10.1016/j.molmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. doi:10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohn DB, Dotti G, Brentjens R, et al. CARs on track in the clinic. Mol Ther. 2011;19:432–438. doi: 10.1038/mt.2011.1. doi:10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crompton JG, Sukumar M, Roychoudhuri R, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. doi:10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. doi:10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. doi:10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson MH, Diven MA, Huff LW, Paulos CM. Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res. 2015;2015:368736. doi: 10.1155/2015/368736. doi:10.1155/2015/368736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B, Ribas A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. doi:10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dulos J, Carven GJ, Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. doi:10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 75.Yosef N, Shalek AK, Gaublomme JT, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. doi:10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–706. doi: 10.1016/j.coi.2011.08.007. doi:10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: implications for cancer surveillance and immuno-therapy. Oncoimmunology. 2012;1:717–725. doi: 10.4161/onci.20068. doi:10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 2011;236:567–579. doi: 10.1258/ebm.2011.011007. doi:10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bremnes RM, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, Stenvold H, Camps C, Busund LT. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6:824–833. doi: 10.1097/JTO.0b013e3182037b76. doi:10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- 80.Fialova A, Partlova S, Sojka L, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer. 2013 doi: 10.1002/ijc.27759. doi:10.1002/ijc.27759. [DOI] [PubMed] [Google Scholar]
- 81.Winkler I, Gogacz M, Rechberger T. Do Th17 cells play an important role in the pathogenesis and prognosis of ovarian cancer? Ginekol Pol. 2012;83:295–300. [PubMed] [Google Scholar]
- 82.Munn DH. Th17 cells in ovarian cancer. Blood. 2009;114:1134–1135. doi: 10.1182/blood-2009-06-224246. doi:10.1182/blood-2009-06-224246. [DOI] [PubMed] [Google Scholar]
- 83.Kim JS, Sklarz T, Banks LB, et al. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol. 2013;14:611–618. doi: 10.1038/ni.2607. doi:10.1038/ni.2607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. doi:10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glauben R, Sonnenberg E, Wetzel M, Mascagni P, Siegmund B. Histone deacetylase inhibitors modulate interleukin 6-dependent CD4+ T cell polarization in vitro and in vivo. J Biol Chem. 2014;289:6142–6151. doi: 10.1074/jbc.M113.517599. doi:10.1074/jbc.M113.517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. doi:10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus—targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. doi:10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. doi:10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NYESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. doi:10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muranski P, Borman ZA, Kerkar SP, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. doi:10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology. 2010;130:10–15. doi: 10.1111/j.1365-2567.2010.03260.x. doi:10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Komori HK, Hart T, LaMere SA, Chew PV, Salomon DR. Defining CD4 T cell memory by the epigenetic landscape of CpG DNA methylation. J Immunol. 2015;194:1565–1579. doi: 10.4049/jimmunol.1401162. doi:10.4049/jimmunol.1401162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. doi:10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 94.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. doi:10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. doi:10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hinrichs CS, Borman ZA, Gattinoni L, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. doi:10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. doi:10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu Y, Iclozan C, Yamazaki T, Yang X, Anasetti C, Dong C, Yu XZ. Abundant c-Fas-associated death domain-like interleukin-1—converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. doi:10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. doi:10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+ CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. doi:10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 101.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+ CD4+ CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. doi:10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 102.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. doi:10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Haines CJ, Gutcher I, et al. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. doi:10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 104.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. doi:10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. doi:10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varela-Rohena A, Carpenito C, Perez EE, et al. Genetic engineering of T cells for adoptive immunotherapy. Immunol Res. 2008;42:166–181. doi: 10.1007/s12026-008-8057-6. doi:10.1007/s12026-008-8057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. doi:10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Khami AA, Mehrotra S, Nishimura MI. Adoptive immunotherapy of cancer: gene transfer of T cell specificity. Self Nonself. 2011;2:80–84. doi: 10.4161/self.2.2.15832. doi:10.4161/self.2.2.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liddy N, Bossi G, Adams KJ, et al. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012;18:980–987. doi: 10.1038/nm.2764. doi:10.1038/nm.2764. [DOI] [PubMed] [Google Scholar]
- 110.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. doi:10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 112.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. doi:10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 113.Hombach AA, Chmielewski M, Rappl G, Abken H. Adoptive immunotherapy with redirected T cells produces CCR7-cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther. 2013;24:259–269. doi: 10.1089/hum.2012.247. doi:10.1089/hum.2012.247. [DOI] [PubMed] [Google Scholar]
- 114.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. doi:10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guedan S, Chen X, Madar A, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. doi:10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang J, Burkett PR, Borges CM, Kuchroo VK, Turka LA, Chang CH. MyD88 is essential to sustain mTOR activation necessary to promote T helper 17 cell proliferation by linking IL-1 and IL-23 signaling. Proc Natl Acad Sci U S A. 2013;110:2270–2275. doi: 10.1073/pnas.1206048110. doi:10.1073/pnas.1206048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. doi:10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim KD, Srikanth S, Tan YV, et al. Calcium signaling via Orai1 is essential for induction of the nuclear orphan receptor pathway to drive Th17 differentiation. J Immunol. 2014;192:110–122. doi: 10.4049/jimmunol.1302586. doi:10.4049/jimmunol.1302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joseph N, Reicher B, Barda-Saad M. The calcium feedback loop and T cell activation: how cytoskeleton networks control intracellular calcium flux. Biochim Biophys Acta. 2014;1838:557–568. doi: 10.1016/j.bbamem.2013.07.009. doi:10.1016/j.bbamem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 120.Nelson MH, Kundimi S, Bowers JS, et al. The inducible costimulator augments Tc17 Cell responses to self and tumor tissue. J Immunol. 2015 doi: 10.4049/jimmunol.1401082. doi:10.4049/jimmunol.1401082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sukumar M, Liu J, Ji Y, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. doi:10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;1:11ps12. doi: 10.1126/scitranslmed.3000302. doi:10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graef P, Buchholz VR, Stemberger C, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41:116–126. doi: 10.1016/j.immuni.2014.05.018. doi:10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]