Abstract
Background
Diagnosis of multidrug-resistant tuberculosis and prompt initiation of effective treatment rely on access to rapid and reliable drug-susceptibility testing. Efficient specimen transport systems and appropriate training on specimen referral contribute to optimal and timely access to tuberculosis diagnostic services.
Methods
With support and technical assistance from a public-private partnership (PPP) between Becton Dickinson and the US President’s Emergency Plan for AIDS Relief, the Uganda National TB Reference Laboratory (NTRL) and National TB and Leprosy Program redesigned the tuberculosis specimen transport network and trained healthcare workers with the goal of improving multidrug-resistant tuberculosis detection.
Results
Between 2008 and 2011, the PPP mapped 93% of health facilities and trained 724 healthcare and postal staff members covering 72% of districts. Strengthening the tuberculosis specimen referral system increased referrals from presumptive multidrug-resistant tuberculosis cases by >10-fold, with 94% of specimens reaching the NTRL within the established target transport time.
Conclusions
This study demonstrates the potential of PPP collaborations with ministries of health to positively influence patient care by strengthening laboratory systems through increased access to drug-susceptibility testing in Uganda. Ongoing efforts to integrate specimen transport networks will maximize resources and improve patient management.
Keywords: Mycobacterium tuberculosis, multidrug-resistant tuberculosis, specimen referral, drug suscpectibility testing, Uganda, public-private partnership
Provision of quality-assured human immunodeficiency virus (HIV) and tuberculosis diagnostic testing, coupled with timely linkage to and retention in care and treatment are critical steps toward reducing morbidity, mortality, and the global burdens of these two closely linked diseases [1–3]. Despite their central role in patient care and public health programs, access to accurate testing and clinical monitoring services are significant weaknesses in the HIV and tuberculosis care and treatment cascades, particularly in low-resource settings [4–6].
As described in the Global Plan to Stop TB, universal access to rapid and accurate diagnostic evaluation is essential to achieve global and national tuberculosis control goals [2]. With 9.6 million new cases and 1.5 million deaths in 2014, tuberculosis continues to be one of the leading causes of morbidity and mortality globally [7]. Multidrug-resistant (MDR) tuberculosis (ie, tuberculosis caused by Mycobacterium tuberculosis that is resistant to at least rifampicin and isoniazid) and extensively drug-resistant (XDR) tuberculosis (ie, MDR tuberculosis that is also resistant to any fluoroquinolone and ≥1 of 3 injectable second-line drugs) pose additional substantial challenges to achieving tuberculosis control targets [2]. In resource-limited settings, detection of tuberculosis primarily is carried out through decentralized sputum smear microscopy. Despite its widespread use and availability, this method of detecting acid-fast bacilli (AFB) is hindered by poor sensitivity and inability to either differentiate between live and dead AFB or identify drug resistance. Reference methods for definitive laboratory-confirmation of tuberculosis, determination of full first-line and second-line antituberculosis drug resistance profiles, and treatment monitoring for drug-resistant tuberculosis cases involve culture isolation of M. tuberculosis and phenotypic drug susceptibility testing (DST) conducted at centralized laboratories with specialized infrastructure, equipment, biosafety measures, and human resources [8]. Such centralized testing requires rapid and safe transport of specimens from health facilities or lower-level laboratories to the higher-level laboratory, as well as expedient reporting of results back to clinicians. However, inefficiencies in these specimen referral and result reporting systems contribute to significant diagnostic delays in routine practice. In Malawi, for example, sputum specimens reached the central tuberculosis reference laboratory for only 40% of patients for whom culture and DST were indicated [9], while in Peru, the median time from diagnostic evaluation request to MDR tuberculosis treatment initiation was nearly 5 months [10].
Uganda’s public health laboratory system includes a tiered network of laboratories providing tuberculosis diagnostic services: the national tuberculosis reference laboratory (NTRL), 516 level III health center laboratories, 145 level IV health center laboratories, 113 district laboratories, and 14 regional laboratories. Culture and DST services are provided only at the NTRL, located in Kampala, and specimens requiring these specialized tests must be referred from 900 health facilities located in rural and urban settings throughout the country. Prior to 2008, there was no established referral network in place to provide routine, rapid, and safe transport of patient specimens from health facilities to the NTRL. Rather, specimens were collected and delivered on an ad hoc basis, typically being transported either from sites within Kampala or when an NTLP staff member was traveling back to the capital from a peripheral site. Thus, an underdeveloped specimen transport system and insufficient clinician training on specimen referral contributed to delayed and suboptimal access to MDR tuberculosis diagnostic and treatment services and hampered surveillance activities in Uganda.
In recent years, increased global attention to the suboptimal state of laboratory systems in low-resource settings resulted in a series of renewed calls for and commitment to improving diagnostic services through a variety of approaches. This included development of tiered national public health laboratory networks and integrated strengthening of cross-cutting elements of laboratory systems (eg, quality management, training and retention, specimen referral, and biosafety) [11–14]. Innovative collaborations such as public-private partnerships (PPPs) have been formed to maximize ministry of health (MOH), donor, and partner resources for the development of national public health laboratory policies and strategic plans and to catalyze implementation of laboratory systems strengthening programs and activities. The Uganda MOH leveraged one such PPP, between Becton Dickinson (BD) and the US President’s Emergency Plan for AIDS Relief (PEPFAR), to address laboratory system weaknesses that were hindering patient care and tuberculosis surveillance activities. Through this PPP–Uganda MOH collaboration, the Centers for Disease Control and Prevention, the Uganda National Tuberculosis and Leprosy Program (NTLP), the NTRL, and BD collaborated with the Uganda postal service to develop a formalized, routine, and safe the tuberculosis specimen referral system, focusing on reducing diagnostic delays, increasing access to and use of M. tuberculosis culture and DST services, and improving MDR tuberculosis surveillance to support patient treatment and health outcomes.
METHODS
In 2008, the NTLP was in the process of planning for Uganda’s first national tuberculosis drug resistance survey (DRS), which would necessitate routine, reliable transport of sputum specimens from peripheral health facilities to the NTLP for culture and DST. In support of DRS planning, the Uganda MOH and the PPP engaged in a series of consultations and reviewed past laboratory service assessments and the Uganda national laboratory strategic plan and identified tuberculosis specimen referral as a critical weakness that not only threatened the efficiency and accuracy of the DRS, but also precluded patients’ access to appropriate diagnostic services in routine programmatic practice. Thus, strengthening the tuberculosis specimen referral system was selected as a priority activity for the collaboration between the PPP and the Uganda MOH.
An in-country specimen referral program development and implementation team composed of the NTRL (representing the Uganda MOH), BD, and in-country and US-based Centers for Disease Control and Prevention staff was established. Each partner’s expertise, experience, and resources were leveraged to define roles and responsibilities, and a detailed work plan including phased implementation of the specimen referral system was developed. The NTRL provided leadership and customized training materials and implemented all PPP activities, including provision of support supervision to the national tuberculosis laboratory network. CDC-Uganda provided technical, programmatic, and financial assistance. Through a series of three 21-day in-country visits, 3 subject matter experts from BD provided technical assistance on the development of the training curricula, introduction of geographic information system (GIS) technology, and training laboratory and postal staff members.
NTRL teams composed of a data manager and a logistics and transport coordinator were assigned to collect global positioning system (GPS; Trimble Navigation, Sunnyvale, California) coordinates for sites referring specimens from persons with presumptive MDR tuberculosis. By use of GIS software (ARC GIS, version 9.3; Esri, Redlands, California), the data were analyzed to provide a visual representation of the geographic locations of referring sites, post offices, and testing laboratories; district population densities; and the number of specimens referred per tuberculosis unit.
NTRL staff customized the BD-developed 3-day didactic and hands-on curriculum and standard operating procedures. Training modules included information on the structure of the specimen referral network, proper specimen collection, safe packaging and transport of sputum specimens, specimen tracking, occurrence management, and continuous quality improvement. To aid in the safe transport of specimens, BD procured specimen transport containers with triple packaging capacity (Laboratory Specialist Services, Cape Town, South Africa). Containers were validated by South African national standards to be in compliance with World Health Organization (WHO) recommendations and United Nations Transport of Dangerous Goods specifications [15]. Communication within the referral network was augmented by toll-free telephone lines installed at the main post office and the NTRL.
Beginning with a pilot in Kampala District, program staff members facilitated training workshops for laboratory staff and postal workers. Training materials were then further refined and expanded to additional districts. Program staff members also sensitized clinicians, administrative staff, and other technical staff to the referral system through a series of informal meetings and half-day informational sessions. Key outcomes of these meetings were the identification of key stake holders and focal persons, selection of Posta Uganda outlets to participate in the transport network, and discussion of potential specimen transport challenges and mitigation measures. These activities were coordinated through the NTLP tiered administrative system involving the zonal and district tuberculosis and leprosy program supervisors and facility focal persons.
Program outcomes were assessed by measuring changes in (1) the number of sites referring specimens to the NTRL, (2) the number of specimens referred to the NTRL, (3) specimen transport time (defined as days from specimen collection to receipt at the NTRL; eg, 0–24 hours = day 1 and 24–48 hours = day 2), and (4) the number of MDR tuberculosis cases detected.
RESULTS
Tuberculosis Specimen Transport Network Development and Training
A national tuberculosis specimen referral system was designed in which sputum specimens were collected from presumptive tuberculosis or MDR tuberculosis cases at 900 NTLP tuberculosis units. Trained personnel at the tuberculosis units then packaged sputum specimens and delivered them to the nearest local or main post office within the national Posta Uganda system, typically located ≤20 km from the tuberculosis unit. Specimens were transported at least daily from the tuberculosis units to the post offices, using either a tuberculosis unit vehicle, motor bike, or public transport. The method of transport used the post office depended on the proximity to the tuberculosis unit and the availability of a vehicle, motor bike, or public transport. Most tuberculosis units preferred using public motor bikes for transport because they were typically the most readily available and reliable means of transport in the communities. Once the specimen was received at the post office, the postal service was responsible for transporting the specimens to the NTRL in Kampala for culture and DST.
The partnership conceptualized and developed a customized training course and standard operating procedures for laboratory and postal staff members on the specimen referral system, safe packaging, and transport of infectious material. Prior to the program, no formal training on specimen referral, packaging, or transport was available. Between 2008 and 2011, the team of NTRL and BD facilitators trained 724 individuals, including 649 health facility or laboratory staff members and 75 Posta Uganda staff members. Phased rollout resulted in staff members from 80 of 111 districts (72%) receiving training by the end of 2011 (Table 1).
Table 1.
Improvements in Key Indicators After Introduction of Public-Private Partnership (PPP)–Supported Interventions to Strengthen the Tuberculosis Specimen Referral System
| Indicator | Time | |
|---|---|---|
| Before PPP Interventions | After PPP Interventions | |
| Staff members trained on specimen referral system, no. | 0a,b | 724 (649 health facility and laboratory staff; 75 postal staff)c |
| Districts covered by formal training, proportion (%) | 0/111 (0)b | 80/111 (72)c |
| Referral sites mapped by GIS, proportion (%) | 200/900 (22)b | 835/900 (93)c |
| Number of sites referring specimens to NTRL (%) | 50/900 (6)d | 400/900 (44)e |
| Sputum specimens referred, no. | 655d | 5813e |
| Presumptive MDR tuberculosis specimens referred, no. | 84d | 877e |
| Laboratory-confirmed MDR tuberculosis cases, no. | 47d | 71e |
| Specimen transport time, d, median (range) | 12 (1–240)d | 2 (1–82)e |
| Specimens arriving at NTRL within 3 d of collection, proportion (%) | 58/655 (9)d | 5476/5813 (94)e |
Abbreviations: GIS, geographic information system; MDR, multidrug resistant; NTRL, national tuberculosis reference laboratory.
No formal training was available.
Data are from before 2008.
Data were obtained through 2011.
Data were obtained during January–December 2008.
Data were obtained during January–December 2011.
Mapping and Specimen Referral to the NTRL
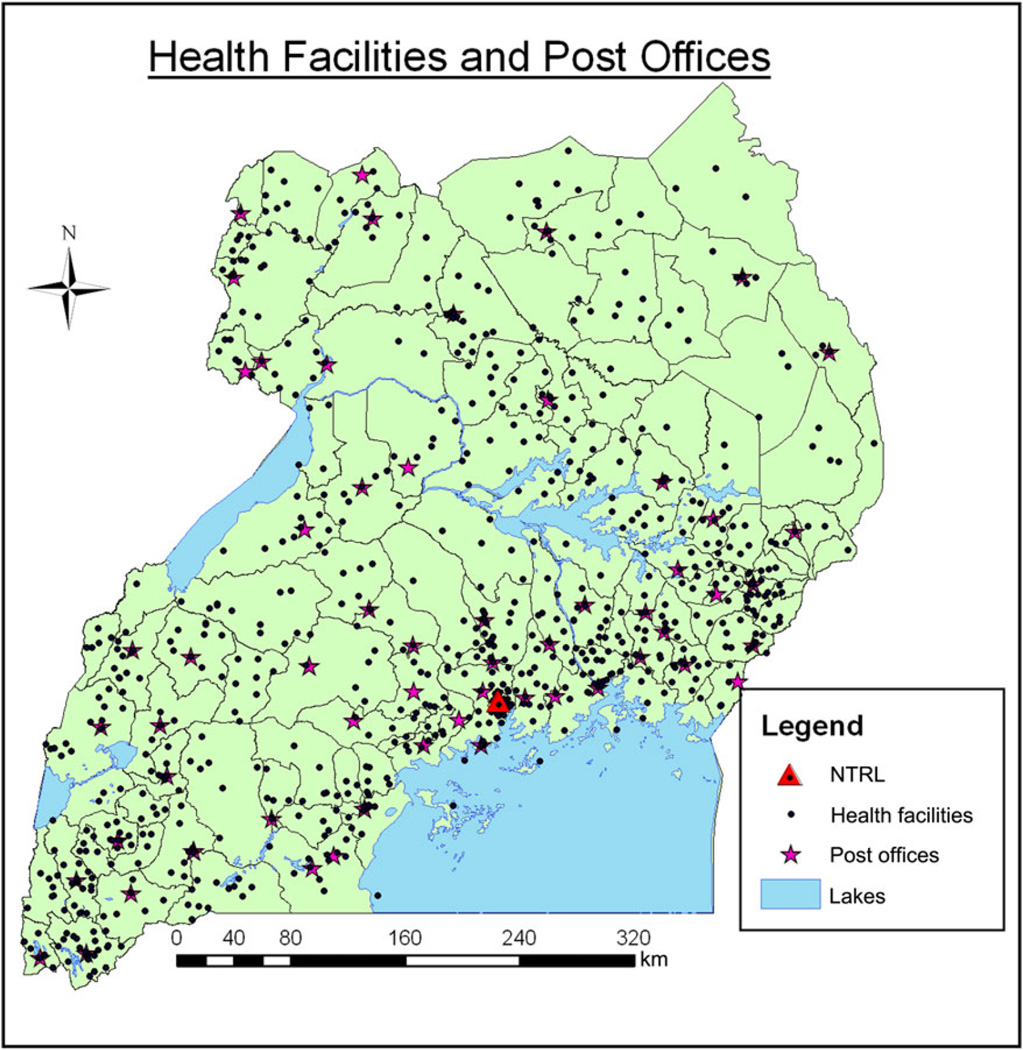
As of the end of 2011, 835 of 900 tuberculosis units (93%) authorized to collect specimens from persons with presumptive tuberculosis or MDR tuberculosis were mapped and visualized using GPS and GIS technology (Table 1, Figure 1). In 2008, prior to efforts to strengthen the specimen transport network, <6% of specimen collection sites (50 of 900) referred any specimens for culture and DST. These 50 tuberculosis units collectively sent 655 specimens to the NTRL in 2008. Of the referred specimens, 84 (13%) were from patients with presumptive MDR tuberculosis. By the end of 2011, there had been an 8-fold increase in the number of referring sites, with 44% of sites (400 of 900) referring 5813 specimens to the NTRL (Table 1). Of these 5813 specimens, 877 (15%) were from patient with presumptive MDR tuberculosis. The total number of laboratory-confirmed cases of MDR tuberculosis in Uganda increased from 47 in 2008 to 71 in 2011 (Table 1). Despite the increased workload, the NTRL maintained its quality, as evidenced by excellent performance on proficiency programs and subsequent receipt of International Organization for Standardization (ISO) 15189 accreditation.
Figure 1.
Distribution of 835 tuberculosis specimen referral sites and 58 post offices throughout Uganda, based on geographic information system mapping through December 2011. Abbreviation: NTRL, national tuberculosis reference laboratory.
Specimen Transport Delays
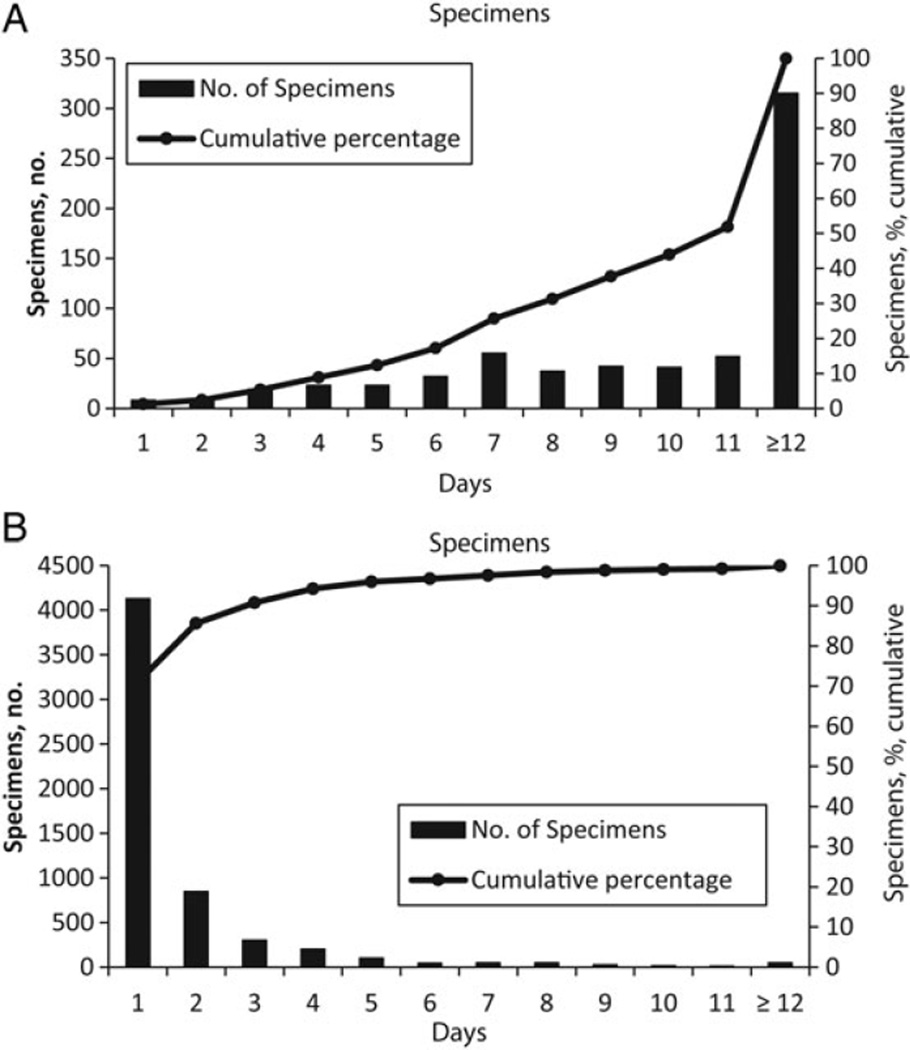
In addition to improvements in numbers of specimens referred, program interventions also yielded reductions in transport times. In 2011, the median time from tuberculosis diagnostic specimen collection to arrival at the NTRL was 2 days (range, 1–82 days) A compared to 12 days (range, 1–240 days) prior to the intervention. Furthermore, 94% of specimens reached the NTRL within the 3-day target transport time, as opposed to 9% in 2008 (Table 1 and Figure 2).
Figure 2.
A, Transit time for all sputum specimens referred to the national tuberculosis reference laboratory (NTRL) for testing before initiation of public-private partnership (PPP)–supported activities to strengthen the tuberculosis specimen referral system (January–December 2008). B, Transit time for all sputum specimens referred to the NTRL after PPP-supported activities to strengthen the tuberculosis specimen referral system (January–December 2011).
Tuberculosis units located close to the NTRL and those with the most routine and efficient transport systems typically delivered specimens to the NTRL on the same day as collection (day 1). In contrast, systematic delays and other outliers were experienced when transporting specimens from difficult-to-access areas, including islands and communities with poor roads, and when specimens were mislabeled or misplaced at any facility in the transport network.
DISCUSSION
Access to rapid and accurate diagnostic evaluation, followed by prompt initiation of effective antituberculosis treatment, is essential to achieve global and national tuberculosis control goals [2, 8]. The PPP collaboration with the Uganda MOH was designed to address one of the most urgent tuberculosis diagnostic service delivery gaps identified through several laboratory and tuberculosis program assessments and in the development of the national laboratory strategic plan. Owing to the significant costs, infrastructure, and technical expertise required to build and maintain safe, quality-assured tuberculosis culture and DST laboratories, the WHO recommends that national tuberculosis laboratory networks are tiered in structure, with culture and phenotypic DST conducted at a limited number of highly proficient laboratories with appropriate biosafety measures [8]. Consistent with this recommendation, MDR tuberculosis can be confirmed in only one public sector laboratory in Uganda, the NTRL, and therefore requires a robust and rapid specimen transport system to deliver specimens collected at peripheral sites to the centralized laboratory.
Prior to the PPP-supported implementation of an optimized standard tuberculosis specimen transport system, relatively few sputum specimens from individuals with presumptive MDR tuberculosis (n = 84), primarily from persons with category 2 treatment failure within the Kampala area, were referred to the NTRL. The project took advantage of the core competencies of each partner in the collaboration, leveraging strengths and building capacity during program implementation to achieve successful outcomes, including safer and more-efficient tuberculosis specimen transport procedures, increased referrals of sputum specimens from a broader range of MDR tuberculosis risk groups (eg, individuals with category 1 or category 2 treatment failure, contacts of persons with MDR tuberculosis, persons with relapsed tuberculosis, and individuals who experienced treatment default), and decreased diagnostic service delays. Furthermore, the NTLP leveraged the specimen transport system to conduct their first national tuberculosis drug resistance survey, the results of which were critical in planning for access to MDR tuberculosis treatment in Uganda. Through referral and conducting culture and DST on smear-positive sputum specimens at the NTRL, the NTLP and WHO revised estimates of MDR tuberculosis prevalence from 0.5% and 4.4% [16] to 1.4% and 12.1% [17] among new and previously treated cases, respectively.
The application of GPS and GIS technologies allowed for mapping and visualization of distances between health facilities, the nearest post office, and the NTRL. Analyzing these data, the partners continuously improved the tuberculosis specimen referral network and customized training program. Ideally, all sites would have been mapped prior to implementation, but the rate of GPS mapping was limited by human resources, particularly for more-remote facilities, and by the number of GPS devices available through the program. Nevertheless, by the end of 2011, 93% of tuberculosis units were mapped. In addition to mapping the specimen transport network, the NTRL has used the GIS database to visualize and improve diagnostic testing quality at individual, lower-level laboratories, and the NTLP has used the database to better understand and monitor the MDR tuberculosis epidemiology in the country by identifying and relating districts and health facilities where cases of MDR tuberculosis are diagnosed.
Protecting shipping personnel, laboratory staff, and the general public from inadvertent exposure to infectious agents and maintaining the integrity of the specimen and its container(s) throughout the entire shipping process were key considerations in the design of the specimen referral system. According to WHO safe-transport guidelines, infectious materials for transport should be triple packed to minimize the risk of leaking or other damage during transport [18]. Prior to the PPP interventions, specimen packaging procedures were not standardized, contributing to postal worker and healthcare staff concerns about safety during transport and reception of specimens. Increasing the number of specimens referred and reducing the transit times through the structured, routine specimen transport network would not have been possible without an emphasis on safety policies and procedures. Through procurement of materials required for triple packaging of the potentially infectious specimens and provision of training to healthcare and postal staff members on how to appropriately package and document specimens for safe transport to the NTRL, the PPP successfully addressed postal and healthcare staff safety concerns and ensured specimen integrity from collection to receipt at the NTRL.
Central to the success of the PPP was the leadership provided for program planning and implementation by the NTRL, as well as the strong collaborative relationships among Centers for Disease Control and Prevention, BD, and Uganda MOH representatives. The PPP faced a number of challenges during implementation, including limited human and financial resources. Operational budgets were insufficiently developed and not able to meet the national scale-up plans, requiring ongoing partner support. The lack of a broadly implemented laboratory information system adapted to local systems affected laboratory result transmission times from the NTRL back to clinicians and patients.
The tuberculosis diagnostic and treatment cascade includes several steps, including symptom identification, specimen collection, specimen transport (unless point-of-care testing is available), testing, result reporting, and linkage to and retention in treatment. As highlighted in previous studies, delays and inefficiencies in any of these steps can lead to delayed treatment initiation, patient attrition, and poor treatment outcomes [9, 10, 19–21]. Improvements in the Uganda tuberculosis specimen referral system led to increased numbers of patients receiving the necessary diagnostic testing and substantial reductions in transport delays. While the increased access to timely culture and DST may ultimately yield improved patient outcomes, the Uganda MOH has continued to support additional program improvements to support patient care. Since 2011, the MOH has taken steps toward increasing access to Xpert MTB/RIF testing for near point-of-care detection of tuberculosis and rifampicin-resistant tuberculosis; supporting quality management systems, which led to successful ISO 15189 accreditation of the NTRL; and integrating the Uganda tuberculosis specimen referral system with the early infant HIV diagnosis specimen transport system [22]. Furthermore, the global health security demonstration project in Uganda catalyzed additional progress toward a national disease-independent integrated referral network, including enhancements such as cold-chain transport, rapid result reporting via short message service technology, and improved information systems for outbreak detection and response [23]. Collectively, these activities in Uganda are serving as a model for integrated diagnostic systems strengthening.
More efforts like these that leverage resources from a variety of multisectoral donors and partners are urgently needed to support sustainable diagnostic network strengthening and capacity building in the areas of quality management systems, laboratory information systems, equipment maintenance systems, and laboratory workforce development.
Acknowledgments
We thank the Uganda MOH, Posta Uganda, BD, and the Centers for Disease Control and Prevention (CDC) management and technical staff, for their support and expertise, which was critical to make this collaboration a success; and the US Agency for International Development and TB Care I project, for additional geographic information system training and support.
Financial support. This work was supported by the US President’s Emergency Plan for AIDS Relief, through the CDC.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.PEPFAR Blueprint: Creating an AIDS-free Generation. 2012 [Google Scholar]
- 2.World Health Organization, Stop TB Partnership. The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. Geneva: World Health Organization; 2010. [Google Scholar]
- 3.Parsons LM, Somoskovi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birx D, de Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, malaria programs: The interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol. 2009;131:849–851. doi: 10.1309/AJCPGH89QDSWFONS. [DOI] [PubMed] [Google Scholar]
- 5.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 6.Nkengasong JN, Nsubuga P, Nwanyanwu O, et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol. 2010;134:368–373. doi: 10.1309/AJCPMPSINQ9BRMU6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 8.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 9.Harries AD, Michongwe J, Nyirenda TE, et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8:204–210. [PubMed] [Google Scholar]
- 10.Yagui M, Perales MT, Asencios L, et al. Timely diagnosis of MDR-TB under program conditions: is rapid drug susceptibility testing sufficient? Int J Tuberc Lung Dise. 2006;10:838–843. [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Regional Office for Africa. The Maputo Declaration on Strengthening of Laboratory Systems. [Accessed 8 February 2016];2008 Jan 24; http://www.who.int/diagnostics_laboratory/Maputo-Declaration_2008.pdf.
- 12.Nkengasong JN, Mesele T, Orloff S, et al. Critical role of developing national strategic plans as a guide to strengthen laboratory health systems in resource-poor settings. Am J Clin Pathol. 2009;131:852–857. doi: 10.1309/AJCPC51BLOBBPAKC. [DOI] [PubMed] [Google Scholar]
- 13.Yao K, McKinney B, Murphy A, et al. Improving quality management systems of laboratories in developing countries: an innovative training approach to accelerate laboratory accreditation. Am J Clin Pathol. 2010;134:401–409. doi: 10.1309/AJCPNBBL53FWUIQJ. [DOI] [PubMed] [Google Scholar]
- 14.Gershy-Damet GM, Rotz P, Cross D, et al. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010;134:393–400. doi: 10.1309/AJCPTUUC2V1WJQBM. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines for the safe transport of infectious substances and diagnostic specimens. Geneva: World Health Organization; 1997. Division of Emerging and other Communicable Diseases Surveillance Control. [Google Scholar]
- 16.World Health Organization. The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance, 2002–2007. Geneva: World Health Organization; 2008. Anti-tuberculosis drug resistance in the world : fourth global report. [Google Scholar]
- 17.Lukoye D, Adatu F, Musisi K, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS One. 2013;8:e70763. doi: 10.1371/journal.pone.0070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Guidelines for the safe transport of infectious substances and diagnostic specimens. Geneva: World Health Organization; 1997. [Google Scholar]
- 19.Pascopella L, Kellam S, Ridderhof J, et al. Laboratory reporting of tuberculosis test results and patient treatment initiation in California. J Clin Microbiol. 2004;42:4209–4213. doi: 10.1128/JCM.42.9.4209-4213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creek TL, Lockman S, Kenyon TA, et al. Completeness and timeliness of treatment initiation after laboratory diagnosis of tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis. 2000;4:956–961. [PubMed] [Google Scholar]
- 21.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 22.Kiyaga C, Sendagire H, Joseph E, et al. Uganda’s new national laboratory sample transport system: a successful model for improving access to diagnostic services for Early Infant HIV Diagnosis and other programs. PLoS One. 2013;8:e78609. doi: 10.1371/journal.pone.0078609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borchert JN, Tappero JW, Downing R, et al. Rapidly building global health security capacity–Uganda demonstration project, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:73–76. [PMC free article] [PubMed] [Google Scholar]