Abstract
Objective
American Indian/Alaska Native (AI/AN) patients are significantly less likely than non-Hispanic whites to receive guideline-concordant cancer care. Our objective was to examine cancer treatment decision making among AI/AN patients and their providers.
Methods
From 2011–14, AI/AN cancer patients and their surgeons were identified through a hospital registry in Washington State. Patients were invited to participate in a mailed survey that queried socio-demographics, cultural affiliation, everyday perceived discrimination, and trust in providers. Both patients and surgeons were queried about decision-making quality (collaboration and satisfaction). The primary outcome was association between patient and provider assessments of decision-making quality. The secondary outcome was non-adherence to treatment.
Results
49 patients (62% response rate) and 14 surgeons (37% response rate) returned surveys. Half of patients had not completed high school; 41% were living in poverty. Half of patients reported a strong tribal affiliation and most reported experiencing some form of discrimination. Patients endorsed high trust in surgeons and a high quality decision-making process; and surgeons’ rated decision-making quality even more highly than patients did in every domain. Non-adherence to treatment recommendations was common (26%) and was significantly associated with lower patient-reported collaboration and satisfaction with decision making.
Conclusions
Given the importance of adherence to cancer treatment for survival, the many non-clinical reasons for non-adherence, and the currently demonstrated association between decision-making quality and adherence, it would be worthwhile to investigate how to increase AI/AN patient satisfaction with decision making and whether improving satisfaction yields improved adherence to the cancer treatment plan.
Keywords: cancer, oncology, disparities, decision making, patient reported outcomes, American Indian
INTRODUCTION
For each of the most common solid cancers, American Indians and Alaska Natives (AI/AN) experience higher mortality than non-Hispanic whites.[1] Reasons for these disparities among AI/AN patients are likely similar to the reasons for poorer survival among every economically disadvantaged racial or ethnic group—some combination of decreased access to care, disadvantageous health behaviors, increased comorbid disease, later stage at diagnosis, and poorer quality of care.[2] However, AI/AN patients are different from other groups in important ways. Unlike many other minority populations, AI/AN cancer patients have not yet benefited from recent trends toward improved cancer survival.[3]
In addition, AI/AN patients are significantly less likely than others to receive guideline concordant cancer care.[4] Guideline concordant care, or the ability of the clinician and patient to successfully adhere to evidence- based treatment guidelines established by nationally recognized experts and clinical organizations, has been shown to improve patient outcomes.[5, 6] Among the AI/AN cohort in the Surveillance, Epidemiology, and End Results-Medicare database, we found that non-adherence to guideline concordant surgical care and separately non-adherence to guideline concordant chemotherapy (defined as delayed initiation, interruption, or premature discontinuation) were associated with reduced cancer-specific survival for breast, colon, and prostate cancer.[4] However, while we were able to adjust for comorbid disease and to discern that access to insurance was not causal (all patients had Medicare), the dataset was too limited to further clarify the systems, provider, or patient-driven mechanisms of non-adherence to guideline-concordant care. While individual health systems or providers no doubt contribute to inadequate care, we postulated that aspects of the patient-provider relationship and its impact on treatment decision making may also play a role.[7]
Little is known of cancer treatment decision making among AI/AN patients and their providers. In a systematic review of the literature, we found no published literature pertaining specifically to aspects of cancer treatment decision making among AI/AN patients.[8] Nor were data available regarding provider perspectives on decision making with AI/AN patients. We did find evidence of pervasive miscommunication between health care providers and AI/ANs, which contravenes shared decision-making and could provoke treatment non-adherence or interruption. As well, cultural affiliation with an AI/AN way of life has been associated with use of traditional AI/AN health practices [9] and may inhibit engagement with treatment that is seen as a product of the dominant culture.[10] We accepted the definition of trust in a medical care encounter offered by Hall as “the optimistic acceptance of vulnerability”.[11] Furthermore, low trust and perceived discrimination are linked to poor overall health and cancer outcomes in AI/ANs and First Nations/Inuit/Métis populations in Canada.[12]
In order to better understand perceptions of cancer treatment decision making among AI/AN cancer patients and their providers, we surveyed AI/AN patients and their cancer surgeons within a statewide hospital collective about aspects of their relationship and treatment decision making. We examined patient-reported socio-demographic and psychosocial attributes including cultural affiliation, perceived discrimination, and trust in providers and the association between patient- and provider-reported aspects of decision making. Finally, we examined associations between patient attributes, aspects of decision making (collaboration and satisfaction), and patient reported interruptions or discontinuation of cancer care.
METHODS
Study population
Between 2011–14, potential study subjects (patients and their providers) were identified from surgical case lists across participating hospitals through a collaborative hospital surgical quality improvement registry, the Surgical Care and Outcomes Assessment Program, throughout Washington State. Inclusion criteria for patients were: AI/AN ethnicity identified through the hospital’s electronic medical records and verbally confirmed by the participant prior to obtaining informed consent; age ≥ 18 years; receipt of surgery for one of the following cancers: lung, breast, prostate, colorectal, uterine, liver, pancreatic, kidney, or esophageal; and ability to provide informed consent. Inclusion criteria for providers were: provision of surgical treatment within the same hospital network for at least one of the following cancers: lung, breast, prostate, colorectal, uterine, liver, pancreatic, kidney, or esophageal, and the ability to provide informed consent.
Patients
Potential patient participants were mailed a study packet that included an introductory letter; an information statement; a copy of the study survey; and a self-addressed, stamped envelope. The letter also advised participants that they would receive a gift of $50.00 at survey completion. Ten business days after the study packet was mailed, the nurse scientist began telephone outreach (maximum 5 attempts) to recruit and enroll patients for the study. If outreach was unsuccessful, a final contact attempt letter was sent to the patient. Upon contacting the patient, the nurse scientist used Institutional Review Board (IRB)-approved telephone scripts to confirm eligibility and obtain informed consent. Patients were given the option of completing the survey over the phone with the study nurse or self-administered on paper using the survey included in the study packet and mailing it back to the nurse scientist.
Providers
Once patients were enrolled and surveyed, their surgeons were identified for recruitment into the study in order to gain insight from the provider perspective. The providers were asked to participate in a brief interview and survey. Provider participants were sent a study packet, which included a study introductory letter and an information statement. Ten business days after the study packet was mailed, research staff began telephone outreach to recruit and enroll providers for the study. Provider participants were interviewed by the nurse scientist, using an interview guide. Each interview lasted approximately 30 minutes and was audio-recorded and transcribed. At the time of the interview, providers were offered a gift of $50 for participation.
Human subjects
The University of Washington’s (UW) Human Subjects’ Division (HSD) served as the study’s IRB of record. IRB approval was obtained from the UW HSD and participating study site’s IRB prior to initiating any research activity. We adhered to the principals and guidelines for research in tribal communities,[12, 13] including ascertainment of approval by the tribal community research review officials in additional to the formal IRB.
Measures
The primary outcome in this study was the association between the mean patient score ± standard deviation and the mean provider score ± standard deviation on specific measures of collaboration in treatment decision making (the quality of interaction) and satisfaction with the decision-making process in the health setting. To assess these patient- and provider-reported outcomes, all participants completed the Collaboration and Satisfaction about Care Decision scale (CSACD).[14] The CSACD is scored on a 9-item 7-point Likert scale with response options ranging from “Strongly disagree” to “Strongly agree” for each of nine domains. A score of “4” was “neutral”. The CSACD has face and content validity with AI/AN cancer survivors, and has a Chronbach’s alpha of 0.93.
A secondary outcome was patient-reported non-adherence to treatment, defined as interruption or premature discontinuation of cancer care, and the reason for non-adherence. All reasons for non-adherence were queried and were not considered mutually exclusive. Open-ended responses to an “other” category were categorized by the research team, determined by consensus.
Independent variables included socio-demographic items and previously validated psychosocial instruments as follows. Socio-demographic items were age, sex, marital status, education, annual household income, and number of household members. Based on annual income and number of household members, we identified participants living in poverty, defined as ≤ 100% of the Federal Poverty Level (FPL), and those living in deep poverty, defined as < 50% FPL and associated with deprivation of basic human needs, including food, safe drinking water, sanitation facilities, health, shelter, education and information.[15]
We measured the strength of cultural affiliation in daily life using the previously validated Ethnic Identity Scale,[16] which uses questions such as “How important is it to you that you, yourself, keep your Tribal identity, and your Tribe’s values and practices?” with a 4-point Likert response scale ranging from “Not at all” to “Very important”. Participants respond to questions within 6 domains that summarize how they observe their cultural heritage, covering cultural activities, life ways, values, family traditions, family values, and language practices. Their responses on the Likert-type scale are clustered into specific ethnic identity groups indicating cultural affiliation for white, tribal, both, or neither.
Respondents indicated perceived discrimination using the Everyday Discrimination scale,[17] which has been associated with greater negative impact on health and quality of life than major events of discrimination and has an alpha above 0.89. We scored the Everyday Discrimination scale both numerically and as ever/never experienced.[17, 18] Patient-provider relationships were measures as patient-reported trust in the provider using the Wake Forest Trust scale, a 5-point Likert-type scale with 5 items pertaining to domains of fidelity, competence, honesty, confidentiality, and global trust, with an alpha above 0.85. [11] For analyses in which trust responses were dichotomized, we grouped “neutral” with reduced trust because the Wake Forest Trust scale skews strongly toward high trust levels.[19]
Statistical analysis
Categorical variables were evaluated using frequency distributions; continuous variables were evaluated using mean (standard deviation). The overall association between patient-reported collaboration and provider-reported collaboration was calculated from patient and provider responses by comparing the mean and standard deviation of the collaboration summary scores from each group. Similarly, the overall association between patient-reported satisfaction and provider-reported satisfaction was calculated by comparing the mean and standard deviation of the satisfaction summary scores from each group. In addition, we dichotomized responses for each domain indicating a high quality decision making process as 1–4=not high quality and 5–7=high quality. We then compared the proportion of patients who endorsed each individual process as high quality to the proportion of providers that did the same using chi squared analysis as well as comparing the means and standard deviations between patient and provider groups.
We compared patients whose cancer treatment was interrupted or discontinued and patients who reported uninterrupted care using Fischer’s exact test for categorical variables (patients rating of their cancer surgical care) and Wilcoxon rank-sum test for continuous variables (collaboration, satisfaction, trust, and discrimination). In addition, we used Fischer’s exact test to measure patient and provider associations on the CSACD. Pearson’s correlation was used to identify associations between metrics with continuous values (collaboration, satisfaction, trust, and discrimination) and ANOVA was used for associations between the continuous variables and the categorical (Identity). All statistical tests were 2-sided. A p-value ≤ 0.05 was considered statistically significant. All analyses were performed using STATA version 12 (STATA Corp, College Station, TX).
RESULTS
Study subjects
We initially identified 153 eligible AI/AN patients across 8 hospitals. Among these, 7 did not meet eligibility requirements at screening, 67 could not be reached, and 26 declined participation. Fifty-three patients consented to participate and 4 did not return the survey, resulting in a final sample of 49 patients (response rate 62%). The majority of patient participants were female (65.3%) with a mean age of 60.8 years (SD ± 9.7) (Table 1). Among the 38 surgeons statewide who cared for these patients, 14 completed the provider survey (response rate 37%). Most provider participants were male (71.4%), white (57.1%), and had completed medical school a mean of 25 years previously (data not shown).
Table 1.
Characteristics of American Indian/Alaska Native (AI/AN) survey participants.
| Age, n=49 | Mean | Std. Dev. |
|---|---|---|
| 60.8 | 9.7 | |
| Gender | N | % |
| Female | 32 | 65.3 |
| Male | 17 | 34.7 |
| Cancer type | ||
| Breast | 22 | 44.9 |
| Colorectal | 8 | 16.3 |
| Prostate | 5 | 10.2 |
| Liver | 5 | 10.2 |
| Kidney | 4 | 8.2 |
| Lung | 3 | 6.1 |
| Uterine | 2 | 4.1 |
| Marital status* | ||
| Living with Partner/Married | 23 | 46.9 |
| Divorced/Separated | 11 | 22.4 |
| Single | 10 | 20.4 |
| Widowed | 4 | 8.2 |
| Annual income* | ||
| Less than $12,000 | 16 | 32.7 |
| $12,000 to 24,999 | 11 | 22.5 |
| $25,000 to 49,999 | 3 | 6.1 |
| $50,000 and Greater | 7 | 14.3 |
| Education* | ||
| Less than high school | 4 | 8.2 |
| High School Degree or GED | 24 | 49.0 |
| At least some college | 18 | 36.8 |
| Cultural affiliation | ||
| Neither AI/AN | 5 | 10.2 |
| White, only | 8 | 16.3 |
| Both AI/AN and White | 7 | 14.3 |
| AI/AN, only | 24 | 49.0 |
| Perceived discrimination | Mean | Std. Dev. |
| 2.8 | 3.0 | |
| N | % | |
| Ever experienced | 31 | 63% |
| Never experienced | 18 | 37% |
some item non-response.
Among patient participants, about half reported that they were living with a partner (47%) and had graduated high school (49%). Forty-one percent of patient participants (16/39; 10 declined to report income) reported annual household income below the Federal Poverty Level (FPL) and 33% reported an annual household income of < $12,000. Among those living in poverty, 44% lived in deep poverty.
Half of patient participants (49%) reported a very strong AI/AN cultural affiliation, indicating that they identified culturally as tribal only and 14% identified culturally as both tribal and white. Overall, the average everyday discrimination score was relatively low at 2.8 (SD ± 3.0, range 0–54). However, 88% of respondents reported experiencing some form of everyday discrimination and only 12% reported never experiencing everyday discrimination. Moreover, there was a trend toward higher scores of perceived discrimination as income decreased (p< 0.03). Although not statistically significant, those who identified culturally as tribal only or both tribal and white reported perceived discrimination more frequently than those who identified culturally as white only or as neither tribal nor white (92% and 100% vs. 63% and 80%; p = 0.129).
Patient-physician relationships and treatment decision making
Overall, patients endorsed neutral to modestly high levels of trust in their providers (mean score 19.2, SD ± 4.5, range 5–25; Table 2). Patients endorsed the domain of fidelity (caring or advocating for the patient’s interest or welfare) least frequently, indicated by 47% reporting that they disagreed or strongly disagreed with the statement that “Sometimes your provider cares more about what is convenient for him/her than about your medical needs”. Patients endorsed the domain of competence (having good practice and interpersonal skills, making correct decisions, and avoiding mistakes) most frequently, indicated by 78% reporting that they strongly agree or agreed with the statement “Your health care provider is extremely thorough and careful.” We found no socio-demographic characteristics that were associated with patient-reported trust. We did find an inverse relationship between perceived discrimination and trust, although this relationship did not achieve statistical significance (p=0.054).
Table 2.
Patient reported trust in providers and quality of decision making.
| Measure | Mean | Std. Dev. |
|---|---|---|
| Trust in physicians* (composite; range 5–25), n=48 | 19.2 | 4.5 |
| Fidelity | 3.16 | 1.45 |
| Competence | 4.10 | 0.94 |
| Honesty | 3.94 | 1.20 |
| Confidentiality | 4.04 | 1.04 |
| Global | 3.98 | 1.18 |
| Collaboration in decision making**(composite; range 7–49), n=45 | 35.5 | 11.8 |
| Satisfaction with decision making**(composite; range 7–14), n=48 | 10.9 | 3.5 |
Wake-Forest Trust Scale [11]; a composite 5-item 5-point Likert scale reflecting trust in physicians; scores 1–3=low trust and 4–5=high trust.
Collaboration and Satisfaction about Care Decision scale [14], a 9-item 7-point Likert scale with domains of Collaboration and Satisfaction reflecting assessment of the quality of shared decision making; scores 1–3=not high quality, 4=neutral, and 5–7=high.
Patients and providers reported generally high perceptions of collaboration in decision making for cancer treatment (Mean (range): 35.5 (10–49) and 39.5(13–49), respectively) and most reported high satisfaction with the decision-making process (Mean (range): 10.9 (2–14) and 11.5 (7 – 14), respectively). While it was not possible to discern a causal relationship, we found that patient trust in providers was significantly correlated with the perception of highly collaborative decision making (R2=0.2683, p<0.001) and with high satisfaction with the decision-making process (R2=0.4319, p<0.001).
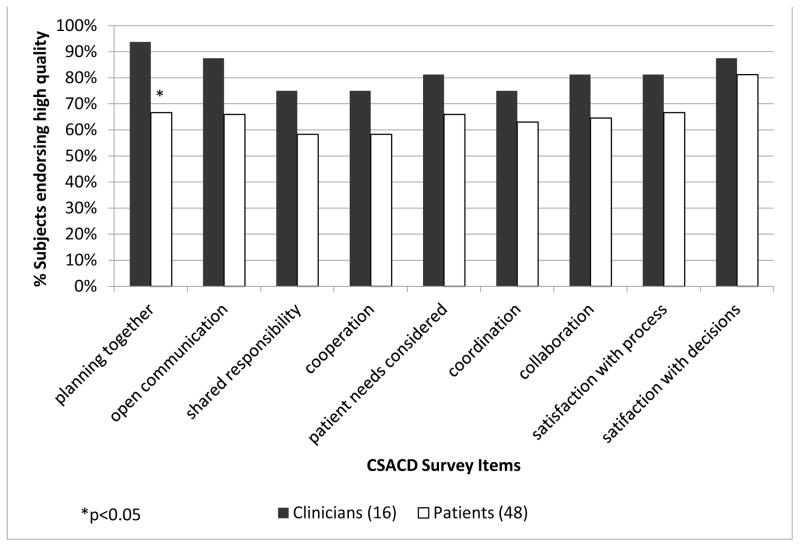
In comparing of patient-provider assessment of the decision-making process, providers scored the quality of the interaction and collaboration more highly than patients did in every individual decision-making domain (Figure 1). However, neither the composite scores for collaboration (mean 39.5 (± SD=10.3) vs. 35.5 (±SD =11.8), p=0.235) nor the composite scores for satisfaction (mean 11.5 (± SD=2.1) vs. 10.9 (± SD=3.5); p=0.542) were significantly different among patients and providers. Patient and provider scores of collaboration were statistically significantly different only in the individual domain of planning together to make cancer treatment decisions, endorsed by 67% patients vs. 94% providers (p=0.048).
Figure 1.
Proportion of patients and providers endorsing high quality* processes of shared decision making using the Collaboration and Satisfaction about Care Decision scale.
*A score of “4” is consistent with the response “neutral”. Processes of shared decision making were dichotomized as “not high quality” (1–4) and “high quality” (5–7).
Non-adherence to treatment
Overall, 76% (n=37) of patients reported adherence to cancer treatment, that is, receipt of an uninterrupted, full course of cancer treatment consistent with national guidelines, while 24% (n=12) reported that their cancer treatment was non-adherent, that is, interrupted or prematurely discontinued. Reasons given for non-adherence ranged from a personal (e.g. “I didn’t think it would help”) or financially-motivated (e.g. “I couldn’t afford it”) decision (n=14), to issues with the healthcare system (e.g., “the machine was broken”) (n=4), or a clinical decision (n=4), defined as intolerable side effects or progression of cancer during chemotherapy treatment.
Relative to those who interrupted or discontinued treatment, patients who reported adherence to treatment reported a greater sense of collaboration with providers (mean 11.6 (SD ± 3.1) vs. mean 8 (SD ± 9.1), p = 0.03, respectively), higher satisfaction with care (mean 11.6 (SD ± 3.1; mean 9.1 (SD ± 4.0), p=0.04), and that their cancer care was very good or excellent (31/37 (83.8%) vs. 3/12 (25%), p <0.001) (Table 3).
Table 3.
Trust, quality of decision making, and non-adherence to treatment.
| Domains (Score range) | Treatment Continuous/Complete | Treatment Interrupted/Discontinued | p | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Dev. | N | Mean | Std. Dev. | ||
| Trust in physicians* (composite; range 5–25) | 36 | 19.4 | 4.2 | 12 | 18.4 | 5.6 | 0.85 |
| Collaboration in decision making**(composite; range 7–49) | 34 | 37.2 | 12.1 | 11 | 30.2 | 9.1 | 0.03 |
| Satisfaction with decision making**(composite; range 7–14) | 36 | 11.6 | 3.1 | 12 | 9.1 | 4.0 | 0.04 |
Wake-Forest Trust Scale [11]; a composite 5-item 5-point Likert scale reflecting trust in physicians; scores 1–3=low trust and 4–5=high trust.
Collaboration and Satisfaction about Care Decision scale [14], a 9-item 7-point Likert scale with domains of Collaboration and Satisfaction reflecting assessment of the quality of shared decision making; scores 1–3=not high quality, 4=neutral, and 5–7=high.
DISCUSSION
In this cohort of American Indian/Alaska Native cancer patients, we found high rates of poverty and high rates of tribal affiliation, and we found that that most participants experienced some form of everyday discrimination although often at a low level—all were directly associated with each other. Nonetheless, trust in physicians was generally high especially in the domain of competence. Patients and their providers endorsed high collaboration during and satisfaction with decision making, although in every respect providers were more satisfied than patients and endorsed collaborative planning significantly more frequently than their patients did. In spite of high trust in their providers, patients also reported common non-adherence to planned treatment—26% overall—and most often for non-clinical reasons. Trust was associated with patients’ collaboration and decision-making satisfaction scores, which in turn were significantly associated with treatment adherence.
Patient trust in physicians is closely related to satisfaction but, while satisfaction reflects a post-treatment perspective, trust is the action of acceptance before treatment is received and therefore may be more useful than satisfaction for understanding uptake of and adherence to cancer care. As well, trust has predominant attributes of a trait (having stability under various conditions) rather than a state (fluctuating under changing conditions).[11] Trust has not been well-studied in cancer care; however, limited data indicate it is a powerful mediator of disparities in the treatment of chronic conditions. For example, in a study of cost burden, trust, and adherence among primary care patients, medication cost was only associated with non-adherence among patients with low trust in physicians.[20] Although trust is stable, it can be positively or negatively affected by interactions and experiences over time and by the quality of patient-physician communication.[19–21]
Our study was subject to several limitations which must be noted. Most importantly, our available sample was small which limited statistical power. Data regarding AI/AN patients are scant due to their very small population size (currently 1.7% of the U.S. population) [22] and due to a reluctance to participate in research studies which may be traced to a legacy of perceived insensitivity and exploitation. [23] Alone among racial/ethnic groups, AI/AN communities have responded to the perceived exploitation by researchers by outlining principles and guidelines for research that take into account the engagement of tribal leadership and the individuality of the tribal community.[12, 13] As noted above, these principles and guidelines have been incorporated into the present study.
In addition to the traditional reluctance to participate in research, other aspects of our cohort may have contributed to the small sample size. Many of our cohort lived in poverty or deep poverty and the complications of daily life in such circumstances may have hindered contact but also had a profound effect on their ability to obtain care. In spite of the small available sample, however, we were able to achieve an excellent response rate among available patients due to the multi-modal approach to contact which included contact by nurse scientists who were well known in the AI/AN communities. The patient population was limited to patients undergoing surgery for cancer and included a variety of cancer types. It is possible that if we had focused on a more limited number of cancer types, we may have had more robust associations; however, such an effort to standardize the cohort would be compromised by very low sample size. Our study also focused on patients obtaining surgical care in the state of Washington, thus generalizability to other areas of the United States is limited.
Finally, our results would be more compelling if we could rate concordance among patient-surgeon dyads. Although the participating surgeons were identified because they had operated on patient participants, the provider response rate was low which limited the statistical power of dyadic pairing. Therefore we accepted association rather than concordance as the primary study outcome. We believe these data support the examination of patient-provider dyads as a future research effort. An alternative research design would be a descriptive correlational study. This design, however, would not provide the insight into both the patient and the provider cancer treatment experience. Although the low participation among surgeons limited our ability to generalize our results, these preliminary findings could be used to direct further research that includes larger samples.
In spite of these limitations, we have previously demonstrated that adherence to treatment guidelines in cancer care is lower among AI/AN patients than non-Hispanic whites and that non-adherence has an inverse relationship to survival in this cohort.[4] Given the importance of adherence to cancer treatment for survival, the many non-clinical reasons for non-adherence, and the currently demonstrated association between decision-making quality and adherence, it would be worthwhile to investigate conditions that increase patient satisfaction with decision making, as well as increasing patient-provider collaboration, and whether enabling such conditions could lead to improved adherence to the cancer treatment plan.
Acknowledgments
Research reported in this publication was supported by the National Institute of Health under Award Number 3P50CA148110.
Footnotes
There are no author conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104(Suppl 3):S377–87. doi: 10.2105/AJPH.2013.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–13. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 3.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 4.Javid SH, Varghese TK, Morris AM, et al. Guideline-concordant cancer care and survival among American Indian/Alaskan Native patients. Cancer. 2014;120(14):2183–90. doi: 10.1002/cncr.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Boland GM, Chang GJ, Haynes AB, Chiang YJ, Chagpar R, Xing Y, Hu CY, Feig BW, You YN, Cormier JN. Cancer. 2013 Apr 15;119(8):1593–601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahtsevani C, Uden G, Willman A. Outcomes of evidence-based clinical practice guidelines: a systematic review. Int J Technol Assess Health Care. 2004;20:427–433. doi: 10.1017/s026646230400131x. [DOI] [PubMed] [Google Scholar]
- 7.Haozous EA, Doorenbos AZ, Alvord LA, Flum DR, Morris AM. Rough waters: The cancer journey for American Indians and Alaska Natives. Oncology Nursing Forum. doi: 10.1188/16.ONF.625-635. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mead EL, Doorenbos AZ, Javid SH, et al. Shared decision-making for cancer care among racial and ethnic minorities: a systematic review. Am J Public Health. 2013;103(12):e15–29. doi: 10.2105/AJPH.2013.301631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald D, Beals J, Manson SM. Use of traditional health practices among Native Americans in a primary care setting. Med Care. 2000;38(12):1191–9. doi: 10.1097/00005650-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Canales MK, Rakowski W, Howard A. Traditionality and cancer screening practices among American Indian women in Vermont. Health Care Women Int. 2007;28(2):155–81. doi: 10.1080/07399330601128544. [DOI] [PubMed] [Google Scholar]
- 11.Hall MA, Dugan E, Zheng B, Mishra AK. Trust in physicians and medical institutions: what is it, can it be measured, and does it matter? Milbank Q. 2001;79(4):613–39. doi: 10.1111/1468-0009.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haozous EA, Neher C. Best Practices for Effective Clinical Partnerships with Indigenous Populations of North America (American Indian, Alaska Native, First Nations, Métis, and Inuit) Nurs Clin North Am. 2015 Sep;50(3):499–508. doi: 10.1016/j.cnur.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell JY, Davis JD, Du Bois B, et al. Culturally competent research with American Indians and Alaska Natives: findings and recommendations of the first symposium of the work group on American Indian Research and Program Evaluation Methodology. Am Indian Alsk Native Ment Health Res. 2005;12(1):1–21. doi: 10.5820/aian.1201.2005.1. [DOI] [PubMed] [Google Scholar]
- 14.Baggs JG. Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–82. doi: 10.1046/j.1365-2648.1994.20010176.x. [DOI] [PubMed] [Google Scholar]
- 15.Lei S. [accessed May 15, 2015];The Unwaged War on Deep Poverty. 2015 Available from URL: http://www.urban.org/features/unwaged-war-deep-poverty.
- 16.Moran J, Fleming CM, Somervell P, Manson SM. Measuring bicultural ethnic identity among American Indian adolescents: A factor analytic study. Journal of Adolescent Research. 1999;14:405–426. [Google Scholar]
- 17.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997;2(3):335–51. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 18.Clark R, Coleman AP, Novak JD. Brief report: Initial psychometric properties of the everyday discrimination scale in black adolescents. J Adolesc. 2004;27(3):363–8. doi: 10.1016/j.adolescence.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Regenbogen SE, Veenstra CM, Hawley ST, et al. The effect of complications on the patient-surgeon relationship after colorectal cancer surgery. Surgery. 2014;155(5):841–50. doi: 10.1016/j.surg.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piette JD, Heisler M, Krein S, Kerr EA. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–55. doi: 10.1001/archinte.165.15.1749. [DOI] [PubMed] [Google Scholar]
- 21.Keating NL, Gandhi TK, Orav EJ, Bates DW, Ayanian JZ. Patient characteristics and experiences associated with trust in specialist physicians. Arch Intern Med. 2004;164(9):1015–20. doi: 10.1001/archinte.164.9.1015. [DOI] [PubMed] [Google Scholar]
- 22.Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010. [accessed May 1, 2015];2010 Census Briefs [serial online] 2012 Available from URL: http://www.census.gov/prod/cen2010/briefs/c2010br-10.pdf.
- 23.Buchwald D, Mendoza-Jenkins V, Croy C, McGough H, Bezdek M, Spicer P. Attitudes of urban American Indians and Alaska Natives regarding participation in research. J Gen Intern Med. 2006;21(6):648–51. doi: 10.1111/j.1525-1497.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]