Abstract
Infection of the central nervous system (CNS) by neurotropic viruses represents an increasing worldwide problem in terms of morbidity and mortality for people of all ages. Although unique structural features of the blood-brain-barrier (BBB) provide a physical and physiological barrier, a number of neurotropic viruses are able to enter the CNS resulting in a variety of pathological outcomes. Nonetheless, antigen-specific lymphocytes are ultimately able to accumulate within the CNS and contribute to defense by reducing or eliminating the invading viral pathogen. Alternatively, infiltration of activated cells of the immune system may be detrimental, as these cells can contribute to neuropathology that may result in long-term cellular damage or death. More recently, myeloid cells e.g. neutrophils have been implicated in contributing to both host defense and disease in response to viral infection of the CNS. This review highlights recent studies using coronavirus-induced neurologic disease as a model to determine how neutrophils affect effective control of viral replication as well as demyelination.
Keywords: neutrophils, mouse hepatitis virus, chemokines, chemokine receptors
1. Introduction
Intracranial infection of susceptible mice with the JHM strain of mouse hepatitis virus (JHMV) causes an acute encephalomyelitis followed by a chronic demyelinating disease similar to the human demyelinating disease multiple sclerosis (MS). Early following JHMV infection of the CNS, the virus targets ependymal cells lining the ventricles, replicates, and rapidly disseminates into the brain parenchyma at which point the virus infects and replicates within astrocytes, oligodendroglia, and microglia throughout the brain and spinal cord (Wang et al., 1992). In response to viral infection of glial cells, a rapid and orchestrated expression of chemokines occurs that contribute to attracting inflammatory cells into the CNS. In terms of host defense, secretion of chemokines derived from the CNS, including CXCL10 and CCL5, promote the migration and accumulation of virus-specific CD4+ and CD8+ T cells that control viral replication via secretion of IFN-γ and cytolytic activity. While inflammatory T cells are effective in eliminating virus, sterile immunity is not achieved; viral protein and/or RNA persist within astrocytes and oligodendroglia resulting in chronic expression of chemokine genes leading to chronic neuroinflammation and demyelination. Histological features associated with viral persistence include the development of an immune-mediated demyelinating disease similar to the human demyelinating disease MS in that both T cells and macrophages are critical mediators of disease severity and contribute to myelin damage (Cheever et al., 1949; Perlman et al., 1999).
Through the course of both acute and chronic JHMV-induced neurologic infection, there is a coordinated expression of chemokines and chemokine receptors that regulate inflammation contributing to both host defense and disease exacerbation. Among the chemokines expressed during infection are members of the ELR(+) chemokine family CXCL1, CXCL2, and CXCL5. CXCL1 and CXCL2 are potent chemoattractants for neutrophils via binding and signaling through the receptor CXCR2 (Moser et al., 1990; Schumacher et al., 1992; Wolpe et al., 1989). Moreover, PMNs have been shown to enhance CNS inflammation by disrupting blood brain barrier (BBB) integrity in various animal models of chronic neuroinflammation including spinal cord injury (SCI) (Gorio et al., 2007; Tonai et al., 2001) and autoimmune demyelination (Carlson et al., 2008) while blocking or silencing of CXCR2 signaling mutes inflammation and tissue damage in mouse models in which PMN infiltration is critical to disease initiation (Belperio et al., 2005; Carlson et al., 2008; Gorio et al., 2007; Kielian et al., 2001; Londhe et al., 2005a, b; Strieter et al., 2005; Wareing et al., 2007).
2. Neutrophils and acute viral-induced encephalomyelitis
Neutrophils represent a component of the innate immune response and provide an essential role in killing invading pathogens through an arsenal of defense mechanisms including release of microbicidal granules and release of reactive oxygen/nitrogen species (Borregaard, 2010; Nathan, 2006). While a clear role for neutrophils in combating bacterial pathogens is documented (Borregaard, 2010; Nathan, 2006), how these cells contribute to host defense and disease in response to CNS viral infection is less well characterized. McGavern and colleagues (Kim et al., 2009) employed two-photon microscopy to elegantly demonstrate that neutrophils, along with monocytes, were responsible for vascular leakage and acute lethality following lymphocytic choriomeningitis virus (LCMV) infection of the CNS. Human immunodeficiency virus-1 (HIV-1) infection of monocyte-derived macrophages increases expression of CXCL5 that serves to attract neutrophils that may augment neuropathology by contributing to neuron death (Guha et al., 2015). Experimental infection of mice with West Nile virus (WNV) in which neutrophil trafficking to the CNS is impaired results in increased protection from WNV encephalitis by limiting immune cell access to the CNS thus diminishing neuropathology (Wang et al., 2012). With regards to JHMV-induced encephalomyelitis, early work by Stohlman and colleagues (Zhou et al., 2003) highlighted a previously unrecognized role for neutrophils in effectively controlling viral replication within the CNS. The underlying mechanisms by which neutrophils contribute to an effective host defense are related to neutrophil-mediated permeabilization of the blood-brain-barrier (BBB) through release of matrix metalloproteinase 9 (MMP-9) (Zhou et al., 2003) although other factors independent of MMP-9 may also be involved (Savarin et al., 2010). In addition, monocytes can also enhance T cell accumulation within the CNS of JHMV-infected mice through the glia limitans (Savarin et al., 2010). Neutrophils are rapidly mobilized from the bone-marrow and into the blood in response to CNS infection by JHMV and this most likely reflects the precipitous increase in expression of the neutrophil chemoattractants CXCL1, CXCL2, and CXCL5 that all bind to their cognate receptor CXCR2 with high binding (Hosking et al., 2009). Indeed, treatment of JHMV-infected mice with a blocking antibody specific for CXCR2 resulted impaired migration of CXCR2-bearing neutrophils to the CNS and this resulted in increased mortality that was associated with impaired ability to control viral replication within the CNS (Hosking et al., 2009). Blocking neutrophil accumulation within the CNS resulted in reduced expression of MMP-9, limited permeabilization of the BBB, and diminished infiltration of virus-specific T cells (Hosking et al., 2009). Collectively, these findings illustrate that neutrophils are an important component of an effective host defense following CNS infection with a neurotropic virus.
3. Neutrophils and viral-induced demyelination
Neutrophil infiltration into the CNS has been associated with neurologic disease in pre-clinical animal models (Christy et al., 2013; Howe et al., 2012; Ransohoff and Brown, 2012; Sayed et al., 2010; Sewell et al., 2004). Herz and colleagues (Herz et al., 2015) have recently demonstrated that CXCR2 antagonization reduced neurological deficits and infarct volumes following middle cerebral artery occlusion and this was associated with reduced neutrophil infiltration into the CNS. Similarly, depletion of neutrophils following subarachnoid hemorrhage was found to improve memory in a model of aneurysmal subarachnoid hemorrhage (SAH) (Provencio et al., 2016). Additionally, Zenaro et al. (Zenaro et al., 2015) have demonstrated a role for the adhesion molecule lymphocyte function-associated antigen 1 (LFA-1) in promoting neutrophil accumulation within the CNS and amplifying AD-like pathology in transgenic models of Alzheimer’s disease (AD). Depletion of neutrophils and/or a deficiency in LFA-1 resulted in protection from cognitive decline and reduced gliosis arguing that blocking neutrophil trafficking may be beneficial in AD (Zenaro et al., 2015). Within models of spinal cord injury/trauma, neutrophils are among the first cells to accumulate within the site of injury and a number of studies argue for a pathogenic role for these cells through limiting tissue sparing and motor recovery while increasing expression of pro-inflammatory cytokines (Bao et al., 2012; Saiwai et al., 2010). Collectively, these studies demonstrate that in animal models of chronic neuroinflammation/neurodegeneration neutrophils can amplify the severity histologic disease and argue that blocking entry into the CNS may limit the severity of neurologic disease.
A role for neutrophils in immune-mediated demyelination remains to be well characterized. Ransohoff and colleagues (Liu et al., 2010), have shown that CXCR2-positive neutrophils are essential for cuprizone-induced demyelination and potentially contribute to oligodendrocyte cell loss. Questions remain regarding the importance of neutrophils in the pathogenesis of MS given the paucity of these cells in active lesions; however, elevated neutrophil numbers within the cerebrospinal fluid (CSF) of MS patients have been correlated with clinical relapse (Kostic et al., 2014). Administration of granulocyte-colony-stimulating factor (G-CSF), a neutrophil activating molecule, to MS and neuromyelitis optica (NMO) patients resulted in disease exacerbation arguing for a role for these cells in amplifying disease severity (Jacob et al., 2012; Openshaw et al., 2000). Additionally, neutrophils have been reported to be more numerous and exhibit a more primed state in MS patients (Naegele et al., 2012). Recent studies (Huber et al., 2014; Rumble et al., 2015) highlight the importance of CXCL1 as well as other myeloid-chemoattractant molecules as having a possible role in potentiating disease in patients with either relapsing-remitting or progressive forms of MS, suggesting that soluble factors that attract neutrophils and/or neutrophil-related molecules may be important therapeutic targets for MS patients. Support for this notion is derived from studies employing experimental autoimmune encephalomyelitis (EAE) as a model for MS in which disease onset is mute when neutrophil trafficking to the CNS is disrupted (Carlson et al., 2008; McColl et al., 1998). More recently, Stoolman et al. (Stoolman et al., 2014) have expanded on these findings to show that enriched expression of CXCL2 within the brainstem attracts neutrophils that substantially contribute to atypical EAE. Similarly, mice in which neutrophils lack suppressor of cytokine signaling 3 (SOCS3) exhibit an increase in susceptibility to the atypical EAE and this correlates with preferential recruitment of neutrophils into the cerebellum and brainstem (Liu et al., 2015). The site of neutrophil recruitment may be critical in terms of amplifying histopathology as neutrophil accumulation within the brain, but to a limited extent in the spinal cord, contribute to tissue injury (Simmons et al., 2014). Collectively, these findings indicate that neutrophils can affect the severity of clinical disease and neuroinflammation in EAE.
4. A transgenic model to study viral-induced neutrophil-mediated neuropathology
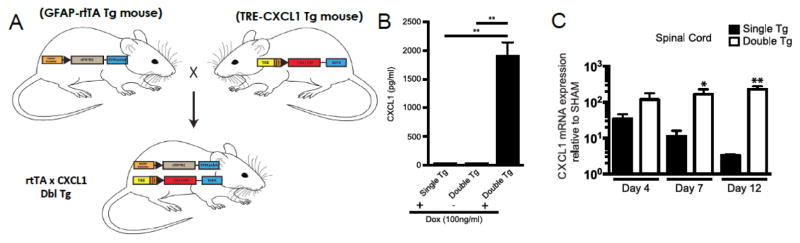
In attempt to better understand how neutrophils influence both host defense and disease following CNS viral infection, we have recently engineered transgenic mice to utilize the tetracycline-controlled transcriptional activation system in which the human glial fibrillary acidic protein (hGFAP) promoter drives expression of a modified version of the reverse tetracycline transactivator protein (rtTA*M2) (Marro et al., 2016) (Figure 1A). Astrocytes were chosen for targeted expression of CXCL1 as previous studies (Glabinski et al., 1999; Glabinski et al., 1998; Kang et al., 2013) have shown that JHMV-infected astrocytes express CXCL1 (Hosking et al., 2010; Lane et al., 1998). In the presence of doxycycline (Dox), transcription initiates at a tet-operon and leads to production of recombinant CXCL1 mRNA transcripts. Double transgenic (tg) mice (pBI-CXCL1-rtTA) and single tg mice (pBI-CXCL1) were generated; characterization of double tg mice revealed Dox-dependent expression of CXCL1 from cultured astrocytes as determined by ELISA (Figure 1B) (Marro et al., 2016). I.c. infection of Dox-treated double tg mice with JHMV resulted in a selective increased expression of CXCL1 mRNA transcripts and protein within the brain and spinal cords when compared to Dox-treated single tg mice infected with JHMV (Figure 1C) (Marro et al., 2016). Dox-induced overexpression of CXCL1 did not enhance control of viral replication within the CNS as both infected double and single tg mice exhibited similar viral titers at defined times post-infection (p.i.) nor were there differences in either frequency or numbers of virus-specific CD4+ and CD8+ T cells within the CNS of double tg mice compared to single tg mice (Marro et al., 2016). However, Dox-treatment of JHMV-infected double tg mice resulted in increased clinical disease and mortality when compared to infected single tg mice (Marro et al., 2016).
Figure 1. Derivation and characterization of a mouse model in which CXCL1 expression within the CNS is under control of a Doxycycline promoter.
(A) Cartoon depiction of experimental strategy to generate double (dbl) transgenic (tg) mice in which expression of mouse CXCL1 is under control of the GFAP promoter upon doxycycline treatment. (B) Cortex tissue from double tg and single tg post-natal day 1 (P1) mice was dissociated and enriched for astrocytes. Following 24-hours of Dox (100ng/ml) treated double tg astrocyte cultures, immunofluorescence confirmed CXCL1 expression within GFAP-positive astrocytes while vehicle treatment yielded no CXCL1 fluorescence. (C) Within the SC, dox-treated double tg mice had statistically significant increases in CXCL1 mRNA expression over Dox-treated single tg mice at days 7 and 12 p.i. Images adapted from Marro et al., 2016 with permission.
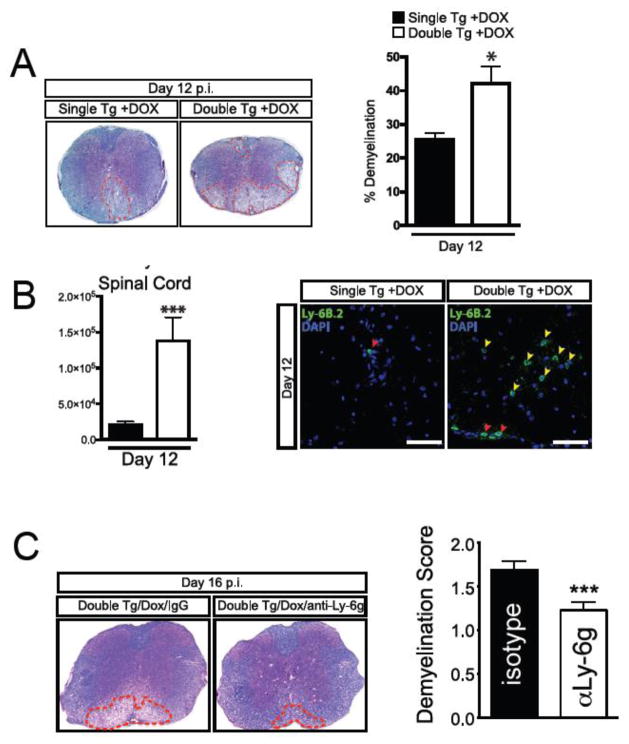
In conjunction with increased expression of CXCL1 initiated within the CNS of Dox-treated double tg mice infected with JHMV, there was a rapid increase in CXCL1 protein levels in serum (Marro et al., 2016). Correspondingly, there is a rapid increase in neutrophils within the blood at days 4 (p<0.05) and 7 (p<0.001) in double tg mice compared to infected single tg controls (Marro et al., 2016). Dox-induced CXCL1 production in JHMV-infected double tg mice also resulted in an increase in neutrophil frequency within the brain at days 4 and 7 p.i. (Marro et al., 2016). Similarly, there was an increase in neutrophil frequency within spinal cords of double tg mice at days 4 (p<0.01) and 7 (p<0.05) p.i. compared to single tg mice (Marro et al., 2016). Immunofluorescence staining for neutrophils (Ly-6B.2) supported the flow cytometric data and revealed increased numbers of neutrophils accumulating within the meninges of double tg mice at day 7 p.i. (Marro et al., 2016). The increased presence of neutrophils within the CNS of double tg mice suggested that there would also be a corresponding increase in blood-brain-barrier (BBB) permeability. Surprisingly, no differences were observed in BBB permeability within the brain or spinal cord at day 4 p.i. as measured by sodium fluorescein (NaF) uptake (Marro et al., 2016). Examination of spinal cords from JHMV-infected Dox-treated double tg mice revealed an overall increase (p<0.05) in the severity of demyelination when compared to infected single tg animals (Fig. 2A) (Marro et al., 2016). The increase in demyelination in double tg mice was associated with a significant (p<0.05) loss of mature oligodendrocytes (as determined by expression of GST-π) within the spinal cords and increased numbers of microglia in Dox-treated JHMV-infected double tg mice compared to infected single tg mice (Marro et al., 2016). Flow cytometric data indicated that neutrophil frequencies within the spinal cords of infected double tg were significantly increased (p<0.01) as well as their total numbers (p<0.001) at day 12 p.i. compared to single tg mice (Fig. 2B) (Marro et al., 2016). Additionally, neutrophils were detected within the spinal cord parenchyma of double tg mice compared to single tg mice (Fig. 2B) (Marro et al., 2016). Elimination of neutrophils via administration of anti-Ly6g monoclonal antibody injection into JHMV-infected double tg mice treated with Dox resulted in a reduction in the severity of demyelination when compared to mice treated with isotype control antibody (Fig. 2C) thus demonstrating that neutrophils are capable of augmenting the severity of white matter damage (Marro et al., 2016)
Figure 2. Elevated CXCL1 expression is associated with increased demyelination. Histopathological analysis of spinal cords of double tg mice reveals an increase in demyelination.
(A) Representative luxol fast blue (LFB)-stained spinal cords reveals increased (p<0.05) demyelination in JHMV-infected Dox-treated double tg mice compared to single tg controls. (B) Flow cytometric analysis revealed a significant increase in the frequency and total number of neutrophils within the spinal cord of JHMV-infected Dox-treated double tg mice compared to single tg mice. Representative immunofluorescence staining further demonstrated a significant increase in the number of Ly6B.2-positive neutrophils (yellow arrowheads) within the spinal cord parenchyma of JHMV-infected double tg compared to single tg mice; red arrowheads indicate neutrophils located within the spinal cord meninges. Quantification of neutrophils within the spinal cords indicated an overall increase (p<0.05) in Dox-treated double tg mice compared to Dox-treated single tg mice. (C) Representative LFB-stained spinal cord sections from JHMV-infected double tg mice treated with either control IgG2a or anti-Ly6G antibody between days 3–15 p.i. Quantification of the severity of demyelination revealed reduced white matter damage in mice treated with anti-Ly6G antibody compared to mice treated with isogenic IgG2a control antibody. Images adapted from Marro et al., 2016 with permission.
5. Perspectives
Although a role for neutrophils in host defense following infection with bacterial pathogens has been appreciated for a number of years, how neutrophils affect host defense in response to viral infection of the CNS has not been as well studied. However, it is now clear that neutrophils are capable of enhancing control of viral replication within the CNS through increasing the permeabilization of the BBB thereby allowing antigen-specific lymphocytes access to sites of infection. Equally interesting is how neutrophil infiltration into the CNS contributes to neuropathology e.g. demyelination. Compelling new information derived from clinical studies from MS patients as well as preclinical animal models of MS have emphasized a potential role for these cells in amplifying white matter damage opening the possibility of targeting neutrophil migration into the CNS as a therapeutic strategy to limit CNS damage.
Highlights.
Neutrophils are rapidly mobilized from the bone-marrow to the central nervous system following infection with neurotropic viruses.
Neutrophils respond to chemokine ligands including CXCL1 and CXCL2 by binding to the receptor CXCR2 expressed upon the neutrophil cell surface.
Contributions to host defense by neutrophils include increasing the permeability of the blood-brain-barrier to allow access by virus-specific lymphocytes.
Neutrophils can also increase the severity of neuropathology e.g. demyelination as evidenced through use of transgenic mouse model systems.
Acknowledgments
This work was supported by NIH grant R01NS041249 to T.E.L. B.S.M. was supported by NIH T32 Training Grant 5T32A1007319.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Literature Cited
- Bao F, Omana V, Brown A, Weaver LC. The systemic inflammatory response after spinal cord injury in the rat is decreased by alpha4beta1 integrin blockade. J Neurotrauma. 2012;29:1626–1637. doi: 10.1089/neu.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Gomperts BN, Xue YY, Hong K, Mestas J, Zisman D, Ardehali A, Saggar R, Lynch JP, 3rd, Ross DJ, Strieter RM. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175:6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. Journal of Exerimental Medicine. 1949;90:181–194. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. Journal of autoimmunity. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Krakowski M, Han Y, Owens T, Ransohoff RM. Chemokine expression in GKO mice (lacking interferon-gamma) with experimental autoimmune encephalomyelitis. Journal of neurovirology. 1999;5:95–101. doi: 10.3109/13550289909029750. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Tuohy VK, Ransohoff RM. Expression of chemokines RANTES, MIP-1alpha and GRO-alpha correlates with inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 1998;5:166–171. doi: 10.1159/000026333. [DOI] [PubMed] [Google Scholar]
- Gorio A, Madaschi L, Zadra G, Marfia G, Cavalieri B, Bertini R, Di Giulio AM. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharmacol Exp Ther. 2007;322:973–981. doi: 10.1124/jpet.107.123679. [DOI] [PubMed] [Google Scholar]
- Guha D, Klamar CR, Reinhart T, Ayyavoo V. Transcriptional Regulation of CXCL5 in HIV-1-Infected Macrophages and Its Functional Consequences on CNS Pathology. J Interferon Cytokine Res. 2015;35:373–384. doi: 10.1089/jir.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of Neutrophils in Exacerbation of Brain Injury After Focal Cerebral Ischemia in Hyperlipidemic Mice. Stroke. 2015;46:2916–2925. doi: 10.1161/STROKEAHA.115.010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS pathogens. 2009;5:e1000648. doi: 10.1371/journal.ppat.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking MP, Tirotta E, Ransohoff RM, Lane TE. CXCR2 signaling protects oligodendrocytes and restricts demyelination in a mouse model of viral-induced demyelination. PloS one. 2010;5:e11340. doi: 10.1371/journal.pone.0011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Lafrance-Corey RG, Sundsbak RS, Lafrance SJ. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. Journal of neuroinflammation. 2012;9:50. doi: 10.1186/1742-2094-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AK, Wang L, Han P, Zhang X, Ekholm S, Srinivasan A, Irani DN, Segal BM. Dysregulation of the IL-23/IL-17 axis and myeloid factors in secondary progressive MS. Neurology. 2014;83:1500–1507. doi: 10.1212/WNL.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Saadoun S, Kitley J, Leite M, Palace J, Schon F, Papadopoulos MC. Detrimental role of granulocyte-colony stimulating factor in neuromyelitis optica: clinical case and histological evidence. Multiple sclerosis. 2012;18:1801–1803. doi: 10.1177/1352458512443994. [DOI] [PubMed] [Google Scholar]
- Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, Li X. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013;16:1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic M, Dzopalic T, Zivanovic S, Zivkovic N, Cvetanovic A, Stojanovic I, Vojinovic S, Marjanovic G, Savic V, Colic M. IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scandinavian journal of immunology. 2014;79:181–186. doi: 10.1111/sji.12147. [DOI] [PubMed] [Google Scholar]
- Lane TE, Asensio VC, Yu N, Paoletti AD, Campbell IL, Buchmeier MJ. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- Liu L, Belkadi A, Darnall L, Hu T, Drescher C, Cotleur AC, Padovani-Claudio D, He T, Choi K, Lane TE, Miller RH, Ransohoff RM. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nature neuroscience. 2010;13:319–326. doi: 10.1038/nn.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Preferential Recruitment of Neutrophils into the Cerebellum and Brainstem Contributes to the Atypical Experimental Autoimmune Encephalomyelitis Phenotype. Journal of immunology. 2015;195:841–852. doi: 10.4049/jimmunol.1403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J Inflamm (Lond) 2005a;2:4. doi: 10.1186/1476-9255-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2/CXCR2 ligand biological axis impairs alveologenesis during dsRNA-induced lung inflammation in mice. Pediatr Res. 2005b;58:919–926. doi: 10.1203/01.PDR.0000181377.78061.3E. [DOI] [PubMed] [Google Scholar]
- Marro BS, Grist JJ, Lane TE. Inducible Expression of CXCL1 within the Central Nervous System Amplifies Viral-Induced Demyelination. Journal of immunology. 2016;196:1855–64. doi: 10.4049/jimmunol.1501802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology. 1998;161:6421–6426. [PubMed] [Google Scholar]
- Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. Journal of neuroimmunology. 2012;242:60–71. doi: 10.1016/j.jneuroim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews Immunology. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP, Kashyap A, McSweeney P, Forman S. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology. 2000;54:2147–2150. doi: 10.1212/wnl.54.11.2147. [DOI] [PubMed] [Google Scholar]
- Perlman SR, Lane TE, Buchmeier MJ. Coronaviruses: Hepatitis, peritonitis, and central nervous system disease. In: Cunningham MW, Fujinami RS, editors. Effects of Microbes on the Immune System. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 331–348. [Google Scholar]
- Provencio JJ, Swank V, Lu H, Brunet S, Baltan S, Khapre RV, Seerapu H, Kokiko-Cochran ON, Lamb BT, Ransohoff RM. Neutrophil depletion after subarachnoid hemorrhage improves memory via NMDA receptors. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM. Neutrophil-related factors as biomarkers in EAE and MS. The Journal of experimental medicine. 2015;212:23–35. doi: 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiwai H, Ohkawa Y, Yamada H, Kumamaru H, Harada A, Okano H, Yokomizo T, Iwamoto Y, Okada S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. The American journal of pathology. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C, Stohlman SA, Atkinson R, Ransohoff RM, Bergmann CC. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. Journal of virology. 2010;84:4878–4888. doi: 10.1128/JVI.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? Journal of immunology. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci U S A. 1992;89:10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, Fabry Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. Journal of neuroimmunology. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SB, Liggitt D, Goverman JM. Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis. Journal of immunology. 2014;193:555–563. doi: 10.4049/jimmunol.1400807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman JS, Duncker PC, Huber AK, Segal BM. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. Journal of immunology. 2014;193:564–570. doi: 10.4049/jimmunol.1400825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Keane MP, Burdick MD, Sakkour A, Murray LA, Belperio JA. The role of CXCR2/CXCR2 ligands in acute lung injury. Curr Drug Targets Inflamm Allergy. 2005;4:299–303. doi: 10.2174/1568010054022178. [DOI] [PubMed] [Google Scholar]
- Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- Wang FI, Hinton DR, Gilmore W, Trousdale MD, Fleming JO. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Laboratory investigation; a journal of technical methods and pathology. 1992;66:744–754. [PubMed] [Google Scholar]
- Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR, Flavell RA, Town T, Fikrig E. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PloS one. 2012;7:e44153. doi: 10.1371/journal.pone.0044153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing MD, Shea AL, Inglis CA, Dias PB, Sarawar SR. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007;20:369–378. doi: 10.1089/vim.2006.0101. [DOI] [PubMed] [Google Scholar]
- Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nani S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nature medicine. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- Zhou J, Stohlman SA, Hinton DR, Marten NW. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. Journal of immunology. 2003;170:3331–3336. doi: 10.4049/jimmunol.170.6.3331. [DOI] [PubMed] [Google Scholar]