SUMMARY
The physiologic importance of autophagy proteins for control of mammalian bacterial and parasitic infection in vivo is unknown. We show that expression of the essential autophagy protein Atg5 in granulocytes and macrophages is required for in vivo resistance to infection with L. monocytogenes and T. gondii. In primary macrophages, Atg5 was not required for IFNγ/LPS-mediated transcription, induction of nitric oxide, or inhibition of T. gondii replication. However, Atg5 was required for IFNγ/LPS-induced damage to the T. gondii parasitophorous vacuole membrane and parasite clearance. While we did not detect autophagosomes enveloping T. gondii, Atg5 was required for recruitment of the IFNγ-inducible p47 GTPase IIGP1 (Irga6) to the vacuole membrane. This work shows that Atg5 expression in phagocytic cells is essential for cellular immunity to intracellular pathogens in vivo and that an autophagy protein can participate in immunity and intracellular killing of pathogens via autophagosome-independent processes such as GTPase trafficking.
INTRODUCTION
Classical cellular immunity to intracellular bacteria and parasites, first described by Mackaness more than 40 years ago (Mackaness, 1964), requires the activation of monocyte/macrophages by IFNγ. The lysosomal system is critical for this type of cellular immunity via its role in killing pathogens and digesting pathogen corpses. The gram-positive bacteria Listeria monocytogenes (L. monocytogenes), mycobacteria such as Bacillus Calmette-Guerin (BCG), and apicomplexan protozoa such as Toxoplasma gondii (T. gondii) are well-studied intracellular pathogens that survive in non-activated macrophages by escaping the phagolysosomal system [L. monocytogenes, (Edelson and Unanue, 2000)], survival in a specialized parasitophorous vacuole which resists fusion with lysosomes [T. gondii (Mordue and Sibley, 1997; Sibley, 2003)], or inhibition of phagosomes acidification [mycobacteria (Gutierrez et al., 2004; Sturgill-Koszycki et al., 1994)]. Activation of macrophages by IFNγ or IFNγ plus bacterial lipopolysaccharide (LPS) overcomes these pathogen survival mechanisms, resulting in blockade of pathogen replication, killing, and clearance of the pathogen from the cell. IFNγ is essential for resistance of mice to infection with L. monocytogenes (Buchmeier and Schreiber, 1985), T. gondii (Suzuki et al., 1988), and mycobacteria (Flynn and Chan, 2001; Dalton et al., 1993; Cooper et al., 1993; Flynn et al., 1993). Resistance to acute T. gondii infection relies primarily on monocytes/macrophages (Robben et al., 2005) following activation by IFNγ (Suzuki et al., 1988). However, the effector mechanisms responsible for IFNγ-induced killing and clearance of intracellular pathogens from activated macrophages are not completely defined. Importantly, a series of IFNγ- inducible p47 GTPases have been implicated in the control of a range of bacterial and parasitic infections including T. gondii (Taylor et al., 2007; Taylor et al., 2000; Ling et al., 2006; Butcher et al., 2005; Halonen et al., 2001).
Autophagy involves the concerted action of cytoplasmic proteins that generate curved isolation membranes to envelop cytoplasm and cytoplasmic organelles. In the canonical pathway, the resulting 0.5 to 1.5 µm double-membrane bound vesicles fuse with lysosomes to deliver their cytoplasmic cargo for degradation and recycling (Levine and Kroemer, 2008). Autophagy requires the action of two Atg5-dependent ubiquitin-like conjugation systems. One conjugation system generates Atg5-Atg12 conjugates which complex with Atg16 to associate with the elongating isolation membrane (Mizushima et al., 2002). The second conjugation system modifies the free C-terminal glycine of Atg8/LC3 (LC3-I) with phosphatidylethanolamine, generating the lipidated LC3-II form of Atg8/LC3, which becomes associated with autophagosomes. Atg5 is essential for conversion of LC3-I to LC3-II and for localization of LC3-II to autophagosomes (Mizushima et al., 2002). LC3 can also be found associated with other cellular structures including aggregates of ubiquitinated proteins and newly forming phagosomes (Sanjuan et al., 2007; Kuma et al., 2007), raising the possibility that autophagy proteins may participate in cellular processes in addition to classical autophagy. Atg5 may have autophagy-independent functions (Codogno and Meijer, 2006).
Many studies have demonstrated co-localization of LC3 to structures either induced by, or containing bacteria or parasites such as T. gondii (Andrade et al., 2006; Martens et al., 2005; Checroun et al., 2006; Amer and Swanson, 2005; Birmingham et al., 2006; Ogawa et al., 2005; Gutierrez et al., 2004; Nakagawa et al., 2004; Py et al., 2007; Gutierrez et al., 2005; Romano et al., 2007; Schnaith et al., 2007), and this has suggested a role for autophagy in these processes. Given that LC3 can co-localize with various types of structures inside the cell, the physiologic meaning of co-localization perse is not clear (Sanjuan et al., 2007; Kuma et al., 2007; Klionsky et al., 2008). For example, Atg5 and autophagy play no role in coronavirus replication in primary macrophages despite the co-localization of LC3 with viral replication compartments in cell lines (Zhao et al., 2007b). However, autophagy may be an important pathogen control mechanism (Levine and Kroemer, 2008; Levine and Deretic, 2007) since, in cultured cells, the presence of Atg5 delays the growth of L. monocytogenes by about two hours (Py et al., 2007), decreases the number of viable Streptococcus pyogenes by about three-fold at four hours after infection (Nakagawa et al., 2004), and decreases intracellular Salmonella typhimurium about two-fold at eight hours after infection (Birmingham et al., 2006). Furthermore, the induction of autophagy by starvation or treatment with rapamycin decreased mycobacterial viability 40–70% three hours after infection (Gutierrez et al., 2004). One study in transformed fibroblasts suggests a role for Atg5 in IFNγ-mediated control of T. gondii (Konen-Waisman and Howard, 2007). Indeed, studies in Drosophila clearly indicate that autophagy has a role in control of L. monocytogenes infection in primary hemocytes (Yano et al., 2008). Such studies have not yet been performed in mammals.
In contrast to the potential protective role for autophagy against bacterial and parasite infection, other studies suggest that both bacteria and parasites may subvert the autophagic process or autophagy proteins for their own benefit resulting in enhanced replication in cultured cells (Schnaith et al., 2007; Swanson and Isberg, 1995; Romano et al., 2007). In addition, it is clear that some pathogens, such as herpes simplex virus, have evolved elegant mechanisms for inhibiting both signaling processes that induce autophagy and autophagy effector mechanisms (Orvedahl et al., 2007). Such mechanisms may contribute to the apparent lack of a role for autophagy in a specific situation. Thus, autophagy and autophagy proteins may play complex roles in vivo, indicating the importance of studies in intact animals and primary cells to determine the physiologic importance of autophagy and individual autophagy proteins in vivo. A role for an autophagy protein in a specific process in immunity may reflect a role for classical autophagosomes in control of infection (Yano et al., 2008). However, it is also possible that these proteins play cellular roles in addition to their role in generation of classical autophagosomes.
T. gondii provides a unique opportunity to define mechanisms of cellular immunity since elimination of parasites in activated macrophages is well studied (Ling et al., 2006; Taylor et al., 2000; Zhao et al., 2007a; Mordue and Sibley, 1997; Sibley, 2003; Sibley et al., 1991). Two mechanisms of macrophage activation result in killing and clearance of T. gondii in cultured cells, one dependent on IFNγ/LPS and the other on ligation of CD40. These two pathways are completely independent (Zhao et al., 2007a; Subauste and Wessendarp, 2006; Andrade et al., 2005; Andrade et al., 2006). Mice lacking CD40 signaling fail to control of chronic T. gondii infection, dying more than 50 days after infection (Reichmann et al., 2000). Mice lacking IFNγ succumb within 10 days of infection (Suzuki et al., 1988; Scharton-Kersten et al., 1996) indicate the greater importance of the IFNγ-mediated pathway for control of acute infection. In macrophages or astrocytes activated by the IFNγ, T. gondii succumbs to damage to the parasitophorous vacuole membrane, followed by stripping of the vacuolar membrane, killing of the parasite, and clearance of parasite corpses (Ling et al., 2006; Martens et al., 2005). For CD40-dependent killing, a role for autophagy is supported by the demonstration that inhibition of expression of the essential autophagy protein Atg6/beclin1 inhibits killing (Andrade et al., 2006). The role of autophagy proteins in IFNγ-dependent killing and clearance of T. gondii is less clear; experiments to date indicate that IFNγ-mediated killing is dependent on p47 GTPases (Ling et al., 2006; Martens et al., 2005; Taylor et al., 2007) and independent of beclin 1/Atg6 (Andrade et al., 2006). Importantly, in astrocytes the GTPase IIGP1 localizes to the membrane of the parasitophorous vacuole very early after infection of IFNγ-activated cells, overexpression of IIGP1 increases damage to the parasitophorous vacuole membrane, and expression of a dominant negative form of IIGP1 inhibits IFNγ-mediated killing of T. gondii (Martens et al., 2005). Thus, IIGP1 is an important component of the cellular machinery that results in control of T. gondii infection.
Together these emerging data on the role of autophagy in control of bacterial and parasitic infection in cultured cells, and for the activation of autophagy by IFNγ (Gutierrez et al., 2004; Singh et al., 2006) beg fundamentally important questions: How important are autophagy proteins for cellular immunity during infection of a living mammalian host? If autophagy proteins are important, do they act downstream of IFNγ to control infection with intracellular pathogens in primary cells and, if so, how? Is the role of autophagy proteins in the control of intracellular pathogens reflective of a role for autophagosomal envelopment of pathogens, or is some other function of autophagy proteins involved? In this paper we address these questions using infection of mice and studies of infection in primary macrophages.
RESULTS AND DISCUSSION
Role of Atg5 in autophagy in primary macrophages
Mice lacking Atg5 entirely die immediately after birth due to developmental defects (Kuma et al., 2004). We therefore deleted the ATG5 gene from monocyte/macrophages and granulocytes (Kuma et al., 2004; Hara et al., 2006; Zhao et al., 2007b) by breeding ATG5flox/flox mice (Hara et al., 2006) to mice expressing the Cre recombinase from the endogenous lysozyme M locus to generate ATG5flox/flox-Lyz-Cre mice (Lyz-Cre throughout this manuscript) (Clausen et al., 1999; Steed et al., 2007). Deletion of the ATG5 gene in these cells resulted in a deficit in autophagy. Peritoneal macrophages and bone marrow derived macrophages from these mice lack Atg5 and fail to efficiently convert LC3-I to LC3-II [ref. (Zhao et al., 2007b) and Supplemental Data Fig. S1A]. To confirm that the autophagy deficiency in primary macrophages from ATG5flox/flox-Lyz-Cre mice is sufficient to alter control of infection with an intracellular pathogen in vitro, we took advantage of the observation that starvion induced autophagy limits survival BCG in a macrophage cell line (Gutierrez et al., 2004) (Fig. 1A). Atg5-deficient macrophages were less effective than control macrophages in killing BCG, confirming a functional deficiency in autophagy protein-dependent control of an intracellular pathogen in these cells (Gutierrez et al., 2004).
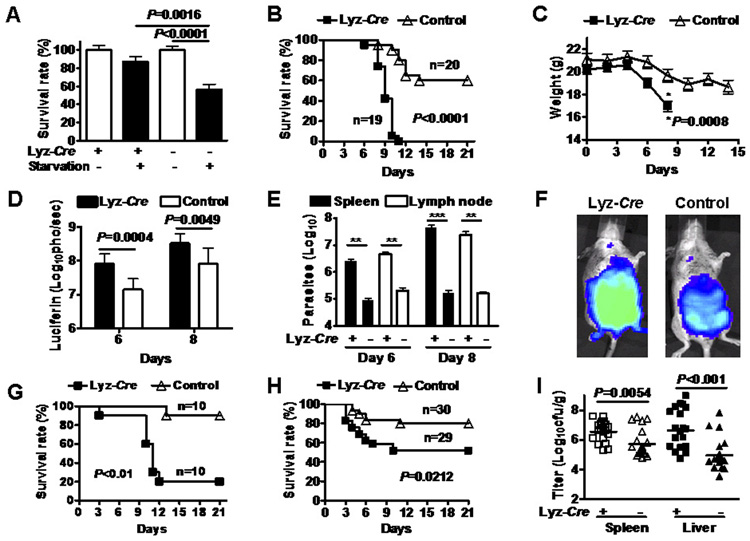
Fig. 1. Atg5 is required for cellular immunity to Toxoplasma gondii and L. monocytogenes in vivo.
(A) Survival of BCG in Atg5 deficient and control macrophages after starvation (Gutierrez et al., 2004). Results are pooled from three independent experiments.
(B) Survival of female mice after infection with 100 T. gondii parasites expressing luciferase. These data were pooled from 4 independent experiments.
(C) Weight of mice in Fig. 1B over the course of T. gondii infection.
(D) Light emission from mice in Fig. 1B after injection of luciferin.
(E) Quantification of parasites in the indicated tissues using the methods and standard curve in Supplemental Figure S3. These data were pooled from 2 independent experiments. ** P<0.001, *** P<0.0001.
(F) Representative images of mice 8 days after infection with T. gondii. The full data set of which these are representatives is provided in Supplemental Figure S2.
(G) Survival of male mice after infection with 200 T. gondii parasites. This dose is higher than that used in female mice in 1B.
(H) Survival of mice after inoculation with 2×105 CFUs of L. monocytogenes.
(I) L. monocytogenes colony forming units in spleen or liver three days after infection. These data were pooled from at least three independent experiments (20 control mice and 19 ATG5flox/flox-Lyz-Cre mice).
Role of Atg5 in vivo for cellular immunity to intracellular bacteria and parasites
To determine the physiologic importance of Atg5 in vivo, we challenged ATG5flox/flox-Lyz- Cre and ATG5flox/flox mice (hereafter referred to as control mice) with T. gondii expressing firefly luciferase and followed infection over time (Fig. 1B to 1F). ATG5flox/flox-Lyz-Cre female mice were more susceptible to T. gondii infection (Fig. 1B, P<0.0001) and exhibited greater weight loss (Fig. 1C, P=0.0008), than control mice. As measured by light detected in whole animals after luciferin injection (Saeij et al., 2005), ATG5flox/flox-Lyz-Cre mice were unable to control T. gondii replication normally (Fig. 1D, P=0.0004, P=0.0049). Quantification of T. gondii parasites in spleen and mesenteric lymph nodes revealed the increased parasite numbers in ATG5flox/flox-Lyz-Cre mice (Fig. 1E, F, Supplemental Fig. S2, S3). Experiments in male mice using the relevant dose of T. gondii confirmed the critically important role of Atg5 expression to resistance to T. gondii (Fig. 1G, P<0.01). The majority of both Atg5-deficient and control mice infected with T. gondii expressed detectable IFNγ in serum, indicating that Atg5 expression in macrophages and granulocytes is not required for induction of IFNγ in vivo (data not shown). Therefore, Atg5 is essential for resistance to T. gondii in vivo. The fact that mice succumb rapidly suggests a failure in the innate immune response, which is highly dependent on IFNγ activation of macrophages, but not granulocytes (Robben et al., 2005).
To determine whether the role of Atg5 in resistance to T. gondii represents a more general role of Atg5 in resistance to intracellular pathogens in vivo, we challenged mice with a second intracellular pathogen, L. monocytogenes. ATG5flox/flox-Lyz-Cre mice were more susceptible to lethal L. monocytogenes infection than control mice (Fig. 1H, P=0.02), and L. monocytogenes replicated to higher levels in both spleen and liver of ATG5flox/flox-Lyz-Cre than in control mice (Fig. 1I, P=0.0054, P<0.001). These data show that Atg5 is essential for effective cellular immunity to intracellular pathogens in vivo.
Atg5 is essential for IFNγ/LPS-induced clearance of T. gondii from primary macrophages
We next defined the cellular mechanisms responsible for the essential role of Atg5 in control of intracellular pathogen infection. The importance of IFNγ and macrophages for resistance to T. gondii infection in vivo suggested that Atg5 is important for IFNγ-dependent control of T. gondii infection in activated primary macrophages. Activation of macrophages by treatment with IFNγ plus a second signal such as LPS optimally restricts T. gondii growth and results in clearance of T. gondii from infected cells (Sibley et al., 1991). We therefore determined the role of Atg5 in IFNγ/LPS-induced inhibition of T. gondii growth in, and clearance from, primary macrophages by measuring the proportion of macrophages infected (Ling et al., 2006) as a measure of clearance, and the number of parasites per parasitophorous vacuole as a measure of replication (Murray et al., 1985a; Murray et al., 1985b; Ling et al., 2006; Mordue and Sibley, 2003).
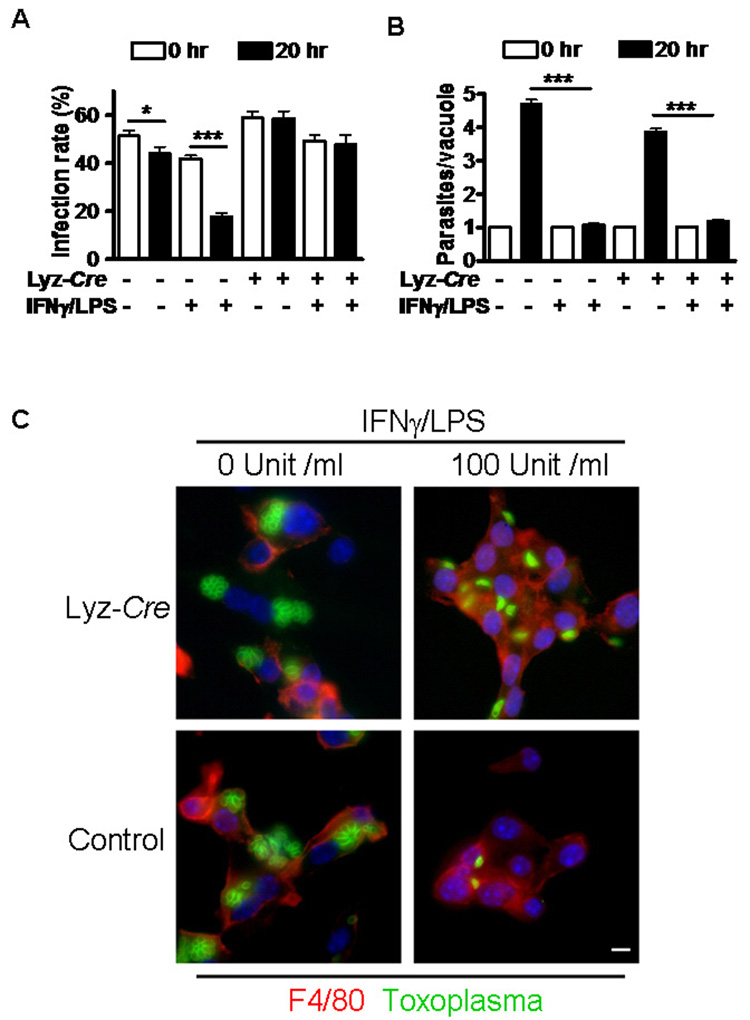
T. gondii efficiently infected and replicated in non-activated control and Atg5-deficient macrophages (Fig. 2). IFNγ/LPS treatment significantly decreased the proportion of T. gondii infected control macrophages (Fig. 2A and C; P<0.0001) twenty hours after infection, reflecting the capacity of these cells to clear infection. In contrast, Atg5-deficient macrophages treated with IFNγ/LPS failed to clear T. gondii infection (Fig. 2A and C, P>0.72). The observation that Atg5 is essential clearance of T. gondii from activated primary macrophages provides a likely explanation for the rapid death of T. gondii infected mice lacking Atg5 expression in macrophages (Fig. 1), which are essential for resistance to acute T. gondii infection (Robben et al., 2005).
Fig. 2. Atg5 is required for IFNγ-induced clearance of Toxoplasma gondii from macrophages.
(A) The proportion of macrophages containing at least one T. gondii parasite; *P <0.05, ***P<0.0001.
(B) The number of T. gondii parasite per vacuole in infected cells; ***P<0.0001. For (A) and (B) data were pooled from at least three independent experiments in which at least 210 cells were counted per condition.
(C) Representative immunofluorescence images of T. gondii-infected macrophages 20 hr after infection stained with anti-mouse F4/80 (red), anti-T. gondii (green), and DAPI (blue). Bar= 100 µm.
Atg5 is not required for IFNγ or IFNγ/LPS signaling, inhibition of T. gondii replication within vacuoles, or induction of nitric oxide in primary macrophages
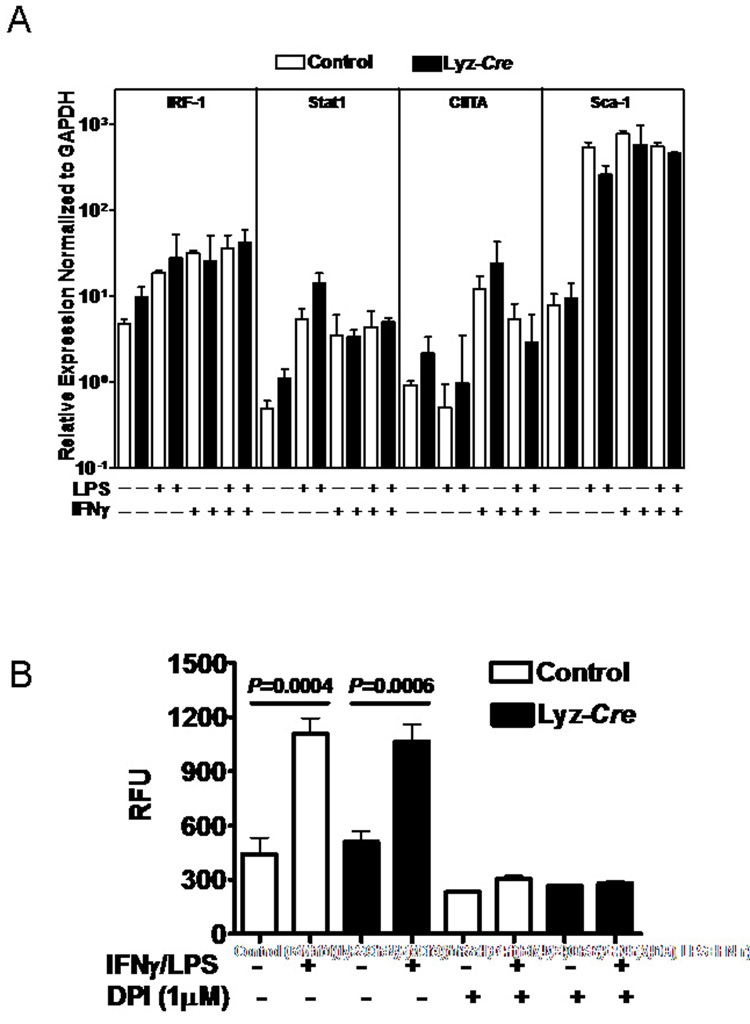
We next defined the role of Atg5 in control of T. gondii replication within the parasitophorous vacuole. Untreated control cells had ca. four parasites per vacuole, reflecting replication within the vacuole. In contrast, IFNγ/LPS treated control cells that had not cleared infection had ca. one parasite per vacuole (Fig. 2B and C; P<0.001). Activation of Atg5-deficient macrophages with IFNγ/LPS also reduced the number of T. gondii parasites per vacuole from ca. three to four to ca. one (P<0.0001), indicating that inhibition of replication of T. gondii within the parasitophorous vacuole did not require Atg5 (Fig. 2B and C, P<0.0001). This shows that IFNγ/LPS efficiently generates Toxoplasma-static responses in the absence of Atg5, indicating Atg5 deficiency does not globally inhibit IFNγ/LPS-induced macrophages activation. To evaluate the possible role of Atg5 in IFNγ-induced transcription and macrophage activation, we quantified several IFNγ-induced transcripts using qRT-PCR (Fig. 3A). IRF-1, Stat1, CIITA, and Sca-1 were all induced comparably in Atg5-deficient as compared to control macrophages after stimulation with IFNγ, LPS, or IFNγ/LPS. Inhibition of T. gondii replication has been assigned to reactive nitric oxide (NO) generated by iNOS (Adams et al., 1990). The induction of NO by IFNγ/LPS was comparable between control and Atg5-deficient macrophages (Fig. 3B). Thus, Atg5 is not required for clearance of T. gondii based on a role in IFNγ-dependent transcription or induction of NO. We therefore evaluated the role of Atg5 in proximal events in the killing of T. gondii by IFNγ/LPS activated macrophages.
Fig. 3. Atg5 is not globally required for IFNγ-induction of transcription.
(A) Shown is the induction of expression of the indicated genes as measured by qRT-PCR at 16 hours after treatment of control or Atg5-deficient macrophages with LPS, IFNγ, or the combination of IFNγ/LPS. Data was pooled from two independent experiments. There were no statistically significant differences between control and Atg5-deficient macrophages.
(B) Shown are the levels of NO produced by macrophages stimulated by IFNγ/LPS for 18 hours. DPI is an inhibitor of NO production. Data were pooled from two independent experiments.
Atg5 is required for disruption of the parasitophorous vacuole membrane in IFNγ activated macrophages
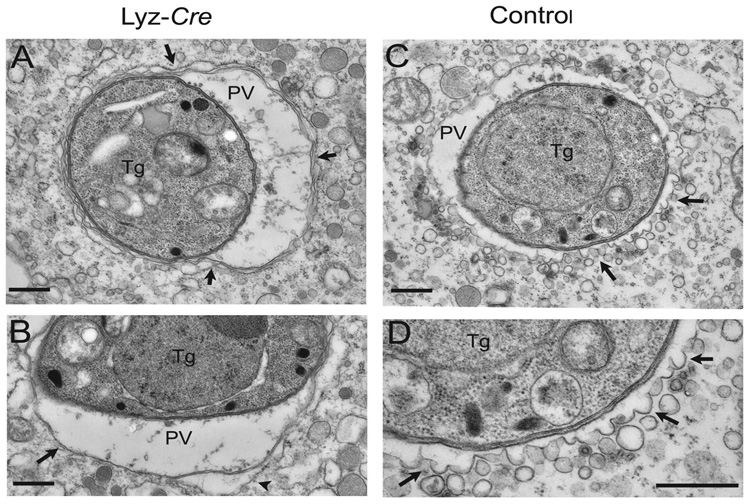
Killing of T. gondii by activated macrophages is associated with blebbing and ultimately stripping of the parasitophorous vacuole membrane early after cell entry followed by parasite destruction (Ling et al., 2006). We therefore defined the role of Atg5 in IFNγ/LPS induced damage to the membrane of the parasitophorous vacuole in activated macrophages five hours after infection. Electron microscopy (EM) revealed marked differences in the vacuole occupied by T. gondii in IFNγ-treated control macrophages versus Atg5-deficient macrophages (Fig. 4). The majority of parasites were found within intact vacuoles in untreated cells (10/10 control and 8/8 Atg5-deficient cells). In control cells treated with IFNγ/LPS, the majority of parasites were found in partially or fully disrupted vacuoles (10/12 control cells). In contrast, the majority of parasites in IFNγ/LPS treated Atg5-deficient cells remained in intact vacuoles (7/8 cells). Parasites within Atg5-deficient IFNγ/LPS-activated macrophages were found within typical parasitophorous vacuoles bound by a single membrane and often surrounded with host ER. The vacuole membrane remained intact and the intracellular membranes of the parasite showed no signs of damage (Fig. 4A, B). There was no consistent difference in the amount of open space within parasitophorous vacuoles between control and Atg5-deficient cells. Inspection revealed that regions of the parasitophorous vacuole membrane that appeared to have more than one bilayer (e.g. Fig. 4A) represented a single parasitophorous vacuole membrane apposed to endoplasmic reticulum. This tight apposition of the endoplasmic reticulum to the parasitophorous vacuole membrane has been well described (Sibley, 2003; Jones et al., 1972; Jones and Hirsch, 1972).
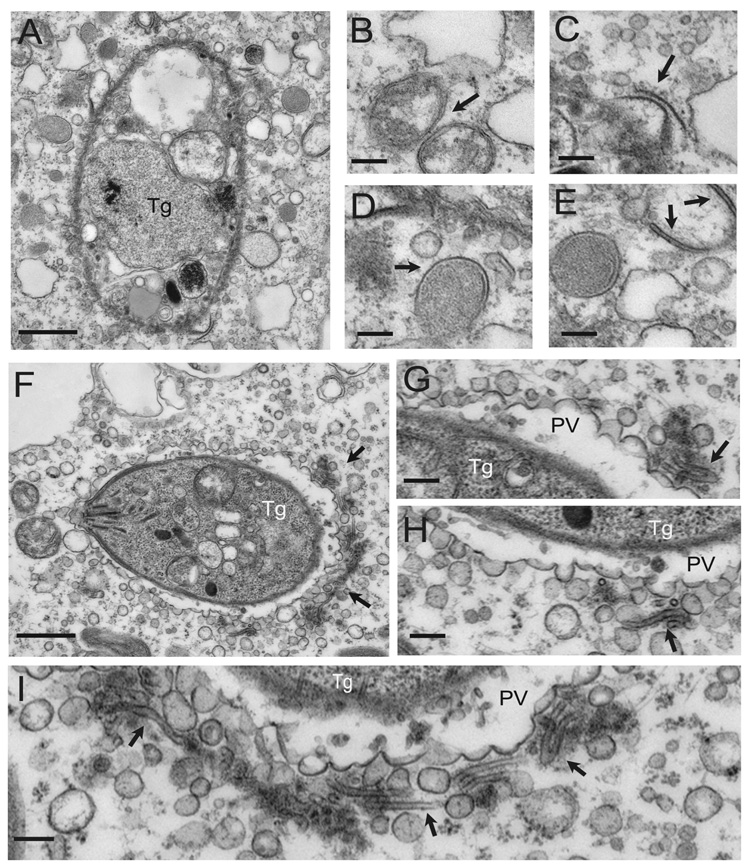
Fig. 4. Electron microscopic analysis of the clearance of T. gondii by IFNγ-activated macrophages.
Macrophages treated as indicated were infected for 5 hours and then analyzed by EM. All images shown are from IFNγ/LPS activated macrophages.
(A) Parasites (Tg) within Atg5 deficient cells, reside within conventional parasitophorous vacuoles (PV) bounded by host ER (arrow heads).
(B) The parasitophorous vacuole membrane is a single unit membrane (arrow) surrounded in places by host ER (arrow head).
(C) Parasites within activated control macrophages are found within vacuoles that are undergoing membrane blebbing and vesiculation (arrows).
(D) Enlarged view from (C) shows membrane blebs protruding from the parasitophorous vacuole membrane (arrows). Scale bars = 0.5 micrometers.
In contrast, the parasitophorous vacuole membrane surrounding parasites within IFNγ/LPS-activated control macrophages showed extensive vesiculation and blebbing with clusters of small vesicles in the vicinity of the vacuole (Fig. 4C, D). Membrane vesiculation and damage to the parasitophorous vacuole was not observed in the absence of IFNγ/LPS treatment (data not shown). This is similar to previous reports of activated macrophages or astrocytes infected with T. gondii (Ling et al., 2006; Martens et al., 2005). Also similar to these previous reports, parasites in IFNγ/LPS-activated control macrophages were often found free in the cytosol and showed extensive membrane damage (Fig. 5A). Frequently, double membrane bound compartments and even membrane crescents were observed in the vicinity of such damaged parasites (Fig. 5B–E). Occasionally, vacuoles that showed extensive vesiculation were adjacent to distinctive flattened membrane stacks (Fig. 5F–I). These flattened membrane structures were not observed associated with the normal-appearing parasitophorous vacuoles in Atg5-deficient IFNγ/LPS activated macrophages, suggesting a role for Atg5 in the generation of these structures. Notably, we did not observe envelopment within double membranes of either the parasitophorous vacuole or partially degraded parasites in the cytosol, suggesting that Atg5-dependent damage to the parasitophorous vacuole did not involve envelopment within autophagosomes. This is consistent with results obtained in activated primary astrocytes (Martens et al., 2005). Collectively, these results show that Atg5 is required for IFNγ/LPS-induced damage to the parasitophorous vacuole 11 membrane and stripping of the membrane away from the parasite. While these observations do not rule out a role for classical autophagy in clearance of debris from damaged parasites, such events would appear to be downstream of a critical Atg5-dependent step in damaging the parasitophorous vacuole. Therefore, these data are consistent with a critical role for Atg5 in a process that does not represent classical autophagy.
Fig. 5. Electron microscopic analysis of damage to the parasitophorous vacuole in IFNγ/LPS activated control macrophages.
Clearance of T. gondii infection by control macrophages treated with IFNγ/LPS is associated with a process of vacuolar membrane damage including vesiculation and blebbing 5 hours after infection. Findings here were specific for control macrophages and were not observed in Atg5-deficient macrophages.
(A) Heavily damaged parasite within the cytosol following dissolution of the parasitophorous vacuole membrane. The parasite plasma membrane shows evidence of damage. Scale bar = 0.5 micrometers.
(B–E) Examples of double-membrane bound compartments forming in the vicinity of the degraded parasite. Scale bars = 0.1 micrometer.
(F) Parasite residing within a parasitophorous vacuole that is undergoing extensive membrane blebbing and vesiculation. A prominent cluster of membrane vesicles and flattened cisternae are found at the posterior end (arrows). Scale bar = 0.5 micrometers.
(G–H) Enlarged views of the membrane vesicles showing flattened cisternae (arrows). Scale bars = 0.1 micrometer.
Role of Atg5 in recruitment of IIGP1 to the parasitophorous vacuole
The failure of IFNγ/LPS-activated, but Atg5-deficient macrophages, to damage and strip the parasitophorous vacuole membrane (Fig. 4, Fig. 5) suggested that Atg5 might be required for recruitment of IFNγ-inducible p47 GTPases, several of which are required for efficient IFNγ-mediated clearance of T. gondii in vitro and/or in vivo (Taylor et al., 2007). We therefore determined whether IIGP1 (Irga6) is properly recruited to the parasitophorous vacuole membrane in IFNγ/LPS-activated, but Atg5-deficient cells. We focused on early times after infection concurrent with or preceding damage to the parasitophorous vacuole membrane (Fig. 6). IIGP1 protein expression was induced by IFNγ/LPS treatment normally in Atg5-deficient macrophages (Supplemental Figure S1B). Within one hour, IIGP1 was recruited to the parasitophorous vacuole in control cells activated by treatment with IFNγ/LPS (Fig. 6A). We did not observe efficient recruitment of IGTP to the parasitophorous vacuole in similar experiments (data not shown). In contrast to control cells, recruitment of IIGP1 was abrogated in Atg5-deficient cells (Fig. 6A, B). It is interesting that at each time point evaluated less than 10% of vacuoles were IIGP1-positive in control cells. This is consistent with rapid transit of the parasitophorous vacuole through a process involving IIGP1 recruitment. Together these data identify a mechanism, recruitment of a key GTPase, by which Atg5 plays an essential role in control of T gondii in activated primary macrophages.
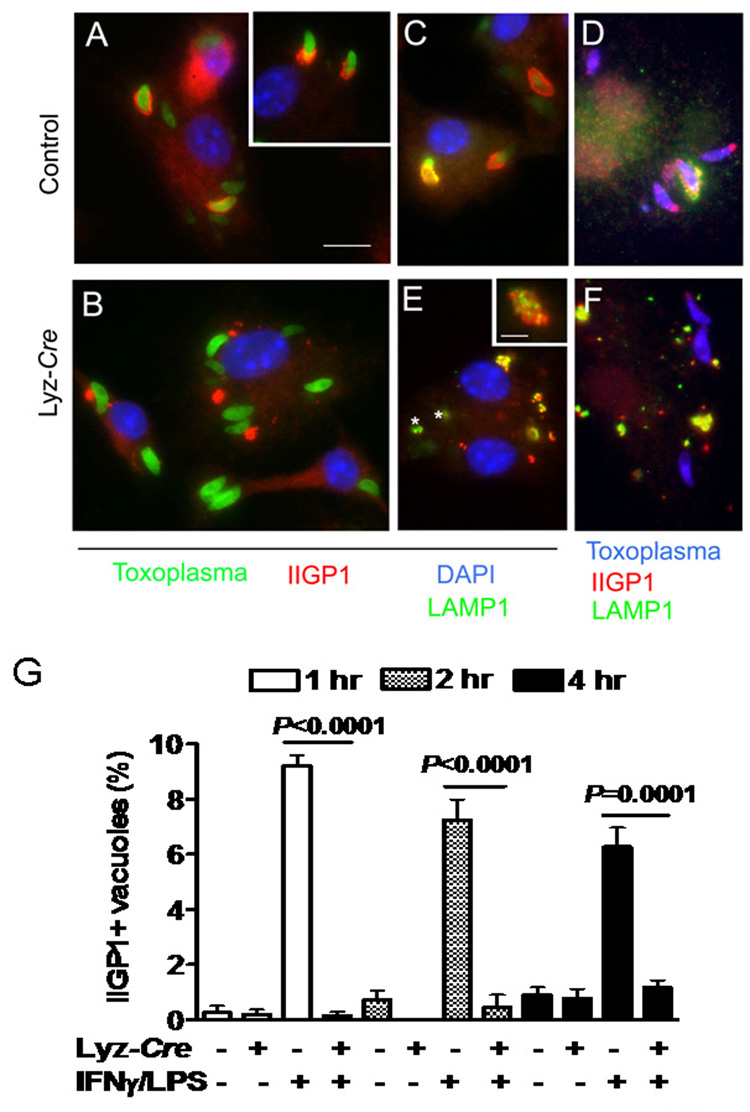
Fig. 6. Atg5 is required for IFNγ/LPS-induced targeting of IIGP1 to the T. gondii parasitophorous vacuole.
Macrophages were stimulated with IFNγ/LPS and infected with T. gondii as in Fig. 2, and then stained for the indicated markers 2 hour after infection. Scale bar = 10 microns for A–F.
(A, B) Localization of IIGP1 (red) with GFP-expressing T. gondii (green) after infection of IFNγ/LPS activated control or Atg5-deficient macrophages. DAPI (blue) staining of nuclei. In control cells IIGP1 decorates the parasitophorous vacuole in a cap, while in Atg5-deficient cells IIGP1 (red) was shunted to large intracellular inclusions and was not recruited to the parasite containing vacuole.
(C) Intracellular fate of GFP-expressing parasites in IFNγ/LPS activated control cells. LAMP-1 (green) was recruited selectively to IIGP1 (red) positive vacuoles and often formed a partial cap, associated with material sloughing from the vacuole surface. DAPI (blue) stains nuclei.
(D) Similar to C except wild type parasites were detected with rabbit anti-Toxoplasma and secondary antibodies conjugated to Alexa355 (blue).
(E) Parasites within Atg5 deficient cells did not recruit IIGP1 or become LAMP1 positive. * GFP expressing parasites. However, IIGP1 positive clusters (red in 1B) were strongly associated with LAMP1. Insert shows enlarged view of intracellular cluster. IIGP1 (red) and LAMP1 (green) were closely associated but not strictly colocalized. (F) Similar to E except wild type parasites were detected with rabbit anti-Toxoplasma and secondary antibodies conjugated to Alexa355 (blue).
G) Quantitation of IIGP1 co-localization with T. gondii parasites in IFNγ/LPS activated macrophages 1, 2, and 5 hours post-infection. Data were collected from two independent experiments counting at least 600 vacuoles.
Role of Atg5 in recruitment of lysosomes
Previous studies have indicated that in vivo activated macrophages infected in vivo with GFP-expressing parasites contain parasitophorous vacuoles that undergo fusion with LAMP-1 positive vesicles, resulting in a significant percentage of vacuoles that are uniformly LAMP-1 positive (Ling et al., 2006). However, we did not observed parasite-containing vacuoles that were uniformly LAMP-1 positive in either control cells or Atg5-deficient cells activated with IFNγ/LPS (data not shown). This difference between our results and the results of Ling et al. (Ling et al., 2006) may be the result of analysis of different types of macrophages, or the possibility that opsonization that occurs in vivo can direct the parasite to a fusigenic vacuole (Mordue and Sibley, 1997; Joiner et al., 1990).
Instead of direct fusion with the vacuole, prominent clusters of lysosomes were observed to colocalize with IIGP1 positive regions of vacuoles containing T. gondii in control but not Atg5-deficient cells (Fig. 6C–F). LAMP-1 signal was often associated with material that was sloughed from the parasite-containing vacuole. We speculate that in our experiments LAMP-1 positive vesicles do not fuse with the vacuole resulting in uniform staining, but rather fuse with the remnants of the membrane that is stripped off by IIGP1 and possibly captured nearby by autophagosomes, resulting in shunting of this material to lysosomes. We failed to observe efficient recruitment of LAMP-1 positive vesicles to region of parasites containing vacuoles in Atg5-deficient cells, consistent with a failure of IIGP1 to be recruited (Fig. 6D,F) and the fact that parasite containing vacuole remain intact in the absence of Atg5 (Fig. 4).
One possible explanation for the failure of IIGP1 to be recruited in Atg5 deficient cells is that it was sequestered in intracellular inclusions (Fig. 5B). These intracellular inclusions consisted of clusters of small vesicles that were IIGP1 positive and which occurred in close association with IIGP1-negative but LAMP-1-positive vesicles, despite the fact that they did not precisely colocalize in the same vesicular structure (Fig. 6E insert). The nature of these inclusions is presently unknown, but their existence suggests a failure for IIGP1 to correctly traffic in the absence of Atg5, thus disrupting its cellular function in control of intracellular pathogens.
Coda: the role of Atg5 in cellular immunity
Together these studies establish Atg5 as a crucial in vivo mediator of cellular immunity to intracellular pathogens and provide new mechanistic insight into the roles of Atg5 in the cell. The impressive increase in susceptibility of mice lacking Atg5 in phagocytic cells argues for serious consideration of drugs that activate relevant Atg5-dependent processes as anti-infectives. With regard to mechanism, it is important to note that the parasitophorous vacuole is not a phagosome, but rather a pathogen driven derivative of the plasmalemma which is autonomous in formation and fate within the cell (Dobrowolski and Sibley, 1996; Joiner et al., 1990; Suss-Toby et al., 1996). However, our results are strikingly similar to recent findings for newly formed phagosomes containing latex beads coated with Toll receptor ligand (Sanjuan et al., 2007). In both cases, Atg5 is important for targeting critically important proteins to recently formed membrane structures.
In our case, Atg5 is important for recruitment of a critical p47 GTPase to the parasitophorous vacuole. The molecular mechanism responsible for this role of Atg5 in recruitment of a GTPase is currently unknown. However, it is interesting that classical autophagosomes were not observed as involved in either fusion of lysosomes to phagosomes (Sanjuan et al., 2007) or in Atg5-dependent damage to the parasitophorous vacuole membrane in the present study. This indicates that Atg5 can play a previously undescribed role in intracellular membrane dynamics that is independent of classical autophagosome formation.
Our data show that the critical role of Atg5 in killing of T. gondii in cultured primary macrophages reflects at least in part a role for Atg5 in damage to the parasitophorous vacuole, a process that is in addition to the role of Atg5 in classical autophagy. It will therefore be important to determine in future studies whether the results we have obtained here for Atg5 in damage to the parasitophorous vacuole and GTPase recruitment also apply to other components of the ubiquitin-like conjugations systems involved in classical autophagy. Since IFNγ-induced small GTPases are implicated in resistance to a variety of pathogens (Taylor et al., 2007), it is possible that the role of Atg5 in targeting of GTPases may be of general importance.
EXPERIMENTAL PROCEDURES
Cells, pathogens, and mice
Peritoneal exudate cells were obtained by lavage, plated at 5×105 cells/ml/well (Edelson and Unanue, 2001) for 4 hr at 37 °C, and washed vigorously to purify adherent macrophages prior to incubation with complete or starvation medium for 2 hr. Cell lysates were then analyzed by western blot for expression of Atg5, LC3-I/LC3-II, and actin (Edelson and Unanue, 2001; Zhao et al., 2007b). Primary macrophages were prepared from bone marrow of male mice as described (Zhao et al., 2007b; Kim et al., 2001). T. gondii (type-II strains): wild type (PTG), expressing luciferase (PRU-LUC), or expressing GFP (GFP-PTG) were maintained in HFF cells (Fux et al., 2007). L. monocytogenes strain EGD was prepared and quantified as described (Edelson and Unanue, 2001). Macrophages were infected with M. tuberculosis var. bovis (BCG, MOI=1) for 1 hr and chased for 4 hr in either complete DMEM or starvation media prior to enumeration of viable bacteria (Gutierrez et al., 2004). ATG5flox/flox (control) mice and ATG5flox/flox Lyz-Cre mice were bred and genotyped as described (Zhao et al., 2007b; Hara et al., 2006). IFNγ levels in serum were determined using a Becton-Dickinson CBA mouse inflammation kit. Female mice 8 to 12 weeks of age were used for in vivo studies.
In vitro and in vivo infections and immunofluorescence microscopy
Macrophages were pre-treated with 100 U/ml recombinant murine IFN-γ (R&D Systems, Minneapolis MN) plus 1ng/ml LPS (Salmonella, Sigma, ST. Louis, MO) for 18 hr, infected with T. gondii tachyzoites (PRU-LUC strain, MOI=5), incubated at 37°C in 5% CO2 for 3 hr, washed, and then either fixed immediately or incubated for 20 hr prior to fixation. T. gondii infection was assessed by indirect immunofluorescence using antibodies to F4/80 (macrophages), SAG1 (mouse monoclonal antibody DG52) for T. gondii, LAMP-1 (rat monoclonal antibody 1D4B) or staining with DAPI (Fux et al., 2007; Nagamune et al., 2008; Ling et al., 2006). IIGP1 was detected using monoclonal antibody 5D9 at a 1:500 dilution (Zerrahn et al., 2002). Alexa Fluor350 conjugated goat anti-rabbit IgG (Invitrogen), Alex Fluor488 conjugated goat anti-rat IgG and Alexa Fluor594 conjugated goat anti-mouse IgG antibodies (Bioscience) were used at a 1.2000 dilution. L. monocytogenes (2×105 CFUs) or T. gondii strain PRU-LUC were inoculated into mice intraperitoneally. L. monocytogenes replication was quantified as cfus in spleen and liver three days after infection (Barton et al., 2007). T. gondii replication was quantified by light emission after injection of 0.15 mg/kg of firefly Dluciferin (Biosynth AG, Switzerland) by a Xenogen IVIS 100 (Saeij et al., 2005; Nagamune et al., 2008). To measure the T. gondii titers in spleen and mesenteric nodes organs were harvested 6 or 8 days after infection, homogenized with 1 ml PBS, and strained (100 μm cell strainer) prior to distribution into 96 well plates (4 wells/tissue sample). To detect luciferase expression 2 µl of firefly D-luciferin (30mg/ml) was added per well, incubated for 10 min at room temperature, and light emission was measured (Xenogen IVIS 100). To prepare a standard curve (Supplemental Figure S3), serial dilutions of PRU-LUC (106 to 102 parasites per well) were made in 96 well plates and luciferase assessed as for spleen and lymph node samples.
Transmission electron microscopy
For ultrastructural analysis T. gondii-infected macrophages were fixed in 1% glutaraldehyde (Polysciences Inc., Warrington, PA)/1% osmium tetroxide (Polysciences Inc.) in 50 mM phosphate buffer, pH 7.2 for 1hr at 4° C. This low osmolarity fixation was used to remove dense soluble cytoplasmic components, allowing for unobscured membrane analysis. Cells were washed in phosphate buffer, rinsed extensively in dH20 prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 hr. Following several rinses in dH20, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 70–80 nm were cut, stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA, Peabody, MA) at an accelerating voltage of 80kV.
Western blotting and assay of NO production
2×105 macrophages were treated with LPS, IFNγ, or IFNγ/LPS in a 96-well plate for 18 hr. Cells were washed with PBS twice prior to western analysis (Zhao et al., 2007b). NO production was detected using the NO synthase detection system (Sigma). 5×104 macrophages were treated with LPS, IFNγ, or IFNγ/LPS for 18 hr, washed, and incubated with 200 µl of reaction buffer or 200 µl inhibition reaction buffer containing 1 µM diphenyleneiodoum chloride (DPI), and incubated at room temperature for 2 hr in dark prior to detection of fluorescence.
Measurement of gene expression
Total RNA was isolated from bone-marrow derived macrophages using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA (2ug) was treated with DNase I (Ambion, Austin, TX) before subject to reverse transcriptase cDNA synthesis using Oligo(dT)12–18 and Superscript II (Invitrogen, Carlsbad, CA) as per manufacture’s protocol. Quantitative RT-PCR was performed with SYBR-Green (Invitrogen, Carlsbad, CA) and the thermal cycler iCycler (Biorad, Hercules, CA). Primer sequences are as follows: CIITA 5’CACCCCCAGATGTGTATGTGC and 5’CGAGGTTTCCCAGTCCAGAAG, GAPDH 5’TGCCCCCATGTTTGTGATG and 5’TGTGGTCATGAGCCCTTCC, IRF-1 5’ACACTAAGAGCAAAACCAAGAG and 5’TTTCCATATCCAAGTCCTGA, Sca-1 5’CTTGCCCATCAATTACCTGCCC and 5’GGAGGGCAGATGGGTAAGCAAA, Stat-1 5’CTCTTAGCTTTGAAACCCAGTT and 5’TTGTACCACAGGATAGACGC. Transcript levels were normalized to GAPDH within each sample and data was calculated using the delta-delta Ct method (Livak and Schmittgen, 2001). Two independent experiments were performed with each qRT-PCR reaction repeated in triplicate.
Statistics
All data were analyzed with Prism software (Graphpad, San Diego, CA) using two-tailed unpaired Student’s t tests. Unless otherwise indicated all experiments were performed at least three times and the data pooled for presentation +/− S.E.M.
Supplementary Material
The supplemental data include Supplemental figures S1–3 and can be found with this article on line at: XXXXXXXXX.
ACKNOWLEDGMENTS
Supported by Project 6 of U54 AI057160 to HWV, and AI036629 and AI071299 to LDS, and funding from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Toray Science Foundation to NM. IRD was supported by a fellowship from the Deutsche Forschungsgemeinschaft, Germany. We thank Wandy Beatty, Microbiology Imaging Facility, for the EM work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cellular Microbiology. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade RM, Portillo JA, Wessendarp M, Subauste CS. CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect. Immun. 2005;73:3115–3123. doi: 10.1128/IAI.73.5.3115-3123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonellacontaining vacuole. J. Biol. Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Buchmeier NA, Schreiber RD. Requirement of endogenous interferongamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. U. S. A. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect. Immun. 2005;73:3278–3286. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U. S. A. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Codogno P, Meijer AJ. Atg5: more than an autophagy factor. Nat. Cell Biol. 2006;8:1045–1047. doi: 10.1038/ncb1006-1045. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–431. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect. Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux B, Nawas J, Khan A, Gill DB, Su C, Sibley LD. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect. Immun. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Halonen SK, Taylor GA, Weiss LM. Gamma interferon-induced inhibition of Toxoplasma gondii in astrocytes is mediated by IGTP. Infect. Immun. 2001;69:5573–5576. doi: 10.1128/IAI.69.9.5573-5576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Jones TC, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J. Exp. Med. 1972;136:1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TC, Yeh S, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J. Exp. Med. 1972;136:1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Eaton MS, Schubert W, Wu S, Tang J. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 2001;113:309–313. doi: 10.1016/s0166-6851(01)00212-2. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di SF, Dice JF, Difiglia M, nesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van dK, I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen-Waisman S, Howard JC. Cell-autonomous Immunity to Toxoplasma gondii in mouse and man. Microbes and Infection. 2007;9:1652–1661. doi: 10.1016/j.micinf.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy. 2007;3:323–328. doi: 10.4161/auto.4012. [DOI] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the Pathogenesis of Disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mackaness GB. The immunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–120. [PubMed] [Google Scholar]
- Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct. Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- Murray HW, Rubin BY, Carriero SM, Harris AM, Jaffee EA. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygenindependent activity against intracellular Toxoplasma gondii. J. Immunol. 1985a;134:1982–1988. [PubMed] [Google Scholar]
- Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J. Immunol. 1985b;134:1619–1622. [PubMed] [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara J, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group a Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell Host and Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Py BF, Lipinski MM, Yuan J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy. 2007;3:117–125. doi: 10.4161/auto.3618. [DOI] [PubMed] [Google Scholar]
- Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun. 2005;73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signaling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- Sibley LD. Toxoplasma gondii: perfecting an intracellular life style. Traffic. 2003;4:581–586. doi: 10.1034/j.1600-0854.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J. Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Steed A, Buch T, Waisman A, Virgin HW. Interferon gamma blocks {gamma}-herpesvirus reactivation from latency in a cell type specific manner. J. Virol. 2007;81:6134–6140. doi: 10.1128/JVI.00108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Subauste CS, Wessendarp M. CD40 restrains in vivo growth of Toxoplasma gondii independently of gamma interferon. Infect. Immun. 2006;74:1573–1579. doi: 10.1128/IAI.74.3.1573-1579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci U. S. A. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW, Tessarollo L, Bray M, McVicar DW, Komschlies KL, Young HA, Biron CA, Sher A, Vande Woude GF. Pathogen-specific loss of host resistance in mice lacking the IFNgamma- inducible gene IGTP. Proc Natl Acad Sci U. S. A. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes. Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, Yoshimori T, Kurata S. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerrahn J, Schaible UE, Brinkmann V, Guhlich U, Kaufmann SH. The IFN-inducible Golgi- and endoplasmic reticulum- associated 47-kDa GTPase IIGP is transiently expressed during listeriosis. J. Immunol. 2002;168:3428–3436. doi: 10.4049/jimmunol.168.7.3428. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect. Immun. 2007a;75:4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Thackray LB, Miller BC, Lynn TM, Becker MM, Ward E, Mizushima NN, Denison MR, Virgin HW. Coronavirus Replication Does Not Require the Autophagy Gene ATG5. Autophagy. 2007b;3:581–585. doi: 10.4161/auto.4782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplemental data include Supplemental figures S1–3 and can be found with this article on line at: XXXXXXXXX.