Abstract
Objective
Angiotensin receptor blockers (ARBs) produce a lower sodium (Na) balance, and the natriuretic effect is enhanced under Na deprivation, despite falls in blood pressure (BP) and glomerular filtration rate (GFR).
Methods
The effect of additional hydrochlorothiazide (HCTZ; 12.5 mg/day) to ARB treatment (valsartan; 80 mg/day) on glomerulotubular Na balance was evaluated in 23 patients with chronic kidney disease.
Results
Add-on HCTZ decreased GFR, tubular Na load, and tubular Na reabsorption (tNa), although 24-hour urinary Na excretion (UNaV) remained constant. Daily urinary angiotensinogen excretion (UAGTV, 152±10→82±17 μg/g Cre) reduced (p=0.02). Changes in tubular Na load (r2=0.26) and tNa (r2=0.25) correlated with baseline 24-hour UAGTV. Changes in filtered Na load correlated with changes in nighttime systolic BP (r2=0.17), but not with changes in daytime systolic BP. The change in the tNa to filtered Na load ratio was influenced by the change in daytime UNaV (β=−0.67, F=16.8), rather than the change in nighttime UNaV.
Conclusions
Lower Na balance was produced by add-on HCTZ to ARB treatment without an increase of intra-renal renin-angiotensin system activity, leading to restoration of nocturnal hypertension. A further study is needed to demonstrate that the reduction of UAGTV by additional diuretics to ARBs prevents the progression of nephropathy or cardiovascular events.
Keywords: Angiotensin receptor blocker, chronic kidney disease, hydrochlorothiazide, sodium, angiotensinogen
Introduction
In the mid-1960s, Guyton and Coleman developed computer models of renal function curve, so-called Guyton’s pressure–natriuresis curve.1 The curve can be depicted by plotting mean arterial pressure (MAP; mmHg) and urinary sodium (Na) excretion rate (mmol/day) at steady state Na balance, which was defined at the time when Na intake and urinary Na excretion were in balance. They found that so long as renal function curve remained immutable and Na intake remained constant, changing any other variable (such as cardiac output or total peripheral resistance) did not change the steady-state blood pressure (BP) value, and they called the BP level as equilibrium level. In other words, renal function curve must be altered in the genesis of hypertension. Our study group proposed that the slope of pressure–natriuresis curve was decreased in patients with salt-sensitive hypertension.2–5
Salt-sensitive hypertension may be attributable to impaired renal capacity for Na excretion, which originates in a diminished glomerular ultrafiltration coefficient (KF) and/or enhanced fractional tubular Na reabsorption (FRNa; tubular reabsorption to filtered load ratio).2–5 Na retention may occur during the day in patients with impaired renal Na excretion, which prevents the “physiologic nocturnal BP dip (a 10–20% decline compared with daytime BP, dipper type of circadian BP rhythm)”6 and may exert nighttime natriuresis.2,7 Patients who do not exhibit a BP dip at night are classified as non-dippers.7 For example, with reduced KF as renal function deteriorates, nighttime BP is elevated to enhance urinary Na excretion in subjects with glomerular diseases;8 and patients with renal dysfunction require a longer time until BP dips at night.9 Examples of enhanced FRNa have been described in patients with IgA nephropathy, including our report of inappropriately activated intrarenal angiotensin II (Ang II) stimulated FRNa and impaired renal Na excretion, resulting in a non-dipper type of circadian BP rhythm.10
Our group has shown that dietary Na restriction,11 diuretics that inhibit FRNa,12,13 and angiotensin receptor blocker (ARB) therapy can restore the non-dipper type of circadian BP rhythm in patients with chronic kidney disease (CKD).14–17 In exerting this effect, ARBs achieve a lower steady state Na balance by inhibition of FRNa to enhance daytime Na excretion, especially in patients with baseline nighttime BP falls that are more diminished.15
In clinical practice, we have experienced patients who exhibit a synergic antihypertensive effect with combination treatment with an ARB and hydrochlorothiazide (HCTZ). These patients serve as a reminder that in a state of chronic Na deprivation, inhibition of the renin-angiotensin system (RAS) can excessively enhance natriuresis, resulting in a Na-wasting state, despite falls in BP and glomerular filtration rate (GFR).18–20
RAS inhibitors (i.e. angiotensin-converting enzyme inhibitors and ARBs) are first-line antihypertensive agents for patients with CKD.21 Diuretics and calcium channel blockers are used as second-line antihypertensives.22 Therefore, we examined if add-on HCTZ administration achieves a lower Na balance than that already present in patients with CKD under ARB treatment. We also investigated the pathophysiological conditions under which add-on HCTZ to ARB therapy exerts further natriuresis. We hypothesized that this effect would be greater in patients with reduced intrarenal RAS activity during preceding ARB treatment because the reduced intrarenal RAS can be interpreted as a sign that preceding ARB therapy could already greatly assuaged intrarenal RAS activity to enhance natriuresis. This effect might be similar to combination treatment with different classes of diuretics. Na deprivation caused by HCTZ might also enhance the natriuretic effect of ARB.
Material and methods
Subjects
A single-arm, open label study was performed in 25 consecutive patients with CKD (12 men and 13 women; 64±14 years; body mass index (BMI): 24.0±3.5 kg/m2). To be eligible, patients had to fulfill the following criteria: (a) age≥20 years; (b) diagnosis of CKD based on Kidney Disease Outcomes Quality Initiative (K/DOQI) criteria23 (GFR<60 ml/min/1.73 m2, or GFR≥60 ml/ min/1.73 m2 with accompanying proteinuria, defined as >300mg per gram creatinine; (c) treatment with an ARB (valsartan, 80 mg/day) for at least eight weeks prior to enrollment; and (d) office BP>130/80 mmHg (or 125/75 mmHg if proteinuria ≥1 g/day on at least one occasion). Patients were excluded if they had (a) previous treatment with ARBs other than valsartan or diuretics two months before enrollment; (b) contraindication to valsartan or HCTZ (history of allergic reactions to these drugs, bilateral renal artery stenosis); (c) presence or possibility of pregnancy; (d) haemoglobin A1C (HbA1c) ≥9.0%; (e) GOT>100 or GPT>85; (f) endocrine hypertension; (g) accelerated or malignant hypertension (progressive renal dysfunction with diastolic BP (DBP)>120–130 mmHg; (h) serious conditions with congestive heart failure, coronary disease, arrhythmia or systemic diseases; (i) nephrotic syndrome (serum albumin<2.5 g/dl); (j) dialysis therapy; and (k) any reason for ineligibility suggested by the attending doctor. All subjects were enrolled after providing informed consent to participate in the study. The study was approved by the Institutional Review Board (IRB) of Nagoya City University Hospital (IRB approval number: 45-10-0031, UMIN registration number: 000005601) and was conducted in accordance with the Declaration of Helsinki.
Study protocol
Measurements were made at baseline, which was defined as the time at which subjects had taken valsartan (80 mg/ day) for at least eight weeks, and eight weeks after initiation of add-on HCTZ (12.5 mg/day). Twenty-four hour BP monitoring and urine collection separately for daytime (06:00–21:00) and nighttime (21:00– 06:00) were conducted on the same day during normal daily activities to compare the circadian rhythms of BP and urinary excretion of Na (UNaV), albumin (UAlbV) and angiotensinogen (UAGTV). Collected urine was combined to calculate 24-hour creatinine clearance (Ccr, ml/min), which was used as an index for GFR. Filtered Na load was calculated as the product of the GFR and plasma Na concentration (SNa), and tubular Na reabsorption (tNa) was calculated as the difference between filtered Na load and absolute urinary Na excretion.14,24 FRNa was then calculated as the tNa to filtered Na load ratio. Blood samples were collected once at 06:00, the marginal point between day and night. For evaluation of plasma renin activity (PRA), plasma aldosterone concentration (PAC), angiotensin I (Ang I), and Ang II, the samples were centrifuged at 3000 rpm for 10 min at 4°C, and then frozen immediately and stored at −35°C until assay. PRA, PAC, Ang I, and Ang II were determined by radioimmunoassay at an external analysis center (SRL, Inc., Hachioji, Japan). Plasma Na (ion-selective electrode method) and creatinine (enzymatic method); and urinary concentrations of Na (ion-selective electrode method), creatinine (enzymatic method) and albumin (tur-bidimetric immunoassay) were measured at the institutional central laboratory. Urinary angiotensinogen (AGT) was measured using a Human Total AGT ELISA Kit (Immuno-Biological Laboratories Co. Ltd, Takasaki, Japan).25 The intra- and inter-assay coefficients of the AGT measurements were 4.4% and 4.3%, respectively.25,26
During 24-hour BP monitoring, BP was monitored non-invasively every 30 min with a validated automatic device (model ES-H531; Terumo, Tokyo, Japan). BP and heart rate (HR) values were not considered valid for analysis if data were missing continuously for two hours or if the patients awoke during the night and had difficulty falling asleep again. MAP was calculated as DBP plus one-third of the pulse BP. Daytime BP was calculated as the average of the 30 readings between 06:00 and 21:00, and nighttime BP was determined as the average of the remaining 18 readings. Patients whose nocturnal fall in MAP was ≥10% from day to night were classified as dippers and those with nocturnal MAP fall <10% were as non-dippers. Nocturnal hypertension was defined as nighttime BP ≥120/70 mmHg. The patients received nutritional instructions to eat a Na-restricted diet containing <6 g/day of salt for at least four weeks before enrollment; and were asked to get up at 06:00 and to start bed-rest at 21:00 during the study measurements. The adequacy of 24-hour urine collection was judged by the amount of urinary creatinine excretion: males aged <50 years, 18.5–25.0; females aged <50 years, 16.5–22.4; males aged ≥50 years, 15.7–20.2; and females aged ≥50 years, 11.8–16.1 mg/kg body weight per day, respectively. Incomplete or excessive urine collection in daytime or nighttime samples was judged based on a night/day urinary creatinine excretion ratio of <0.5 or >2.0, respectively.
After baseline examinations, a single daily dose of HCTZ (12.5 mg/day) in the morning was added to the val-sartan therapy. The goal for office BP was <130/80 mmHg (<125/75 mmHg if daily proteinuria ≥1.0 g) for patients with BP above these values. During the eight-week study period, a change in the valsartan dosage or additional administration of other antihypertensives was not allowed; and if these were needed, the study discontinued for the patient. When office or home systolic BP (SBP) fell below 100 or 95 mmHg, respectively, or a patient felt postural dizziness, the dose of antihypertensive agent was decreased and the patient was excluded from the study.
Statistical analysis
Results are expressed as mean±standard deviation (SD) or as median (interquartile range (IQR)) according to the data distribution, which was tested using a Kolmogorov-Smirnov test. Variables that were not normally distributed were analyzed after log transformation. Differences in parameters between baseline and ARB plus HCTZ treatment were examined by Student t-test for paired samples or by Wilcoxon signed-rank test, as appropriate. Correlations among quantitative variables were evaluated by the least-squares method. Forward stepwise multiple regression analysis was conducted to compare the contribution of changes in daytime and nighttime UNaV to the change in FRNa. A value of p<0.05 was considered to be significant. Statistical analyses were performed using SPSS Statistics 22 (IBM Corp., New York, USA).
Results
Baseline measurements during ARB therapy
Of the 25 eligible patients, one had excess BP reduction during add-on HCTZ therapy and another was judged as excessive urine collection, and they were excluded from the study. Therefore, changes in clinical variables were evaluated in 23 patients (11 males and 12 females; 64±14 years; BMI, 24.0±3.6 kg/m2). At baseline under ARB therapy, the median (IQR) for albuminuria was 624 (205–1780) mg/g Cre (geometric mean±SD, 419±6 mg/g Cre), and the mean GFR was 66±47 ml/min (Table 1). BP and HR at baseline are shown in Table 2. Office, 24 h, daytime, and nighttime BP were 147/83, 132/79, 134/81, 127/73 mmHg; and night/day ratios of SBP, DBP, MAP, and HR were 0.95±0.06, 0.91±0.06, 0.93±0.06 and 0.88±0.10, respectively. Of 23 patients, eight exhibited dipper type of circadian BP rhythm, and 15 non-dipper rhythm. Among eight patients with dipper BP rhythm, four patients exhibit nocturnal hypertension, and four did not; among 15 patients with non-dipper BP rhythm, 12 patients exhibit nocturnal hypertension, and three did not.
Table 1.
Clinical variables before and during add-on hydrochlorothiazide (HCTZ) treatment.
| ARB | ARB+HCTZ | p Value | |
|---|---|---|---|
| Body weight (kg) | 61.9±15.2 | 61.5±15.0 | 0.2 |
| GFR (ml/min) | 66±47 | 59±38 | 0.04 |
| SNa (mEq/l) | 141±2 | 139±3 | 0.002 |
| SK (mEq/l) | 4.4±0.6 | 4.3±0.5 | 0.1 |
| SCr (mg/dl) | 1.7±1.3 | 1.8±1.4 | 0.02 |
| SUA (mg/dl) | 6.5±1.3 | 7.1±1.3 | 0.006 |
| HbA1c (%) | 5.9±0.6 | 6.1±0.8 | 0.05 |
| PRA (ng/ml/h) | 2.5±3.2 | 4.3±3.3 | 0.01 |
| PAC (pg/ml) | 121±61 | 155±99 | 0.09 |
| Ang I (pg/ml) | 215±3 | 382±4 | 0.009 |
| Ang II (pg/ml) | 16±2 | 21±3 | 0.08 |
Ang I: angiotensin I; Ang II: angiotensin II; ARB: angiotensin receptor blocker; GFR: glomerular filtration rate; HbA1c, haemoglobin A1C; PAC: plasma aldosterone concentration; PRA: plasma renin activity; SNa, SK, SCre, SUA: serum concentrations of sodium, potassium, creatinine, uric acid, respectively; SD: standard deviation.
Values are means±SD (n=23).
Table 2.
Blood pressures, heart rates, and urinary excretion of albumin, sodium, and angiotensinogen before and during add-on hydrochlorothiazide (HCTZ) treatment.
| Variable | ARB | ARB+HCTZ | p Value | |
|---|---|---|---|---|
| SBP | Office (mmHg) | 147±9 | 124±13 | <0.0001 |
| 24-Hour (mmHg) | 132±14 | 118±15 | <0.0001 | |
| Day (mmHg) | 134±13 | 120±15 | 0.0003 | |
| Night (mmHg) | 127±17 | 113±19 | 0.0003 | |
| Night/day ratio | 0.95±0.06 | 0.94±0.10 | 0.7 | |
| DBP | Office (mmHg) | 83±12 | 76±14 | 0.04 |
| 24-Hour (mmHg) | 79±11 | 72±10 | 0.0003 | |
| Day (mmHg) | 81±11 | 75±10 | 0.0008 | |
| Night (mmHg) | 73±12 | 67±11 | 0.002 | |
| Night/day ratio | 0.91±0.06 | 0.90±0.08 | 0.7 | |
| MAP | Office (mmHg) | 104±9 | 92±13 | 0.0002 |
| 24-Hour (mmHg) | 96±10 | 87±11 | 0.0001 | |
| Day (mmHg) | 98±10 | 89±11 | 0.0003 | |
| Night (mmHg) | 91±12 | 82±13 | 0.0009 | |
| Night/day ratio | 0.93±0.06 | 0.92±0.08 | 0.6 | |
| HR | Office (rpm) | 71±15 | 74±12 | 0.2 |
| 24-Hour (rpm) | 68±8 | 68±8 | 0.9 | |
| Day (rpm) | 71±9 | 72±9 | 0.4 | |
| Night (rpm) | 61±6 | 61±8 | 0.8 | |
| Night/day ratio | 0.88±0.10 | 0.85±0.08 | 0.3 | |
| UAlbV | 24-Hour (mg/g Cre) | 419±6 | 237±7 | 0.0007 |
| Day (mg/h) | 16.07±6.14 | 9.40±6.43 | 0.003 | |
| Night (mg/h) | 16.52±4.99 | 8.79±6.44 | 0.001 | |
| Night/day ratio | 1.55±1.82 | 1.12±0.79 | 0.7 | |
| UNaV | 24-Hour (mmol/g Cre) | 152±63 | 164±63 | 0.3 |
| Day (mmol/h) | 6.45±3.77 | 6.60±2.79 | 0.9 | |
| Night (mmol/h) | 6.66±4.09 | 6.55±3.55 | 0.9 | |
| Night/day ratio | 1.23±0.90 | 1.11±0.62 | 0.9 | |
| UAGTV | 24-Hour (μg/g Cre) | 152±10 | 82±17 | 0.02 |
| Day (μg/h) | 7.06±0.01 | 2.81±0.02 | 0.001 | |
| Night (μg/h) | 3.21±0.01 | 2.73±0.01 | 0.4 | |
| Night/day ratio | 0.80±0.88 | 2.97±6.96 | 0.05 |
ARB: angiotensin receptor blocker; /gCre: per gram creatinine; DBP: diastolic blood pressure; HR: heart rate; MAP: mean arterial pressure; SBP: systolic blood pressure; SD: standard deviation; UAlbV, UNaV, and UAGTV: urinary excretions of albumin, sodium, and angiotensinogen, respectively.
Values are means±SD (n=23).
GFR was inversely correlated with daytime SBP (r=−0.53, r2=0.28, p=0.009), nighttime SBP (r=−0.55, r2=0.30, p=0.007), and 24 h SBP (r=−0.54, r2=0.29, p=0.008); but not significantly correlated with 24-hour, daytime, and nighttime DBP and MAP; night/day ratios of SBP, DBP, and MAP; or nocturnal dips of SBP, DBP, and MAP.
Urinary Na excretion (UNaV, mmol/g Cre) was inversely correlated with PRA (r=−0.48, r2=0.23, p=0.02), Ang I (r=−0.47, r2=0.22, p=0.03), Ang II (r=−0.54, r2=0.29, p=0.01), but not with PAC. UNaV correlated positively with night/day ratios of SBP (r=0.49, r2=0.24, p=0.02) and MAP (r=0.52, r2=0.27, p=0.01), but not with the night/day DBP ratio (r=0.40, r2=0.16, p=0.06). UNaV correlated inversely with nocturnal dips of SBP (r=−0.48, r2=0.23, p=0.02) and MAP (r=−0.51, r2=0.26, p=0.01), but not with nocturnal DBP dip (r=−0.40, r2=0.16, p=0.05). These findings are consistent with greater Na intake causing elevated BP, a switch of circadian rhythm to the non-dipper type, and decreased plasma renin activity (due to body fluid retention).
There were positive (but not significant) relationships of the night/day ratio of UNaV (mmol/h) with those of SBP, DBP, and MAP; and the night/day ratio of UAlbV with those of SBP, DBP, and MAP. Urinary excretion of AGT (UAGTV) was 152±10 μg per g Cre. UAGTV correlated inversely with GFR (r=−0.74, p<0.0001); but not with UNaV (p=0.06), 24-hour SBP (p=0.1), daytime SBP (p=0.1), daytime DBP (p=0.3), daytime MAP (p=0.9), nighttime SBP (p=0.2), nighttime DBP (p=0.5), nighttime MAP (p=0.9); night/day ratios of SBP (p=0.8), DBP (p=0.7), and MAP (p=0.9); or nocturnal dips (%) of SBP (p=0.8), DBP (p=0.7), and MAP (p=0.9).
Effects of add-on HCTZ to ARB therapy
Clinical variables before and during add-on treatment with HCTZ are shown in Tables 1 and 2, respectively. Body weight, serum potassium concentration, HbA1c, PAC, and Ang II did not change significantly; PRA, and serum concentrations of creatinine, uric acid, and Ang I increased; and GFR and serum Na decreased after addition of HCTZ. Daytime, nighttime, 24 h, and office SBP, DBP, and MAP were all lowered; daytime, nighttime, and 24-hour albuminuria was reduced; urinary Na excretion did not change; and 24-hour and daytime UAGTV decreased, but nighttime UAGTV did not change. Circadian BP rhythm and nighttime BP profiles were changed as follows. Among four patients with dipper BP rhythm without nocturnal hypertension at baseline, three patients remained with dipper BP rhythm without nocturnal hypertension and one patient changed to non-dipper without nighttime hypertension. Among four patients with dipper BP rhythm with nocturnal hypertension, three changed to dipper without nocturnal hypertension and one patient changed to non-dipper with nocturnal hypertension (but nighttime BP was lowered 131/68 to 127/70 mmHg). Among 12 patients with non-dipper BP rhythm and nocturnal hypertension, five patients changed to dipper without nocturnal hypertension, one dipper with nocturnal hypertension, and six remained non-dipper with nocturnal hypertension.
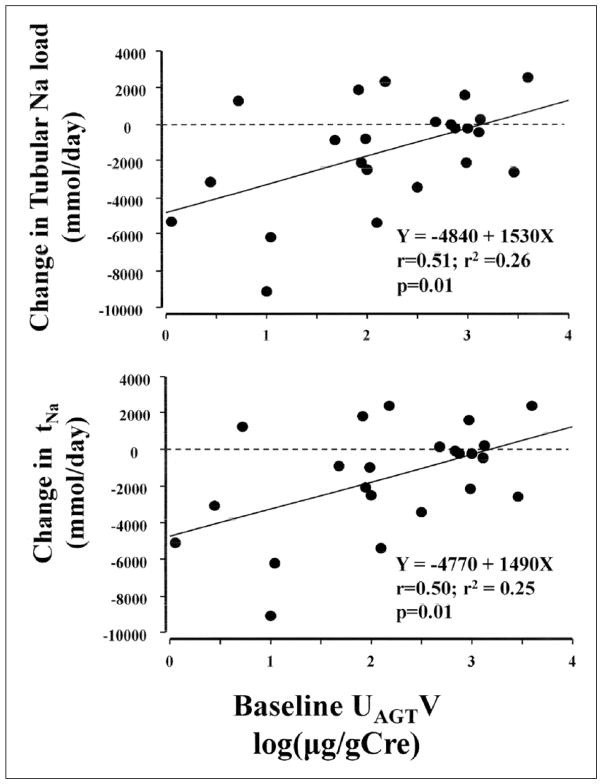
The glomerulotubular balances of Na before and during addition of HCTZ to ARB therapy are summarized in Table 3. Filtered Na (i.e. tubular Na load, p=0.03), and tNa (p=0.03) were both significantly reduced by add-on HCTZ, but 24-hour UNaV remained constant (p=0.6), indicating that a lower steady Na balance had been achieved. As mentioned above, HCTZ significantly decreased daily excretion of urinary AGT (p=0.02). Changes in tubular Na load (r=0.51, r2=0.26, p=0.01) and tNa (r=0.50, r2=0.25, p=0.01) correlated positively with baseline 24-hour UAGTV (Figure 1). The change in filtered Na load correlated positively with changes in 24-hour SBP (r=0.40, r2=0.16, p=0.05), DBP (r=0.47, r2=0.22, p=0.02) and MAP (r=0.46, r2=0.21, p=0.03) and with changes in nighttime SBP (r=0.41, r2=0.17, p=0.04), DBP (r=0.46, r2=0.21, p=0.02), and MAP (r=0.45, r2=0.21, p=0.03); but did not correlate with changes in daytime SBP (p=0.1), DBP (p=0.06), and MAP (p=0.05).
Table 3.
Glomerulotubular balance of sodium (Na) before and during add-on hydrochlorothiazide (HCTZ) treatment.
| Variable | ARB | ARB+HCTZ | p Value |
|---|---|---|---|
| Tubular Na load (mmol/day)a | 13350±9400 | 11840±7600 | 0.03 |
| tNa (mmol/day) | 13190±9400 | 11680±7630 | 0.03 |
| UNaV (mmol/day) | 156±81 | 158±58 | 0.6 |
ARB: angiotensin receptor blocker; tNa: tubular Na reabsorption; UNaV: urinary Na excretion rate.
Values are means±SD (n=23).
Amount of Na filtered from the glomerulus and loaded to renal tubules was calculated as SNa×GFR, where SNa and GFR are serum Na concentration and glomerular filtration rate, respectively.
Figure 1.
The effect of intrarenal renin-angiotensin system (RAS) activity during angiotensin receptor blocker (ARB) treatment on the changes in glomerulotubular sodium (Na) balance by additional treatment with hydrochlorothiazide. In patients, whose intra-renal RAS activity was suppressed during ARB therapy, greater decrease of changes in tubular Na load and tubular Na reabsorption was shown. Intra-renal RAS activity was indicated by daily urinary angiotensinogen excretion. AGT: angiotensinogen; tNa: tubular Na reabsorption (mmol/day); UAGTV: urinary angiotensinogen excretion (log(μg/g Cre)).
The primary role of HCTZ is reduction in FRNa at the distal convoluted tubules, the connecting segment at the end of the distal tubule, and the early cortical collecting tubule via neutral Na-Cl cotransport.27,28 Change in FRNa correlated inversely with changes in 24-hour (r=−0.64, r2=0.41, p=0.0009) and daytime (r=−0.67, r2=0.45, p=0.0005) UNaV, but not with nighttime UNaV (p=0.3). In stepwise multiple regression analysis (R2=0.42, p=0.0005), the main determinant of the change in FRNa was the change in daytime UNaV (β= −0.67, F=16.8, p=0.0005), rather than the change in nighttime UNaV.
Discussion
Our results show that addition of HCTZ to preceding ARB therapy can achieve a lower steady state Na balance. An increase in daytime UNaV contributed to the lower Na balance, and this balance resulted in BP lowering, especially at night. This is consistent with the following postulate. Thus, when patients with diminished renal function go about daily activities of life, their renal perfusion pressure is reduced, resulting in insidious Na retention in the daytime.6 At night, when these patients lie supine, the effective circulating volume increases, resulting in pressure natriuresis. In fact, daytime UNaV is greater than nighttime UNaV in healthy individuals, whereas daytime UNaV decreases and nighttime UNaV increases as renal Na excretion capacity is diminished.8,29–32 This postulate is supported by the persistence of high BP until excess Na is excreted as renal function deteriorates,6,9 and by the shortening of the time until nocturnal BP falls below 90% of the daytime average by treatment with diuretics or ARB, both of which reduce FRNa.6,12–15 This is the reason why the increase in daytime UNaV brought the circadian rhythm back to normal (a non-dipper to dipper trend) in the current study.
In experimental models, inappropriate activation of intrarenal Ang II impairs renal Na excretion through various mechanisms. Ang II stimulates FRNa in various segments along the pathway from the proximal to the collecting ducts33–35 and enhances tubuloglomerular feedback sensitivity to sustain FRNa.36 ARBs inhibit these anti-natriuretic effects via the Ang II type 1 receptor (AT1R) in animal models.37,38 In humans, ARBs suppress FRNa and increase renal Na excretion,14,15 similarly to diuretics. Of note, ARBs can excessively enhance renal Na excretion under conditions of Na deprivation.18,19 Pretreatment with ARBs also impairs adaptation of renal Na conservation in response to abrupt withdrawal of dietary Na.20 These two findings are consistent with our findings that add-on treatment with diuretics causes greater natriuresis in patients in whom preceding ARB therapy already greatly assuaged intrarenal RAS activity. Given the antinatriuretic effects of ARBs, addition of HCTZ to ARB therapy may have a synergistic effect similar to that of enhanced diuresis following administration of a thiazide diuretic after chronic therapy with a loop diuretic agent. In clinical practice, we have seen a synergic antihypertensive effect in combination treatment with ARBs and HCTZ, resulting in appropriate BP lowering and sometimes hypotensive adverse events, even in patients without a beneficial natriuretic effect with the ARB alone or HCTZ alone. Thus, suppressed UAGTV under ARB treatment may be a predictor for patients in whom an antihypertensive effect will occur with add-on diuretics.
Of 338 patients with CKD but not yet on dialysis (stages 3–5), more than half had volume overload accompanied by higher SBP and increased arterial stiffness, both of which can contribute to future cardiovascular diseases.39 Na restriction and treatment with diuretics are both expected to restore volume overload, but are also known to activate systemic renin-angiotensin-aldosterone activity.40–42 However, dietary Na restriction does not augment intrarenal RAS activity, as indicated by UAGTV measurements in an experimental model43,44 and a clinical study.45 For instance, urinary AGT excretion was significantly higher with an ordinary salt diet containing 10 g of NaCl daily compared with a low-salt diet containing 5 g of NaCl daily in patients with IgA nephropathy.45 Therefore, in this study, we investigated whether treatment with HCTZ can activate or assuage intrarenal RAS activity, as represented by UAGTV.
We found that UAlbV was reduced in the daytime and at night; and that UAGTV decreased in the daytime, but did not change at night. The different effects on UAGTV and UAlbV may support our suggestion that the genesis of urinary AGT is not the same as that of urinary albumin, which is filtered through glomerular capillary walls.
Dietary Na restriction enhances the effects of ARBs on renal and cardiovascular outcomes, compared with a high-Na diet, in patients with CKD.46,47 We found that treatment with HCTZ bore a resemblance to dietary Na restriction in restoring a non-dipper circadian BP rhythm,12,13 which was known as the risk for cardiovascular events.48 In the present study, treatment with HCTZ assuages intrarenal RAS activity, similar to dietary Na restriction. Moreover, it has been found that a RAS inhibitor, perindopril, cannot reduce the risk for stroke without combination treatment with diuretics,49 and increased UAGTV is associated with a risk of CKD.50 Therefore, we speculate that add-on diuretics can intensify the effect of RAS inhibitors on risk reduction for cardiovascular events and CKD progression, similarly to dietary Na restriction.
In conclusion, as baseline intrarenal RAS activity is reduced by ARB therapy, add-on HCTZ administration achieves a lower Na balance and resultant restoration of nocturnal hypertension without an increase in intrarenal RAS activity. Further studies are needed to determine whether reduction of UAGTV induced by Na restriction or add-on treatment with diuretics in patients under treatment with RAS inhibitors could be a marker of predisposition to prevention of progression to nephropathy or cardiovascular events, and whether addition of diuretics to ARBs is useful for patients with decreased UAGTV under preceding treatment with RAS inhibitors.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Guyton AC. Renal function curve–a key to understanding the pathogenesis of hypertension. Hypertension. 1987;10:1–6. doi: 10.1161/01.hyp.10.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda M, Kimura G. Salt sensitivity and nondippers in chronic kidney disease. Curr Hypertens Rep. 2012;14:382–387. doi: 10.1007/s11906-012-0286-3. [DOI] [PubMed] [Google Scholar]
- 3.Kimura G. Sodium, kidney, and circadian rhythm of blood pressure. Clin Exp Nephrol. 2001;5:13–18. [Google Scholar]
- 4.Kimura G, Brenner BM. Implications of linear pressure–natriuresis relationship and importance of sodium sensitivity in hypertension. J Hypertens. 1997;15:1055–1061. doi: 10.1097/00004872-199715100-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kimura G, Brenner BM. The renal basis for salt sensitivity in hypertension. In: Laragh JH, Brenner BM, editors. Hypertension pathophysiology, diagnosis, and management. 2. New York: Raven Press; 1995. pp. 1569–1588. [Google Scholar]
- 6.O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet. 1988;2:397. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Uzu T, Kimura G. Duration until nighttime blood pressure fall indicates excess sodium retention. Chronobiol Int. 2012;29:1412–1417. doi: 10.3109/07420528.2012.728663. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Munemura M, Usami T, et al. Nocturnalblood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Mizuno M, Yamanaka T, et al. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–1160. doi: 10.1161/HYPERTENSIONAHA.108.115329. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Urushihara M, Wakamatsu T, et al. Proximal tubular angiotensinogen in renal biopsy suggests nondipper BP rhythm accompanied by enhanced tubular sodium reabsorption. J Hypertens. 2012;30:1453–1459. doi: 10.1097/HJH.0b013e328353e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzu T, Ishikawa K, Fujii T, et al. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. doi: 10.1161/01.cir.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 12.Uzu T, Harada T, Namba T, et al. Thiazide diuretics enhance nocturnal blood pressure fall and reduce proteinuria in immunoglobulin A nephropathy treated with angiotensin II modulators. J Hypertens. 2005;23:861–865. doi: 10.1097/01.hjh.0000163156.37363.47. [DOI] [PubMed] [Google Scholar]
- 13.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M, Wakamatsu-Yamanaka T, Mizuno M, et al. Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am J Physiol Renal Physiol. 2011;301:F953–F957. doi: 10.1152/ajprenal.00167.2011. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda M, Yamanaka T, Mizuno M, et al. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J Hypertens. 2008;26:583–588. doi: 10.1097/HJH.0b013e3282f2fded. [DOI] [PubMed] [Google Scholar]
- 16.Miura T, Watanabe S, Urushihara M, et al. The natriuretic effect of angiotensin receptor blockers is not attributable to blood pressure reduction during the previous night, but to inhibition of tubular sodium reabsorption. J Renin Angiotensin Aldosterone Syst. 2014;15:316–318. doi: 10.1177/1470320313518253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogiyama Y, Miura T, Watanabe S, et al. Circadian rhythm of urinary potassium excretion during treatment with an angiotensin receptor blocker. J Renin Angiotensin Aldosterone Syst. 2014;15:509–514. doi: 10.1177/1470320313475909. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, Guyton AC, Smith MJ, Jr, et al. Chronic blockade of angiotensin II formation during sodium deprivation. Am J Physiol. 1979;237:F424–F432. doi: 10.1152/ajprenal.1979.237.6.F424. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Mizelle HL, Hildebrandt DA, et al. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15:547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 20.Jover B, Saladini D, Nafrialdi N, et al. Effect of losartan and enalapril on renal adaptation sodium restriction in rat. Am J Physiol. 1994;267:F281–F288. doi: 10.1152/ajprenal.1994.267.2.F281. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 22.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 24.Fukuda M, Kimura G. Can calcium channel blockers preserve renal function better than diuretics during antihy-pertensive treatment? Arch Intern Med. 2005;165:1312. doi: 10.1001/archinte.165.11.1312-a. [DOI] [PubMed] [Google Scholar]
- 25.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzaki Y, Ozawa Y, Kobori H. Quantification of human angiotensinogen by a novel sandwich ELISA. Peptides. 2006;27:3000–3002. doi: 10.1016/j.peptides.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Yoshitomi K, Nakamura M, et al. Site and mechanism of action of trichlormethiazide in rabbit distal nephron segments perfused in vitro. J Clin Invest. 1988;82:721–730. doi: 10.1172/JCI113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velázquez H, Wright FS. Effects of diuretic drugs on Na, Cl, and K transport by rat renal distal tubule. Am J Physiol. 1986;250:F1013–F1023. doi: 10.1152/ajprenal.1986.250.6.F1013. [DOI] [PubMed] [Google Scholar]
- 29.Bankir L, Bochud M, Maillard M, et al. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 30.Centonza L, Castoldi G, Chianca R, et al. Short-term analysis of the relationship between blood pressure and urinary sodium excretion in normotensive subjects. Clin Sci (Lond) 2000;98:495–500. [PubMed] [Google Scholar]
- 31.Koopman MG, Koomen GC, Krediet RT, et al. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989;77:105–111. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 32.Staessen JA, Birkenhäger W, Bulpitt CJ, et al. The relationship between blood pressure and sodium and potassium excretion during the day and at night. J Hypertens. 1993;11:443–447. doi: 10.1097/00004872-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Olsen ME, Hall JE, Montani JP, et al. Mechanisms of angiotensin II natriuresis and antinatriuresis. Am J Physiol. 1985;249:F299–F307. doi: 10.1152/ajprenal.1985.249.2.F299. [DOI] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 35.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulatessodium transport in rabbit proximal convoluted tubules. J Clin Invest. 1984;73:507–515. doi: 10.1172/JCI111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braam B, Navar LG, Mitchell KD. Modulation of tubuloglomerularfeedback by angiotensin II type 1 receptors during the development of Goldblatt hypertension. Hypertension. 1995;25:1232–1237. doi: 10.1161/01.hyp.25.6.1232. [DOI] [PubMed] [Google Scholar]
- 37.Kobori H, Prieto-Carrasquero MC, Ozawa Y, et al. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuo JL, Imig JD, Hammond TG, et al. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: Role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 39.Hung SC, Kuo KL, Peng CH, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 40.Ingert C, Grima M, Coquard C, et al. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol. 2002;283:F995–F1002. doi: 10.1152/ajprenal.00321.2001. [DOI] [PubMed] [Google Scholar]
- 41.Schunkert H, Hense HW, Bröckel U, et al. Differential effects of antihypertensive drugs on neurohormonal activation: Insights from a population-based sample. J Intern Med. 1998;244:109–119. doi: 10.1046/j.1365-2796.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 42.Zusman RM, Spector D, Caldwell BV, et al. The effect of chronic sodium loading and sodium restriction on plasma prostaglandin A, E, and F concentrations in normal humans. J Clin Invest. 1973;52:1093–1098. doi: 10.1172/JCI107274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobori H, Nishiyama A, Abe Y, et al. Enhancement of Intrarenal Angiotensinogen in Dahl Salt-Sensitive Rats on High Salt Diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao W, Seth DM, Prieto MC, et al. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am J Physiol Renal Physiol. 2013;304:F505–F514. doi: 10.1152/ajprenal.00587.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konishi Y, Nishiyama A, Morikawa T, et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–211. doi: 10.1161/HYPERTENSIONAHA.110.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 47.Vegter S, Perna A, Postma MJ, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers Circulation. 1990;81:700–702. doi: 10.1161/01.cir.81.2.700. [DOI] [PubMed] [Google Scholar]
- 49.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 50.Mills KT, Kobori H, Hamm LL, et al. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012;27:3176–3181. doi: 10.1093/ndt/gfs011. [DOI] [PMC free article] [PubMed] [Google Scholar]