Abstract
BACKGROUND
The recent development of MRI-guided laser-induced thermal therapy (LITT) offers a minimally invasive alternative to craniotomies performed for tumor resection or for amygdalohippocampectomy to control seizure disorders. Current LITT therapies rely on linear stereotactic trajectories that mandate twist-drill entry into the skull and potentially long approaches traversing healthy brain. The use of robotically-driven, telescoping, curved needles has the potential to reduce procedure invasiveness by tailoring trajectories to the curved shape of the ablated structure and by enabling access through natural orifices.
OBJECTIVE
To investigate the feasibility of using a concentric tube robot to access the hippocampus through the foramen ovale to deliver thermal therapy and thereby provide a percutaneous treatment for epilepsy without drilling the skull.
METHODS
The skull and both hippocampi were segmented from dual CT/MR image volumes for 10 patients. For each of the 20 hippocampi, a concentric tube robot was designed and optimized to traverse a trajectory from the foramen ovale to and through the hippocampus from head to tail.
RESULTS
Across all 20 cases, the mean distances (error) between hippocampus medial axis and backbone of the needle were 0.55 mm, 1.11 mm, and 1.66 mm for best, mean, and worst case, respectively.
CONCLUSION
These curvilinear trajectories would provide accurate transforamenal delivery of an ablation probe to typical hippocampus volumes. This strategy has the potential to both decrease the invasiveness of the procedure and increase the completeness of hippocampal ablation.
Keywords: Skull base, epilepsy surgery, robotic needle, ablation, interventional MRI, nonlinear trajectory
Epilepsy has a point prevalence of about 0.6% globally and accounts for 1% of the global burden of disease in terms of disability-adjusted life years.1,2 Forty-seven percent of patients are seizure-free with their first anti-epileptic drug (AED), and an additional 13% are seizure-free with their second AED.3 Thus, about 40% of patients are refractory to medical therapy, and many of these are potential surgical candidates. Although epilepsy surgery outcomes reflect seizure-free rates of 70% to 80%,4-6 only a small fraction of potential surgical candidates are referred for treatment, due in part to physician bias against the highly invasive surgery.7,8 Furthermore, the invasiveness of current surgical procedures likely causes many otherwise eligible patients to forgo surgery, despite the high likelihood of a seizure-free outcome.
In recent years, needle-based thermal ablation has been investigated as a minimally-invasive alternative to selective amygdalohippocampectomy (SAH). An SAH resection is less aggressive than standard anterior temporal lobectomy (ATL) but has demonstrated equivalent or near-equivalent outcomes.5 Parrent and Blume reported amygdalohippocampotomy by MRI-guided radiofrequency ablation (RFA); seizure outcomes were worse than ATL.9 However, more recent clinical trials using MRI-guided laser ablation systems have more positively indicated the efficacy of percutaneous thermal ablation for epilepsy. Curry et al reported seizure freedom at 3-month follow-up for 5 pediatric patients treated using the Visualase Thermal Therapy System (Medtronic, Dublin, Ireland).10 For a small series of procedures using the Visualase laser, Willie et al reported 6-month seizure freedom for 7 of 13 patients and substantial improvement for an additional 3 patients.11 Hawasli et al effectively treated 1 case of medically refractory epilepsy using the Monteris Neuroblate System (Monteris, Plymouth, Minnesota).12 Despite some promising results, these ablation procedures to date have not matched the success of SAH. One of the primary limitations of current laser-induced thermal therapy (LITT) procedures is that the laser probes used with currently available devices are restricted to linear trajectories, which cannot lesion the entire hippocampus, even with directionally aiming probes. Additionally, these procedures still require full operating room preparation for twist-drilling the skull.
This paper investigates a novel approach for accessing the hippocampus through the foramen ovale using helical concentric tube needles. We address the question of whether concentric tube needles with this geometry can, in principle, guide an ablator along the curved shape of the hippocampus when delivered through the foramen ovale. Through medical image analysis and computer simulation, we determine optimized, curvilinear needle trajectories specific to the anatomy of 20 patients. This is the first paper to consider optimal design of concentric tube needles for a transforamenal approach for accessing the hippocampus and the first paper to consider variations in patient anatomy when considering helical needle design.
METHODS
The Transforamenal Ablation Concept
Our transforamenal approach is enabled by nonlinear probe trajectories that can also traverse the natural curvature of the hippocampus. These trajectories may offer more complete lesioning than linear trajectories, potentially improving seizure outcomes to equivalency with SAH. The proposed procedure accesses the mesial temporal lobe by cannulation of the foramen ovale. This would eliminate the need for twist-drilling the skull and allow for real-time course correction as the needle insertion is performed in the MRI.
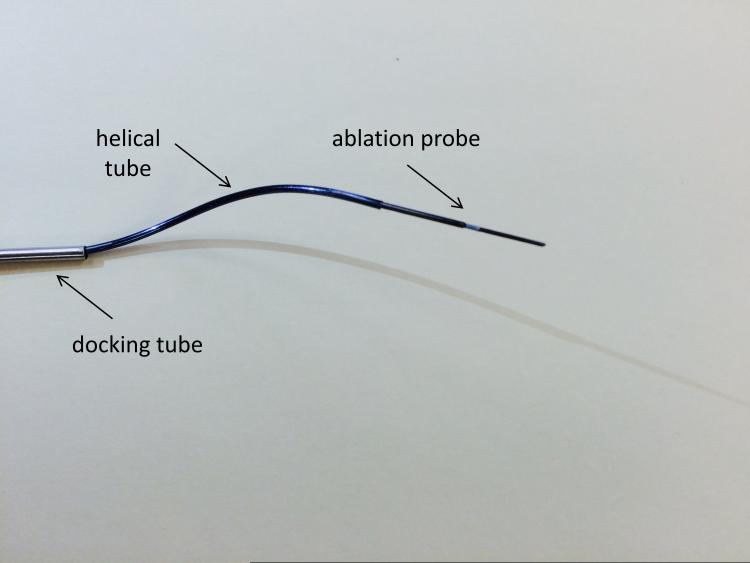
The curvilinear trajectories required are realized using a concentric tube needle. This device consists of nested tubes of superelastic nitinol. Controllable, curvilinear motion is realized when axial translations and rotations are applied to the tube bases.13-16 A photograph of the concentric tube needle as designed for transforamenal ablation is shown in Figure 1. The technique used for creating this prototype needle is discussed by Gilbert and Webster.17 Deploying this needle through a soft tissue media in a helical trajectory requires precise coordination of both insertion and axial rotation of the component tubes, as was recently described by Gilbert et al,18 and is hence typically achieved by attaching motors to tube bases to enable computer control of tube motions. Several particular MRI-compatible robot designs have recently been proposed to accomplish this motion control.19-21 Gilbert et al also explored in simulation the potential benefits of using helical trajectories with a concentric tube needle to access the hippocampus from a burr hole in the rear of the skull,18 but did not consider transforamenal deployment or variation in patient anatomy, both of which we investigate in this paper.
Figure 1.
Photograph of concentric tube needle concept for transforamenal ablation.
Cannulation of the foramen ovale is often performed for placement of foramen ovale electrodes to monitor the mesial temporal structures.22,23 Several research groups have also performed frameless stereotactic cannulation of the foramen ovale for trigeminal rhizotomy.24,25 Similarly, for diagnosis and surgical treatment of drug-refractory epilepsy, Ortler et al reported an optically-tracked aiming device and noninvasive maxillary fixation system called the Vogele-Bale-Hohner (VBH) head holder.26,27 This setup facilitates placement of foramen ovale depth electrodes and also mitigates the risk of entering no-go zones like the carotid artery during cannulation.
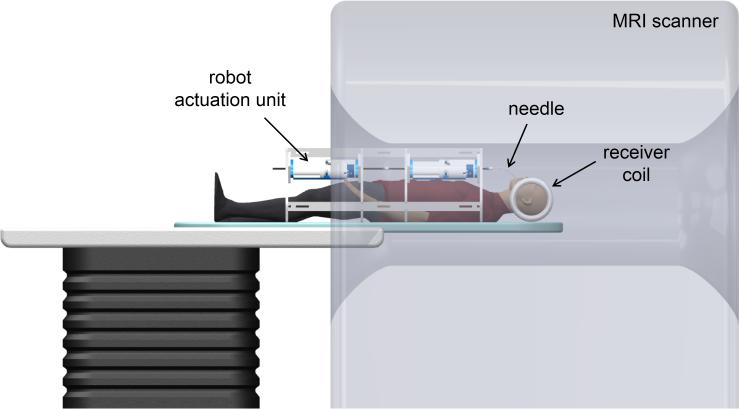
To enable the transforamenal ablation concept, we envision a robotic, MRI-guided system (see Figure 2). The system comprises an MR-compatible actuation unit, a concentric tube robot, an ablation probe, and MRI guidance. We anticipate the procedure could take place from start to finish in a radiology suite and not require use of a surgical suite. In our envisioned procedure, a robot actuation unit is positioned within a standard MRI scanner with the patient under general anesthesia. The robot is suspended above the patient's torso using an arch-shaped frame affixed to the scanner gantry. The needle in the system is made up of 2 parts: a docking tube and a robotically actuated concentric tube needle, as shown in Figure 1. The docking tube (a 14-gauge needle, a size typically available in trigeminal rhizotomy kits) is an outer cannula manually placed by the neurosurgeon under fluoroscopic guidance to cannulate the foramen ovale prior to transferring the patient to the MRI scanner. The concentric tube needle, consisting of an ablation probe contained within a helically curved superelastic tube, is withdrawn inside the docking tube during cannulation of the foramen ovale. After cannulation of the foramen ovale, the concentric tube needle is deployed by the robot actuation unit (under surgeon control) and guided using real-time MRI for visualization. The helical tube (with the ablation probe retracted inside) passes as close as possible along the medial axis of the hippocampus towards its tail. The ablation probe is then extended a short distance beyond the tip of the helical tube to deliver thermal therapy. Spatial thermal energy deposition is monitored using MR thermal imaging. When sufficient thermal dose is reached at this first position, the ablator is retracted within the helical tube, the helical tube is retracted a short distance along its entry trajectory, and the ablator is re-deployed. This process can be repeated as many times as desired by the physician to achieve desired thermal dose to the hippocampus. The amygdala can also be lesioned if desired by fully retracting the concentric tube robot into the docking tube and then re-inserting along a second trajectory targeting the amygdala.
Figure 2.
Illustration of robotic transforamenal ablation system concept.
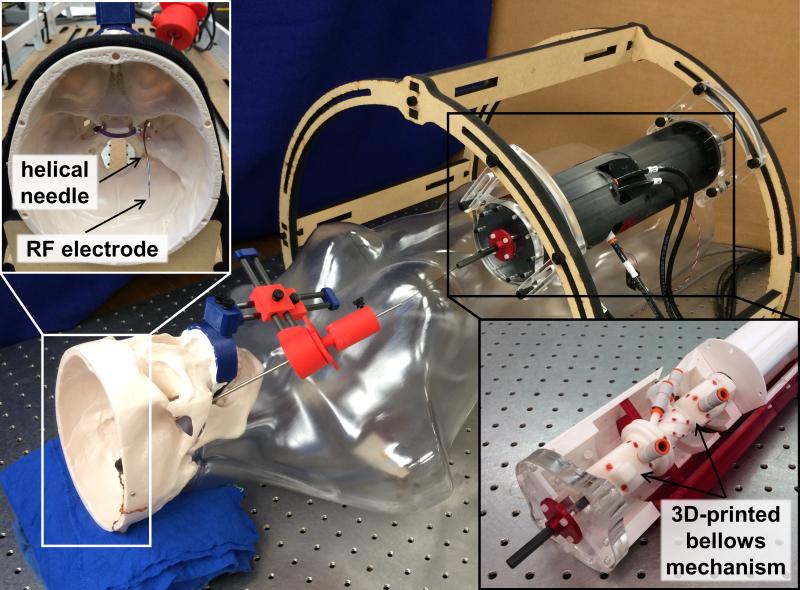
Previously, we have developed a fail-safe, pneumatic, MRI-compatible robot actuation unit.20 Figure 3 shows a photograph of our current prototype assembled for a benchtop simulation of the procedure using an anatomically accurate skull model. Insets in Figure 3 show the deployed helical tube inside the skull and the 3D-printed bellows mechanism inside the actuation unit. This prototype fits inside a 60-centimeter MRI scanner bore. During needle deployment, the pneumatic robot actuators grasp both the helical tube and the ablation probe at their proximal ends and apply necessary insertion and axial rotation motions to deliver the helical tube and ablation probe along the desired trajectory. Detailed information on the design, control, and performance of the robot actuation unit can be found in Comber et al.20
Figure 3.
MRI-compatible robot actuation unit prototype in benchtop mockup of transforamenal ablation procedure.
We note that some aspects of this system concept are not yet fully integrated. In particular, we have developed the MRI-compatible robotic hardware and the concentric tube device and have conducted initial experiments to verify MRI compatibility, but we have not yet integrated MRI thermometry or demonstrated use of MRI images to guide the needle to desired points. We also have not yet tested our envisioned procedure in cadaveric or animal models. These are elements of future work.
Medical Image Analysis
To characterize the required workspace and constraints for the concentric tube robot, a medical image analysis was conducted. As a retrospective study with exemption approval from the Vanderbilt University Institutional Review Board, dual CT/MR image volumes were obtained and de-identified. Image sets for 10 patients were selected based only on whether a sufficient amount of mandible and maxillary bone were included in the CT scan. This was to ensure adequate information to inform the design of cannula orientation angles for cannulation of the foramen ovale. No prior indication as to whether the hippocampus would be convenient to reach through the foramen ovale influenced patient selection.
Visualization and analysis of the image volumes were performed using the open-source 3D Slicer software (www.slicer.org). The CT and MR image volumes were registered by a rigid transformation plus scaling using Slicer's BRAINSFit module.28 The skull was segmented from the CT volume using an automated threshold. The hippocampi and amygdala were segmented manually from the MRI volume and reviewed and confirmed by an experienced neurosurgeon (Dr Neimat, who is a co-author on this paper).
Concentric Tube Needle Design
The skull, hippocampus, and foramen ovale models were used to design a concentric tube needle trajectory for each patient. Our design objective was to cause the ablator to pass as close to the medial axis as possible. The medial axis is the locus of the centers of all maximal spheres inscribed within the object, where maximal spheres touch more than one point on the object boundary.29 The rationale for this approach is that if the ablator radiates heat evenly in all directions, it will be most likely to achieve uniform coverage of the hippocampus if it travels along the medial axis. However, we note that the ablator need not travel perfectly along the axis, as there are examples of successful ablations being performed substantially away from the medial axis.10-12
In this application, the concentric tube needle trajectory is fully defined by 7 parameters: helix curvature, helix torsion, helix maximum insertion length, helix initial rotation, ablation probe maximum insertion length, and the 2 orientation angles of the docking tube. Note that the curvature and torsion define the geometry of the helical tube, whereas the other 5 parameters describe the needle and ablator placement. To achieve our design objective, we performed a numerical optimization to determine the set of 7 parameters that minimized the mean distance from each point on the medial axis of the hippocampus of each patient to the closest point on the concentric tube needle's backbone. The segmented anatomical models were imported to MATLAB so that they could be overlaid with possible trajectories of the concentric tube needle. The medial axis was computed using the skeletonizing method of Lee et al.29 To generate an initial set of parameters for the numerical optimization, a needle trajectory capable of closely following the medial axis was manually designed using the forward kinematic equations of the helical concentric tube robot. An example of this manual design is shown for patient 4 in Figure 4A. Numerical optimization of the trajectory parameters was then performed using the well-established Nelder-Mead simplex method, which was implemented with the MATLAB fminsearch function.30 During optimization, a limitation was placed on the helix curvature to ensure that the needle would not plastically deform when straightened inside the docking tube. A maximum of 8% strain was allowed for helical tube curvature; this is the often-quoted recoverable strain limit for nitinol in the literature. Additional constraints were placed on the orientation angles of the docking tube to ensure that the needle trajectory did not exceed the safe insertion region. Specifically, using the skull model, we defined a coordinate frame for the docking tube such that its central axis lies in a plane of maximum adjustability in the space between maxilla and mandible. This plane is approximately sagittal, and the corresponding range of motion is illustrated by the larger circular sector in Figure 4B. This range of allowable motion was constrained in the optimization to ±10°, and for the orthogonal plane shown in Figure 4C, a constraint of ±5° was imposed. All parameter limits were enforced via cost conditions in the numerical optimization.
Figure 4.
(A) Initial transforamenal trajectory before numerical optimization. (B,C) Docking tube range of allowable motion.
RESULTS
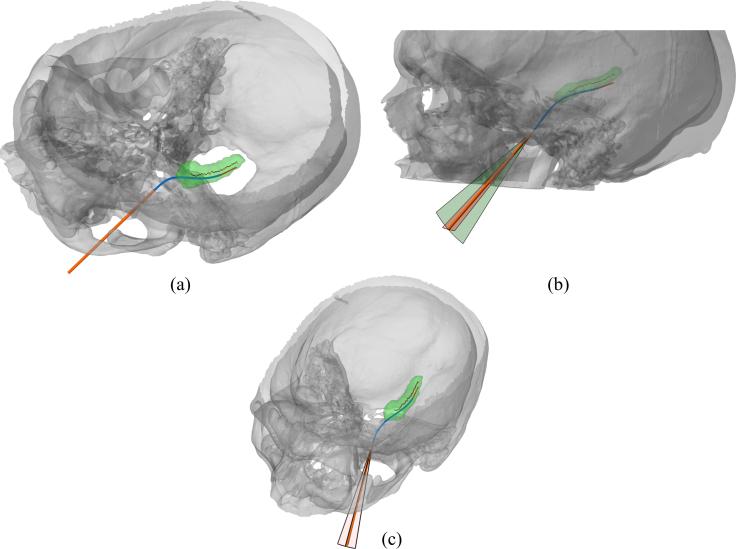
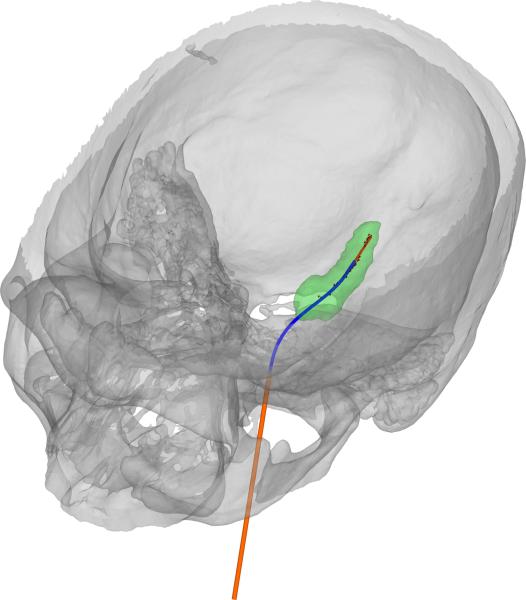
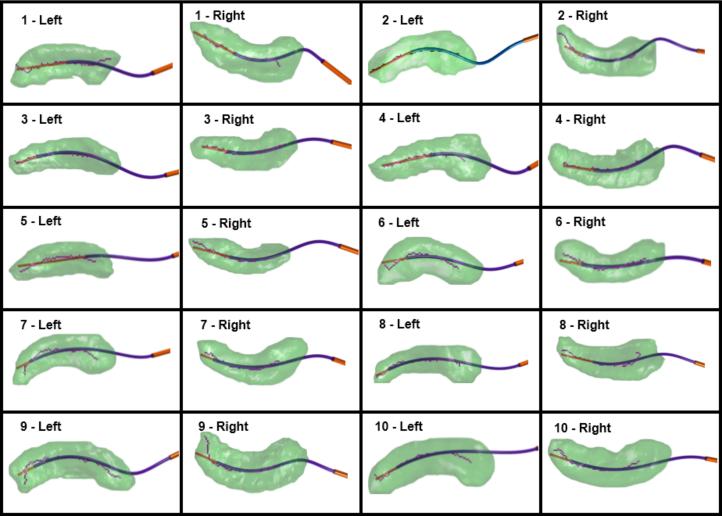
The optimized concentric tube robot design for patient 4 is shown in Figure 5. A comparison of initial (Figure 4) to optimized designs shows a substantial improvement in the accuracy of the trajectory to traverse the medial axis of the hippocampus. A summary of all 20 optimized designs is provided in Figure 6 and Table 1. Figure 6 shows the medial axis of each hippocampus and each trajectory at its maximum path distance from the foramen ovale. Across all 20 cases, the mean distances (error) between hippocampus medial axis and backbone of the needle were 0.55 mm, 1.11 mm, and 1.66 mm for best, mean, and worst case, respectively. For each case, a helical curvature was found that resulted in strains less than the recoverable limit.
Figure 5.
Optimized concentric tube robot design for patient 4.
Figure 6.
Optimized concentric tube robot designs for 20 hippocampi, axial view.
Table 1.
Concentric tube robot design parameters and corresponding error predictions.
| Helically Pre-curved Tube | Ablation Probe | ||||
|---|---|---|---|---|---|
| Patient No. | Max Length (mm) | Curvature (m−1) | Torsion (m−1) | Max Length (mm) | Mean Error (mm) |
| 1 Left | 42.0 | 40.7 | 95.2 | 60.8 | 0.84 |
| 1 Right | 47.6 | 60.8 | −110.4 | 65.6 | 0.67 |
| 2 Left | 56.2 | 78.3 | 107.0 | 71.2 | 0.81 |
| 2 Right | 56.0 | 83.9 | −118.0 | 65.0 | 1.26 |
| 3 Left | 52.8 | 49.7 | 94.9 | 60.6 | 0.74 |
| 3 Right | 42.6 | 44.9 | −112.9 | 51.9 | 1.14 |
| 4 Left | 46.3 | 50.6 | 84.1 | 63.1 | 0.59 |
| 4 Right | 50.0 | 55.1 | −102.8 | 61.1 | 0.55 |
| 5 Left | 33.8 | 43.6 | 92.5 | 57.4 | 0.86 |
| 5 Right | 45.5 | 57.0 | −111.3 | 58.9 | 1.26 |
| 6 Left | 48.3 | 39.9 | 102.7 | 58.1 | 1.49 |
| 6 Right | 47.2 | 29.9 | −101.5 | 54.9 | 1.62 |
| 7 Left | 58.7 | 32.4 | 72.2 | 64.8 | 1.43 |
| 7 Right | 56.5 | 34.1 | −84.7 | 62.0 | 0.90 |
| 8 Left | 58.2 | 39.5 | 77.8 | 63.6 | 1.17 |
| 8 Right | 48.5 | 48.9 | −114.0 | 62.3 | 1.64 |
| 9 Lett | 58.4 | 62.3 | 102.1 | 64.9 | 1.59 |
| 9 Right | 52.6 | 59.0 | −125.8 | 62.4 | 1.66 |
| 10 Left | 55.0 | 32.9 | 62.9 | 63.5 | 0.90 |
| 10 Right | 55.2 | 36.8 | −91.2 | 63.3 | 1.03 |
Considering the summary of the optimized design parameters given in Table 1, there are several reasons why patient-specific designs were required to achieve sufficient accuracy. First, the helical tube torsion is positive for the left hippocampus and negative for the right hippocampus, so at least two different needles are required. Second, optimized torsion varied by ±25% from the mean (89.1 m-1 rad-1) for the set of right-handed helices and by ±19% from the mean (107.3 m-1 rad-1) for the left-handed helices. If a generic shaped needle were used instead, this amount of variation from optimal torsion would result in additional trajectory error of 3 mm to 5 mm.
It is feasible to rapidly and accurately fabricate patient-specific needles using shape setting of superelastic nitinol. For this work, we used a laboratory-grade electric heating method developed by our lab17 to rapidly prototype a concentric tube needle for patient 1, using a superelastic nitinol tube of outer and inner diameters 1.14 mm and 0.97 mm, respectively. The fabricated prototype had a curvature of 41.2 m-1 and a torsion of 84.8 m-1. The geometry of this prototype deviates slightly from the optimized geometry given in Table 1; however, we note that this deviation results from current limitations of the rapid prototyping technique. Albeit more expensive, industrial methods for shape setting of nitinol would be able to achieve desired geometry with substantially greater accuracy on appropriate timescales for our envisioned procedure. Using the measured geometry of the prototype needle, we repeated the optimization to find an optimal set of 5 insertion parameters for the prototype needle. In simulation, this new trajectory follows the medial axis of the hippocampus at a mean error distance of 1.80 mm and is illustrated in Figure 7.
Figure 7.
Optimized trajectory using measured curvature and torsion of prototype.
CONCLUSION
This paper has described a novel approach to access the hippocampus through the foramen ovale using a concentric tube robot for the purpose of hippocampotomy by ablation. We have presented a computer simulation of optimized helical needle trajectories for accurate traversal of the curvilinear medial axis of 20 hippocampi. This is the first paper to consider optimal design of concentric tube needles for a transforamenal approach for accessing the hippocampus. This is also the first paper to address variations in patient anatomy when considering helical tube optimization. A prototype needle with sufficiently accurate pre-curvature for this application was also manufactured. These trajectories potentially enable ablation of tissue—in particular, in the tail of the hippocampus—that cannot typically be reached by linear trajectories. In light of correlation between higher resection volume and better clinical outcomes,31 the potential to achieve more complete ablation could improve the efficacy of hippocampotomy by ablation for the treatment of epilepsy. Though significant additional testing is necessary to confirm the feasibility of this procedure, the results of this study preliminarily suggest that MRI-guided transforamenal ablation could be a less-invasive alternative to current ablation treatments for epilepsy and may ultimately provide a more complete ablation.
The eventual realization of our envisioned procedure will require much future work in both technical and clinical aspects. One area of work in concentric tube robots is to explore the effects of friction between tubes, especially in light of the relatively long transmission length required for our approach. One prior paper on this topic suggests modelling friction as an axial torque located at the end of the straight transmission section of the tubes.32 This friction model can be integrated into the mechanics-based model of the tubes,14,15 and we expect that it will be straightforward to compensate for friction with our actuators or to mitigate it with stiffer straight transmissions33 and/or with low-friction coatings. Further areas of technical work for our system include evaluating imaging protocols for MR thermometry and integrating MRI guidance for our robot. A variety of ablation technologies are possible, and additional work will also be needed to choose the best technology for this application. An important clinical concern to be addressed in future design work is the ability to extract a concentric tube needle in the event of a failure of the robotic actuation system after insertion in the temporal lobe. Many other clinical aspects of our envisioned procedure will also need to be extensively evaluated in future cadaver and animal studies.
Acknowledgments
This research was supported in part by NIH grant number 1R21NS091735-01 and NSF grant number EEC-0540834. Joseph Neimat does limited consulting work for Monteris Inc.
Footnotes
Disclosures: The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of Epilepsy: The Ontario Health Survey. Canadian J Neurol Sci. 1999;26:263–270. doi: 10.1017/s0317167100000354. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Jr, Wiebe S, French J, et al. Practice Parameter: Temporal Lobe and Localized Neocortical Resections for Epilepsy. Epilepsia. 2003;44(6):741–751. doi: 10.1046/j.1528-1157.2003.48202.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 4.Hori T, Yamane F, Ochiai T, Hayashi M, Taira T. Subtemporal Amygdalohippocampectomy Prevents Verbal Memory Impairment in the Language-Dominant Hemisphere. Stereotact Funct Neurosurg. 2003;80(1-4):18–21. doi: 10.1159/000075154. [DOI] [PubMed] [Google Scholar]
- 5.Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological Outcome after Selective Amygdalohippocampectomy with Transsylvian versus Transcortical Approach: A 11 Randomized Prospective Clinical Trial of Surgery for Temporal Lobe Epilepsy. Epilepsia. 2004;45(7):809–816. doi: 10.1111/j.0013-9580.2004.54003.x. [DOI] [PubMed] [Google Scholar]
- 6.Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:7510–763. doi: 10.3171/jns.2003.98.4.0751. [DOI] [PubMed] [Google Scholar]
- 7.Uijl SG, Leijten FSS, Moons KGM, Veltman EPHM, Ferrier CH, van Donselaar CA. Epilepsy surgery can help more adult patients with intractable seizures. J Epilepsy Research. 2012;101:210–216. doi: 10.1016/j.eplepsyres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.de Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol. 2010;17(4):619–625. doi: 10.1111/j.1468-1331.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- 9.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia. 1999;40(10):1408–1416. doi: 10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 10.Curry DJ, Gowdy A, McNichols RJ, Wilfong AA. MR-Guided Stereotactic Ablation of Epileptogenic Foci in Children. Epilepsy and Behavior. 2012;24(4):408–414. doi: 10.1016/j.yebeh.2012.04.135. [DOI] [PubMed] [Google Scholar]
- 11.Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74(6):569–85. doi: 10.1227/NEU.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawasli AH, Bandt SK, Hogan RE, Werner N, Leuthardt EC. Laser Ablation as Treatment Strategy for Medically Refractory Dominant Insular Epilepsy: Therapeutic and Functional Considerations. Stereotact Funct Neurosurg. 2014;92:397–404. doi: 10.1159/000366001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster RJ, III, Romano JM, Cowan NJ. Mechanics of Precurved-Tube Continuum Robots. IEEE Trans Robotics. 2009;25(1):67–78. [Google Scholar]
- 14.Rucker DC, Jones BA, Webster RJ., III A geometrically exact model for externally loaded concentric tube continuum robots. IEEE Transactions on Robotics. 2010;26(5):769–780. doi: 10.1109/TRO.2010.2062570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont PE, Lock J, Itkowitz B, Butler E. Design and control of concentric-tube robots. IEEE Trans Robotics. 2010;26(2):209–225. doi: 10.1109/TRO.2009.2035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert HB, Rucker DC, Webster RJ., III Concentric Tube Robots: State of the Art and Future Directions. Springer Tracts in Advanced Robotics. 2016;114:253–269. [Google Scholar]
- 17.Gilbert HB, Webster RJ., III Rapid, Reliable Shape Setting of Superelastic Nitinol for Prototyping Robots. IEEE Robotics and Automation Letters. 2016;1(1):98–105. doi: 10.1109/LRA.2015.2507706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert HB, Neimat J, Webster RJ., III Concentric Tube Robots as Steerable Needles: Achieving Follow-The-Leader Deployment. IEEE Trans. Robotics. 2015;31(2):246–258. doi: 10.1109/TRO.2015.2394331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comber DB, Barth EJ, Webster RJ., III Design and control of a magnetic resonance compatible precision pneumatic active cannula robot. ASME J Med Dev. 2014;8(1):011003. [Google Scholar]
- 20.Comber DB, Slightam JE, Neimat JS, Gervasi VR, Barth EJ. Design, Additive Manufacture, and Control of a Pneumatic, MR-Compatible Needle Driver. IEEE Trans Robotics. 2016;32(1):138–149. doi: 10.1109/TRO.2015.2504981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Cardona DC, Shang W, et al. A MRI guided concentric tube continuum robot with piezoelectric actuation: a feasibility study. 2015 IEEE International Conference on Robotics and Automation (ICRA) 2015:1939–1945. [Google Scholar]
- 22.Wieser HG, Elger CE, Stodieck SR. The ‘foramen ovale electrode’: a new recording method for the preoperative evaluation of patients suffering from mesio-basal temporal lobe epilepsy. Electroencephalography and Clinical Neurophysiology. 1985;61(4):314–322. doi: 10.1016/0013-4694(85)91098-3. [DOI] [PubMed] [Google Scholar]
- 23.Sheth SA, Aronson JP, Shafi MM, et al. Utility of foramen ovale electrodes in mesial temporal lobe epilepsy. Epilepsia. 2014;55(5):713–724. doi: 10.1111/epi.12571. [DOI] [PubMed] [Google Scholar]
- 24.Bale R, Laimer I, Martin A, et al. Frameless Stereotactic Cannulation of the Foramen Ovale for Ablative Treatment of Trigeminal Neuralgia. Neurosurgery. 2006;59(4):394–402. doi: 10.1227/01.NEU.0000232770.97616.D0. [DOI] [PubMed] [Google Scholar]
- 25.Lin M, Lee M-H, Wang T-C, et al. Foramen ovale cannulation guided by intra-operative computed tomography with integrated neuronavigation for the treatment of trigeminal neuralgia. Acta Neurochir. 2011;153:1593–1599. doi: 10.1007/s00701-011-1009-2. [DOI] [PubMed] [Google Scholar]
- 26.Ortler M, Widmann G, Trinka E, et al. Frameless Stereotactic Placement of Foramen Ovale Electrodes in Patients with Drug-Refractory Temporal Lobe Epilepsy. Neurosurgery. 2008;62(2):481–489. doi: 10.1227/01.neu.0000326038.00456.f3. [DOI] [PubMed] [Google Scholar]
- 27.Ortler M, Trinka E, Dobesberger J, et al. Integration of multimodality imaging and surgical navigation in the management of patients with refractory epilepsy. A pilot study using a new minimally invasive reference and head-fixation system. Acta Neurochir. 2010;152:365–378. doi: 10.1007/s00701-009-0386-2. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JH, Harris G, Williams K. BRAINSFit: Mutual Information Registrations of Whole-Brain 3D Images, Using the Insight Toolkit. The Insight Journal. 2007 Available at http://hdl.handle.net/1926/1291.
- 29.Lee T-C, Kashyap R-L, Chu C-N. Building Skeleton Models via 3-D Medial Surface/Axis Thinning Algorithms. CVGIP: Graphical Models and Image Processing. 1994;56(6):462–478. [Google Scholar]
- 30.Lagarias JC, Reeds JA, Wright MH, Wright PE. Convergence Properties of the Nelder-Mead Simplex Method in Low Dimensions. SIAM Journal of Optimization. 1998;9(1):112–147. [Google Scholar]
- 31.Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: A randomized prospective study. Neurosurgery. 1995;37(5):982–991. doi: 10.1227/00006123-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Lock J, Dupont PE. Friction Modeling in Concentric Tube Robots. 2011 IEEE International Conference on Robotics and Automation (ICRA) 2011:1139–1146. doi: 10.1109/ICRA.2011.5980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin F-Y, Bergeles C, Yang G-Z. Biometry-based concentric tubes robot for vitreoretinal surgery. 2015 International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015:5280–5284. doi: 10.1109/EMBC.2015.7319583. [DOI] [PubMed] [Google Scholar]