Abstract
Background and Purpose
Elastase-induced aneurysms in rabbits have been proposed as a useful preclinical tool for device development. The object of this study is to report rates of morbidity and mortality associated with creation and embolization of the elastase-induced rabbit aneurysm, and to assess the impact of operator experience on these rates.
Methods
Elastase-induced model aneurysms were created in New Zealand White rabbits (n=700). One neuroradiologist/investigator, naïve to the aneurysm creation procedure at the outset of the experiments, performed all surgeries. All morbidity and deaths related to aneurysm creation (n=700) and embolization procedures (n=529) were categorized into acute and chronic deaths. Data were analyzed with single regression analysis and ANOVA. To assess the impact of increasing operator experience, the number of animals was broken into 50 animal increments.
Results
There were 121 (17%) deaths among 700 subjects. Among 700 aneurysm creation procedures, 59 deaths (8.4%) were noted. Among 529 aneurysm embolization procedures, 43 deaths (8.1%) were noted. Nineteen additional deaths (2.7% of 700 subjects) were unrelated to procedures. Simple regression indicated mortality associated with procedures diminished with increasing operator experience (R2=0.38; p=0.0180) and that for each 50 rabbit increment mortality is reduced on average by 0.6 percent.
Conclusions
Mortality rates of approximately 8% are associated with both experimental aneurysm creation and with embolization in the rabbit, elastase-induced aneurysm model. Increasing operator experience is inversely correlated with mortality and the age of the rabbit is positively associated with morbidity.
Keywords: elastase-induced aneurysms, aneurysm creation, rabbit model
Introduction
The development of endovascular occlusion devices has been facilitated through use of various animal models of saccular aneurysms. Detachable, platinum coils have been tested using surgically created aneurysms in swine, canines, rabbits and primates1–7. More recently, elastase-induced aneurysms in rabbits have been proposed as a useful preclinical tool for device development8–14.
Previous studies9, 10, 15 have shown that elastase-induced aneurysms in rabbits are similar to that of human aneurysms in size and shape. A recent study of the rabbit model’s histopathology16 has shown that it mimics well the healing seen in human aneurysms, especially when compared to the swine model. Further, as these experimental aneurysms are in close proximity to the aorta and are situated on a curved vessel, they also are exposed to substantial hemodynamic stress.
We report rates of morbidity and mortality associated with model aneurysm creation and correlate these rates with operator experience and type of procedure.
Materials and Methods
All animals employed in this study underwent surgeries that were approved by this institution’s Animal Care and Use Committee. Seven hundred female, white, New Zealand rabbits were employed in this retrospective study. The single investigator who performed all the surgeries was a trained neuroradiologist who was naïve to the aneurysm creation procedure at the outset of the experiments. All the rabbits employed in this study were originally used as part of another investigation. The original investigation was entirely unrelated to this project.
Aneurysm Creation
The surgical procedures used to create an aneurysm have been described elsewhere9, 17. Briefly, female New Zealand white rabbits (2–4 kg) were anesthetized with ketamine (74 mg/kg), xylazine (5 mg/kg) and acepromazine (1 mg/kg). Using sterile technique through a vertical, midline incision in the neck, the trachea and the right carotid artery were identified. The vagus nerve and jugular vein were gently dissected free from the right common carotid artery (CCA). A 5 French sheath (Cordis Endovascular, Miami Lakes, FL) was advanced retrograde in the CCA to a point approximately 3 cm cephalad to the CCA origin. Through this indwelling sheath, a 3 French Fogarty balloon (Baxter Healthcare Corporation, Irvine, CA) was advanced to the origin of the right CCA at its junction with the subclavian artery. The balloon was inflated with iodinated contrast with just enough pressure to achieve flow arrest in the CCA. Porcine elastase (5.23u/mgP, 40.1mgP/ml, approximately 200 U/ml; Worthington Biochemical Corporation, Lakewood, NJ) mixed with saline and iodinated contrast was incubated in the dead space of the CCA for approximately 20 minutes, above the inflated balloon. Following incubation of the elastase solution the balloon and sheath were removed, the CCA was ligated below the sheath entry site and the incision was closed in two layers. The fascial layers were closed with interrupted 4-0 Vicryl suture. Interrupted subcuticular sutures as well as running skin suture, both Vicryl, were used to close the wound.
Embolization Procedure
There was a minimum of 21 days between the aneurysm creation10 and the embolization. The animal was induced for the embolization procedure using the same dose of cocktail as in aneurysm creation, with an intramuscular injection of ketamine/xylazine followed by maintenance anesthesia with ketamine and xylazine. Using sterile technique, surgical exposure of the right common femoral artery (CFA) was performed. The artery was ligated distally using 4-0 silk suture, and a 22 g angiocatheter was advanced retrograde into the artery. A 0.018 guidewire was passed through the angiocatheter followed by placement of a 5 F vascular sheath. Heparin (100 U/kg) was administered intravenously. As described previously8, a 5 F catheter (Envoy, Cordis Neurovascular Systems, Miami Lakes, FL) was advanced into the brachiocephalic artery. Using coaxial technique, with continuous heparinized saline flush, an 2-marker microcatheter was advanced into the aneurysm cavity. The size of the aneurysm cavity was assessed using direct comparison to radiopaque sizing devices during digital subtraction angiography (DSA). Where possible, maximal packing of devices at the parent artery/neck interface was achieved. Following the embolization, a final control DSA was performed. The catheters and sheath were removed, the femoral artery ligated, and the incision closed, as in the aneurysm creation, with fascial layers closed with interrupted as well as running skin suture, both 4-0 Vicryl.
Data Set
The 700 animals with surgeries, aneurysm creations and embolizations, spanning four years, were subdivided into groups of 50. Within each group of fifty, two categories were created, including those who survived until their scheduled sacrifice and those who did not. Those who did not survive were further subdivided into categories describing cause of death.
Statistics
Analysis of variance (JMP; SAS Inc, Cary NC) coupled with Student –t post hoc tests when necessary, was used to determine if there were any differences between any of the categories/causes of death. Correlations (JMP; SAS Inc, Cary NC) were run on the number of incidents in the category in question by the number of creations in 50 animal increments. Finally, multiple regression analysis (JMP; SAS Inc, Cary NC) were run on all the independent variables within each group (acute and chronic aneurysm creation and acute and chronic embolization) to determine which variable was the largest contributor to the r-square value and if there were additive effects with more than one independent variable.
Results
There were 121 (17%) deaths among 700 subjects, which reflected a total of 1259 procedures (700 aneurysm creation procedures and 529 embolizations). Causes of death included (see table 1) anesthesia-related deaths, device failure, failure to thrive (FTT) which included animals that would not eat after surgery and became lethargic, self-mutilation which included animals that chewed their incisions or owing to an injection too close to the sciatic nerve, chewed their foot, paralysis, unknown causes of death and other which included those that we believed had strokes but were unable to fully document. Unrelated to the procedures, we sacrificed 12 subjects because of unfavorable aneurysm morphology, such as being too small to embolize and seven animals because of morbidities such as hairballs, a lacerated liver, a corneal ulcer and a gastric ulcer.
Table 1.
Breakdown of rabbit mortality by category
| Acute Aneurysm Creation | Acute Aneurysm Embolization | Chronic Aneurysm Creation | Chronic Aneurysm Embolization | Non-Procedural | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
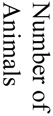 |
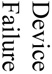 |
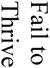 |
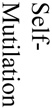 |
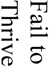 |
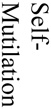 |
||||||||||||
| 50 | 0 | 8 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 1 |
| 150 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| 200 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 0 |
| 250 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| 300 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 3 | 0 | 2 | 0 | 0 | 2 | 1 |
| 350 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 400 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 450 | 0 | 0 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 500 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 4 |
| 550 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 600 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 650 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 700 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total number of Deaths by Category | 4 | 19 | 3 | 8 | 10 | 9 | 3 | 4 | 10 | 11 | 8 | 6 | 2 | 3 | 2 | 12 | 7 |
| Percent of Procedural Deaths by Category | 4% | 19% | 3% | 8% | 10% | 9% | 3% | 4% | 10% | 11% | 8% | 6% | 2% | 3% | 2% | 63% | 37% |
| Percent of Total Deaths by Category | 3% | 16% | 2% | 7% | 8% | 7% | 2% | 3% | 8% | 9% | 7% | 5% | 2% | 2% | 2% | 10% | 6% |
The two categories related to procedural deaths, aneurysm creation and embolization, were divided into acute, at the time of surgery, and chronic, post-surgical complications. Of the 121 deaths, 102 were procedure-related and 19 were not related to the procedure. Fifty-nine (58% of 102 procedure related deaths) of the procedure-related deaths were associated with both acute and chronic aneurysm creation, yielding a mortality rate of 8.4% for aneurysm creation procedures. Forty-three (42% or 102 procedure related deaths) were related to both acute and chronic aneurysm embolization, yielding a mortality rate of 8.1% for aneurysm embolization procedures.
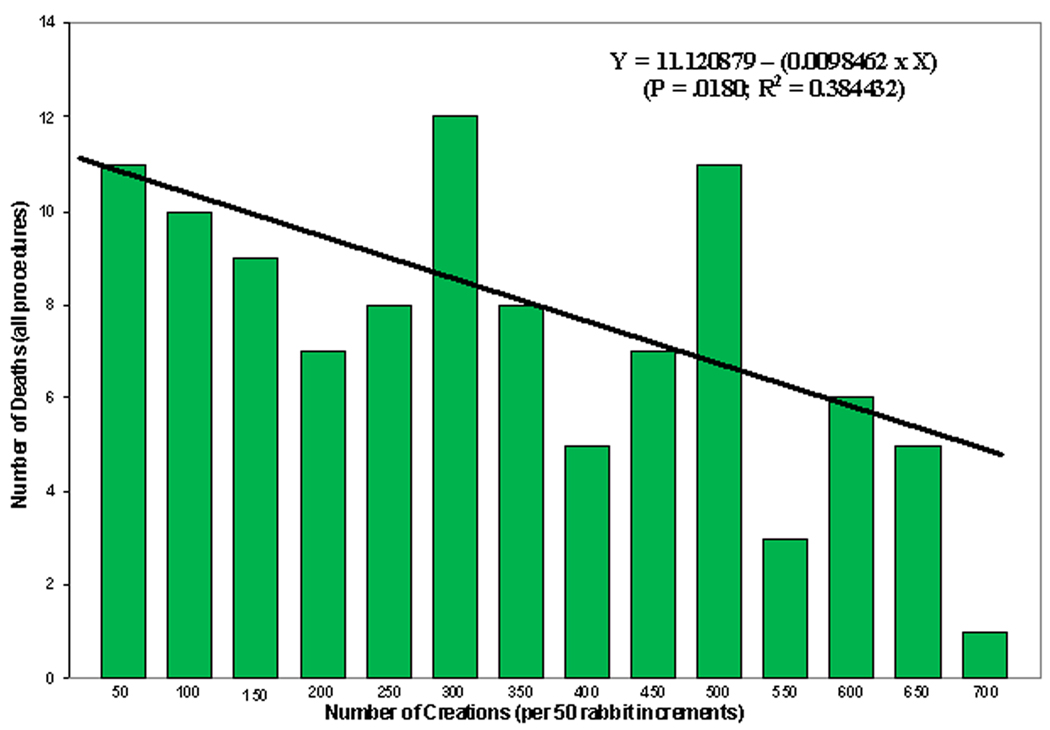
Breakdown of all deaths by category (creation, embolization and non-procedural deaths, showed that the single greatest cause of death was aneurysm creation. If deaths due to aneurysm creation are plotted over fifty rabbit increments, the data show that the number of deaths due to aneurysm creation are diminished by more than half after 100 creations and remain fairly constant thereafter regardless of the time between surgeries.
A Pearson Product Moment correlation (JMP; SAS Inc, Cary NC) shows a significant (p=0.0246; R2=0.354738) negative relationship between the number of aneurysm creation deaths and the number of creations in 50 rabbit increments. If all procedural deaths (aneurysm creations and embolizations) are correlated with the number of creations, the significant negative relationship remains (p=0.0180; R2=0.384432; Figure 1). When the number of days between the start of each fifty-surgery increment and the finish of that increment were examined in relationship to the same factors, there were no significant correlations. Finally, the factor “unknown cause of death” was the single largest contributor to the multiple regression equations for both the acute (p=0.301; R2=0.3352) and chronic (p=0.0279; R2=0.3424) aneurysm creation equations as well as for the acute (p=0.0471; R2=0.2896) embolization equation. The single greatest contributor to the chronic embolization equation was the failure to thrive (FTT) variable (p=0.0175; R2=0.3873).
Figure 1.
A bar chart showing the number of deaths due to all procedures (aneurysm creation and embolization) over fifty-rabbit increments. The line indicates the linear regression line for these data. In addition, the linear regression equation (Y = 11.120879 - (0.0098462 * X)) is noted along with the p value (p=0.0180) and R2 (R2=0.384432) value for the equation.
The failure to thrive group, as defined above, was the group that was unable to return to normal eating habits, etc after embolization. When the average age of these animals was compared to the average of the other animals that had embolization procedures it was discovered that the FTT animals were significantly older (591 ± 362 days) than the other animals (159 ± 298 days; p= 0.0004).
Discussion
The rabbit model of elastase-induced aneurysm creation is being utilized by multiple research laboratories18–22. With more laboratories trying to employ the model, have come anecdotal reports of high mortality/morbidity rates and one published report23 of a 54% death rate. This current, retrospective study shows an approximately 8% death rate for both aneurysm creation procedures and for embolization procedures. Further, we noted that, as might be expected, mortality/morbidity rates are inversely related to the number of surgeries performed per operator. These current data suggest that the rabbit model can be applied in experienced hands with low mortality, but that a learning curve is present. These data are important in guiding researchers new to the technique regarding expected numbers of total subjects that will be needed to complete a desired sample size. The data may also inform researchers that their own mortality rates may be excessive, as noted previously, and that low rates are indeed attainable.
Multivariate analysis of variance showed that the only factor that correlated with both acute and chronic aneurysm creation deaths was what was termed unknown causes of death (19 of 36 acute creation deaths and eight of 33 chronic creation deaths). We suspect that most of these deaths were due to stroke. However, conclusive evidence for this diagnosis was not available, thus the unknown category. The reason why the aneurysm creation surgery may be more conducive to stroke can only be based upon conjecture. The surgery requires a complete block of flow to the brain in the right carotid artery. While the rabbit does have a Circle of Willis, it may be that there is some biological variability in the adequacy of the re-routed flow in some of the animals.
Acute and chronic embolization deaths accounted for 42% of the 121 total deaths. Multivariate analysis showed the factors making an impact on acute embolization deaths were unknown causes of death (nine of 30) and also device failure (ten of 30). Factors making a significant impact on chronic embolization deaths were failure to thrive (six of 13) and paralysis (three of 13). Interestingly, the average time from embolism creation until sacrifice in the failure to thrive group was significantly older (p= 0.0004) than the other animals that had embolization procedures. This indicates that the younger the animals are, the better chance they have to survive chronic embolization.
One study (Thiex et al.23) cites hemorrhagic tracheal necrosis as a leading cause of death (nine of 24 rabbits). In this study the authors found an arterial branch coming off the common carotid artery and providing blood to the trachea. When this branch was accommodated the authors had no further complications due to tracheal necrosis. While this issue was not a problem in this study, it does point out the need to be mindful of anatomic variations not only in the creation phase but through out any treatment phase as well.
The fact that the exact cause of death was not known in every case was one limitation in this study. The other limitation was the fact that while one operator enabled us to provide a unique examination of changes in death rates over time, it also means that we cannot say that every operator will have diminishing mortality rates over the same period of time.
Conclusion
Mortality rates of approximately 8% are associated with both experimental aneurysm creation and with embolization in the rabbit, elastase-induced aneurysm model. Increasing operator experience is inversely correlated with mortality and the age of the rabbit is positively associated with morbidity.
Supplementary Material
Acknowledgments
Funding in part came from NIH grants NS42646 and HL72247.
References
- 1.Reul J, Weis J, Spetzger U, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol. 1997;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Bocher-Schwarz H, Ringel K, Bohl J, et al. Histological findings in coil-packed experimental aneurysms 3 months after embolization. Neurosurgery. 2002;50:379–384. doi: 10.1097/00006123-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Ji C, Massoud T, et al. Experimental saccular aneurysms. Ii. A new model in swine. Neuroradiology. 1994;36:547–550. doi: 10.1007/BF00593518. [DOI] [PubMed] [Google Scholar]
- 4.Graves V, Partington C, Rufenacht D, et al. Treatment of carotid artery aneurysms with platinum coils: An experimental study in dogs. AJNR Am J Neuroradiol. 1990;11:249–252. [PMC free article] [PubMed] [Google Scholar]
- 5.Graves V, Strother C, Rappe A. Treatment of experimental canine carotid aneurysms with platinum coils. AJNR Am J Neuroradiol. 1993;14:787–793. [PMC free article] [PubMed] [Google Scholar]
- 6.Mawad M, Mawad J, Cartwright JJ, et al. Long-term histopathologic changes in canine aneurysms embolized with guglielmi detachable coils. AJNR Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Tenjin H, Fushiki S, Nakahara Y, et al. Effect of guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke. 1995;26:2075–2080. doi: 10.1161/01.str.26.11.2075. [DOI] [PubMed] [Google Scholar]
- 8.Kallmes D, Helm G, Hudson S, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology. 1999;213:217–222. doi: 10.1148/radiology.213.1.r99oc16217. [DOI] [PubMed] [Google Scholar]
- 9.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: A model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;174:349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara N, Cloft H, Marx W, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: Evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol. 2001;22:698–703. [PMC free article] [PubMed] [Google Scholar]
- 11.de Gast A, Altes T, Marx W, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: An animal study. Neurosurgery. 2001;49:690–694. doi: 10.1097/00006123-200109000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Marx W, Cloft H, Helm G, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: Coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol. 2001;22:323–333. [PMC free article] [PubMed] [Google Scholar]
- 13.Kallmes D, Fujiwara N. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 14.Kallmes D, Fujiwara N, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand white rabbit model. AJNR Am J Neuroradiol. 2003;24:591–596. [PMC free article] [PubMed] [Google Scholar]
- 15.Short J, Fujiwara N, Marx W, et al. Elastase-induced saccular aneurysms in rabbits: Comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol. 2001;22:1833–1837. [PMC free article] [PubMed] [Google Scholar]
- 16.Dai D, Ding Y, Danielson M, et al. Histopathologic and immunohistochemical comparison in human, rabbit, and swine aneurysms embolized with platinum coils. AJNR American Journal Neuroradiology. 2005;26:2560–2568. [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Danielson M, Kadirvel R, et al. Modified technique to create morphologically reproducible elastase-induced aneurysms in rabbits. Neuroradiology. 2006;48:528–532. doi: 10.1007/s00234-006-0093-0. [DOI] [PubMed] [Google Scholar]
- 18.Onizuka M, Miskolczi L, Gounis M, et al. Elastase-induced aneurysms in rabbits: Effect of postconstruction geometry on final size. AJNR Am J Neuroradiol. 2006;25:1129–1131. [PMC free article] [PubMed] [Google Scholar]
- 19.Krings T, Hans F, Moller-Hartmann W, et al. Treatment of experimentally induced aneurysms with stents. Neurosurgery. 2005;56:1347–1359. doi: 10.1227/01.neu.0000159887.03290.d1. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H, Kuroda S, Nanba R, et al. A novel coating biomaterial for intracranial aneurysms: Effects and safety in extra- and intracranial carotid artery. Neuropathology. 2005;25:66–76. doi: 10.1111/j.1440-1789.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 21.Hans F, Moller-Hartmann W, Brunn A, et al. Treatment of wide-necked aneurysms with balloon-expandable polyurethane-covered stentgrafts: Experience in an animal model. Acta Neurochir (Wein) 2005;147:871–876. doi: 10.1007/s00701-005-0503-9. [DOI] [PubMed] [Google Scholar]
- 22.Doerfler A, Becker W, Wanke I, et al. Multimodal imaging in the elastase-induced aneurysm model in rabbits: A comparative study using serial DSA, MRA and CTA. Rofo. 2004;176:590–596. doi: 10.1055/s-2004-812753. [DOI] [PubMed] [Google Scholar]
- 23.Thiex R, Hans F, Krings T, et al. Haemorrhagic tracheal necrosis as a lethal complication of an aneurysm model in rabbits via endoluminal incubation with elastase. Acta Neurochir (Wien) 2004;146:285–289. doi: 10.1007/s00701-003-0198-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.