Abstract
Adoptive T cell immunotherapy is a promising approach to cancer treatment that currently has limited clinical applications. DNA methyltransferase inhibitors (DNAMTi) have known potential to affect the immune system through multiple mechanisms that could enhance the cytotoxic T cell responses, including: upregulation of tumor antigen expression, increased MHC class I expression, and blunting of myeloid derived suppressor cells (MDSCs) expansion. In this study, we have investigated the effect of combining the DNAMTi, decitabine, with adoptive T cell immunotherapy in the murine 4T1 mammary carcinoma model. We found that expression of neu, MHC class I molecules, and several murine cancer testis antigens (CTA) was increased by decitabine treatment of 4T1 cells in vitro. Decitabine also increased expression of multiple CTA in two human breast cancer cell lines. Decitabine-treated 4T1 cells stimulated greater IFN-gamma release from tumor-sensitized lymphocytes, implying increased immunogenicity. Expansion of CD11b + Gr1 + MDSC in 4T1 tumor-bearing mice was significantly diminished by decitabine treatment. Decitabine treatment improved the efficacy of adoptive T cell immunotherapy in mice with established 4T1 tumors, with greater inhibition of tumor growth and an increased cure rate. Decitabine may have a role in combination with existing and emerging immunotherapies for breast cancer.
Keywords: T lymphocytes, Immunotherapy, Decitabine, Epigenetic, Myeloid derived suppressor cells
Introduction
Despite substantial improvement in breast cancer therapy over the past three decades, with an improvement in 5-year survival from 76 to 90 % [1], invasive disease continues to affect over 200,000 American women every year and 40,290 women were expected to succumb to breast cancer in the USA in 2015 alone [2]. Advances in adjuvant therapy have contributed to the improvements of the last 30 years, but therapies available for metastatic disease remain palliative.
Adoptive cellular immunotherapy (AIT), the expansion and infusion of tumor specific lymphocytes into tumor-bearing hosts, has been studied in human cancers for the last three decades [3, 4]. AIT using tumor infiltrating lymphocytes has been effective for melanoma, leading to objective responses in 50 % of patients in some clinical trials [5, 6]. Attempts to use this therapy against epithelial cancers, including breast cancer, have mostly been unsuccessful to date, with several barriers to success [7–10].
Success of immunotherapy depends, in part, on the immunogenicity of the tumor, a function of its production of tumor antigens recognized by the immune system [11]. Immunoediting, or the elimination through genetic or epigenetic changes of tumor antigen expression with a reduction in immunogenicity, is a normal process that cancer cells undergo in order to evade and escape the immune system [12]. Epigenetic alterations include excessive deacetylation of histones as well as excessive methylation of the genome, thereby silencing or inactivating numerous genes that are involved in tumor suppression, apoptosis, antigen presentation, and those that serve as tumor antigens in de-differentiated cells. Indeed, loss of Her-2/neu, under immune pressure, has been shown to be related to genome methylation [13]. In previous studies, MHC class I molecules have also been shown to be silenced through this mechanism, severely diminishing the immune response [14, 15]. The DNAMTi, 5-aza-2′-deoxycytidine (decitabine or Dec) is a global hypomethylating agent, and it has been suggested that through its liberation of silenced tumor antigens it may increase responses to immunotherapy [15–18].
Strategies utilizing DNA methyltransferase inhibition in combination with immunotherapy have been tested in clinical trials. Odunsi et al. [19] recently published their findings that a New York esophageal squamous cell carcinoma 1 (NY-ESO-1) targeting cancer vaccine combined with decitabine treatment resulted in enhanced immune responses in advanced platinum-resistant ovarian cancer. We have used a similar strategy utilizing the DNAMTi azacytidine (Aza) in combination with lenalidomide to increase CTA-specific immune responses to multiple myeloma in patients [20].
Tumors can also escape from immune attack by inducing immunosuppressive responses in the form of an increase in splenic and circulating myeloid derived suppressor cells (MDSCs, classically characterized as CD11b + Gr1 + cells of myeloid origin) and regulatory T cells [21–25]. Strategies that serve to overcome these barriers may increase the efficacy of immune therapies for breast cancer. Recruitment of MDSCs by tumors has also been shown to be altered by demethylating agents [18]. Based on these known effects of DNAMTi, we hypothesized that treatment of mice bearing weakly immunogenic 4T1 mammary tumors with Dec would increase expression of tumor antigens and antigen presenting molecules, increase immunogenicity, decrease MDSC burden, and increase the efficacy of AIT with T cells in 4T1 tumor-bearing syngeneic mice.
Methods
Mice
Balb/c mice (National Cancer Institute, Bethesda, MD) between 8 and 12 weeks of age, caged in groups of five, were used for all animal experiments. Transgenic (Tg) mice overexpressing a disintegrin and metalloproteinase 10 (ADAM10) in hematopoietic cells were kindly provided by Dr. Daniel Conrad at Virginia Commonwealth University [26]. All experimental protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee, which conforms to the American Association for Accreditation of Laboratory Animal Care and the U.S. Department of Agriculture recommendations for the care and ethical treatment of research animals.
Tumor cell lines
The 4T1 mammary tumor cell line used was provided by Dr. Jane Tsai of the Michigan Cancer Foundation, Detroit, Michigan. Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) with 10 % heat-inactivated fetal calf serum (Hyclone, Logan, UT), 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis MO) (modified DMEM). Tumor cells were harvested for inoculation of mice with 0.05 % trypsin–EDTA (Fisher, Pittsburgh, PA).
Preparation of tumor-sensitized lymphocytes
Donor mice were vaccinated in the left hind footpad with 0.5 × 106 viable 4T1 cells. Popliteal draining lymph nodes (DLN) were harvested under sterile conditions 10 days later and dispersed into a single cell suspension in complete RPMI medium at 1 × 106 cells/ml. The cells were activated by incubation with 5 nM bryostatin and 1 μM ionomycin (Calbiochem, San Diego, CA) (B/I), and 80 U/ml of recombinant interleukin-2 (IL-2, Chiron, Emeryville, CA) at 37 °C for 18 h, as we have described previously [27, 28]. After activation, cells were washed × 3 with warm complete RPMI, then brought back to 1 × 106 cells/ml with the addition of either 40 U/ml of rIL-2 or IL-7 and IL-15 (10 ng/ml each) (Peprotech, Rocky Hill, NJ). The cells were allowed to proliferate in culture for an additional 6 days and were split every 2–3 days to maintain 1 × 106 cells/ml concentration with new media and fresh cytokines added at each split.
Adoptive immunotherapy and DNAMTi treatment
To establish 4T1 flank tumors, 2.5 × 104 4T1 cells were injected in PBS suspension i.d. over the flank. On day 3 after tumor inoculation, mice received cyclophosphamide (CYP, Mead Johnson, Princeton, NJ), 100 mg/kg i.p. On post-tumor inoculation day 4, expanded DLN lymphocytes were washed twice in PBS, filtered through a 70-μm nylon mesh strainer (Invitrogen, Carlsbad, CA), and injected i.v. through the tail vein in 0.5 ml. Mice receiving Dec or Aza were injected i.p. with 15 μg in 0.3 ml daily for 4 doses from day 3 to day 6. Each group generally consisted of 5–7 mice for each experiment. For experiments to evaluate induction of MDSCs, mice received Dec or Aza daily, starting on day 10 or every other day starting on day 8.
Flow cytometry assessment of decitabine effect on 4T1 and on tumor-induced expansion of MDSCs
For assessment of decitabine's direct effects on 4T1 cells, tumor cells were cultured for 24 or 48 h without decitabine or with decitabine at concentrations from 0.75 to 3 μM and then stained with antibodies of interest. For experiments to assess tumor-induced MDSC expansion in vivo, tumor-bearing mice were either untreated or treated with decitabine 15 μg i.p daily on days 10–13; mice were euthanized on day 15. Blood was collected in prepared tubes with 100U heparin in 100 μl, and spleens and liver tissue were macerated and strained with a 40-μm filter then stained with antibodies of interest. Tumors were harvested, minced in RPMI and then digested at 37 °C in collagenase (1 mg/ml, Sigma-Aldrich, St. Louis, MO) for 30 min. The cell suspension was then filtered through a 70 micron strainer, rinsing through with 2 % FBS in PBS and washed twice with 2 % FBS in PBS before staining for flow cytometry. Analysis was performed by multicolor flow cytometry for surface marker expression on a Canto Beckman Coulter (Brea, CA) flow cytometer. Fluorescent labeled Abs directed against the following markers were obtained from Biolegend (San Diego, CA): MHC class I (H-2KD) (PE conjugated, clone SF 1-1.1), CD11b (PE conjugated, clone M1/70), Gr-1 (FITC conjugated, clone RB6-8C5), Ly6C (APC conjugated, clone HK1.4), Ly6G (PE conjugated, clone 1A8). Ab against Neu (Anti-mErbB2, PE conjugated, clone 666521) was obtained from R&D Systems (Minneapolis, MN). Fc blocking antibody (CD16/32, Biolegend) was used to prevent non-specific staining. Fluorescence of unstained cells and cells incubated with appropriately labeled isotype control antibodies were analyzed as negative controls, and gates were set based on unstained cells.
Cytokine release assays
Interferon-gamma (IFN-γ) release in supernatants from tumor-sensitized and B/I-activated and expanded lymphocytes in response to stimulation with irradiated 4T1 cells was assayed using BD OptEIA mouse IFN-γ ELISA sets from BD Biosciences (San Jose, CA). Stimulation of T cells with syngeneic MethA sarcoma cells was used as a control for antigen specificity. Prior to being used to stimulate T cells in these assays, tumor cells, with or without previous culture in Dec, for 24 h, were irradiated in a Gammacell 40 Exactor (MDS Nordion) with 14,000 rads.
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)
Investigation of the induction of murine CTAs was performed using 4T1 cells which were cultured for 48 h with concentrations from 0 to 3 μM decitabine, while the induction of human CTAs was done using MCF-7 and Skbr-3 cell lines in the presence of 0 or 3 μM decitabine for 72 h. RNA was then extracted from tumor cells using TRIzol reagent (Invitrogen), according to the manufacturer's protocol. Complementary DNA (cDNA) was prepared as previously described [29]. qRT-PCR was assayed by SensiMix™ SYBR® & Fluorescein Kit (BIOLINE) using a CFX96™ Real-Time PCR Detection System. Murine and human primers (Table 1) were designed using NCBI/Primer-BLAST. To normalize for the amount of source RNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts from the same samples were measured and used as internal reference. Transcript expression was validated using the comparative Ct method for relative quantification (ΔΔCt) referenced to the amount of a common reference gene (GAPDH), as previously described [30].
Table 1.
List of primers used for RT-pCR assays of murine and human cancer testis antigens
| Sense or antisense strand | Gene | Size (base pairs) | |
|---|---|---|---|
| Murine primers 5′–3′ | |||
| ACTGGAACCATCCGCACCAGC | Sense | Sperm autoantigenic protein 17 (spa 17) | 448 |
| CACGTGTCCCCGGAAGAGGGA | Antisense | ||
| CCGCGCTCCGGTCATGGAAT | Sense | Extraembryonic spermatogenesis homeobox 1 (Esx1) | 392 |
| CCCCCAGCTGAGCGTTGGAC | Antisense | ||
| GCCCTGGTAGGCAGTGGCTC | Sense | A-kinase anchor protein 4 (akap4) | 329 |
| GCCATGTTGCCCACGGCTTC | Antisense | ||
| GTAGTCACCATGCCCAGGGGT | Sense | Melanoma antigen, family B, 5 (mageb5) | 269 |
| CCACAAACAGTGGCAGGCGA | Antisense | ||
| AAGGCAGTGCTCGGAGCCAA | Sense | Melanoma antigen, family A, 4 (magea4) | 194 |
| AGCTTCCTCAGATGGGCCTTCA | Antisense | ||
| GAGCGGTGGGAACAGAAGGCG | Sense | Reproductive homeobox 5 (rhox5) | 203 |
| GAGGGGCATCTGCCTACCCCC | Antisense | ||
| ACCACAGTCCATGCCATCAC | Sense | Glyceraldehyde-3-phosphate dehydrogenase (gapdh) | 452 |
| TCCACCACCCTGTTGCTGTA | Antisense | ||
| Human primers 5′–3′ | |||
| CTGAGGGGCCTGCCCAGTCT | Sense | Melanoma antigen family C, 1 (mage-C1) | 346 |
| CGCGCCAACTCGTCCACCTT | Antisense | ||
| CATGCCCAAGGCAGGCCTCC | Sense | Melanoma antigen family A, 3 (mage-A3) | 343 |
| CAAAACCCACTCATGCAGGGGT | Antisense | ||
| GAGCAGACAGGCCAACCG | Sense | Melanoma antigen family A, 4 (mage-A4) | 446 |
| AAGGACTCTGCGTCAGGC | Antisense | ||
| AGATCAGCAGAGGGGAATGGGCC | Sense | Melanoma antigen family A, 5 (mage-A5) | 97 |
| TGGGGATCCTCTGGAGCTTCCTGGT | Antisense | ||
| TCGGTGAGGAGGCAAGTCCTG | Sense | Melanoma antigen family A, 6 (mage-A6) | 204 |
| CTGGTCAGGGCAACAGGCGG | Antisense | ||
| CAGGGCTGAATGGATGCTGCAGA | Sense | Cancer/testis antigen 1A/B (ny-eso-1) | 332 |
| GCGCCTCTGCCCTGAGGGAGG | Antisense | ||
| TGGGATCATGTTGTTGGCCCTGG | Sense | Sperm acrosome associated 3 (sllp1) | 187 |
| AGCAGCTGCGTTGAAACCGC | Antisense | ||
| TGAAGGGCTGACACGCGAGA | Sense | Sperm surface protein Sp17 (sp17) | 322 |
| TCCCCGGAAGGCAGCTTGGAT | Antisense | ||
| GCAGTCAAGGCTGTAGGAGGGC | Sense | A-kinase anchoring protein 4 (akap4) | 190 |
| TGCACACACCCCTGTGGCTG | Antisense | ||
| ATTGCCCTCAACGACCACTTTG | Sense | Glyceraldehyde-3-phosphate dehydrogenase (gapdh) | 264 |
| TTGATGGTACATGACAAGGTGCGG | Antisense |
Myeloid cell isolation and T cell suppression assays
Spleens from ADAM10Tg or 4T1 tumor-bearing BALB/c mice were isolated, crushed between two frosted microscope slides, and filtered through a 40 μm cell strainer. Erythrocytes were lysed using ACK Lysis buffer (Quality Biological Inc., Gaithersburg, MD), T cells were depleted using magnetic bead depletion with anti-CD90.2 antibody and Gr1+ cells were enriched by anti-Gr1 magnetic bead selection, both according to the manufacturer's instructions (Miltenyi Biotec, Gergisch, Gladbach Germany). Purity was assessed by flow cytometry using fluorochrome labeled antibodies (PE anti-CD11b (clone M1/70) and FITC anti-Gr1 (clone FB6-8C5), Biolegend, San Diego, CA) following isolation and were confirmed to be >90 % CD11b + Gr1 +. The putative ADAM10 Tg MDSCs were cultured for 1 week with 0, 2.5, 5, or 10 μM decitabine in complete RPMI (cRPMI, RPMI 1640 containing 10 % heat-inactivated fetal bovine serum (Atlanta Biological Inc., Norcross, GA), 2 mM l-glutamine, 50 μg/ml penicillin, 50 μg/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 1× non-essential amino acids, 20 mM HEPES buffer (all from Invitrogen, San Diego, CA), and GM-CSF (10 ng/ml, Peprotech, Rocky Hill, NJ) with media changes on days 3 and 5. For the T cell suppression assays, T cells were isolated by anti-CD90.2 magnetic bead selection (Miltenyi Biotec) from wild-type C57Bl/6 or BALB/c spleens and labeled with Track-It Violet proliferation dye (Biolegend). Putative MDSCs were enumerated using trypan blue exclusion, and viable cells were incubated with T cells at indicated ratios in cRPMI with anti-CD28 (2 μg/ml, clone 37.5, Biolegend) on plates coated with anti-CD3ε (1 μg/ml, clone 145-2C11, Biolegend). After 96 h, cells were Fc blocked with unlabeled anti-CD16/32 (clone 2.4G2) and then stained with PE anti-CD3ε (Biolegend). Cells were analyzed on a BD LSR-Fortessa-X20 and percent divided was assessed by dilution of proliferation dye using FlowJo software (version 7.6, TreeStar, Ashland, OR).
Results
Decitabine treatment increased expression of Her-2, CTA and MHC class I in vitro
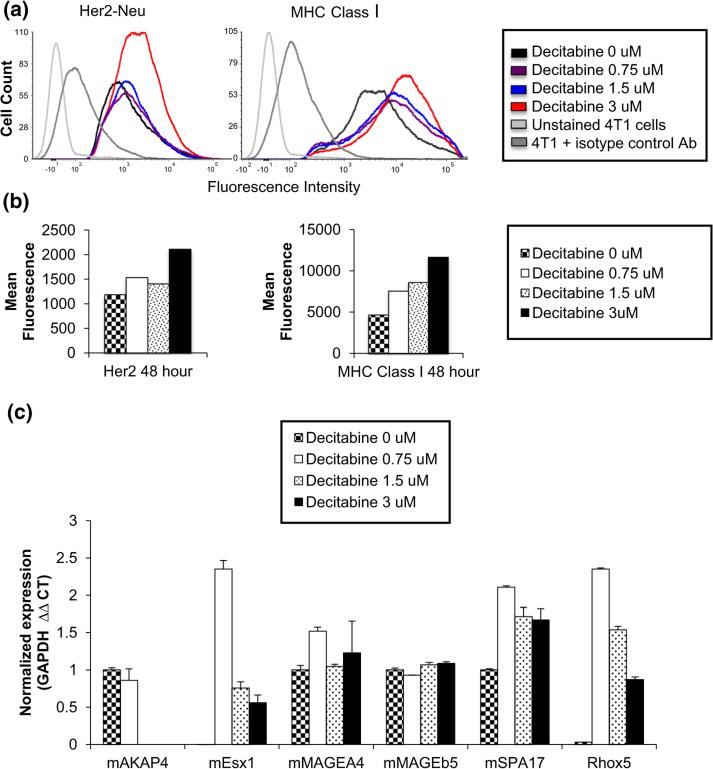
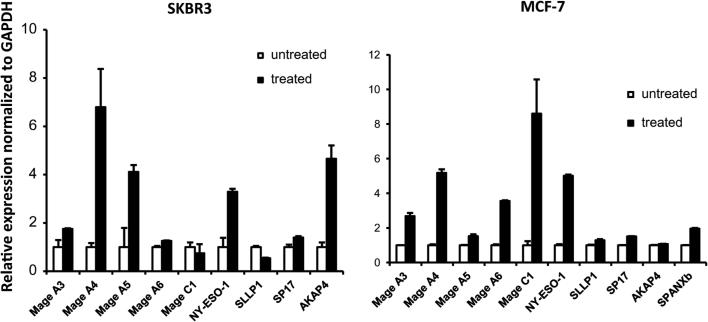
In order to determine whether Dec induces increased expression of 4T1 tumor antigens, 4T1 cells were cultured with varying concentrations (0, 0.75, 1.5, and 3.0 μM) of decitabine for 24 and 48 h. We had shown previously, in a HER-2 transgenic murine mammary carcinoma model, that HER-2 antigen-negative variants emerged in vivo as a result of immunoediting and that the mechanism of suppressed HER-2 gene expression was gene methylation [13]. Moreover, HER-2 gene expression was restored by treating those cells with Dec. Therefore, we hypothesized that Dec treatment of 4T1 cells might raise the level of HER-2 expression on the cell surface of 4T1 cells. Cells were collected at the relevant time points and stained with anti-neu antibody labeled with phycoerythrin (PE). Mean fluorescence intensity (MFI) was calculated. 4T1 cells cultured with 3 μM decitabine had higher MFI after 48 h of culture (2109 vs. 1186) than cells cultured without decitabine (Fig. 1a, b). 4T1 cells cultured as above were also stained with anti-MHC class I antibody labeled with PE. Mean fluorescence intensity was increased by 161, 184, and 250 % compared to control cells by culture with 0.75, 1.5, and 3.0 μM decitabine, respectively (Fig. 1a, b). Dec treatment of 4T1 cells in vitro did slow cell proliferation, but did not result in cytotoxicity except at high concentrations (Supplemental Figure S1). Similar results were obtained in four separate experiments. When 4T1 cells were incubated with equally growth inhibitory concentrations of cytosine arabinoside (CA), a non-DNAMTi nucleoside analog, MHC class I expression did not increase compared to controls (MFI/% positive cells = 10,393/54 % for controls, 17,569/95 % for 3 μM Dec and 10,790/67 % for 0.187 μM CA). In a separate experiment, expression of a panel of CTAs was assayed using CTA mRNA expression measured by qRT-PCR. Expression was found to be increased for 4 out of the 6 CTAs in our panel; specifically, expression of murine extraembryonic spermatogenesis homeobox 1 (mEsx1), murine sperm autoantigenic protein 17 (mSpA17), reproductive homeobox 5 (Rhox5), and murine melanoma-associated antigen A4 (mMAGEA4) was found to increase as a result of Dec treatment (Fig. 1c). We also confirmed upregulation of human CTAs in two human breast cancer cell lines, as shown in Fig. 2.
Fig. 1.
Decitabine treatment increased expression of tumor antigens and antigen presentation molecules in vitro. a Expression of Her-2-neu and MHC class I molecules on untreated and decitabine-treated 4T1 cells was determined by flow cytometry after staining with PE-labeled probes. b Mean fluorescence intensity (geometric mean) was significantly increased after 48 h in decitabine for cells stained with both anti-Her2-neu or anti-MHC class I. c mRNA was purified from untreated 4T1 cells and 4T1 cultured with decitabine, and qPCR was performed to quantify expression of a panel of cancer testis antigens. Expression of 4 out of the 6 CTAs tested was increased by decitabine treatment. See Table 1 for full names of all CTAs
Fig. 2.
RT-PCR assay of human cancer testis antigens in two breast cancer cell lines. Expression was normalized to internal GAPDH expression. Cells were treated with 3 μM decitabine, or left untreated. After 3 days of treatment, RNA was isolated and cDNA was prepared. Error bars represent standard error of the means (SEM). Results shown are representative of 4 independent experiments. See Table 1 for full names of all CTAs
Decitabine treatment of 4T1 cells increased stimulation of interferon-gamma release from tumor-sensitized lymphocytes
In order to assess the effects of Dec on the immunogenicity of 4T1 cells, tumor reactive lymphocytes were produced as described above with ex vivo expansion using IL-2 and co-cultured with irradiated 4T1 cells that had previously been incubated with various concentrations of decitabine. After 24 h of tumor cell-T cell co-culture, supernatants were harvested and assayed for interferon-gamma levels using ELISA. IFN-γ release was significantly higher from cells cultured with 1.5 or 3.0 μM decitabine (Fig. 3a). Co-culture of 4T1-sensitized T cells with the MethA sarcoma cells resulted in little or no IFN-γ release, regardless of whether those cells were treated with decitabine or not (Fig. 3a). When DLN lymphocytes were expanded in IL-7 + IL-15 instead of IL-2, the effect of decitabine treatment on IFN-γ release was similar (Fig. 3b). These experiments were repeated 3 times to validate results.
Fig. 3.
Decitabine treatment of 4T1 cells increased the release of interferon gamma from tumor-sensitized lymphocytes. a ELISA IFN-γ release assay. IL-2 treated lymphocytes exposed to irradiated 4T1 cells cultured in various levels of decitabine have increased IFN-γ release compared to 4T1 cultured without decitabine and to the MethA specificity control [±standard deviation (SD)]. b IL-7/IL-15 treated lymphocytes exposed to irradiated 4T1 cells cultured in various concentrations of decitabine release increased IFN-γ release compared to 4T1 cells not exposed to decitabine and to the MethA specificity control. *p < 0.05
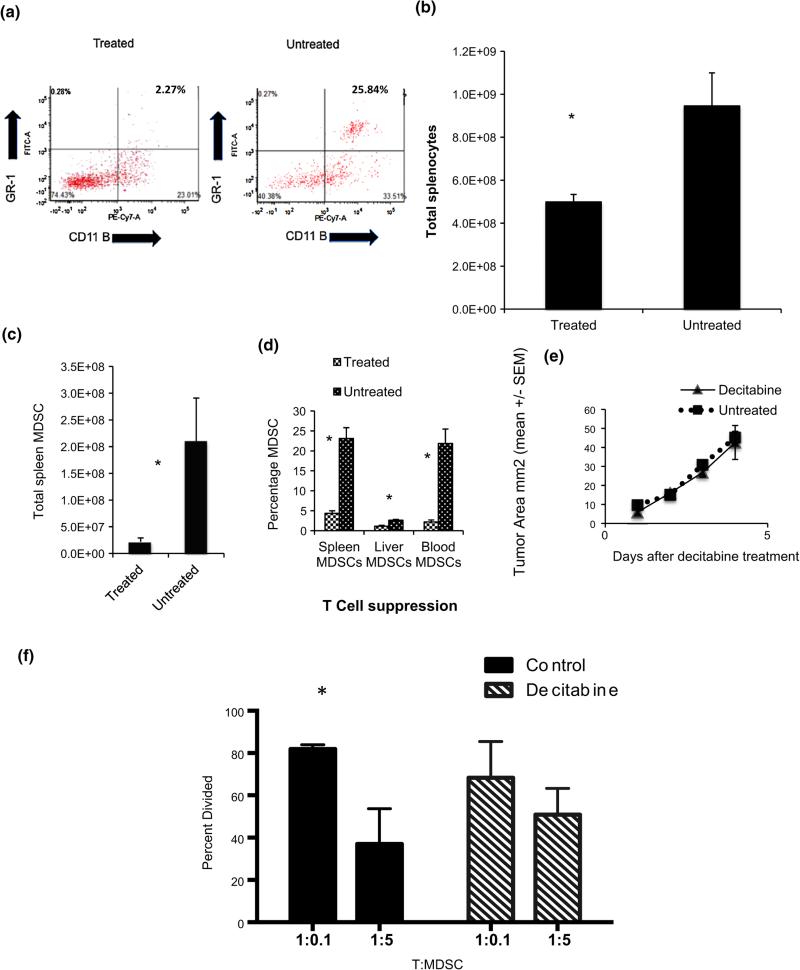
Decitabine treatment resulted in decreased splenic and circulating CD11b + Gr1 + cells, without a concomitant reduction in tumor burden
The 4T1 model is known to induce significant MDSC accumulation that inhibits anti-tumor immune function [24, 25, 31]. Balb/c mice (n = 5/group) underwent flank tumor injection of 50,000 4T1 cells, and tumor growth was permitted for 10 days. Starting on day 10, the treatment group received i.p. injections of 15 μg decitabine daily for 4 days. Mice were euthanized on day 15, and spleen, liver, and blood were harvested and processed into single cell suspensions. Staining for Gr1 and CD11b was performed, and cells were analyzed with flow cytometry (Fig. 4a). The proportion of CD11b + Gr1 + cells was markedly reduced in the Dec-treated mice, and total splenocyte counts were also significantly decreased in Dec-treated tumor-bearing mice compared to untreated tumor-bearing mice (4.9 × 108 vs. 9.5 × 108, p = 0.04) (Fig. 4b). Thus, total spleen CD11b + Gr1 + cell counts were significantly lower in 4T1 tumor-bearing mice treated with decitabine compared to untreated tumor-bearing mice (22 × 106 vs. 21 × 107, p = 0.006) (Fig. 4c). Similar results were observed in 5 repeat experiments of this type. CD11b + Gr1 + cell percentages were also significantly different between treated and untreated mice in spleen, liver, and blood (Fig. 4d). Decitabine treatment also reduced the proportions of CD11b + Gr1 + cells in the tumors themselves (8.8 % in control tumors vs. 1.7 % in tumors from decitabine-treated mice in one experiment and 26.6 vs. 9.8 %, respectively, in another). Importantly, tumor size was not altered between treated and untreated mice in this experiment (Fig. 4e), indicating that the reduction in CD11b + Gr1 + cells was likely a direct effect of decitabine rather than a secondary effect of reducing tumor burden. Flow cytometric analysis for CD11b plus Ly6C and Ly6G (markers for monocytic and granulocytic cells, respectively) demonstrated that decitabine treatment of 4T1 tumor-bearing mice (3 in each group) decreased both populations in the spleen (6.7 ± 0.72 vs. 2.4 ± 0.4 % for Ly6C and 6.9 ± 0.92 vs. 1.2 ± 0.23 % for Ly6G) and the tumors (15.8 ± 4.4 vs. 7.3 ± 4.3 % for Ly6C and 10.8 ± 3.2 vs. 2.5 ± 1.6 % for Ly6G). Gr1 + cells sorted from the spleens of control 4T1 tumor-bearing mice expressed high levels of arginase mRNA, normalized to GAPDH (Supplemental Figure S2). Interestingly, when the same number of Gr1 + cells from Dec-treated tumor-bearing mice was tested, the level of arginase mRNA was barely detectable. We showed previously that in vitro depletion of Gr1 + cells from the spleens of 4T1 tumor-bearing mice increased the proliferative response of the remaining lymphocytes to B/I and that adding back similar numbers of Gr1 + cells enriched from the spleens of these mice profoundly inhibited proliferation of lymphocytes [25]. Similarly, Gr1 + cells enriched from the spleens of 4T1 tumor-bearing mice suppressed proliferation of normal splenic T lymphocytes stimulated with anti-CD3 + anti-CD28 (Fig. 4f). Again, the number of splenic CD11b + Gr1 + cells was markedly reduced in the spleens of tumor-bearing mice treated with decitabine (means of 34.8 vs. 18.5 % per spleen in this experiment), and when the remaining Gr1 + cells were enriched from these spleens, the Gr1 + cells from the decitabine group did not significantly suppress T cell proliferation. Moreover, we found that in vitro Dec treatment of CD11b + Gr1 + cells sorted from the spleens of ADAM10 transgenic mice, which have very high levels of these cells, and which we have shown to behave similarly to tumor-induced MDSCs [32], abrogated their ability to suppress proliferation of T lymphocytes (Supplemental Figure S3). We have also tested a different DNAMTi, azacytidine (Aza) for its effect on CD11b + Gr1 + cells in vivo, and although it did inhibit expansion of CD11b + Gr1 + cells in 4T1 tumor-bearing mice, it was consistently less effective than Dec in two separate experiments. (Representative results of one such experiment are shown in Supplemental Table S1).
Fig. 4.
Decitabine treatment resulted in decreased splenic and circulating CD11b + Gr1 + cells, without a concomitant reduction in tumor burden. Balb/c mice were injected with 5 × 104 4T1 cells s.c. into the right flank. On day 10 after tumor inoculation, treatment began with decitabine 15 μg i.p. × 4 days or no treatment. Mice were killed on day 15. a Spleen, liver, blood, and tumor were harvested and sorted for Gr-1 and CD11b expression by flow cytometry. b Total splenocytes differed greatly between the two groups, c as did total spleen CD11b + Gr1 + cell numbers. d Percentage of cells with co-expression of Gr-1 and CD11b was significantly different between the two groups in liver, spleen, and blood. e Decitabine alone had no statistically significant effect on tumor burden (n = 5/group). f Suppression of T cell proliferation by Gr1 + cells from spleens of 4T1 tumor-bearing mice; mice were either untreated or treated with decitabine (3 mice per group). 1 × 105 Tag-It Violet labeled CD90.2 + T cells were co-cultured with MDSCs at indicated ratios with anti-CD3/28 stimulation. Proliferation was assessed by flow cytometry after 96 h of culture. For all graphs, error bars are ±SEM; *p < 0.05
Adoptive immunotherapy in combination with decitabine resulted in greater efficacy than either therapy alone
We next tested whether combining Dec with AIT in the 4T1 breast cancer model would enhance the efficacy of AIT when conditions for the effect of AIT alone were suboptimal. Animals were injected with 50,000 4T1 cells in the right flank and cyclophosphamide (100 mg/kg i.p.) was administered on day 3, as previously described [33]. Mice began treatment with decitabine on day 3 and underwent AIT with B/I-activated and expanded T lymphocytes via the tail vein on day 4 (n = 5–7/group). In an experiment using AIT with 20 million cells expanded with IL-2, a regimen established as suboptimal by our previous experiments [28], addition of Dec to AIT resulted in statistically significantly greater impact on tumor size than CYP + AIT alone (Fig. 5a, b). A Kaplan–Meier curve of survival to humane endpoint also demonstrated a substantial improvement for the AIT + Dec combination group compared to AIT alone (Supplemental Figure S4) (representative experiment shown). This was also notable for an increase in “cured” mice (defined as no detectable tumors at 52 days). Repeated experiments using IL-7 + IL-15 expanded lymphocytes resulted in similar findings, with the Dec + AIT combination groups having smaller tumors (Fig. 5c). These sets of experiments were repeated 10 times with varying numbers of adoptively transferred cells expanded in either IL-2 or IL-7/15, with similar results in every experiment. In four of these experiments, we have also compared Dec with Aza, as shown in Fig. 5d and Supplemental Figure S5. In contrast to the marked effect of adding Dec, Aza was consistently less effective at augmenting AIT, using T cells grown in either IL-2 or IL-7/15.
Fig. 5.
Adoptive immunotherapy in combination with decitabine resulted in a higher cure rate and greater efficacy than AIT alone. Tumor diameters ± SEM are shown in plots versus days after tumor inoculation. Numbers at end of each plot are the number of mice whose tumors regressed completely over the total # of mice in each group. a, b Decitabine (15 micrograms per mouse i.p. on days 3–6) added to AIT + cyclophosphamide (CYP), with IL-2 expanded DLN cells (2 × 107 cells/mouse) resulted in a significant decrease in 4T1 tumor size compared to AIT + CYP alone (70.1 ± 19.8 vs. 6.1 ± 4.3 mm2 on day 24 and 72.66 ± 19.5 vs. 8.66 ± 6.3 on day 27 for experiment shown in a and 27.7 ± 4.1 vs. 0 ± 0 on day 24 and 42.9 ± 9.1 mm2 vs. 0 ± 0 mm2 on day 28 for b). c Augmentation of AIT effect by adding Dec to AIT with 2 × 107 lymphocytes expanded in IL-7 + IL-15. Tumor sizes for AIT + CYP alone were 21.6 ± 8.1 mm2 versus 2.2 ± 2.2 for AIT + CYP + Dec at day 27 and 41.6 ± 16.3 versus 0 ± 0 at day 38. Similar results were seen in a total of 10 separate experiments, including 4 experiments comparing Decitabine and azacytidine, as shown in d. For the experiment shown in d, mice were inoculated with 50,000 4T1 cells and AIT consisted of 2 × 107 lymphocytes expanded in IL-2 for 7 days. At day 27, mean tumor sizes were 29.9 ± 15.7 mm2 for CYP/AIT, 1.4 ± 0.9 for CYP/AIT + DEC and 16.3 ± 8.1 for CYP/AIT + AZA. At day 31, sizes were 31.7 ± 16.7, 2.5 ± 1.9 and 17.1 ± 8.1, respectively
Discussion
Epigenetic modulation has the potential to alter antigen expression and presentation to T cells, as well as the immunosuppression induced by tumors, thus improving the efficacy of immunotherapy. We have previously demonstrated that immunoediting can suppress expression of HER-2/neu in a transgenic murine mammary carcinoma and that this occurs by methylation of the gene. Moreover, treatment of the HER-2/neu negative cells that emerged under immune pressure with Dec increased expression of Her-2/Neu [13, 34]. We found that treatment of 4T1 cells with Dec at the highest dose tested moderately increased neu expression, suggesting that methylation may account for low neu expression in these cells. Cancer testis antigens (CTA) have also been shown to be increased by hypomethylation and have been shown to increase the efficacy of CTA vaccines in eliciting an immune response [15, 16, 19, 35]. We found that Dec increased mRNA expression for several, but not all, murine CTA in 4T1 cells and human CTA in two human breast cancer cell lines. However, we cannot state with certainty the baseline levels of messages on which these increases are based, although these were normalized to GAPDH message. Most importantly, decitabine treatment of 4T1 cells in vitro increased their ability to stimulate tumor-sensitized T cells to secrete IFN-γ, in an antigen-specific manner.
Defects in antigen presentation pathways are a well-known phenomenon in cancer, with lower expression of MHC class I occurring frequently as a result of immunoediting processes [36]. Other authors have demonstrated increases in MHC class I expression in human sarcoma cell lines using flow cytometry based methods as a result of treatment with Dec [15]. Using flow cytometry, we showed that Dec treatment of 4T1 breast cancer cells increased MHC class I expression, based on assessment of MFI. Though our results and that of other authors are clear that MHC class I cell surface expression is increased by Dec therapy, the mechanisms underlying this increase are not clear at present. In contrast, a recent study by Kim et al. [37], using azacytidine, did not demonstrate increased MHC class I expression by quantitative polymerase chain reaction (qPCR) in 4T1 cells as a result of epigenetic therapy. The disparity between our results and those with Aza may result from different methods of analysis. The increased MHC class I cell surface expression we observed here could be the result of post-transcriptional events rather than increased mRNA levels or may reflect post-translational modification and transport of MHC class I molecules to the cell membrane. For example, DNAMTi may increase MHC surface expression by increasing beta-2 microglobulin levels or peptide processing by TAP transporters, both of which are required for MHC class I molecules to reach the cell surface [38, 39]. Kim et al. did not see any increases in mRNA expression for these genes, but did not measure intracellular or cell surface proteins. Moreover, Aza, as opposed to Dec, is also known to cause an overall blunting of protein expression from its ability to intercalate into RNA as well as in DNA, which also may prevent increased MHC mRNA and protein levels [39]. In future experiments, we will explore whether DNAMTi treatment also increases expression of other molecules important for immune recognition, such as CD80, CD86, adhesion molecules such as ICAM-1, and we will also examine 4T1 tumors after treatment of tumor-bearing mice in vivo for antigen expression.
The relationship of MDSC proliferation to methylation of the genome is complex, with multiple studies demonstrating a profound effect on myeloid cell differentiation [18, 40], but the genes activated or suppressed by decitabine that lead to this response are still not clearly understood. 4T1 breast cancer cells are known to promote a significant immunosuppressive MDSC response in host mice [24, 25, 31]. In the previously mentioned report from Kim et al. [37], reduction in MDSCs and potent anti-tumor effects against 4T1 were only observed when epigenetic modulators were combined with dual checkpoint inhibition. In their in vivo studies, in contrast to the results shown here, they saw no significant effect on MDSC levels from treatment of 4T1-bearing mice with Aza alone or with Aza + entinostat [37]. To test the hypothesis that these somewhat contradictory results may have been related to using different compounds, regardless of their similarity, we have now compared Dec and Aza “head-to-head,” and we found that Aza did suppress CD11b + Gr1 + cells in 4T1 tumor-bearing mice, but not as effectively as Dec. The difference between our results with single agent DNAMTi treatment in 4T1-bearing mice and those of Kim et al. are difficult to explain, but may relate to the differing metabolism and intracellular effects of these two drugs.
We consistently observed that the addition of Dec to AIT which was carried out in a “suboptimal” fashion, dramatically increased the efficacy of the treatment, with complete regression of tumors in the vast majority of treated mice, regardless of whether the T cells used for AIT were grown in IL-2 or IL7/15. Somewhat surprisingly, Aza did not consistently or significantly augment AIT efficacy, which was consistently observed with Dec in our model. Whether this difference in efficacy between Dec and Aza when combined with AIT results entirely from the differences we observed in inhibition of MDSC expansion or reflects other differences in the effects of these two drugs is not clear.
Dec has been shown previously to have negative effects on anti-tumor immunity and the cellular immune response to cancer, with some authors showing a decrease in Th1-associated anti-tumor effects [18]. Controversially, some authors have also demonstrated a simultaneous increase in T regulatory cells after Dec treatment, though this has not been a consistent finding [18]. By activating and expanding T cells ex vivo using a protocol that we have shown to preferentially expand central memory T cells [28], we may have avoided the potential negative effects that decitabine might have had on host immunity. Additionally, the use of CYP prior to AIT, which is now often used in clinical applications of cellular therapy [41] has a lymphodepleting effect and its selective effect on T regulatory cells may have augmented the effects of Dec with AIT in this model [42, 43].
The impressive and consistent combination effect seen with CYP, AIT, and decitabine represents a favorable impact on immunological checks and balances. In our studies, Dec treatment of 4T1 cells in vitro modestly increased neu expression on tumor cell membranes and CTA mRNA expression, as well as expression of cell surface MHC class I molecules, and, most significantly, by whatever mechanism, Dec-treated tumor cells were able to elicit a higher level of IFN-gamma release from activated cytotoxic T cells, implying an increase in immunogenicity. In addition, Dec treatment of 4T1 tumor-bearing mice decreased systemic levels of CD11b + Gr1 + cells (both Ly6C and Ly6G subsets) induced by 4T1 tumor progression. Similar findings on anti-MDSC effects were recently identified as the major factor in the ability of a combination of DNAMTi, HDAC inhibition and immune checkpoint inhibition to cure advanced 4T1 tumors, although as mentioned above, Aza alone did not modulate MDSC levels in those studies [37]. Interestingly, we found that CD11b + Gr1 + cells from decitabine-treated mice had markedly decreased levels of arginase mRNA, and when Gr1 + cells were enriched from the spleens of these mice, these cells did not significantly suppress T cell proliferation. Moreover, in vitro decitabine treatment of CD11b + Gr1 + cells from spleens of ADAM10 transgenic mice inhibited their ability to suppress T cell proliferation. Future experiments are planned to delineate the role of the various proposed mechanisms by which MDSC (from both tumor-bearing and transgenic mice) inhibit T cells and to determine which, other than their numbers, are most affected by decitabine.
Future trials of immunotherapy in breast cancer may benefit from combination approaches such as were demonstrated in this study. Combining therapies that promote anti-cancer immune responses with treatments that may help to overcome tumor-induced immunosuppression may improve the efficacy of immunotherapy for breast cancer and other epithelial malignancies.
Supplementary Material
Acknowledgments
The Flow Cytometry Shared Resource Core was supported in part by Grant P30CA16059 from the National Institutes of Health to the Massey Cancer Center. Dr. Terracina was supported by a training grant from the National Institutes of Health (T32CA085159). Ms. Damle was supported by a fellowship grant from the National Cancer Institute (F30CA192836-01A1)
Abbreviations
- ADAM10
A disintegrin and metalloproteinase 10
- AIT
Adoptive immunotherapy
- Aza
Azacytidine
- CTA
Cancer testis antigen
- CYP
Cyclophosphamide
- Dec
Decitabine
- DLN
Draining lymph nodes
- DNAMTi
DNA methyl transferase inhibitor/inhibition
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- mEsx1
Murine extraembryonic spermatogenesis homeobox 1
- mMAGEA4
Murine melanoma-associated antigen A4
- mSpA17
Murine sperm autoantigenic protein 17
- NY-ESO-1
New York esophageal squamous cell carcinoma 1
- qPCR
Quantitative polymerase chain reaction
- qRT-PCR
Quantitative reverse-transcriptase polymerase chain reaction
- Rhox5
Reproductive homeobox 5
- SD
Standard deviation
- SEM
Standard error of the mean
- Tg
Transgenic
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00262-016-1868-8) contains supplementary material, which is available to authorized users.
The data shown here were presented, in part, at the Society of Surgical Oncology Annual Cancer Symposium, Houston, TX USA, March, 2015. Published online in Ann. Surgical Oncol. 22 (Suppl. 1) P78, S69, 2015.
Compliance with ethical standards
Conflicts of interest None of the authors have any relevant conflicts of interest to disclose.
References
- 1.Howlader N, Noone A, Krapcho M. SEER cancer statistics review, 1975–2010. National Cancer Institute. National Cancer Institute; Bethesda: 2013. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. doi:10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. doi:10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. doi:10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 5.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. doi:10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 6.Itzhaki O, Hovav E, Ziporen Y. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 7.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Investig. 2002;110:1415–1417. doi: 10.1172/JCI17214. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne KK, Zoon CK, Wan W, Marlar K, Keim RC, Kenari MN, et al. Peripheral blood mononuclear cells of patients with breast cancer can be reprogrammed to enhance anti-HER-2/neu reactivity and overcome myeloid-derived suppressor cells. Breast Cancer Res Treat. 2013;142:45–57. doi: 10.1007/s10549-013-2733-5. doi:10.1007/s10549-013-2733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. doi:10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domschke C, Ge Y, Bernhardt I, Schott S, Keim S, Juenger S, et al. Long-term survival after adoptive bone marrow T cell therapy of advanced metastasized breast cancer: follow-up analysis of a clinical pilot trial. Cancer Immunol Immunother. 2013;62:1053–1060. doi: 10.1007/s00262-013-1414-x. doi:10.1007/s00262-013-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. doi:10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immuno-surveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 13.Kmieciak M, Knutson K, Dumur C, Manjili M. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–685. doi: 10.1002/eji.200636639. doi:10.1002/eji.200636639.HER-2/neu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Símová J, Polláková V, Indrová M, Mikyšková R, Bieblová J, Stěpánek I, et al. Immunotherapy augments the effect of 5-azacytidine on HPV16-associated tumours with different MHC class I-expression status. Br J Cancer. 2011;105:1533–1541. doi: 10.1038/bjc.2011.428. doi:10.1038/bjc.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnadas DK, Bao L, Bai F, Chencheri SC, Lucas K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol. 2014;35:5753–5762. doi: 10.1007/s13277-014-1764-9. doi:10.1007/s13277-014-1764-9. [DOI] [PubMed] [Google Scholar]
- 16.Cruz CR, Gerdemann U, Leen AM, Shafer JA, Ku S, Tzou B, et al. Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGEA4. Clin Cancer Res. 2011;17:7058–7066. doi: 10.1158/1078-0432.CCR-11-1873. doi:10.1158/1078-0432.CCR-11-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–120. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triozzi PL, Aldrich W, Achberger S, Ponnazhagan S, Alcazar O, Saunthararajah Y. Differential effects of low-dose decitabine on immune effector and suppressor responses in melanoma-bearing mice. Cancer Immunol Immunother. 2012;61:1441–1450. doi: 10.1007/s00262-012-1204-x. doi:10.1007/s00262-012-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res. 2014;2:37–49. doi: 10.1158/2326-6066.CIR-13-0126. doi:10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toor AA, Payne KK, Chung HM, Sabo RT, Hazlett AF, Kmieciak M, et al. Epigenetic induction of adaptive immune response in multiple myeloma: sequential azacitidine and lenalidomide generate cancer testis antigen-specific cellular immunity. Br J Haematol. 2012;158:700–711. doi: 10.1111/j.1365-2141.2012.09225.x. doi:10.1111/j.1365-2141.2012.09225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545. doi:10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. doi:10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. doi:10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. doi:10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le HK, Graham L, Cha E, Morales JK, Manjili MH. Bear HD Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Internat Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. doi:10.1016/j.intimp2009.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Gibb DR, Saleem SJ, Kang DJ, Subler MA, Conrad DH. ADAM10 overexpression shifts lympho-and myelopoiesis by dysregulating site2/site3 cleavage products of Notch. J Immunol. 2011;186:4244–4255. doi: 10.4049/jimmunol.1003318. doi:10.4049/jimmunol.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:941–953. doi: 10.1007/s00262-008-0609-z. doi:10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha E, Graham L, Manjili MH, Bear HD. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res Treat. 2010;122:359–369. doi: 10.1007/s10549-009-0573-0. doi:10.1007/s10549-009-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier J, Roberts C, Avent K, Hazlett A, Berrie J, Payne K, et al. Fractal organization of the human T cell repertoire in health and after stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:366–377. doi: 10.1016/j.bbmt.2012.12.004. doi:10.1016/j.bbmt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang X-Y, et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med. 2013;11:145. doi: 10.1186/1479-5876-11-145. doi:10.1186/1479-5876-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunt SK, Sinha P, Clements VK, Ostrand-rosenberg S, Leips J. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 32.Saleem SJ, Martin RK, Morales JK, Sturgill JL, Gibb DR, Graham L, et al. Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J Immunol. 2012;189:511–515. doi: 10.4049/jimmunol.1200647. doi:10.3039/jimmunol.1200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CHT, Graham L, Bear HD. Phenotype, functions and fate of adoptively transferred tumor draining lymphocytes activated ex vivo in mice with an aggressive weakly immunogenic mammary carcinoma. BMC Immunol. 2010;11:54. doi: 10.1186/1471-2172-11-54. doi:10.1186/1471-2172-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kmieciak M, Payne KK, Idowu MO, Grimes MM, Graham L, Ascierto M-L, et al. Tumor escape and progression of HER-2/neu negative breast cancer under immune pressure. J Transl Med. 2011;9:35. doi: 10.1186/1479-5876-9-35. doi:10.1186/1479-5876-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y, et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–1113. doi: 10.1158/0008-5472.CAN-05-3020. doi:10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampen MH, van Hall T. Strategies to counteract MHC-I defects in tumors. Curr Opin Immunol. 2011;23:293–298. doi: 10.1016/j.coi.2010.12.005. doi:10.1016/j.coi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. doi:10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. doi:10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heninger E, Krueger TE, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol. 2015;6:29. doi: 10.3389/fimmu.2015.00029. doi:10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2′-deoxycytidine. Cancer Immunol Immunother. 2010;59:697–706. doi: 10.1007/s00262-009-0786-4. doi:10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raval RR, Sharabi AB, Walker AJ, Drake CG, Sharma P. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J Immunother Cancer. 2014;2:14. doi: 10.1186/2051-1426-2-14. doi:10.1186/2051-1426-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25 + CD4 + regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. doi:10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4 + CD25 + regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. doi:10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.