Abstract
The primary purpose of this study was to determine whether the electrically evoked compound action potential (ECAP) can be used to predict psychophysical electrical-field interaction patterns obtained with simultaneous stimulation of intracochlear electrodes. A second goal was to determine whether ECAP patterns are affected by recording location, because differences might influence the relation between ECAP and psychophysical measures. The third goal was to investigate whether symmetrical threshold shifts are produced with phase inversion of the interaction stimulus. Nine adults with Advanced Bionics cochlear implants participated. ECAP and psychophysical thresholds were obtained for basal, middle, and apical probe electrodes in the presence of a sub-threshold interaction stimulus delivered simultaneously to each of seven to eight interaction electrodes per probe. Results showed highly significant correlations between ECAP and psychophysical threshold shifts for all nine subjects, which suggests that the ECAP can adequately predict psychophysical electrical-field interaction patterns for sub-threshold stimuli. ECAP thresholds were significantly higher for recordings from the basal (versus apical) side of the probe, which suggests that recording location may affect relations between ECAP and psychophysical measures. Interaction stimulus phase inversion generally produced symmetrical threshold shifts for psychophysical measures but not for half of ECAP measures.
I. INTRODUCTION
Present cochlear implant (CI) speech-processing strategies use pulsatile stimulation to circumvent the electrical-field interaction problems associated with analog stimulation. With pulsatile stimulation, current pulses are interleaved across electrodes so that they do not overlap temporally. The primary disadvantage of fully sequential stimulation is that the per-channel and overall rates of stimulation are limited, thus reducing the amount of information that can be presented to the auditory neurons. As a compromise, the stimulation rate can be increased by presenting pulsatile stimulation simultaneously to two or more far-spaced electrodes chosen to minimize current field overlap. Overlap of current fields can produce summation or subtraction of the electrical field, depending upon the relative phase of each current field. Perceptual results can be either a change in loudness (and consequently threshold) or a shift in pitch (Bierer, 2007; Boëx et al., 2003; Buechner et al., 2008; de Balthasar et al., 2003; Donaldson et al., 2005; Favre & Pelizzone, 1993; Firszt et al., 2007; Shannon, 1983, 1985; Stickney et al., 2006; Townshend et al., 1987; Wilson et al., 2003). It is therefore of interest to determine the spatial extent to which electrical fields spread, which may help to determine how closely spaced electrodes can be if they are to be simultaneously activated. Further, it is of interest to determine whether the effects of electrical-field interaction are the same for psychophysical and physiological measures. If both measures show similar effects, then physiological measures would have practical utility in predicting time-consuming behavioral measures of electrical-field interaction, which would be particularly valuable for patients who are unable to provide reliable behavioral information. This latter issue was the focus of the present study.
Several earlier studies have evaluated the psychophysical effects of simultaneous stimulation of two intracochlear electrodes. Results show behavioral thresholds on a single electrode are lower when a sub-threshold stimulus is presented simultaneously in phase to a second electrode. Conversely, thresholds are higher when the added stimulus is inverted in phase (Boëx et al., 2003; de Balthasar et al., 2003; Favre & Pelizzone, 1993; Stickney et al., 2006). These findings indicate summation or subtraction of the electrical fields prior to neural activation. Favre and Pelizzone (1993) used psychophysical threshold to measure electrical-field interaction patterns with in-phase and inverted-phase monopolar stimuli for two subjects implanted with the Ineraid device, and noted asymmetrical threshold shifts between the two phase conditions. Although not specifically reported, it appears from their Fig. 2 that the in-phase condition tended to yield more interaction than the inverted-phase condition. Boëx et al. (2003) reported symmetrical shifts between in-phase and phase-inverted conditions for 12 subjects tested with monopolar stimulation and two subjects tested with bipolar stimulation. Statistical analyses were not reported, however, and a visual inspection of their Figs. 5 and 7 shows what appear to be asymmetrical shifts for in-phase and inverted-phase conditions for half of the subjects tested in the monopolar condition and for both subjects tested in the bipolar condition. Specifically, there appears to be larger threshold shifts (i.e., more interaction) for the inverted-phase conditions in most of those cases. In a similar experiment, de Balthasar et al. (2003) reported symmetrical threshold shifts between in-phase and inverted-phase conditions for three of four subjects. The subject with asymmetrical threshold shift showed more interaction with the inverted-phase condition. Other studies that used measures of loudness have also shown examples of asymmetrical shifts; in those cases, it appears that more interaction was measured for the in-phase condition (Shannon, 1983; 1985). In summary, it appears that phase inversion can produce asymmetrical shifts with no clear outcome as to which phase combination produces more interaction.
Relatively few studies have evaluated physiological effects of simultaneous electrode stimulation in human CI users. Results obtained using the electrically evoked auditory brainstem response (EABR) have shown larger amplitudes and lower thresholds for simultaneous, in-phase stimulation of two electrodes, compared with smaller amplitudes and higher thresholds when the phase of one stimulus was inverted relative to the other (Abbas and Brown, 1988; Gardi, 1985; White et al., 1984). Cortical potentials from animal preparations also have shown lower thresholds with simultaneous in-phase stimulation (Bierer and Middlebrooks, 2004; Middlebrooks, 2004) compared with phase-inverted stimulation (Bierer and Middlebrooks, 2004). Therefore, physiological and psychophysical studies of threshold shifts with stimulus phase have generated similar results. Very few studies have examined current-field interaction from simultaneous electrode activation using the electrically evoked compound action potential (ECAP) in human CI users (e.g., Abbas et al., 2003).
The majority of psychophysical and physiological studies pertaining to electrical-field interaction have focused on examining the effects of stimulus polarity for fixed electrode pairs (as discussed above). Few studies have evaluated spatial interaction patterns along the length of the cochlea, where one stimulus location is fixed and the location of the other stimulus is systematically varied across many electrodes. A few psychophysical studies have examined the effects of electrical-field interaction as a function of location of the interaction stimulus. Favre and Pelizzone (1993) examined electrical-field interaction patterns for psychophysical thresholds from two Ineraid subjects using monopolar stimulation. Results showed that interaction effects decreased as the distance between simultaneously stimulated electrodes increased. For both subjects, a sub-threshold stimulus fixed on the most apical electrode (E1) had a negligible effect for the largest electrode spacing (E1 and E5; 14.4 mm apart). White et al. (1984) reported similar findings for psychophysical thresholds in a single subject implanted with an early version of the 16-electrode Clarion device, where an electrode separation of 14 mm produced very little interaction with monopolar stimulation. More recently, Stickney et al. (2006) showed less interaction for greater electrode separations for both monopolar and bipolar stimulation, with less interaction overall for bipolar stimulation. Finally, loudness estimates from single subjects in two different studies (Shannon, 1983, 1985) showed less interaction with greater separation between the two stimulated electrodes. In some of those examples, there was little to no interaction for electrode separations of 10–12 mm. In summary, psychophysical results show negligible effects of electrical field interaction for electrode separations of 10–14 mm with monopolar stimulation.
Studies evaluating physiological spatial interaction patterns with simultaneous stimulation along the length of the cochlea are also sparse. There do not appear to be any published reports that comprehensively evaluate physiological electrical-field interaction as a function of electrode separation. Two studies presented physiological data for limited conditions. Abbas and Brown (1988) reported EABR threshold shifts for several Ineraid patients, where stimuli were presented simultaneously to adjacent monopolar (E1 and E2) or overlapping bipolar (E1-3 and E2-4) electrode pairs. Threshold shifts were also measured for a slightly wider separation of monopolar electrodes (E1 and E3) and for adjacent bipolar pairs (E1-2 and E3-4). Larger threshold shifts (i.e., more interaction) typically occurred for the adjacent monopolar and overlapping bipolar pairs compared with the other respective pair spaced slightly farther apart. Cortical measures obtained by Bierer and Middlebrooks (2004) from guinea pigs showed a similar trend; larger threshold shifts were obtained for 1.5-mm separation between bipolar electrode pairs compared with 2.25-mm separations. In summary, the limited physiological data suggest less electrical-field interaction with larger separations between simultaneously stimulated electrode pairs, which is consistent with psychophysical findings. We are unaware of any studies that have directly compared physiological and psychophysical electrical-field interactions within individual subjects.
The primary purpose of this study was to determine whether physiological electrical-field interaction patterns measured with the ECAP are predictive of patterns measured psychophysically. If the measures are strongly correlated, then the ECAP may provide an efficient way to predict behavioral electrical-field interaction patterns for simultaneous stimulation. A secondary goal was to examine the effect of the recording electrode location on ECAP electrical-field interaction patterns, as previous studies have shown that ECAP amplitude varies with position of the intracochlear recording electrode (Abbas et al., 1999; Cohen et al., 2004; Frijns et al., 2002). If recording electrode location affects ECAP electrical-field interaction patterns, then the relation between ECAP and psychophysical measures would also be affected by recording electrode location. The third and final goal was to investigate whether phase inversion of the interaction stimulus produces threshold shifts that are symmetrical to the in-phase condition, as well as to determine whether phase inversion affects physiological and psychophysical electrical-field interaction patterns in the same way. If phase inversion produces symmetrical threshold shifts that are the same for both physiological and psychophysical measures, then it could be presumed that electrical fields sum and subtract in a simple, linear manner. If phase inversion produces asymmetrical threshold shifts and/or affects physiological and psychophysical measures differently, then it could be presumed that mechanisms other than simple field summation are also actively involved.
II. METHODS
A. Subjects
Nine adult CI recipients participated. Six patients were implanted with the Advanced Bionics CII and three received the HiRes 90K device (Advanced Bionics Corporation, Sylmar, CA). Both devices have the same internal chip and 16 electrodes in the intracochlear array. The primary difference between the two devices is that the CII housing is ceramic and the 90K housing is titanium. Both devices had the HiFocus electrode array. Electrodes are numbered sequentially from apex (E1) to base (E16), with a full insertion length of 21 mm and 1.1 mm between electrodes (center to center). Subject C15 had an open circuit on E16; otherwise all subjects had normal electrode impedance as measured with the clinical programming software (Sound Wave). Table I lists subject number, gender, internal device type, whether subjects had an electrode positioner, the ear implanted, age at implant in years (y) and months (m), duration of implant use at the time of participation in the study, duration of deafness prior to implantation, and etiology of deafness for each subject. Subject C16 had only used the implant minimally for the first 2 y following surgery and had been a non-user for approximately 4½ y at the time of participation in this study.
Table I.
Demographic information for the subjects participating in this study.
| Subject Number | Gender | Internal Device | Positioner | Implant Ear | Age at Implant (y, m) | Duration CI Use (y, m) | Duration Deafness (y, m) | Etiology |
|---|---|---|---|---|---|---|---|---|
| C1 | F | CII | Yes | Right | 18, 4 | 4, 10 | 16, 0 | Unknown |
| C6 | M | CII | No | Left | 64, 4 | 4, 0 | 21, 0 | Unknown |
| C7 | F | CII | No | Left | 57, 2 | 4, 2 | 5, 0 | Genetic- unspecified |
| C8 | M | CII | No | Right | 55, 7 | 3, 10 | 0, 3 | Sudden |
| C10 | M | 90K | No | Right | 51, 10 | 3, 5 | 14, 0 | Unknown |
| C11 | M | 90K | No | Right | 58, 1 | 3, 5 | 15, 0 | Unknown |
| C13 | F | CII | No | Left | 77, 1 | 4, 5 | 20, 0 | Unknown |
| C15 | F | 90K | No | Left | 39 | 3, 1 | 34, 0 | Unknown |
| C16 | F | CII | Yes | Left | 13, 11 | Minimal use 2y; non-use 4.5y | 13, 11 | Connexin 26 |
F = female, M = male, y = years, m = months
B. Equipment setup
The Bionic Ear Data Collection System (BEDCS; Advanced Bionics Corporation, Sylmar, CA) research platform was used for both physiological (ECAP) and psychophysical stimulus generation and data collection. BEDCS controlled a Platinum Series Processor (PSP) through a clinical programming interface (CPI II) connected to a laptop computer. The PSP was connected to the subject using a Platinum Headpiece. The subject’s own processor and headpiece were not used for any portion of data collection.
C. Psychophysical measures
Prior to data collection, an ascending procedure was used to estimate behavioral threshold and upper comfort levels to determine approximate starting and ending current levels for the ECAP measures and for the psychophysical adaptive procedure. The stimulus was a 340-ms pulse train consisting of cathodic-leading, 50-μs/phase, biphasic current pulses with a 10-μs inter-phase gap presented at a rate of 30 pps (total of 10 pulses). Monopolar stimulation was used for all measures in this study (return electrodes were always the case ground, called IE1 in the CII and IE2 in the 90K). Because the pulse rate and overall duration of a pulse train affect the threshold for that stimulus, it was important that the stimulus used for the psychophysical measures was as similar as possible to the stimulus used to elicit the ECAP so that threshold shifts for the two measures could be compared more directly. Each pulse train was delivered once before the current level was increased. Stimulus levels were increased using log-based increments with the following formula:
where a is the starting current level, n is the step size factor (typically set between 0.6 and 0.7), and b is the next current level (substituted for a on the following iteration). The subject was instructed to indicate when the sound was first heard and when the sound was loud but not uncomfortable. These judgments corresponded to ratings of 1 and 8, respectively, on a visual scale of 0 to 10, where 0 was no sound and 10 was too loud.
Psychophysical thresholds were then obtained using an adaptive, 3-interval, 2-alternative, forced choice (3I-2AFC) task. The initial stimulus level for the probe was chosen on an individual basis to be sufficiently audible but well below the estimated upper-loudness level. Thresholds were first obtained for probe electrodes P5, P9, and P12, which represent apical, middle, and basal cochlear positions, respectively. For probe-electrode-only conditions, the stimulus was randomly presented to either interval 2 or 3 and the subject indicated which interval contained the sound. Thresholds were then measured for each probe electrode in the presence of a sub-threshold, fixed-level interaction stimulus systematically applied in phase to each interaction electrode. For the probe-plus-interaction-electrode conditions, the interaction electrode was stimulated in all three intervals, with the probe electrode stimulated simultaneously in either interval 2 or 3. Again, the subject’s task was to choose which interval (2 or 3) contained the sound. Intervals were represented visually with numbered boxes on a computer screen. Each interval was separated by 700 ms. The subject used the computer keyboard to enter the interval number for each response. Feedback was not provided.
Interaction electrodes were chosen to be one, three, and five electrode positions from the probe electrode in both directions, as well as the two most apical and basal electrodes (E1 and E16, respectively). Deviations from this pattern were necessary for interaction electrodes apical to P5 and basal to P12. Table II lists the specific interaction electrodes tested for each probe electrode. E15 was used instead of E16 for subject C15, who had the open circuit on E16.
Table II.
Probe and interaction electrode combinations used in this study.
| Probe Electrode | Interaction Electrodes |
|---|---|
| 5 | 1, 2, 4, 6, 8, 10, 16 |
| 9 | 1, 4, 6, 8, 10, 12, 14, 16 |
| 12 | 1, 7, 9, 11, 13, 15, 16 |
The current level of the interaction stimulus used for each probe electrode was the same for both ECAP and psychophysical measures. Because the goal was to measure electrical field overlap, it was important to use the same current level across all interaction electrodes for a given probe electrode. The interaction stimulus level was fixed at one-half (50%) of the behavioral threshold (in μA) for each probe electrode obtained with the adaptive procedure1. This same current level was also used for the respective ECAP interaction stimulus. In all but one case, the interaction stimulus level was sub-threshold across all interaction electrodes. The exception was C6, whose probe thresholds for P5, P9, and P12 were 65.6 μA, 130.1 μA, and 136.6 μA, respectively. The interaction levels for P9 and P12 were therefore 65 μA and 68 μA, respectively, which was just at or slightly above threshold for E5. In this case, the subject was instructed to choose which interval contained the sound that was different.
Psychophysical thresholds for the interaction condition were determined using a 3-down, 1-up adaptive procedure, which estimates 79.4% correct (Levitt, 1971). Each block consisted of nine reversals. The initial step size was 2 dB for the first three reversals, followed by 1 dB for the next two reversals, and finally 0.5 dB for the remaining four reversals. Threshold for each block was computed as the mean of the last four reversals. Final threshold was an average of three to five blocks.
D. ECAP measures
ECAP amplitude growth functions were obtained using ascending stimulus levels beginning at a current level near the value that the subject indicated as behavioral threshold (rating of 1) and ending at the level indicated as a rating of 8, with the step size as described in the previous section. The stimulus used to evoke the ECAP consisted of 50-μs/phase biphasic current pulses with a 10-μs inter-phase gap presented in monopolar mode (relative to the extracochlear case electrode), repeated at a rate of approximately 30 pps. Each recorded waveform consisted of 120 averages with a gain of 300. Alternating polarity was used to reduce stimulus artifact.
ECAP growth functions were obtained for P5, P9, and P12. A sub-threshold, fixed-level, in-phase interaction stimulus was then delivered simultaneously to another electrode in the array and the ECAP growth function was repeated for each probe electrode. The current level and electrode location for the interaction stimulus was the same as for the psychophysical measures. For each stimulated electrode (or electrode pair, in the case of simultaneous stimulation), two ECAP growth functions were recorded: one from an apical recording electrode and one from a basal recording electrode, each typically located two positions away from the probe electrode. In two cases, excessive stimulus artifact necessitated changing the recording site farther away from the probe. These exceptions were C10, P9 (recorded from E4 instead of E7 for interaction E6) and C16, P9 (recorded from E5 instead of E7 for all interaction electrodes). The recording reference electrode was the case electrode (IE1) for CII subjects and the ring electrode (IE1) for 90K subjects.
ECAP waveforms were read into a custom analysis program written in Matlab (The MathWorks, Inc., Natick, MA). ECAP amplitudes were calculated as the difference between the first negative peak (N1) and the following positive peak or plateau (P2), which were manually marked by the investigators. ECAP threshold was visually determined as the lowest current level that produced a non-zero amplitude that was above the noise floor. The noise floor yielded ECAP thresholds that were approximately 20–40 μV in most cases. Final ECAP threshold was the average of the thresholds for basal and apical recording conditions.
III. RESULTS
A. Effect of recording electrode on ECAP thresholds
Because previous research has shown that relative position of the intracochlear recording electrode can affect ECAP amplitudes (Abbas et al., 1999; Cohen et al., 2004; Frijns et al., 2002), it is reasonable to assume that ECAP thresholds could also potentially be affected. Therefore, it was important to first examine the effect of the recording electrode location on ECAP thresholds obtained with simultaneous stimulation, as this would affect the relation between ECAP and psychophysical measures.
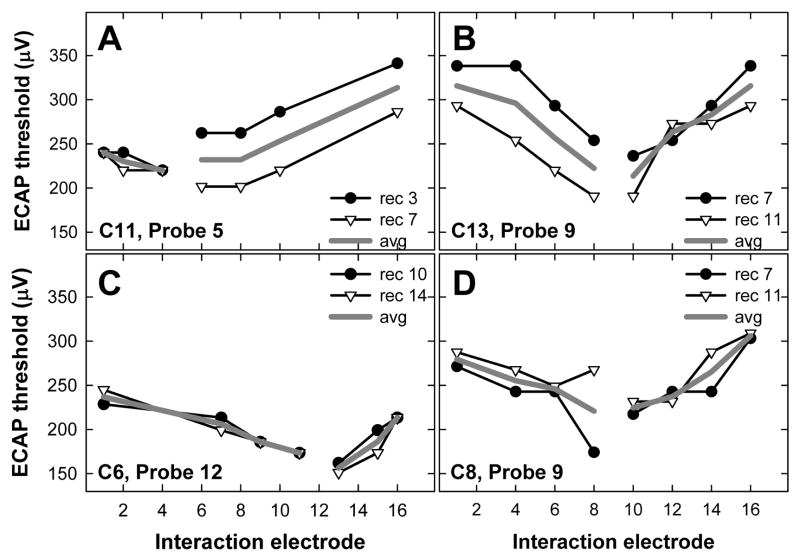
Figure 1 shows four individual examples of ECAP thresholds for a fixed probe electrode (noted in each panel) as a function of interaction electrode location. In each panel, the symbols represent recordings made from an electrode two positions apical to the probe (filled circles) and two positions basal to the probe (open triangles). The solid gray line represents the average of the two recording positions. In Fig. 1A, similar ECAP thresholds were obtained for both recording positions when the interaction stimulus was applied to the apical side of the probe (i.e., low-numbered electrodes), but not for interaction electrodes basal to the probe. In Fig. 1B, the opposite pattern is seen: similar thresholds are obtained for both recording positions when the interaction stimulus was applied basal to the probe, but not for interaction electrodes apical to the probe. In Fig. 1C, similar thresholds were obtained for basal and apical recording sites across all interaction electrodes. In Fig. 1D, similar ECAP thresholds were obtained for all interaction electrodes except for E8. A lower ECAP threshold was obtained when the recording electrode (E7) was adjacent to the interaction electrode (E8). This may be due to stimulus artifact adding to the neural response, making it appear larger. As the examples in Fig. 1 illustrate, there was no systematic pattern across subjects or electrodes for ECAP threshold differences between the two recording sites.
FIG. 1.
Individual examples of recording electrode effects on ECAP electrical-field interaction patterns. Subject number and probe electrode are indicated on each graph. Symbols represent probe thresholds as a function of interaction electrode. Filled circles and open triangles represent apical and basal recording electrodes, respectively. Specific recording electrode numbers are listed in each figure legend. Solid gray lines represent the average of both recording sites.
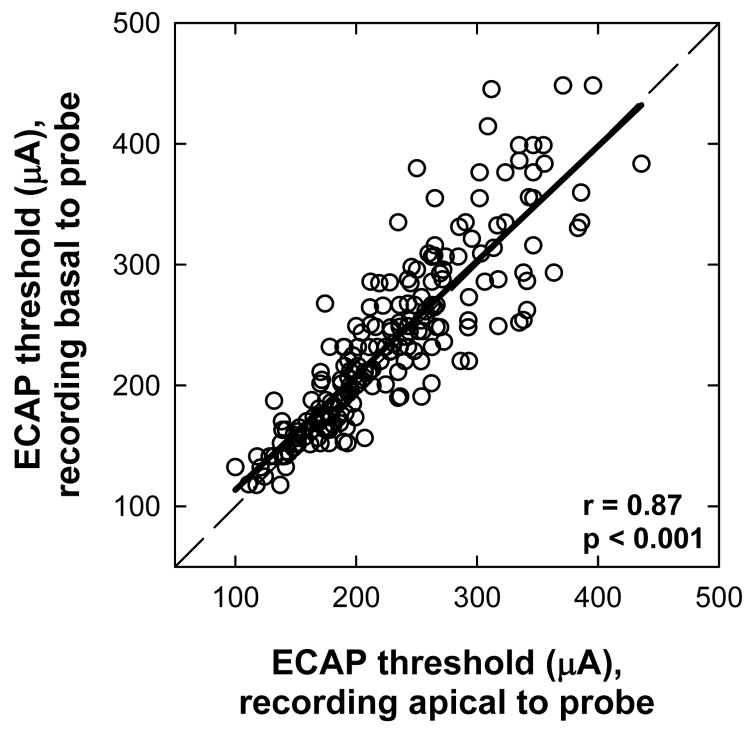
Figure 2 shows ECAP thresholds for basal (ordinate) versus apical (abscissa) recording electrode locations for all stimulating-electrode conditions in all subjects. There was a strong correlation between the two recording sites (r = 0.87, p < 0.001)2. The slope of the linear regression line was 0.95. Because the data sets were not normally distributed, a Wilcoxon Signed Rank test was used to determine whether ECAP thresholds differed significantly for basal versus apical recording sites. A significant difference was found between basal and apical recordings (p = 0.003), with higher thresholds on average for recordings from the basal side of the probe electrode. Figure 2 shows greater differences in ECAP thresholds between the two recording sites when thresholds were higher overall. This likely reflects the larger step sizes used at higher levels due to the log step scale, given data in Fig. 2 are plotted on a linear scale.
FIG. 2.
ECAP thresholds for basal (ordinate) versus apical (abscissa) recording electrode locations (re: probe electrode) for all stimulating-electrode conditions in all subjects. The dashed diagonal represents unity. The bold solid line represents linear regression results.
B. Psychophysical versus physiological electrical-field interaction patterns
The primary goal of this study was to compare psychophysical and physiological electrical-field interaction patterns to determine whether ECAP measures can be used as an effective way to predict behavioral electrical-field interaction patterns. For each probe electrode, psychophysical and ECAP thresholds were measured with and without in-phase simultaneous stimulation of a second (interaction) electrode. The interaction stimulus was identical to the stimulus delivered to the probe. Thresholds obtained in the presence of the interaction electrode were subtracted from the probe-only condition to yield the amount of threshold shift. It was hypothesized that the ECAP threshold shift would not be significantly different from the psychophysical threshold shift as a function of interaction electrode location.
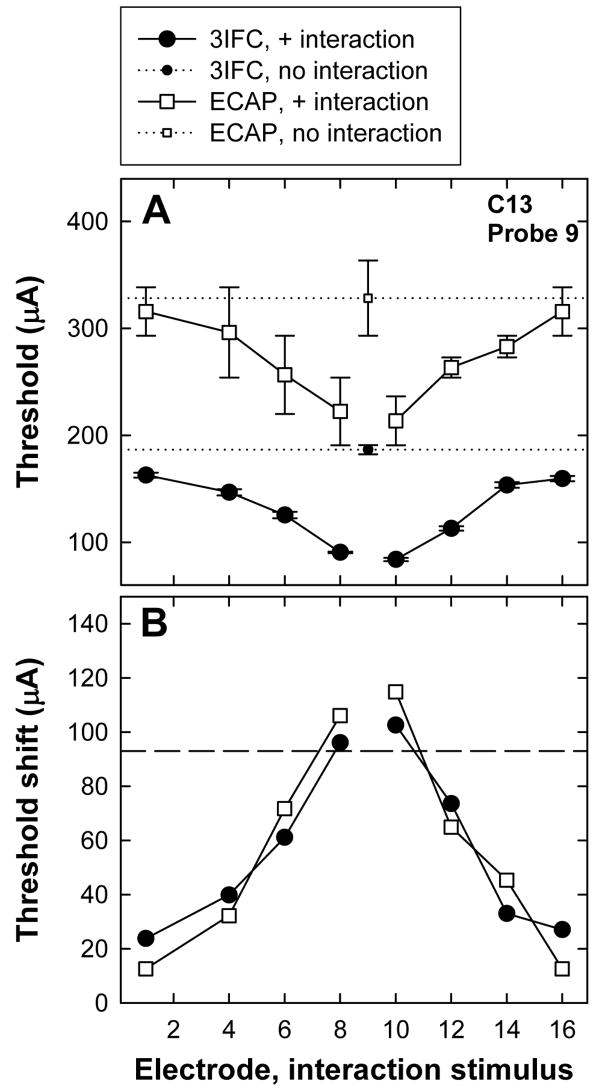
Figure 3A shows an example of ECAP (open squares) and psychophysical (filled circles) thresholds for P9 from subject C13. Small symbols with a horizontal dotted line represent the respective probe-only thresholds. Thresholds obtained in the presence of the interaction stimulus are plotted with larger symbols and solid lines as a function of interaction electrode position. Error bars represent one standard error around the mean (±1 SEM). For both ECAP and psychophysical measures, probe thresholds were lowest when the interaction stimulus was adjacent to the probe electrode, representing the greatest contribution from the interaction electrode and thus the greatest amount of current field summation. Probe thresholds increased with greater separation between interaction and probe electrodes, consistent with less overlap and thus less contribution from the interaction electrode. If the probe threshold in the presence of the interaction stimulus is equal to the probe-alone threshold, it is presumed that there is no overlap of current fields from the two simultaneously activated electrodes. Conversely, if the probe threshold in the presence of the interaction stimulus is equal to the difference between probe-alone threshold and interaction stimulus level, it is presumed that there is complete overlap of current fields from the two simultaneously activated electrodes.
FIG. 3.
A: Example of ECAP (open squares) and psychophysical (3IFC; filled circles) thresholds for P9 from subject C13. Small symbols with a horizontal dotted line represent the respective probe-only conditions. Larger symbols with solid connecting lines represent thresholds obtained in the presence of the interaction stimulus, plotted as a function of interaction electrode position. Error bars represent ±1 SEM. B: Threshold shifts for ECAP (open squares) and psychophysical measures (filled circles) as a function of interaction electrode location. Threshold shifts were calculated as probe-alone threshold minus probe threshold in the presence of the interaction stimulus. The horizontal line represents the current level of the interaction stimulus.
ECAP and psychophysical threshold shifts were calculated by subtracting the mean threshold in the interaction condition from the mean threshold in the probe-only condition. Figure 3B shows the mean threshold shifts for the data presented in Fig. 3A. The horizontal dashed line indicates the current level of the interaction stimulus. Ideally, the threshold shifts should not be greater than the level of the interaction stimulus. (This situation is described further in the Discussion.) In this example, smaller threshold shifts occurred with greater separation between probe and interaction electrodes, as expected. The threshold shifts were not significantly different between ECAP and psychophysical measures in this example (paired t test).
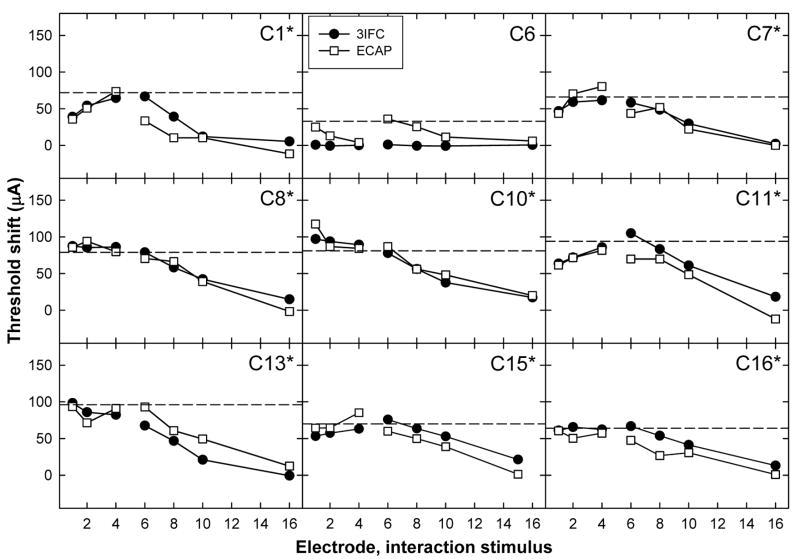
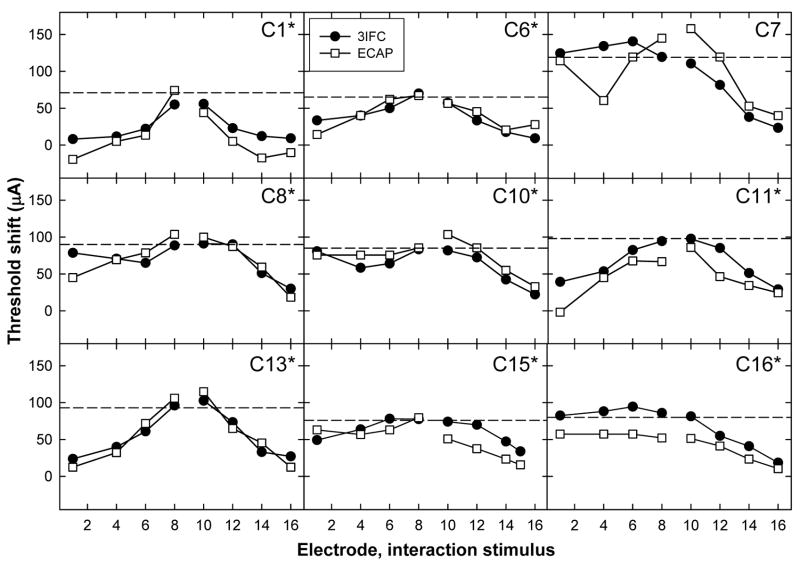
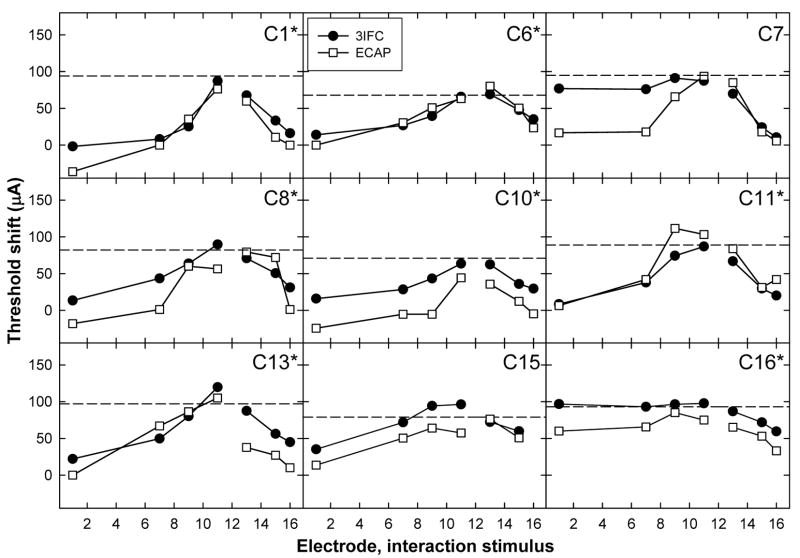
Figures 4,Fig. 5 and 6 show individual threshold-shift patterns for all subjects. Data are plotted as in Fig. 3B. Data for the apical P5 are shown in Fig. 4, the middle P9 in Fig. 5, and the basal P12 in Fig. 6. An asterisk next to the subject number in each panel indicates a significant correlation (Pearson’s r, p < 0.05) between the ECAP and psychophysical threshold shift. Correlation coefficients and p values for each subject are listed in Table III. Across Figs. 4–6, 23 of 27 probe-electrode patterns (85%) exhibited a significant correlation between ECAP and psychophysical data.
FIG. 4.
Psychophysical (3IFC; filled circles) and ECAP (open squares) threshold shifts plotted as a function of interaction electrode location for apical P5. Each graph represents data from a different subject. The horizontal dashed line represents the level of the interaction stimulus. Asterisks next to the subject numbers indicate a statistically significant correlation between ECAP and psychophysical threshold shifts (see Table III).
FIG. 5.
Psychophysical (3IFC; filled circles) and ECAP (open squares) threshold shifts plotted as a function of interaction electrode location for middle P9. Data are plotted as in Fig. 4.
FIG. 6.
Psychophysical (3IFC; filled circles) and ECAP (open squares) threshold shifts plotted as a function of interaction electrode location for basal P12. Data are plotted as in Figs. 4 and 5.
Table III.
Correlation coefficients (r values) and significance (p values) for comparison of threshold shifts between psychophysical and physiological measures (data presented in Figs. 4–6). Asterisks denote statistical significance (p < 0.05).
| Subject | Probe 5 | Probe 9 | Probe 12 | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| C1 | 0.84 | 0.02* | 0.94 | <0.001* | 0.94 | 0.002* |
| C6 | 0.32 | 0.48 | 0.83 | 0.01* | 0.95 | 0.001* |
| C7 | 0.92 | 0.003* | 0.61 | 0.1 | 0.63 | 0.1 |
| C8 | 0.97 | <0.001* | 0.83 | 0.01* | 0.81 | 0.03* |
| C10 | 0.95 | <0.001* | 0.92 | 0.001* | 0.94 | 0.002* |
| C11 | 0.90 | 0.006* | 0.87 | 0.005* | 0.96 | <0.001* |
| C13 | 0.91 | 0.004* | 0.97 | <0.001* | 0.81 | 0.03* |
| C15 | 0.80 | 0.03* | 0.72 | 0.046* | 0.76 | 0.078 |
| C16 | 0.91 | 0.005* | 0.98 | <0.001* | 0.88 | 0.009* |
Figure 4 (apical probe) shows a significant positive correlation between ECAP and psychophysical threshold shifts for eight of the nine subjects. C6 showed measurable threshold shifts with the ECAP, but virtually no threshold shifts psychophysically, resulting in a lack of correlation between the two measures. This subject’s probe-alone threshold for P5 was 65.6 μA, which was significantly lower than all other electrodes in all subjects (range: 128.7 μA to 196.1 μA). Because the interaction stimulus level was calculated as one-half of the probe-alone threshold, the interaction level in this case (33 μA) may have been too small to have a measurable effect. It is possible that the threshold shifts for C6 obtained with ECAP measures were due to the large amount of variability generally associated with ECAP measures (as illustrated in Fig. 3A for C13 by large standard-error bars).
For the middle probe electrode patterns, Fig. 5 shows a significant positive correlation for eight of the nine subjects. The correlation for C7 was not significant, which was most likely due to the large difference between data points for the interaction stimulus on E4. For the basal probe electrode patterns in Fig. 6, there were significant positive correlations for seven of the nine subjects. For subject C7, the two patterns were somewhat similar, but the magnitude of the threshold shift was much larger psychophysically than physiologically for the apical interaction electrodes. For subject C15, the patterns appeared quite similar, but were not statistically significant. This subject also had one less interaction condition than the other subjects due to the open circuit on E16.
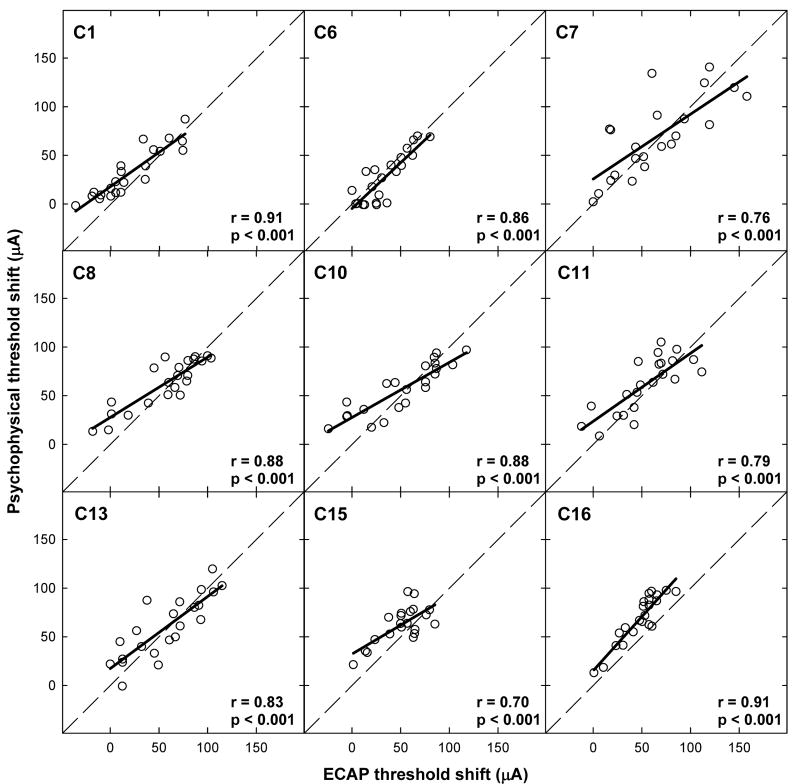
Figure 7 shows psychophysical threshold shifts plotted relative to ECAP threshold shifts for all electrode conditions within each subject. Subject number is indicated in each panel along with correlation coefficients and p values. Bold solid lines indicate linear regression results and the dashed diagonal lines represent unity. Correlations ranged from 0.70 to 0.91 across subjects. For all subjects, there was a highly significant correlation between ECAP and psychophysical threshold shift (p < 0.001). Paired t test results, shown in Table IV, indicate no significant difference between ECAP and psychophysical threshold shift as a function of interaction electrode location for five of the nine subjects (C7, C8, C10, C11, and C13), consistent with the hypothesis that similar patterns would be obtained with both measures. For the remaining four subjects, C6 had significantly larger threshold shifts for the ECAP measures; whereas C1, C15, and C16 exhibited greater threshold shifts psychophysically. When data were pooled across all electrodes and all subjects, a strong correlation was observed between ECAP and psychophysical threshold shift (r = 0.82, p < 0.0001). A paired t test for the group data indicated a statistically significant difference between ECAP and psychophysical threshold shift, with greater shifts occurring for psychophysical thresholds (t = 4.93, p < 0.001, df = 196).
FIG. 7.
Individual scatter plots comparing psychophysical and ECAP threshold shifts across all electrode conditions within a subject. Subject number, correlation coefficient (r value), and significance level (p value) are indicated on each graph. Diagonal dashed lines represent unity and bolded solid lines represent linear regression results.
C. Effect of interaction stimulus phase
The third goal of the present study was to investigate whether phase inversion of the interaction stimulus produced a pattern of threshold shifts that was symmetrical to that obtained with the in-phase interaction stimulus. It was also of interest to determine whether phase inversion affected physiological and psychophysical electrical-field interaction patterns in the same way. If phase inversion produces symmetrical threshold shifts that are the same for both physiological and psychophysical measures, then it could be presumed that electrical fields sum and subtract in a simple, linear manner. If phase inversion produces asymmetrical threshold shifts and/or affects physiological and psychophysical measures differently, then it could be presumed that mechanisms other than simple field summation are also actively involved.
For all but subject C11, ECAP and psychophysical thresholds were measured for P9 in the presence of an inverted-phase interaction stimulus applied to the same electrodes as indicated in Table II. The current level of the inverted-phase interaction stimulus was the same as that used for the in-phase portion of the study. In the inverted-phase condition, pulses delivered to the probe were cathodic leading, while pulses delivered to the interaction electrode were anodic leading. Because ECAP measures used alternating polarity to eliminate stimulus artifact, the stimulus polarity for the interaction electrode was always opposite of that presented to the probe for the inverted-phase condition. All other aspects of data collection were the same as described for the in-phase condition.
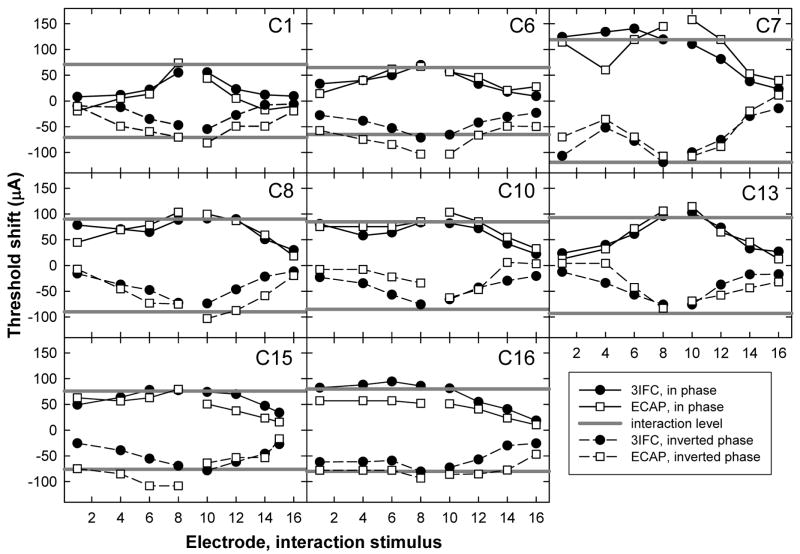
Figure 8 shows threshold shifts for the psychophysical task (filled circles) and for the ECAP (open squares) as a function of interaction electrode for each subject. Solid lines connecting symbols represent data from the in-phase condition (taken from Fig. 5), and dashed lines represent data from the inverted-phase condition. The horizontal solid gray lines represent the current level of the interaction stimulus, where positive values are in phase with the probe and negative values are phase-inverted relative to the probe. Ideally, all symbols should fall between the solid gray lines and should be symmetric about y = 0. In general, most of the data followed these expected trends. However, differences in threshold shift between in-phase and inverted-phase conditions can be seen in several of the individual graphs. For example, ECAP threshold shifts were larger than the magnitude of the interaction stimulus for subjects C6 and C15 in the inverted-phase condition but not for the in-phase condition.
FIG. 8.
Individual interaction patterns for P9; each panel represents data from a different subject. ECAP (open squares) and psychophysical (3IFC; filled circles) threshold shifts are plotted as a function of interaction electrode location. Symbols connected with solid lines represent threshold shifts with the addition of an in-phase interaction stimulus and dashed lines represent the inverted-phase condition. Solid gray lines represent the interaction stimulus level, with negative values representing the inverted phase condition.
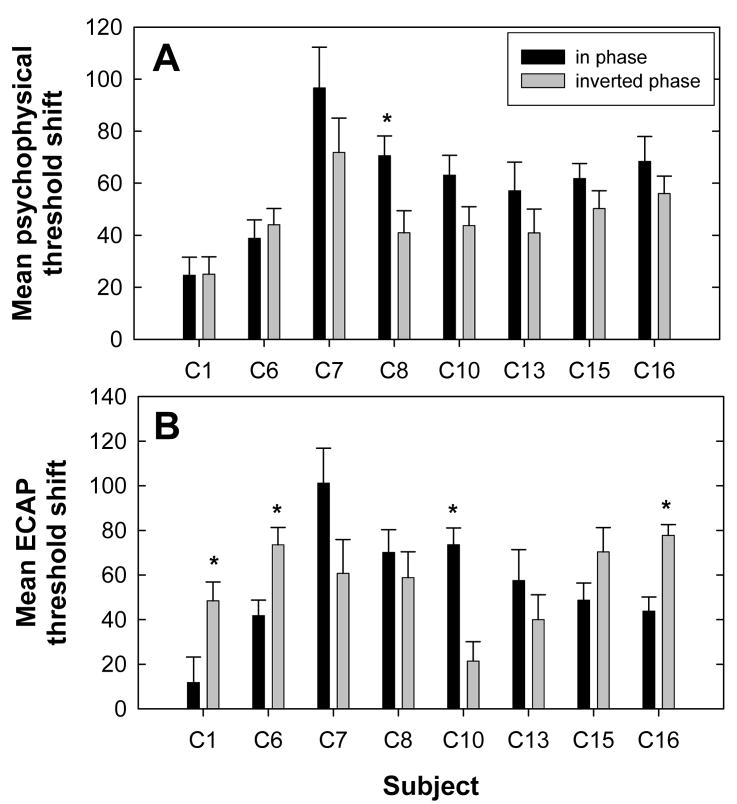
To summarize the data in Fig. 8, the mean threshold shift across all interaction electrodes was calculated for both phase conditions of the ECAP and psychophysical data. Figure 9 shows the mean threshold shifts for each subject. Black bars represent the in-phase condition and gray bars represent the inverted-phase condition. Figure 9A shows psychophysical data and Fig. 9B shows ECAP data. Because threshold shifts were calculated as the no-interaction condition minus the interaction condition, the inverted phase condition typically resulted in negative values whereas the in-phase condition typically yielded positive values. Therefore, the inverted-phase threshold shifts were multiplied by −1 prior to calculating the average across interaction electrodes so that threshold shifts could be compared for the two phase conditions3.
FIG. 9.
Mean threshold shifts (+1 SEM) across P9 interaction electrodes for in-phase (black bars) and inverted-phase (gray bars) conditions for each subject. Data were calculated from Fig. 8. A: psychophysical threshold shifts, B: ECAP threshold shifts. Asterisks indicate a statistically significant difference between phase conditions (p < 0.05).
For each subject, a two-way analysis of variance (ANOVA) with measure and phase as factors was used to evaluate whether threshold shifts across interaction electrodes (data from Fig. 8) were the same for in-phase versus inverted-phase conditions. Asterisks above the bars in Fig. 9 indicate statistically significant differences between in-phase and inverted-phase conditions (p < 0.05). For psychophysical data, only subject C8 demonstrated significantly more interaction (i.e., greater threshold shift) for the in-phase stimulus compared with the inverted-phase stimulus. For ECAP data, subjects C1, C6, and C16 exhibited significantly larger mean threshold shifts for the inverted-phase condition and subject C10 showed a significantly larger mean threshold shift in the in-phase condition. For five subjects (C1, C6, C8, C10, and C16), phase inversion did not affect ECAP threshold shifts and psychophysical threshold shifts in the same way. Specifically, C1, C6, C10 and C16 showed asymmetrical threshold shifts as a function of interaction stimulus phase for ECAP measures but not psychophysically. C8 showed an asymmetrical threshold shift for psychophysical measures but not with the ECAP. The remaining three subjects (C7, C13, and C15) demonstrated no significant difference in threshold shifts for the two phase conditions with either measure.
IV. DISCUSSION
The primary goal of this study was to evaluate the relation between physiological and psychophysical measures of electrical-field interaction in CI recipients. First, to determine whether recording site might affect this relation, we examined the effect of recording electrode location on electrical-field interaction patterns measured with the ECAP. Because recording electrode location was found to significantly affect the ECAP results, ECAP thresholds were averaged for two recording sites for comparison with psychophysical thresholds. Results showed a strong correlation between the ECAP and psychophysical spatial interaction patterns, which suggests that the ECAP has practical utility in predicting psychophysical measures of electrical-field interaction. Finally, the effect of interaction stimulus phase was examined for both ECAP and psychophysical measures. Results showed different effects of phase between the two measures. These results are discussed further in the following sections.
A. Effect of recording electrode on ECAP thresholds
As illustrated by the examples in Fig. 1, the recording electrode location can strongly affect ECAP threshold measures. As Fig. 2 shows, greater discrepancies between apical and basal recordings were seen for higher thresholds, likely due to the larger step sizes used at higher stimulus levels. Several previous studies have reported that ECAP amplitude varies as the position of the intracochlear recording electrode changes relative to the stimulating electrode (Abbas et al., 1999; Cohen et al., 2004; Frijns et al., 2002). Specifically, ECAP amplitude tends to decrease as the recording site is located farther away from the stimulating site. The recording electrode measures the voltage change associated with neural discharge. Because the cochlea is filled with fluid, that voltage is conducted along the length of the cochlea allowing relatively large voltages to be measured from distal recording sites. In some cases, however, the measured voltage can approach zero for the largest separation between stimulating and recording electrode (e.g., Frijns et al., 2002). While these studies have reported measurable effects of recording electrode position on supra-threshold ECAP amplitudes, no studies have assessed the effect of recording electrode position on ECAP thresholds. However, if ECAP amplitudes are smaller at farther recording sites, it is probable that thresholds will be higher at farther recording sites.
The literature is inconsistent in regard to the symmetry of ECAP amplitudes obtained from recording sites that are apical versus basal to the stimulated electrode. Abbas et al. (1999) compared the difference in ECAP amplitudes between recordings made at basal and apical sites that were equidistant from the stimulated electrode and found virtually no difference in amplitude between the two recording positions. In contrast, Frijns et al. (2002) reported a tendency for amplitudes to be larger when recorded from a location apical to the stimulated electrode than from an equidistant basal location. They attributed those findings to the tapered anatomy of the cochlea from base to apex, which likely puts electrodes in closer proximity to neural elements at the apical end. ECAP data from the present study showed higher thresholds occurred more often for recordings from the basal side of the stimulating electrode, which is consistent with the amplitude data reported by Frijns et al. (2002). The variability in ECAP thresholds and amplitudes across recording electrode locations suggests that ECAP recordings should be averaged across more than one recording location for a more robust measurement, particularly if ECAP measures are to be compared with psychophysical measures within subjects.
As shown in Fig. 3A, mean ECAP thresholds typically exhibited a much larger standard error than psychophysical thresholds. This may be due to several measurement-related issues. First, only two ECAP threshold measures were obtained for the average (each from a different recording electrode); whereas psychophysical results were an average of three to five threshold estimates. Second, a log step size was used for both ECAP and psychophysical measures, and since ECAP thresholds were always higher than psychophysical thresholds, the step size was therefore larger for ECAP measures. A third reason is that the psychophysical measures used an adaptive procedure where step size was systematically reduced several times near threshold. ECAP thresholds were obtained using an ascending (rather than adaptive) procedure with a fixed log-based step size. It is possible that a smaller SEM would have resulted from a repeated reduction in step size near threshold, as in the psychophysical procedure. The relatively large SEM for ECAP measures suggests that alternative methods for determining final ECAP thresholds should be considered when relating these measures to psychophysical results. Specifically, it may be worthwhile to investigate the efficacy of using an adaptive procedure to obtain physiological thresholds.
B. Psychophysical versus physiological electrical-field interaction patterns
The present results represent a comprehensive first report of electrical-field interaction patterns obtained with the ECAP in human CI recipients. In general, results from the present study showed smaller threshold shifts (i.e., less interaction) with greater separation between simultaneously activated electrodes for both measures. These trends are consistent with previous psychophysical threshold studies (Favre and Pelizzone, 1993; Stickney et al., 2006; White et al., 1984) and with studies that measured spatial interaction physiologically using either EABR (Abbas and Brown, 1988) or cortical responses (Bierer and Middlebrooks, 2004).
It was hypothesized that ECAP threshold shifts would not be significantly different from psychophysical threshold shifts as a function of interaction electrode location. Paired t tests revealed no significant difference between ECAP and psychophysical threshold shifts for five of the nine subjects. However, for four individual subjects (see Table IV) and for group data, there was a significant difference in the amount of threshold shift between the two measures. Specifically, larger shifts occurred for psychophysical thresholds than for ECAP thresholds for three of those four subjects and for the group data as a whole. It is interesting to note that those three subjects (C1, C15, and C16) were the only subjects in this study who had either been born deaf or became deaf in early childhood. Two of those subjects (C1 and C16) were the only two subjects with a positioner. It seems unlikely, however, that the positioner would affect one measure and not the other.
Table IV.
Statistical results from paired t tests for psychophysical versus ECAP threshold shifts for data collapsed across the three probe electrodes within each subject (see Fig. 7). Asterisks denote statistical significance (p < 0.05). df = degrees of freedom.
| Subject | t | p | df |
|---|---|---|---|
| C1 | 4.05 | <0.001* | 21 |
| C6 | −2.38 | 0.03* | 21 |
| C7 | 0.64 | 0.53 | 21 |
| C8 | 1.57 | 0.13 | 21 |
| C10 | 1.13 | 0.27 | 21 |
| C11 | 1.77 | 0.09 | 21 |
| C13 | 0.57 | 0.58 | 21 |
| C15 | 3.30 | 0.004* | 20 |
| C16 | 9.40 | <0.001* | 21 |
For one subject (C1), ECAP thresholds in the presence of the in-phase interaction stimulus were higher than the probe-alone threshold for 5 of the 22 electrode pairs. This trend is opposite of what is expected, based on previous physiological findings. We would expect thresholds to be lower than the probe-alone condition due to summation of current fields resulting in less current needed from the probe electrode to elicit threshold (e.g., Abbas and Brown, 1988; Bierer and Middlebrooks, 2004; Gardi, 1985; Middlebrooks, 2004; White et al., 1984). Because threshold shifts were calculated as the probe-alone threshold minus the threshold obtained in the presence of the interaction stimulus, this resulted in negative threshold shifts for several of the ECAP measures. As a result, ECAP measures yielded smaller threshold shifts compared with psychophysical measures. It is possible that the higher thresholds obtained in the interaction condition may be attributed to the larger SEM observed with ECAP measures, as shown in Fig. 3A and discussed in the preceding section.
For the remaining two subjects (C15 and C16), psychophysical threshold shifts were not only larger than ECAP threshold shifts, but were also larger than the magnitude of the interaction stimulus for 5 of 21 and 10 of 22 electrode pairs, respectively (see Figs. 4–6). These subjects’ psychophysical responses were highly repeatable across blocks, so measurement variability was not an issue. In theory, a probe threshold shift should not be larger than the magnitude of the interaction stimulus. If it is, this suggests that a mechanism other than simple current summation is contributing to detection of the combined stimuli. In other words, the total amount of current delivered to the probe and interaction electrodes together is less than the amount of current needed to elicit threshold on the probe electrode alone. As the interaction stimulus is moved farther from the probe, the amount of electrical field overlap is reduced. However, the sub-threshold interaction stimulus may recruit enough individual nerve fibers that are adjacent to the region stimulated by the probe, potentially resulting in a broader excitation pattern. It is possible that sub-threshold neural activity in the vicinity of the interaction electrode produces more aggregate neural activity, leading to a lower perceptual detection threshold. This theory can be likened to the difference in neural recruitment patterns between monopolar and bipolar stimulation configurations, where lower current levels are needed for broader stimulation patterns due to an increase in the total number of neurons recruited. Perhaps psychophysical threshold shifts that are larger than the magnitude of the interaction stimulus indicate areas of greater neural survival or possibly a larger number of low-threshold, high-spontaneous-rate fibers in the region of the interaction electrode. Alternatively, the sub-threshold interaction stimulus may change the “center of gravity” of the summed electric field, which shifts the area of excitation toward the interaction electrode. This is the concept used to achieve intermediate or virtual channels using current steering (e.g., Donaldson et al., 2005; Firszt et al., 2007; Townshend et al., 1987; Wilson et al., 2003). It is possible that the threshold of the interaction electrode was lower than the threshold of the probe electrode, and detection was largely based on percepts in the vicinity of the interaction electrode. This was likely the case for C16, whose behavioral thresholds were lowest for the apical electrodes. Recall that the stimulus level for the interaction electrode was fixed at one-half the psychophysical threshold of the probe electrode. If the interaction electrode had a lower threshold than the probe (but still higher than the level of the interaction stimulus), it would take less current from the probe for stimulus detection in the region of the interaction electrode if electrical fields from both electrodes overlapped sufficiently. However, only one subject (C6) had a large enough difference in thresholds across the electrode array such that an interaction stimulus presented at one-half the threshold of one electrode would have been audible on another (interaction) electrode, and that subject did not exhibit psychophysical threshold shifts that were larger than the magnitude of the interaction stimulus on the apical electrodes (see Figs. 4–6).
In a previous study, we compared ECAP and psychophysical spatial interaction patterns obtained with non-simultaneous stimulation using a forward-masking paradigm (Hughes and Stille, 2008). Results showed that while there was a significant correlation between the two measures (r = 0.55, p < 0.0001), the correlation was not strong enough to suggest using ECAP measures alone to predict psychophysical electrical-field interaction patterns. In contrast, the present results showed a highly significant correlation (p < 0.001) between ECAP and psychophysical threshold shifts for all individual subjects (see Fig. 7) and for group data (r = 0.82, p < 0.0001). Two methodological differences between the Hughes and Stille (2008) study and the present study likely contributed to the higher correlation found in the present study. First, the previous study used non-simultaneous stimulation (forward masking) to measure spatial interaction patterns. Spatial interaction patterns measured with forward masking reflect the overlap of neural populations recruited by the masker and probe electrodes, as well as relative temporal aspects of the stimulated neurons (i.e., neural refractory-recovery mechanisms). The use of simultaneous stimulation in the present study allowed more direct assessment of spatial interaction, because it is presumed that electrical fields sum or subtract prior to neural activation. Therefore, temporal aspects related to neural refractory periods do not confound the relationship between ECAP and psychophysical threshold measures as they do with forward masking. Second, Hughes and Stille (2008) used fast-rate pulse trains for psychophysical measures and single pulses repeated at a relatively slow rate for ECAP measures. This resulted in the use of overall higher current levels for the ECAP measures due to less temporal integration. In the present study, care was taken to use stimuli that were as similar as possible (i.e., slow rate) for both ECAP and psychophysical measures to reduce confounding effects of stimulus current level associated with temporal integration. However, it should be emphasized that even if stimuli are similar for both ECAP and psychophysical threshold measures, there are differences in the respective underlying mechanisms. These differences likely contribute to the variance in the relationship between the two measures. The ECAP is a synchronized response of auditory nerve fibers to a single current pulse, which is repeated at a relatively slow rate to avoid neural refractory and adaptation effects in the averaged response. In contrast, psychophysical thresholds involve temporal integration across the duration of the pulse train. The findings of the present study are consistent with other studies that have shown stronger correlations between physiological and psychophysical measures when the same stimulus was used for both measures (e.g., Brown et al., 1994, 1996).
Another difference in results between the present study and the Hughes and Stille (2008) study is that the forward-masking study showed more masking overall for ECAP measures than for psychophysical measures; the opposite result was found for electrical-field interaction in the present study. In the Hughes and Stille (2008) study, the greater amount of masking for ECAP patterns was attributed to the use of higher current levels for ECAP than for psychophysical measures, which may have led to more current spread and thus more interaction (e.g., Eisen and Franck, 2005). In the present study, more interaction was measured psychophysically; however, the difference between ECAP and psychophysical measures was not as large as in the forward-masking study (Hughes and Stille, 2008). In general, the present results for simultaneous stimulation show a strong correlation between ECAP and psychophysical measures, which suggests that ECAP measures can be used as an effective alternative to time-consuming psychophysical measures of electrical-field interaction.
Although it was not the goal of this study to explicitly measure an optimal electrode separation that results in no interaction, it can be seen in Figs. 4–6 that electrode interactions were, in many cases, minimal at the largest electrode spacing. These results are consistent with those reported in several other studies (Favre and Pelizzone, 1993; Shannon, 1983, 1985; White et al., 1984). The partially-simultaneous High-Resolution sound processing (HiRes-P) used in current Advanced Bionics devices effectively doubles the overall stimulation rate by activating two electrodes at the same time that are located half the array apart. When HiRes programs are created, behavioral thresholds (T-levels) are typically set to zero or some small percentage (e.g., 10%) of the most-comfortable levels (M-levels). Thus, the two simultaneously activated electrodes could potentially be activated at sub-threshold levels, as was the case in the present study, or a combination of sub- and supra-threshold levels. Results from the present study can provide insight about channel interactions that may occur with a partially simultaneous strategy. As can be seen in Fig. 5, some subjects had virtually no interaction for electrodes spaced half the array apart (e.g., C1, C11, C13), whereas others had significant interactions (e.g., C10, C15, C16). These differences may account for differences in performance between the partially-simultaneous (HiRes-P) and fully sequential (HiRes-S) versions of HiRes within and across subjects (e.g., Buechner et al., 2005). Further research is needed to determine whether ECAP electrical-field interaction patterns such as those used here have potential clinical utility for determining an optimal processing strategy (e.g., HiRes-P versus HiRes-S) on an individual basis. It is also worth investigating whether these ECAP measures have clinical utility in estimating channel independence in regard to speech perception. That is, can these measures be used to estimate the number of channels that result in optimal speech-perception performance?
Finally, the presence of an electrode positioner did not seem to affect electrical-field interaction patterns for either ECAP or psychophysical measures. Only two subjects (C1 and C16) had an electrode positioner, so there is insufficient power for a statistical analysis. However, interaction patterns in Figs. 4–6 for those two subjects do not appear narrower than the other subjects who did not have a positioner. These results are consistent with those reported by Boëx et al. (2003) and Stickney et al. (2006), who compared electrical-field interactions for various electrode array types, including those with a positioner.
C. Effect of interaction stimulus phase
Several studies have reported results that support the notion of direct vector summation of current for in-phase and phase-inverted simultaneous stimulation of two electrodes; however, examples are seen in many of those studies in which the amount of interaction (or threshold shift) was not equal for in-phase versus phase-inverted conditions (e.g., Boëx et al., 2003; de Balthasar et al., 2003; Favre and Pelizzone, 1993; present study). Asymmetries may be due to differences in how the shape of the electrical field changes with level. The inverted-phase condition essentially produces a smaller resultant electrical field when fields of opposite polarity sum. Therefore, more current must be delivered to the probe electrode to compensate for the cancellation of current from the inverted-phase stimulus on the other electrode in order to achieve threshold. The shapes of the current fields produced by each electrode are likely to be slightly different due to differences in current level, electrode impedance, and geometry and impedance of the tissue in the vicinity of each electrode (e.g., Finley et al., 1990; Frijns et al., 1995, 1996; Kral et al., 1998; Ruddy and Loeb, 1995). Thus, the shape of the summed field may differ depending on the relative current levels of the two contributing electrodes. Differences in anatomical geometry, impedance, etc. across subjects may explain why some individuals showed asymmetry between phase conditions while others did not.
Results from Fig. 9 show that phase inversion did not affect psychophysical and ECAP measures in the same way for more than half of the subjects. Specifically, C8 demonstrated asymmetrical shifts for psychophysical thresholds but not for the ECAP, and four other subjects (C1, C6, C10, and C16) demonstrated asymmetrical threshold shifts for the ECAP but not psychophysically. Recall that psychophysical measures were obtained with cathodic-leading pulse trains presented to the probe electrode, whereas ECAP measures were obtained with pulses of alternating polarity for artifact reduction. Therefore, half of the ECAPs in each averaged response were obtained with anodic-leading pulses. A recent study by Macherey et al. (2008) showed anodic-leading pulses resulted in more effective electrical stimulation in human CI users than cathodic-leading pulses. It is not clear whether electrical fields from two electrodes stimulated with anodic-leading pulses will sum in the same way as electrical fields from two electrodes stimulated with cathodic-leading pulses. However, given the difference in physiological responses between cathodic and anodic stimulus polarity as reported by Machery et al. (2008), it is possible that the alternating polarity paradigm used for artifact reduction in the ECAP measures contributed to the differences seen in Fig. 9 between ECAP and psychophysical measures.
V. CONCLUSIONS
The primary purpose of this study was to determine whether physiological electrical-field interaction patterns measured with the ECAP are predictive of patterns measured psychophysically. Results showed a highly significant correlation (r = 0.82, p < 0.0001) between ECAP and psychophysical threshold shifts obtained with simultaneous stimulation of two electrodes. There was no significant difference between ECAP and psychophysical threshold shift for five of the nine subjects, which was consistent with the hypothesis. However, group data showed statistically significantly larger shifts for psychophysical thresholds than for the ECAP. In general, ECAP data predicted psychophysical data with enough confidence to suggest that ECAP measures can adequately predict psychophysical electrical-field interaction patterns for sub-threshold stimuli. ECAP data from the present study also showed higher thresholds occurred more often for recordings made from the basal side of the stimulating electrode compared with the apical side. The variability in ECAP thresholds and amplitudes across recording electrode locations suggests that ECAP measures should be averaged across more than one recording electrode location, particularly if ECAP measures are to be compared with psychophysical measures within subjects. Finally, phase inversion of the interaction stimulus resulted in asymmetrical threshold shifts for some subjects, particularly for ECAP measures. This finding may be attributed to differences in the relative current levels of the two contributing electrodes and how their respective electrical fields change shape with level. Phase inversion did not affect psychophysical and ECAP measures in the same way for more than half of the subjects. This result may be influenced by the alternating polarity paradigm used to elicit ECAP measures.
Acknowledgments
This research was funded by the NIH/NIDCD, grant R03 DC007017. Human-subjects recruitment was supported by the NIH/NIDCD grant P30 DC04662. The authors thank Hongyang Tan of Boys Town National Research Hospital and Leonid Litvak of Advanced Bionics Corporation for technical assistance with programs used for data analysis and collection, respectively. We also thank Donna Neff, Walt Jesteadt, and two anonymous reviewers for helpful comments on earlier drafts of this manuscript, and the nine subjects who participated in this study.
Footnotes
Portions of this work were presented in: Hughes, M. L. and Stille, L. J. (2007), “Channel interaction patterns with simultaneous stimulation: psychophysical and physiological measures,” in Auditory Research Bulletin, Advanced Bionics: Valencia, CA, pp. 70-71; and Hughes, M. L., Stille, L. J., and Neff, D. L., “Physiological and psychophysical channel interaction with simultaneous stimulation,” Abstracts of the 2007 Conference on Implantable Auditory Prostheses, Lake Tahoe, CA, July 15-20, 2007, p. 105.
PACS numbers: 43.64.Me, 43.64.Pg, 43.66.Cb, 43.66.Nm
Exceptions were P5, P9, and P12 for subject C1 and P9 in C7. For those initial two subjects, the interaction stimulus level was 20% of the difference between behavioral threshold and loudness comfort. This corresponded to 41% of probe threshold for P5 and P9 and 58% for P12 for subject C1, and 68% of P9 threshold for C7. This protocol was subsequently changed to one-half of the probe threshold to ensure the interaction stimulus level was sufficiently below threshold.
All statistical calculations reported in this study were made with Sigma Stat 3.0 (SPSS Inc., Chicago, IL). An alpha level of 0.05 was used to determine statistical significance.
This method was preferred over taking the absolute value because it captured instances where the threshold shift went in the opposite direction from what was expected (e.g., inverted-phase interaction stimulus produced thresholds that were lower than the no-interaction condition).
References
- Abbas PJ, Brown CJ. Electrically evoked brainstem potentials in cochlear implant patients with multi-electrode stimulation. Hear Res. 1988;36:153–162. doi: 10.1016/0378-5955(88)90057-3. [DOI] [PubMed] [Google Scholar]
- Abbas PJ, Brown CJ, Hughes ML, Etler CP, Behrens A, Dunn SM. The electrically evoked compound action potential: channel interaction measures. Abstracts of the 2003 Conference on Implantable Auditory Prostheses; Pacific Grove, CA. 2003. p. 41. [Google Scholar]
- Abbas PJ, Brown CJ, Shallop JK, Firszt JB, Hughes ML, Hong SH, Staller SJ. Summary of results using the nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20:45–49. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Bierer JA. Threshold and channel interaction in cochlear implant users: Evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–1653. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Cortical responses to cochlear implant stimulation: Channel interaction. J Assoc Res Otolaryngol. 2004;5:32–48. doi: 10.1007/s10162-003-3057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boëx C, de Balthasar C, Kós MI, Pelizzone M. Electrical field interactions in different cochlear implant systems. J Acoust Soc Am. 2003;114:2049–2057. doi: 10.1121/1.1610451. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Borland J, Bertschy MR. Electrically evoked whole nerve action potentials in Ineraid cochlear implant users: Responses to different stimulating electrode configurations and comparison to psychophysical responses. J Speech Hear Res. 1996;39:453–467. doi: 10.1044/jshr.3903.453. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Fryauf-Bertschy H, Kelsay D, Gantz BJ. Intraoperative and postoperative electrically evoked auditory brain stem responses in Nucleus cochlear implant users: Implications for the fitting process. Ear Hear. 1994;15:168–176. doi: 10.1097/00003446-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Buechner A, Brendel M, Krüeger B, Frohne-Büchner C, Nogueira W, Edler B, Lenarz T. Current steering and results from novel speech coding strategies. Otol Neurotol. 2008;29:203–207. doi: 10.1097/mao.0b013e318163746. [DOI] [PubMed] [Google Scholar]
- Buechner A, Frohne-Büchner C, Stoever T, Gaertner L, Battmer RD, Lenarz T. Comparison of a paired or sequential stimulation paradigm with Advanced Bionics’ High-Resolution mode. Otol Neurotol. 2005;26:941–947. doi: 10.1097/01.mao.0000185069.27705.f0. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Richardson LM. Spatial spread of neural excitation: Comparison of compound action potential and forward-masking data in cochlear implant recipients. Int J Audiol. 2004;43:346–355. doi: 10.1080/14992020400050044. [DOI] [PubMed] [Google Scholar]
- de Balthasar C, Boëx C, Cosendai G, Valentini A, Sigrist A, Pelizzone M. Channel interactions with high-rate biphasic electrical stimulation in cochlear implant subjects. Hear Res. 2003;182:77–87. doi: 10.1016/s0378-5955(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users (L) J Acoust Soc Am. 2005;118:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrode interaction in pediatric cochlear implant subjects. J Assoc Res Otolaryngol. 2005;6:160–170. doi: 10.1007/s10162-005-5057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre E, Pelizzone M. Channel interactions in patients using the Ineraid multichannel cochlear implant. Hear Res. 1993;66:150–156. doi: 10.1016/0378-5955(93)90136-o. [DOI] [PubMed] [Google Scholar]
- Finley CC, Wilson BS, White MW. Models of neural responsiveness to electrical stimulation. In: Miller JM, Spelman FA, editors. Cochlear implants: Models of the electrically stimulated ear. Springer-Verlag; New York: 1990. pp. 55–93. [Google Scholar]
- Firszt J, Burton Koch D, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol Neurotol. 2007;28:629–636. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, Briaire JJ, de Laat JAPM, Grote JJ. Initial evaluation of the Clarion CII cochlear implant: Speech perception and Neural Response Imaging. Ear Hear. 2002;23:184–197. doi: 10.1097/00003446-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, de Snoo SL, Schoonhoven R. Potential distributions and neural excitation patterns in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res. 1995;87:170–186. doi: 10.1016/0378-5955(95)00090-q. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, de Snoo SL, ten Kate JH. Spatial selectivity in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res. 1996;95:33–48. doi: 10.1016/0378-5955(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Gardi JN. Human brain stem and middle latency responses to electrical stimulation: Preliminary observations. In: Schindler RA, Merzenich MM, editors. Cochlear Implants. Raven Press; New York: 1985. pp. 351–363. [Google Scholar]
- Hughes ML, Stille LJ. Psychophysical versus physiological spatial forward masking and the relation to speech perception in cochlear implants. Ear Hear. 2008;29:435–452. doi: 10.1097/AUD.0b013e31816a0d3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: The electrical field and excitation of auditory afferents. Hear Res. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Macherey O, Carlyon RP, Van Wieringen A, Deeks JM, Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116:452–568. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Ruddy HA, Loeb GE. Influence of materials and geometry on fields produced by cochlear electrode arrays. Med Biol Eng Comp. 1995;33:793–801. doi: 10.1007/BF02523011. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hear Res. 1983;12:1–16. doi: 10.1016/0378-5955(83)90115-6. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Loudness summation as a measure of channel interaction in a cochlear prosthesis. In: Schindler RA, Merzenich MM, editors. Cochlear Implants. Raven Press; New York: 1985. pp. 323–334. [Google Scholar]
- Stickney GS, Loizou PC, Mishra LN, Assmann PF, Shannon RV, Opie JM. Effects of electrode design and configuration on channel interactions. Hear Res. 2006;211:33–45. doi: 10.1016/j.heares.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Townshend B, Cotter N, Van Compernolle D, White RL. Pitch perception by cochlear implant subjects. Acoust Soc Am. 1987;82:106–115. doi: 10.1121/1.395554. [DOI] [PubMed] [Google Scholar]
- White MW, Merzenich MM, Gardi JN. Multichannel cochlear implants: Channel interactions and processor design. Arch Otolaryngol. 1984;110:493–501. doi: 10.1001/archotol.1984.00800340005002. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Wolford R, Schatzer R, Sun X, Lawson D. Speech processors for auditory prostheses. NIH Contract N01-DC-2-1002, 7th Quarterly Progress Report; October 1 – December 31, 2003.2003. [Google Scholar]