Abstract
BACKGROUND
Sorafenib is an oral antiangiogenic agent administered in advanced-stage hepatocellular carcinoma (HCC). Based on preclinical and human studies, we hypothesized that, in addition to its antiangiogenic properties, sorafenib may beneficially reduce the extent of the immunosuppressive network in HCC patients. To test this hypothesis, we examined whether alterations in the immunosuppressive burden of advanced-stage HCC patients correlated with clinical outcome.
METHODS
In before and after sorafenib treatment, blood samples collected from 19 patients with advanced HCC, the frequency of PD-1+ T cells, Tregs, and myeloid derived suppressor cells (MDSC) were quantified by multiparameter FACS. Cytokine levels in plasma were determined by ELISA.
RESULTS
Overall survival (OS) was significantly impacted by the reduction in the absolute number of both CD4+PD-1+ T cells and CD8+PD-1+ T cells following sorafenib treatment. Significant decreases in the frequency and absolute number of Foxp3+ Tregs were also observed, and a statistically significant improvement in OS was noted in patients exhibiting a greater decrease in the number of Foxp3+ Tregs. The ratio of CD4+CD127+PD-1− T effector cells to CD4+Foxp3+PD-1+ Tregs was significantly increased following treatment with sorafenib. Increased frequency of CD4+CD127+ T effector cells in the posttreatment samples significantly correlated with OS.
CONCLUSION
This study is the first to our knowledge to demonstrate the potent immunomodulatory effects of sorafenib therapy on PD-1+ T cells and Tregs and the ensuing correlation with survival. These phenotypes could serve as predictive biomarkers to identify HCC patients who are likely to benefit from sorafenib treatment.
TRIAL REGISTRATION
Registration is not required for observational studies.
FUNDING
This study was supported by NCI Core Grant to RPCI (NIH P30 CA016056) and discretionary funds to Y. Thanavala.
Introduction
Sorafenib, considered as the current backbone of hepatocellular carcinoma (HCC) treatment, is an oral small molecule inhibitor of several tyrosine protein kinases, targeting VEGF receptors (VEGFR), platelet-derived growth factor receptor-β, c-Kit, and Flt-3 (1). VEGF was originally identified as a tumor-secreted factor that increased vascular permeability, promoted angiogenesis, and thus facilitated tumor growth. However, the role of VEGF on the biology of immune cells, particularly Tregs, has only been recently appreciated (2).
Tumors exploit multiple immunosuppressive pathways to actively evade immune recognition, including endogenous “immune checkpoints” that normally terminate immune responses after antigen activation. This knowledge has resulted in a concerted effort to develop targeted immunotherapeutic approaches to cancer by the blockade of immune checkpoint receptors. PD-1 is an inhibitory checkpoint receptor expressed on T cells after chronic antigenic stimulation. Engagement of PD-1 on T cells with PD-L1 on tumor cells downregulates antitumor T cell responses (3, 4). Upregulation of PD-L1 by neoplastic cells allows tumors to escape the antitumor effector T cell responses. Therefore, recent efforts in immunotherapy of cancer have focused on activating the dampened immune system by inhibiting the immune checkpoint pathways responsible for T cell paralysis. Clinical efficacy of the PD-1–PD-L1 axis as a therapeutic target has been validated in several cancers (5–7). However, little information is available on the relative contribution of PD-1 inhibitory pathways to the dampened antitumor T cell responses or the impact of PD-1 blockade on reinvigoration of exhausted T cells in HCC patients. We and others have reported that the magnitude of antitumor T cell responses is severely compromised in advanced HCC patients by redundant immunosuppressive pathways comprising Tregs, myeloid derived suppressor cells (MDSC), PD-1+ T effector cells, and inhibitory cytokines (8–10). We have also demonstrated that high expression of PD-1 on exhausted T cells contributes to ineffective effector T cell function, and selective in vitro depletion of the immunosuppressive cells resulted in moderate improvement of T effector cell function in HCC patients (11).
Sorafenib targets multiple kinase receptors — including VEGF, c-Kit, and Flt-3 — which are abundantly expressed on Tregs and MDSC (2, 12–14). Expression of these receptors on immunosuppressive cell subsets provided the rationale for our hypothesis that, in addition to targeting angiogenesis, sorafenib treatment may also reduce the immunosuppressive burden in HCC patients and thereby invigorate antitumor effector T cell function. Our study demonstrates, for the first time to our knowledge, the immunomodulatory effect of sorafenib in reducing the number of PD-1+ T cells with survival benefit. Furthermore, the measurement of the phenotype and functional activity of T cells before treatment and their modulation following sorafenib therapy could serve as a prognostic biomarker to identify HCC patients who may benefit from this treatment alone or as a combination therapy regimen.
Results
Reduction in PD-1+ T cells following treatment with sorafenib
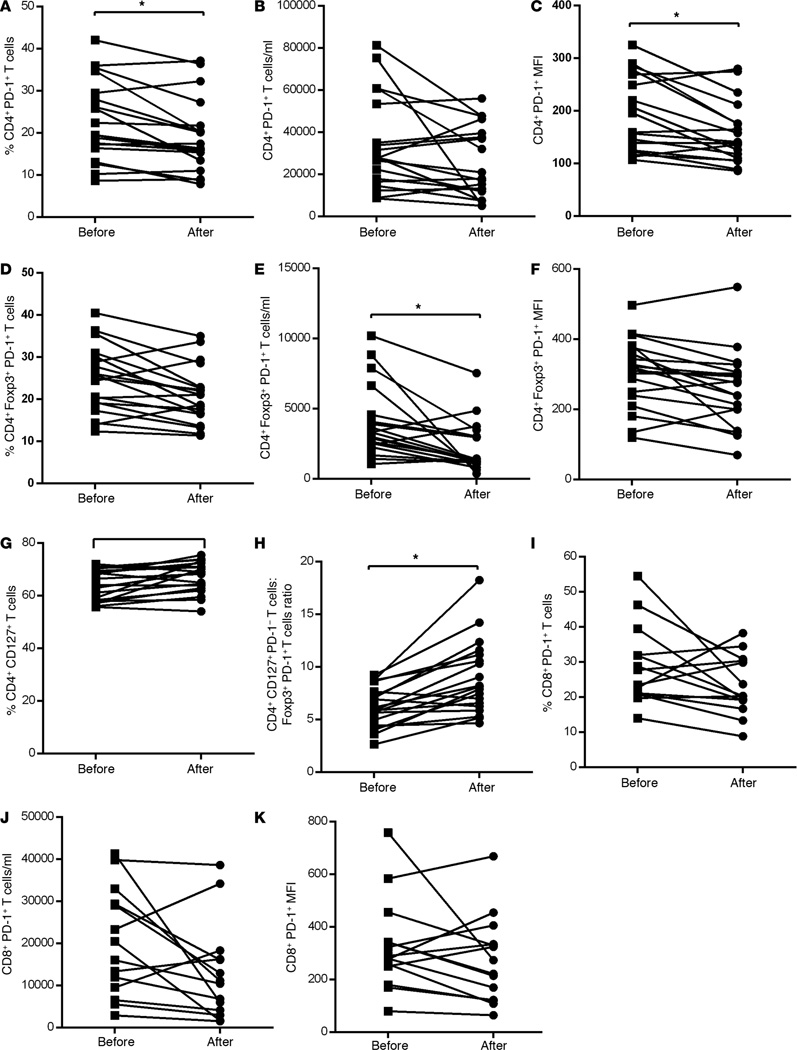
We investigated the effect of sorafenib treatment on PD-1 expression by CD4+ T cells in HCC patients and observed that the frequency of CD4+PD-1+ T cell was significantly reduced (P = 0.01, Figure 1A, and Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/jci.insight.86182DS1.). The reduction in the absolute numbers of this phenotype was not significant (Figure 1B). PD-1 expression levels (mean fluorescence intensity [MFI]) on CD4+ T cells were also significantly decreased (P = 0.02, Figure 1C). The reduction in the frequency of CD4+PD-1+ T cells and PD-1 expression levels on CD4+ T cells did not show significant association with OS (Supplemental Figure 4, G and H). This study is a demonstration of a beneficial reduction in CD4+ T cell PD-1 expression by a multikinase inhibitor. Additionally, we also demonstrate that the frequencies and absolute numbers of CD4+Foxp3+PD-1+ T cells after sorafenib therapy were diminished and the reduction in the absolute numbers was statistically significant (Figure 1, D and E, respectively; P = 0.04). Of note, PD-1 expression levels on Foxp3+ Tregs was also reduced in the posttreatment samples (Figure 1F), suggesting that the mechanism by which sorafenib mediates the reduction in surface PD-1 expression can also be extended among a wide lineage of activated T cells. Nevertheless, reduction in any of the above immune parameters after sorafenib therapy did not correlate with OS of the patients (Supplemental Figure 4, I–K). Sorafenib had a mixed effect on the percentage of CD4+CD127+ T effector cells, with half of the patients showing an increase in frequency (Figure 1G). Importantly, the ratio of the percentage of CD4+CD127+PD-1− T effector cells to the percentage of CD4+Foxp3+PD-1+ Tregs was also significantly increased following treatment (P=0.03, Figure 1H). The frequency and absolute number of CD8+PD-1+ T cells or PD-1 expression on CD8+ T cells measured as MFI showed decreases after treatment but did not reach statistical significance (P = 0.20, P = 0.15, and P = 0.31, respectively, Figure 1, I–K). The decrease in the frequency of CD8+PD-1+ T cells did not correlate with OS of the patients (Supplemental Figure 4L).
Figure 1. Decrease in PD-1+ T cells in HCC patients after sorafenib treatment.
Immunophenotypic analysis of T cells was performed using PBMC isolated from HCC patients before and after sorafenib treatment by multicolor flow cytometry. Frequencies of immune cell subsets were calculated on CD3+CD4+ or CD3+CD8+ population. (A) Frequency of CD4+PD-1+ T cells. (B) Absolute number of CD4+PD-1+ T cells. (C) PD-1 expression levels (MFI) on CD4+ T cells. (D) Frequency of Foxp3+PD-1+ T cells. (E) Absolute number of Foxp3+PD-1+ T cells. (F) PD-1 expression levels (MFI) on Foxp3+ T cells. (G) Frequency of CD4+CD127+PD-1− T effector cells. (H) Ratio of CD4+CD127+PD-1− T cells to CD4+Foxp3+PD-1+ T cells (CD4+CD127+PD-1−/CD4+Foxp3+PD-1+ T cells). (I) Frequency of CD8+PD+ T cells. (J) Absolute number of CD8+PD-1+ T cells. (K) PD-1 expression levels (MFI) on CD8+ T cells. Each symbol represents an individual HCC patient before or after sorafenib treatment. n = 19, * P < 0.05, permutation paired t test.
Twelve of 19 patients included in our study were hepatitis C virus–positive (HCV+), and we observed that the ratio of the percentage of CD4+CD127+ T effector cells to the percentage of CD4+Foxp3+ PD-1+ T cells was higher in the pretreatment samples of HCV+ patients (median, 3.4; range, 1.9–1.6) as compared with HCV− patients (median, 2.5; range, 1.7–3.9), and this was significant (P = 0.05). A comparison of peripheral blood Tregs/T effector cells with intrahepatic Tregs or T effector cells could not be performed in these patients with advanced disease who present with poor liver function, since tumor biopsies for translational studies could impact their current standard of care or quality of life.
Sorafenib treatment reduces number and frequency of Tregs
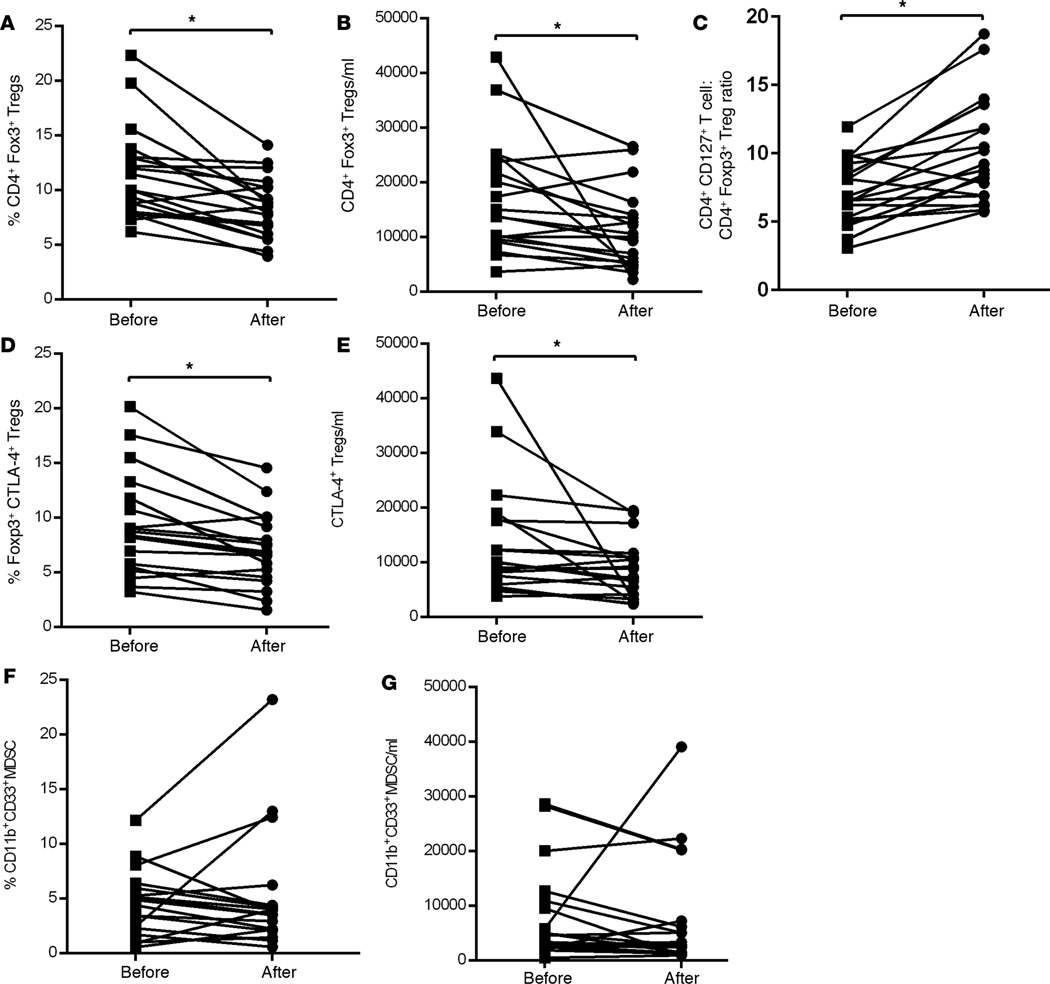
A significant decrease in both the frequency and absolute numbers of CD3+CD4+Foxp3+ Tregs was observed in samples collected after sorafenib treatment (P= 0.02, P = 0.05, respectively; Figure 2, A and B, and Supplemental Figure 1A). However, decrease in the frequency of Tregs did not correlate with OS of the patients (Supplemental Figure 4A). In addition to the decline in Treg frequencies, there was a significant increase in the ratio of CD4+CD127+ T effector cells to Tregs (P = 0.01, Figure 2C).
Figure 2. Decrease in Tregs and MDSCs after sorafenib therapy in HCC patients.
(A) Frequency and (B) absolute numbers of CD4+Foxp3+ Tregs in the peripheral blood of HCC patients. (C) Ratio of CD4+CD127+ T cells to CD4+Foxp3+ T cells (CD4+CD127+/CD4+Foxp3+ T cells). (D) Frequency and (E) absolute numbers of Foxp3+CTLA-4+ Tregs in HCC patients. (F) Frequency and (G) absolute numbers of MDSC in HCC patients. Each symbol represents an individual HCC patient before or after sorafenib treatment. Frequencies of Tregs and T effector cells were calculated based on CD3+CD4+ T cell population and MDSC based on CD14−HLA-DR− population. n = 19, * P < 0.05, permutation paired t test.
While there was marked reduction in both the frequency and the absolute number of Foxp3+CTLA-4+ Tregs following sorafenib therapy (P = 0.02, P = 0.05, respectively, Figure 2, D and E), this did not correlate with any improvement in overall survival (OS; log-rank, P = 0.51, P = 0.30, Supplemental Figure 4, B and C).
Overall, sorafenib treatment has a beneficial effect on reducing PD-1 expression and putative Treg suppressive phenotype, which prompted us to perform further mechanistic experiments described in Figure 3.
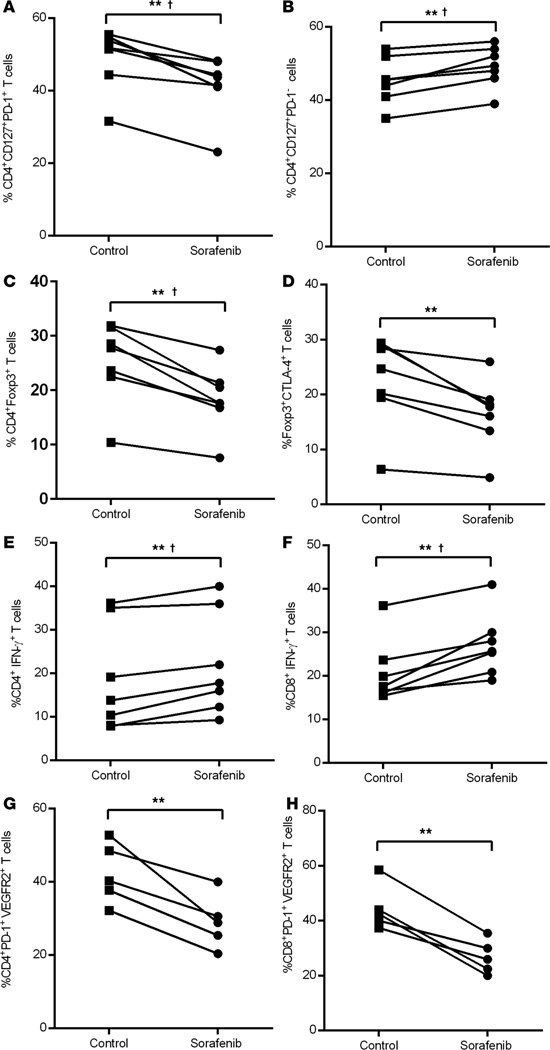
Figure 3. Effect of in vitro treatment of sorafenib on frequencies of T effector cells and Tregs.
Pretreatment samples of PBMCs from HCC patients were stimulated with anti-CD3/CD28 in the presence or absence of 10 μM concentration of sorafenib in vitro for 48 hours, and the frequencies of (A) CD4+CD127+PD-1+ T effector cells, (B) CD4+CD127+PD-1− T cells, (C) CD4+Foxp3+ Tregs, (D) Foxp3+CTLA-4+ Tregs, (E) CD4+IFN-γ+ T cells, (F) CD8+IFN-γ+ T cells, (G) CD4+PD-1+VEGFR2+ T cells, and (H) CD8+PD-1+VEGFR2+ T cells were determined by flow cytometry as described in Methods. Each symbol represents an individual HCC patient: anti-CD3/CD28–treated (control) or sorafenib-treated PBMC. **P < 0.005, paired t test, †P < 0.003 statistically significant after Hommel multiplicity adjustment (n = 7 for A–F, n = 5 for G and H).
Effect of sorafenib treatment on MDSC levels
On average, treatment did not significantly influence either the frequency or the absolute number of CD11b+CD33+ MDSC in HCC patients (P = 0.61, P = 0.92, respectively, Figure 2, F and G). Representative staining and gating of MDSC from HCC patients before and after sorafenib therapy are shown in Supplemental Figure 1B. Reduction in the frequency and absolute number of MDSC following sorafenib treatment did not show significant correlation with OS (log-rank, P = 0.29, P = 0.09, respectively, Supplemental Figure 4, E and F).
Immunomodulatory effect of sorafenib in vitro
To directly address the hypothesis that sorafenib treatment has an unbiased effect on mitigating the immunosuppressive qualities of all T cell lineages, unsorted T cells from peripheral blood monocytes (PBMC) were activated via the T cell receptor (TCR) in the presence or absence of 10 μM sorafenib or 10 μg/ml of anti-human VEGF antibody. Dose response experiments of sorafenib established the optimal in vitro activity at 10 μM concentration of sorafenib, and hence, this concentration was used in subsequent experiments (Supplemental Figure 1C). Examining the impact on these bulk T cells as a model of the nonselective effect of sorafenib revealed a marked reduction in the frequency of CD4+CD127+PD-1+ T cells, whereas the frequency of CD4+CD127+PD-1− T cells was enhanced by sorafenib treatment. This finding confirms that the beneficial effect of sorafenib treatment can be directly attributed to the pathway-specific downmodulation of PD-1 expression on CD4+ T cells rather than nonspecific cytotoxicity of sorafenib on T cells (P = 0.02, Figure 3, A and B). The lack of cytotoxicity was further corroborated by the use of a viability-exclusion dye (FVS V450) that demonstrated equivalent cell viability in both sorafenib-treated and untreated cells (Supplemental Figure 2, A and B). Additionally, we observed a significant reduction in the frequency of Foxp3+ Tregs and CTLA-4+ Tregs following in vitro treatment with sorafenib (P = 0.02 for both, Figure 3, C and D) with concomitant increase in IFN-γ production by CD4+ and CD8+ T cells (Supplemental Figure 2, C–H; P = 0.02, P = 0.05, Figure 3, E and F, respectively). VEGFR2 expression on both CD4+PD-1+ and CD8+PD-1+ T cells was significantly reduced in sorafenib-treated PBMC as compared with control (Figure 3, G and H, respectively, and Supplemental Figure 2 , I and J). The targeted capacity of sorafenib to restrict PD-1+ T cell frequency compared with PD-1− T cells in HCC patients is, to our knowledge, a new immunomodulatory function of this multikinase inhibitor. This finding represents an alternative method to PD-1 blockade in order to prevent accumulation of PD-1+ T cells, while simultaneously promoting increased frequency of effector T cells unburdened with the potential to undergo suppression by cognate PD-1 signaling.
Blocking of VEGF/VEGFR2 pathway downregulates Tregs and PD-1+ T cells
We further examined the mechanism by which sorafenib treatment imparts a beneficial reduction in the PD-1 signaling pathway. Given that sorafenib primarily targets angiogenic signaling, we examined whether the absence of soluble VEGF in cultures of activated T cells recapitulated the effect of sorafenib treatment. We observed significant reduction in the frequency of CD4+CD127+PD-1+ T cells with concomitant enhancement in the frequency of CD4+CD127+PD-1− T cells in cells treated in vitro with anti-VEGF antibody (Supplemental Figure 3, A and B). Furthermore, the frequencies of Foxp3+ and CTLA-4+ Tregs (Supplemental Figure 3, C and D) and expression of VEGFR2 on exhausted T cells declined significantly in anti-VEGF antibody–treated PBMC (Supplemental Figure 3, E and F). These results show that targeting the VEGFA/VEGFR axis using antiangiogenic molecules is sufficient to reduce PD-1 expression on exhausted T cells.
Effect of sorafenib treatment on cytokine levels
Significant decreases in the levels of immunosuppressive cytokines IL-10 and TGF-β1 were noted after treatment (P = 0.03, P = 0.03, respectively, Supplemental Figure 5, A and B). In contrast, the levels of IFN-γ were significantly increased after sorafenib treatment (P = 0.04, Supplemental Figure 5C). Change in IL-1β levels was not statistically significant (P = 0.11, Supplemental Figure 5D). The changes in the levels of cytokines did not translate to changes in OS (log-rank, P = 0.73, P= 0.61, P = 0.62, P = 0.92; Supplemental Figure 4, M–P, respectively.)
Reduction in PD-1+ T cells and Tregs after sorafenib treatment correlates with OS
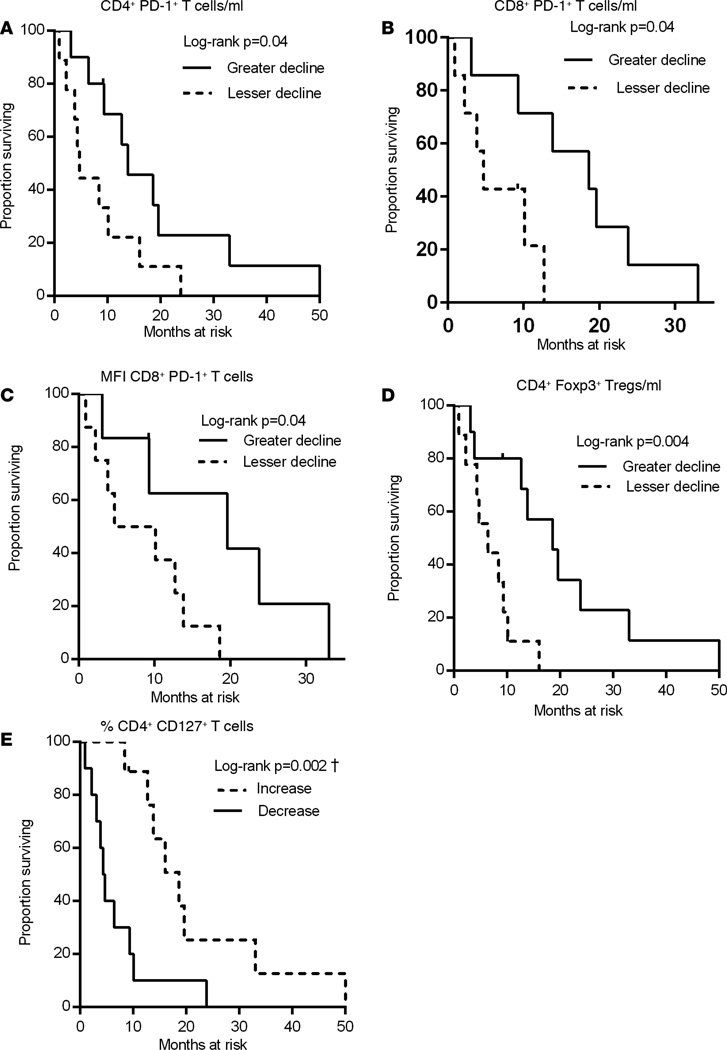
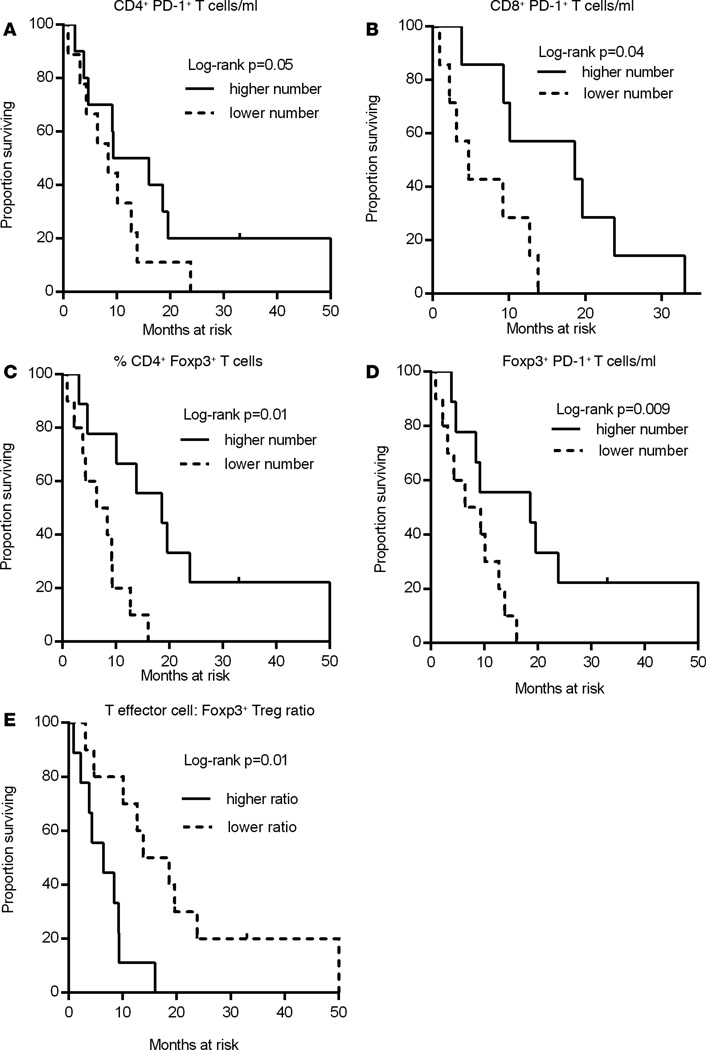
Patients were stratified into 2 groups based on the ratio between before-and-after measurements of PD-1 expression levels on CD4+ T cells following sorafenib treatment. Patients with greater decline in circulating CD4+PD-1+ T cells achieved significantly improved OS compared with patients with lesser decline (log-rank P = 0.04, Figure 4A). There was also a significant association between OS and a greater decline both in the absolute number of CD8+PD-1+ T cells and PD-1 MFI on CD8+ T cells after treatment (log-rank, P = 0.04 for both, Figure 4, B and C). These data suggest that preexisting T cell immunity is suppressed by PD-1 on T cells of HCC patients and T cells may be reinvigorated by downregulation of PD-1 following sorafenib treatment. Our data demonstrate the plasticity of PD-1 expression on activated effector T cells in cancer patients. To our knowledge, this plasticity has not been reported in the literature and offers a novel method to leverage the accumulation of PD-1+ T cells in cancer patients into reinvigorated effector T cells with the capacity to exert an effective antitumor response. Patients having higher numbers of CD4+PD-1+ T cells and PD-1+CD8+ T cells before initiation of sorafenib treatment achieved significant improvement in OS compared with patients with lower numbers of these phenotypes (log-rank, P = 0.05, P = 0.04, Figure 5, A and B, respectively). These results indicate that patients with increased numbers of PD-1+ T cells before treatment initiation are more responsive to sorafenib therapy. Therefore, increased numbers of this phenotype in pretreatment blood samples may be a biomarker of prognostic significance. The most critical aspect of PD-1 reduction is the association with improved OS in patients exhibiting decreased PD-1 expression after sorafenib therapy.
Figure 4. Kaplan-Meier plots illustrating the impact of the sorafenib-induced decrease in PD-1+ T cells or Tregs on OS.
The association between OS and the change in the immune parameters of HCC patients was calculated as described in Methods. (A) Absolute numbers of CD4+PD-1+ T cells. Median Pre = 28.05, Post = 18.08, median of ln (1 + Post) − ln ( 1 + Pre) = −0.24. (B) CD8+PD-1+ T cells. Median Pre = 18.27, Post = 10.84, median of ln (1 + Post) − ln (1 + Pre) = −0.46. (C) Expression levels of PD-1 (MFI) on CD8+ T cells. Median Pre = 285, Post = 248, median of ln (1 + Post) – ln (1 + Pre) = −0.32. (D) Absolute numbers of CD4+Foxp3+ T cells. Median Pre = 13.84, Post = 10.01, median of ln (1 + Post) − ln (1 + Pre) = −0.32. (E) Frequency of CD4+CD127+ T cells. Median Pre = 73.60, Post = 74.40, median of ln (1 + Post) − ln (1 + Pre) = 0.01. †P < 0.003 statistically significant after Hommel multiplicity adjustment.
Figure 5. Kaplan-Meier plots showing the predictive immune correlates of response to sorafenib treatment in HCC patients.
Plots A–E show the correlation between immune parameters before sorafenib treatment and patient outcome. The association between immune markers and OS was calculated as described in Methods. (A) Absolute numbers of CD4+PD-1+ T cells; median = 28.05. (B) Absolute numbers of CD8+PD-1+ T cells; median = 18.27. (C) Frequency of CD4+ Foxp3+ T cells; median = 10.0. (D) Absolute numbers of CD4+Foxp3+ T cells; median =13.84. (E) CD4+CD127+ T effector cell/CD4+ Foxp3+ T cell ratio; median = 6.58.
The reduction in the absolute numbers of Tregs quantified in postsorafenib treatment samples correlated strongly with OS, suggesting that sorafenib reduces Treg numbers in patients who respond to therapy (log-rank, P = 0.004, Figure 4D). Additionally, patients having higher frequencies of CD4+Foxp3+ T cells and greater numbers of Foxp3+PD-1+ T cells before the initiation of sorafenib therapy had significantly improved OS as compared with those patients having lower frequencies or numbers of these phenotypes (log-rank, P = 0.01, P = 0.009, respectively, Figure 5, C and D). Therefore, higher frequencies of Foxp3+ Tregs in the pretreatment samples may represent a predictive immune correlate of responsiveness to sorafenib treatment.
The importance of the enhancement in the T effector/Treg ratio achieved following treatment (Figure 2C) was additionally reflected by the significant association of increased CD4+CD127+ T effector cell frequencies to OS (log-rank, P = 0.002, Figure 4E). Patients having a low ratio of CD4+CD127+ T effector cells to CD4+ Foxp3+ T cells before sorafenib treatment achieved significant improvement in OS as compared with those having higher ratio of these phenotypes (log-rank, P = 0.01, Figure 5E). These results corroborate with improved OS reported in patients with higher frequencies of Foxp3+ Tregs in their pretreatment samples (Figure 5C).
Discussion
Previous studies from our laboratory provided the first demonstration that PD-1+ T cells, Tregs, and MDSC are major factors contributing to immune dysfunction in HCC patients (8, 11). These critically important cells of the immune system are exploited by tumors to maintain an immunosuppressive microenvironment and promote tumor growth. In this study, we investigated the prognostic relevance of these circulating immune cell subsets and their relative impact on OS of advanced HCC patients treated with sorafenib. We have demonstrated that sorafenib therapy reduces the frequency and expression pattern of immune checkpoint receptors, Tregs, MDSC, and the levels of immunosuppressive cytokines.
The critical role of the PD-1 pathway as a deterrent to antitumor immunity has now been validated in clinical studies using monoclonal antibodies blocking PD-1 (5–7). PD-1 blockade mediated rapid and durable regressions in patients with treatment-refractory solid tumors and nonimmunogenic tumors. In this context, our study represents the first demonstration that anti-VEGF therapy using sorafenib resulted in statistically significant reduction of CD4+PD-1+ T cells in a subset of HCC patients. Greater decreases in the numbers of CD4+PD-1+ T cells or CD8+PD-1+ T cells after sorafenib therapy were correlated with better OS. Conversely, sorafenib treatment resulted in significant improvement in the OS of patients having higher numbers of CD4+PD-1+ T cells and CD8+PD-1+ T cells before treatment initiation.
Though a recently published study in a murine HCC model observed a reduction in PD-1+CD8+ T cells via sorafenib treatment (15), our data is the first demonstration to our knowledge in HCC patients and further reveals a reversible aspect of PD-1 expression. Recently, the role of VEGF in increasing PD-1 expression on tumor antigen–specific T cells and downregulation of PD-1 expression by blockade of the VEGFA/VEGFR axis has been demonstrated in an experimental tumor model of colon cancer (16). We have demonstrated the downregulation of VEGFR2 on exhausted human CD4+ and CD8+ T cells after in vitro exposure to sorafenib or anti-VEGF antibody, which suggests that blockade of the VEGF/VEGFR2 pathway may decrease PD-1 expression on T cells. In a separate study, we have observed significant reduction in ERK2 phosphorylation of endothelial progenitor cells (EPCs) in HCC patients after sorafenib therapy (unpublished work). A previous study has shown the downregulation of ERK2 phosphorylation in the PBMC of HCC patients treated with sorafenib alone or in combination with octreotide long-acting release (LAR) (17). ERK is the downstream target of sorafenib, and signaling via VEGFA/VEGFR2 is critical for the phosphorylation of ERK2, which is being inhibited by sorafenib. Based on these findings, we postulate that one potential mechanism of action by which sorafenib regulates PD-1 expression on T cells could be achieved by inhibiting the VEGFA/VEGFR signaling pathway.
In our in vitro experiments, sorafenib treatment resulted in a significant reduction in the frequency of CD4+CD127+PD-1+ T effector cells. Importantly, sorafenib treatment did not cause a decline in the frequency of CD4+CD127+PD-1− T effector cells, which ostensibly may comprise a population of activated Th cells required for sustaining antitumor immune responses by CD8+ T cells. In our in vitro experiments, significant enhancement in IFN-γ production by CD4+ and CD8+ T cells observed after sorafenib treatment supports this notion. The increased ratio of CD4+CD127+PD-1− T effector cells to Tregs also indicates that immunosuppression by Tregs may have been negated; this is further validated by the increased CD4+CD127+ T effector cell frequency after sorafenib treatment correlating with improved OS. These results indicate that the reduced frequency of exhausted CD4+ T effector cells observed after treatment is not due to nonspecific cytotoxicity of sorafenib. Importantly, significant improvement in OS achieved by patients having higher numbers of both CD4+PD-1+ and CD8+PD-1+ T cells before sorafenib therapy indicates that elevated pretreatment levels of these phenotypes are associated with better responsiveness to therapy. Our results suggest that monitoring of the dynamics of PD-1–expressing T cell frequencies could serve as an important biomarker to predict the clinical efficacy of antiangiogenic therapy in HCC patients.
A recent study in renal cell carcinoma patients treated with sunitinib and bevacizumab reported increased infiltration of Tregs into the tumor microenvironment associated with decreased survival of patients (18). However, the above study lacked analysis of paired before-and-after treatment samples to allow conclusions on the kinetics of Tregs or effector T cell infiltration. Increased infiltration of Tregs relative to T effector cells after antiangiogenic therapy reported in the above study contrasts with our findings in sorafenib-treated HCC patients, in whom the frequency of effector T cells is elevated compared with Tregs and the PD-1 expression on the T cells is decreased. Importantly, the unique aspect of our study is the kinetic analysis in all patients of circulating immune cells, revealing the underappreciated impact of anti-VEGF therapy on effector T cell phenotype. Since neither liver nor tumor biopsy are standard of care for patients with advanced HCC, this precluded the analysis of effector T cells within the tumor. The analysis of matched before-and-after treatment circulating T cells used in our study suggests that the effector/Treg ratio is beneficially skewed toward sustained antitumor immunity rather than immunosuppression with important clinical consequences. Reduction in PD-1 expression on T cells is also critical, since antitumor activity of these cells could be potentially compromised within the tumor microenvironment due to the cognate expression of programmed death-ligand 1 (PD-L1) by HCC tumor cells (19). Thus, the immune-mediated beneficial outcome in sorafenib-treated HCC patients is a result of increased effector T cell frequency and the reduction of PD-1 expression on the effector T cells. This favorable change in immune phenotype after sorafenib therapy suggests several potential avenues by which to pursue combination therapy for sustained antitumor immunity, with the potential for tumor regression or cure.
In earlier studies, we showed that T effector cell function in advanced HCC patients can be partially restored by targeted removal of highly suppressive Tregs (8, 11). In the present study, sorafenib treatment decreased the absolute number of Tregs, which correlated with OS. The pivotal role of the VEGF signaling pathway in Treg development and the VEGF-mediated escape mechanism of tumors by directly triggering Treg proliferation has been extensively studied in animal models. With the recognition that Foxp3+ Tregs express receptors for VEGF including VEGFR2 (20), several preclinical studies targeting VEGFR have shown reduced Treg accumulation in the tumor microenvironment (21–24). Furthermore, patients with metastatic colorectal carcinoma treated with bevacizumab showed decreases in the frequency of their Tregs (24). The decrease in the number of Tregs following sunitinib treatment has not consistently been found to correlate with OS of renal cell carcinoma (RCC) patients (25, 26).
In HCC patients treated with sorafenib, a significant reduction in the frequency and number of tumor-infiltrating Tregs with downregulation of TGF-β signaling pathway was reported in liver tissue (27). However, a recent study in RCC patients reported that sorafenib treatment prior to high-dose IL-2 therapy had no significant effect on circulating Tregs or MDSC (28). This is understandable in light of the fact that treatment with high-dose IL-2 promotes Treg accumulation, thereby masking the suppressive effect of sorafenib on Tregs. A subpharmacological concentration of sorafenib in vitro has been shown to enhance CD4+ T effector cell proliferation and IL-2 production with concomitant downregulation of Treg function (29). We have shown significant reduction in the frequency of Tregs with concomitant enhancement in T effector cell function following in vitro treatment with sorafenib. In our study, we have demonstrated that, in patients on a standard regimen, sorafenib therapy was sufficient to achieve reduction in circulating Treg frequency while simultaneously reducing the accumulation of exhausted CD4+PD-1+ effector T cells. The higher ratio of the percentage of CD4+CD127+ T effector cells to the percentage of CD4+Foxp3+PD-1+ T observed in the pretreatment samples of HCV+ HCC patients in our study could be akin to the downregulation of Treg expansion by PD-1 in chronic HCV patients described by Franceschini et al. (30). However, a comparison of Tregs/T effector cells in the PBMC with intrahepatic Tregs or T effector cells was not possible in our study.
Importantly, in our studies, we have shown that redundant immunosuppressive cell types are present at high levels in HCC patients compared with healthy controls (8) and that the number of Tregs, MDSC, and PD-1+ exhausted T cells are reduced following sorafenib treatment. In addition to reduction in the high pretreatment level of these immunosuppressive cell subsets, our most important finding was the association of decrease in PD-1 expression on T cells with improved OS in patients following sorafenib treatment. Furthermore, high pretreatment levels of these phenotypes significantly correlated with achievement of better OS in patients, suggesting that higher pretreatment numbers of these cells represent predictive immune correlates of responsiveness to sorafenib treatment. These signatures could also potentially be used for in vitro assessment of patients most likely to benefit from sorafenib therapy. Additionally, our studies suggest that administration of sorafenib as a potentially useful adjunct in combination with immunotherapeutic approaches may enhance the therapeutic efficacy of immune-based strategies against advanced malignancies.
Methods
Biomarker analysis
Heparinized peripheral blood samples were obtained from HCC patients before the initiation of treatment and after 4 weeks of sorafenib treatment through the Data Bank and Biorepository at RPCI. PBMCs were isolated by Ficoll-Paque density gradient centrifugation and were cryopreserved (8); plasma was stored at −80°C and used for cytokine assays (31); and frequency and phenotype of Tregs, MDSC, and PD-1+ T cells were analyzed by flow cytometry as described in the Supplementary Methods (8, 31). Experiments on in vitro treatment of PBMC with sorafenib are detailed in the Supplementary Methods.
Statistics
Measurements for multiple immune parameters were obtained before and after sorafenib treatment in a single-institution convenience sample of 19 HCC patients. All markers are natural-log (ln) transformed prior to statistical analysis. The effect of sorafenib exposure was quantified as the difference in transformed marker values (ln [1 + post] − ln [1 + pre]). Primary and derived immune marker expression measurements were summarized with common descriptive statistics (mean or ±SD). Associations between the paired before-and-after measurements were described with scatterplots and dot plots. The null hypothesis of no difference in the paired before-and-after treatment measurements was assessed using permutation paired t tests.
OS was defined as the number of months between initiation of sorafenib therapy and death from any cause. Seventeen of the 19 patients died. Two patients who were alive at the time of analysis were censored on their dates of last followup. To illustrate the effects on OS, the patient sample was bifurcated at the median value for the change in marker expression (Figure 4) or median pretreatment expression values (Figure 5). For each marker, the OS distributions of the 2 groups were compared using Kaplan-Meier plots. The null hypothesis of no difference between the groups was assessed with a log-rank test.
Figure 4, A–D, represents markers with a sorafenib-induced reduction of expression and the impact of that reduction on OS. Patients were bifurcated at the median of the observed reduction values. Patients grouped below the median were described as having greater declines, and those above the median were described as having lesser declines.
Sorafenib had a mixed effect on the percentage of CD4+CD127+ T effector cells, with half of the patients showing an increase in expression, and the other half showing a decrease. The effect of this bifurcation on OS is shown in Figure 4E.
These results are considered hypothesis generating. P values less than 0.05 were considered statistically significant. Given the number of comparisons conducted, a correction for multiple testing has been included. For supplemental information, methods developed by Hommel (32) identified statistically significant comparisons that maintained a 0.05 family-wise Type I error rate. The main body of the manuscript includes 36 hypothesis tests. Of these, the 26 considered central to the premise of the manuscript were adjusted for multiple testing. The Hommel adjustment identified 6 comparisons with nominal P values less than 0.003 as being statistically significant, and these are shown in Figure 3 and Figure 4. A complete listing of the nominal and adjusted P values is provided in Supplemental Table 1.
The analyses for the in vitro effects of sorafenib treatment are based on a sample size of 7 patients with available PBMC samples. The VEGFR comparisons are based on 5 patients. The data included paired observations for each patient (before and after sorafenib exposure). Statistical significance of the sorafenib exposure was assessed using paired t tests. The sample size for these comparisons was too small to support permutation-based methods.
All data analyses were generated using SAS/STAT software, Version 9.4. (SAS Institute Inc.).
Study approval
The study is based on a convenience sample of 19 HCC patients treated at RPCI between 11/3/2009 and 8/26/2014. The study protocol (I 67809) was approved by the RPCI IRB. Informed consent was obtained in a manner consistent with the World Medical Association Declaration of Helsinki and RPCI standards. Clinical characteristics of patients are summarized in Table 1.
Table 1.
HCC Patient characteristics (n = 19)
| Sex (M/F) | 16:3 |
| Median age years | 62 (range 45–83) |
| Child Pugh class liver function | A = 11 |
| B = 8 | |
| C = 0 | |
| D = 0 | |
| BCLC class | A = 0 |
| B = 12 | |
| C = 7 | |
| Etiology of liver disease | Hepatitis B = 3 |
| Hepatitis C = 12 | |
| Alcohol = 17 | |
| Prior therapies = 7 | |
BCLC, Barcelona Clinic Liver Cancer
Supplementary Material
Acknowledgments
The authors would like to thank Paul Wallace for his help with FACS experiments. The RPCI Data Bank and Biorepository and Flow Cytometry facility are cancer center support grant (CCSG) shared resources supported, in part, by the NCI Core grant to RPCI (NIH P30 CA016056). Discretionary funds available to Y. Thanavala were also used to support this study.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Author contributions
SGK and YT conceptualized and designed the study. SGK, AAL, and YT developed the methodology. SGK, AAL, and YT acquired the data. SGK, AAL, AM, and YT analyzed and interpreted the data. SGK, AAL, AM, RI, and YT wrote, reviewed, and revised the manuscript.
References
- 1.Wilhelm S, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 2.Voron T, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73(8):2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 10.Hoechst B, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+) CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Lugade AA, Kalathil S, Miller A, Iyer R, Thanavala Y. High immunosuppressive burden in advanced hepatocellular carcinoma patients: Can effector functions be restored? Oncoimmunology. 2013;2(7):e24679. doi: 10.4161/onci.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein O, et al. Flt3 ligand expands CD4+ FoxP3+ regulatory T cells in human subjects. Eur J Immunol. 2013;43(2):533–539. doi: 10.1002/eji.201242603. [DOI] [PubMed] [Google Scholar]
- 13.Lechner MG, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ML, et al. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer. 2014;134(2):319–331. doi: 10.1002/ijc.28362. [DOI] [PubMed] [Google Scholar]
- 16.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caraglia M, et al. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011;2:e150. doi: 10.1038/cddis.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XD, et al. Resistance to Antiangiogenic Therapy Is Associated with an Immunosuppressive Tumor Microenvironment in Metastatic Renal Cell Carcinoma. Cancer Immunol Res. 2015;3(9):1017–1029. doi: 10.1158/2326-6066.CIR-14-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariani E, et al. Immunological and molecular correlates of disease recurrence after liver resection for hepatocellular carcinoma. PLoS ONE. 2012;7(3):e32493. doi: 10.1371/journal.pone.0032493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40(1):197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- 21.Cao M, et al. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest. 2011;91(4):598–608. doi: 10.1038/labinvest.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21. doi: 10.3389/fimmu.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12(22):6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 24.Terme M, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 25.Adotevi O, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33(9):991–998. doi: 10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]
- 26.Finke JH, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, et al. Sorafenib reduces hepatic infiltrated regulatory T cells in hepatocellular carcinoma patients by suppressing TGF-beta signal. J Surg Oncol. 2013;107(4):422–427. doi: 10.1002/jso.23227. [DOI] [PubMed] [Google Scholar]
- 28.Monk P, et al. A phase I study of high-dose interleukin-2 with sorafenib in patients with metastatic renal cell carcinoma and melanoma. J Immunother. 2014;37(3):180–186. doi: 10.1097/CJI.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabrera R, et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62(4):737–746. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franceschini D, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119(3):551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalathil SG, et al. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(1):40–50. doi: 10.1164/rccm.201312-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hommel G. A comparison of two modified Bonferroni procedures. Biometrika. 1989;76:624–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.