Abstract
Background
Commercially available pedometers have been used as tools to measure endpoints in studies evaluating physical activity promotion programs. However, their accuracy in patients recovering from COPD exacerbations is unknown. The objectives of this study were to 1) assess the relative accuracy of different commercially available pedometers in healthy volunteers and 2) evaluate the accuracy of the top-performing commercially available pedometer in patients recovering from COPD exacerbations following hospital discharge.
Methods
Twelve healthy volunteers wore 2 pedometers, 2 smartphones with pedometer apps and an accelerometer for 15 minutes of indoor activity. The top-performing device in healthy volunteers was evaluated in 4 patients recovering from COPD exacerbations following hospital discharge during 6 minutes of walking performed at home. Bland-Altman plots were employed to evaluate accuracy of each device compared with direct observation (the reference standard).
Results
In healthy volunteers, the mean percent error compared to direct observation of the various devices ranged from −49% to +1%. The mean percent error [95% confidence interval (CI)] of the top-performing device in healthy volunteers, the Fitbit Zip®, was +1% [−33 to +35%], significantly lower than that of the accelerometer (−13% [−56 to +29%], p=0.01). The mean percent error [95% CI] for the Fitbit Zip® in patients recovering from COPD exacerbations was −3% [−7 to +12%].
Conclusions
The accuracy of commercially available pedometers in healthy volunteers is highly variable. The top-performing pedometer in our study, the Fitbit Zip,® accurately measures step counts in both healthy volunteers and patients recovering from COPD exacerbations.
Keywords: pedometers, accuracy, COPD exacerbation
Introduction
Deconditioning is common in patients with chronic obstructive pulmonary disease (COPD) exacerbations and leads to excessive fatigue, respiratory symptoms with exertion and reduction in physical activity, which together result in further deconditioning.1,2 Limited physical activity is also associated with decreased quality of life, more severe dyspnea and increased risk of exacerbations, hospitalizations (including 30-day rehospitalizations), and death.3-9 Patients recovering from COPD exacerbations have been shown to have even lower levels of physical activity compared to patients with stable COPD.10,11 Clinical trials have demonstrated that pulmonary rehabilitation programs in clinical settings that include early mobilization to increase physical activity improve functional status and clinical outcomes following COPD exacerbations.12 However, pulmonary rehabilitation programs in hospitals or ambulatory care settings are often inaccessible to patients following COPD exacerbations.13 Moreover, there is a need for validated tools to promote physical activity that can be used outside of pulmonary rehabilitation and other clinical settings.
Pedometers have been shown to be effective tools in promoting physical activity in healthy adults when combined with goal setting, feedback and self-management education.14 Similar programs using commercially-available pedometers designed for patients with stable COPD have also resulted in increased levels of physical activity and improved quality of life.15,16 However, physical activity promotion programs have not been extensively evaluated in patients recovering from COPD exacerbations, a population at particularly high risk of adverse outcomes.17,18
The accuracy of commercially available pedometers (including smartphone applications: apps) in relation to accelerometers has not been evaluated in comparison with direct observation in either healthy adults or patients recovering from COPD exacerbations. As patients with COPD may have differences in gait, including decreased walk intensity, cadence and speed, compared with healthy adults,19 it is crucial that these commercially available pedometers be evaluated for accuracy before being used in physical activity promotion programs.
The objectives of this study were two-fold: 1) to compare, in healthy volunteers, the relative accuracy of commercially available pedometers to both direct observation and an accelerometer commonly used in clinical research, and 2) to evaluate the accuracy of the top-performing commercially available pedometer in patients recovering from COPD exacerbations. The results of this study could aid in selecting a device with sufficient accuracy for objectively monitoring physical activity in patients recovering from COPD exacerbations.
Methods
We conducted 2 sub-studies, each addressing one of the study objectives.
Sub-study 1
We sought to assess the validity of different pedometers in healthy volunteers during indoor activities over 15 minutes, compared with direct observation. The results of this sub-study were intended to guide the selection of a commercially available device for sub-study 2. We did not provide specific instructions for physical activity to these healthy volunteers. A convenience sample of 12 healthy non-smoking adults (age 18 years or older) was recruited for this sub-study. Age and gender of the volunteers were recorded.
We selected a sample of low-cost (<$150) wireless-enabled tri-axial (motion captured in the horizontal, lateral and vertical axes) pedometers, and smartphone-based apps that take advantage of tri-axial accelerometers built into modern smartphones (Table 1). We also measured physical activity using an accelerometer commonly used in clinical research (Actigraph® wGT3X-BT [ActiGraph, Pensacola, Florida]).20 Retail prices of the various devices were obtained from online sources.20-24 The pedometers and accelerometer were used according to the manufacturers’ recommendations and instructions. After the investigator attached the devices, each healthy volunteer was asked to carry out their usual activities indoors while under direct observation by a trained research assistant. Smartphones with pedometer apps (Moves® and Runtastic®) were carried in the front pants pockets, the Actigraph® and Fitbit Zip® were attached to the participant’s waist, and the Fitbit Force® was worn on the participant’s non-dominant wrist. This field study was conducted indoors to approximate the type of physical activity that may be observed in patients recovering from COPD exacerbations.5
Table 1. Device Characteristics.
| Class | Device | Output | Position Worn |
Retail Price |
|---|---|---|---|---|
|
Smartphone
Apps |
Runtastic® | Step count, energy expenditure, distance walked |
$0 | |
| Moves® | Step count, energy expenditure, distance walked |
$0 | ||
| Pedometers | Fitbit Zip® | Step count, energy expenditure, distance walked |
Hip | $60 |
| Fitbit Force® | Step count, energy expenditure, distance walked, stairs climbed |
Wrist | $129 | |
| Accelerometer | Actigraph® | Step count, VMU, energy expenditure, body position |
Hip / Wrist | $225 + $1495 for analysis software |
VMU=vector magnitude units
Sub-study 2
In sub-study 2 we used the device with the lowest error compared to direct observation established in sub-study 1. Sub-study 2 took place in the homes of patients recovering from COPD exacerbations within one month of hospital discharge. Patients were eligible to participate if they had a physician diagnosis of COPD exacerbation, were able to walk unaided (e.g., without a walker or cane) and provided written informed consent. Participants were instructed to walk for up to 6 minutes to mimic the duration of activity promotion used in previous studies.25,26 We also collected age, gender, weight and height (for calculating body mass index [BMI]; kg/m2), post-bronchodilator percent predicted forced expiratory volume in 1 second (FEV1), time walked during a 6-Minute Walk Test (6MWT, minutes), and resting Borg dyspnea and fatigue scores (0-10, higher scores indicate more severe dyspnea and fatigue, respectively).27-29 The study was approved by the institutional review board at the University of Illinois at Chicago.
Analyses
For both sub-studies, directly observed step counts were the reference standard. Descriptive statistics employed proportions, mean (standard deviations [SD]), or median (range), as appropriate. Bland-Altman analyses were performed to calculate the mean percent error (reference standard-calculated/reference standard*100%) and 95% confidence interval (CI) between step counts calculated by each pedometer versus the reference standard.30 Paired t-tests were also used to compare the error for each device with the error of the Actigraph®. All analyses were performed using SAS® (Cary, North Carolina).
Results
Sub-study 1
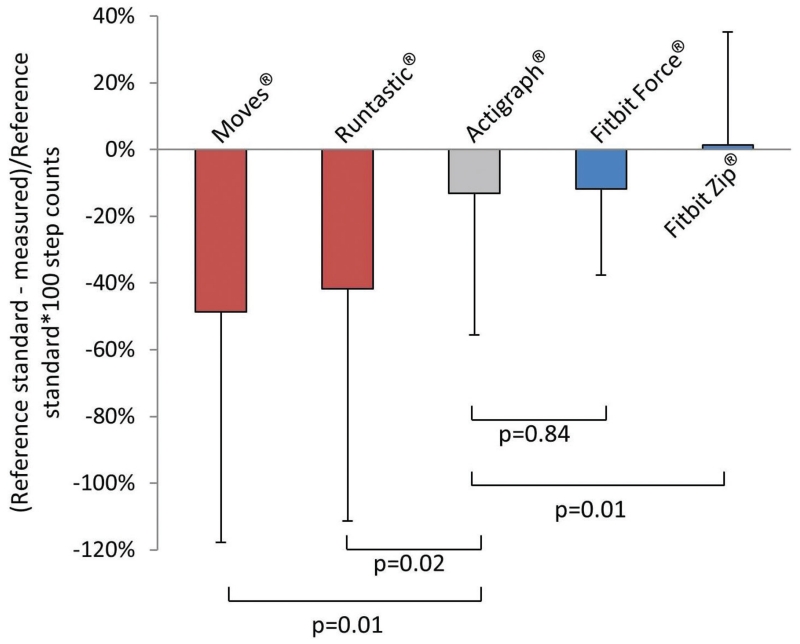
All 12 participants were under the age of 40 years and 7 were female. Assessed by direct observation, healthy volunteers walked a mean of 120 steps (SD = 53) over 15 minutes. The mean percent error for the various devices ranged from −49% to +1% (−62 to −2 steps) (Figure 1). The mean percent error [95% CI] of the accelerometer was −13% [−56 to +29%] or −20 steps [−62 to +21 steps]. The Fitbit Zip® had a significantly lower mean percent error (+1% [−33 to +35%]; −2 steps [−42 to +37 steps]) compared to the accelerometer (p=0.01). The Moves® and Runtastic® apps performed significantly worse than the accelerometer (p<0.05).
Figure 1. Relative Accuracy of the Different Commercially Available Pedometers.
Bars represent standard deviation. Smartphones with pedometer apps (Moves® and Runtastic®) were carried in the front pants pockets, the Actigraph® and Fitbit Zip® were attached to the participant’s waist, and the Fitbit Force® was worn on the participant’s non-dominant wrist. Each of the healthy volunteers wore all devices concurrently.
Sub-study 2
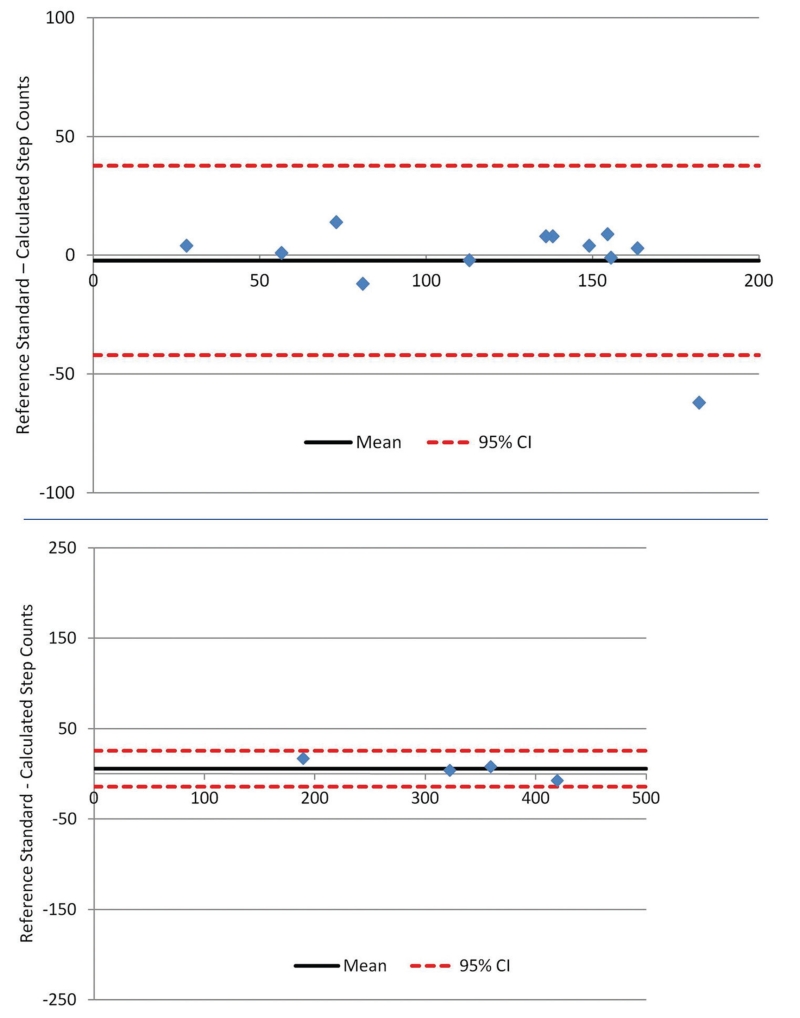
The 4 participants in this sub-study were men with a mean age of 69 years (SD=10) and mean BMI of 23kg/m2 (SD=2). The mean % predicted post-bronchodilator FEV1 for these participants was 33% (SD=5). The participants had a median resting Borg dyspnea and fatigue scores of 2 (range 0.5-4) and 1 (range 1-5), respectively. They walked a mean of 3.8 minutes (SD=0.6), during which they walked a mean of 320 steps (SD=102) as measured by direct observation. The mean percent error (95% CI) for the Fitbit Zip® in this population was −3% (−7 to +12%) (Figure 2), or 6 steps (−14 to +25 steps).
Figure 2. Bland-Altman Plot Showing Accuracy of the Fitbit Zip® Compared to Direct Observation in (A) Healthy Volunteers and (B) Patients Recovering from COPD Exacerbations.
Discussion
Our results indicate that commercially available pedometers (including pedometer apps) have variable levels of step count accuracy when compared to direct observation and an accelerometer. Interestingly, one of the pedometers (the Fitbit Zip®), outperformed an accelerometer commonly used in clinical research studies (Actigraph®). When compared with direct observation, the Fitbit Zip® performed exceedingly well in both healthy volunteers and patients recovering from COPD exacerbations. The increased accuracy of the Fitbit Zip® in patients recovering from COPD exacerbations compared to the healthy volunteers is related to the design of the pedometer’s proprietary algorithm; however, there may be other reasons for the observed findings as well.
Although previous studies have examined the validity of accelerometers,31 commercially available pedometers,32-34 and even a pedometer smartphone app,35 to our knowledge this is the first study to evaluate them concurrently and in comparison with direct observation. Furthermore, while a previous study in patients with stable COPD evaluated energy expenditure estimates using a commercially available device (Fitbit Ultra® [Fitbit Inc, San Francisco, California]) compared to an accelerometer (SenseWear Armband® [BodyMedia Inc, Pittsburgh, Pennsylvania]),34 we are not aware of any studies examining the accuracy of step count measurements in patients recovering from COPD exacerbations.
Our current study focused on step counts, as lower daily step counts in patients with COPD are associated with a range of adverse surrogate clinical outcomes, including elevated inflammatory biomarkers (C-reactive protein and IL-6), lower functional capacity (6-minute walk distance), more severe dyspnea (modified Medical Research Council [mMRC] score) and, higher risk of COPD-related hospitalizations or all-cause mortality.7,36,37 While these commercially available pedometers also measure other markers of physical activity, including energy expenditure, daily distance walked and active time, these other measures are less well studied in terms of their relation with important clinical outcomes, such as rehospitalizations or all-cause mortality.
Our study has a number of limitations. First, healthy volunteers in sub-study 1 were only monitored indoors for a short period of time (15 minutes) and their activities were not standardized. However, the goal of the sub-study was to characterize the step count accuracy of different pedometers that would approximate the level of physical activity that would likely be seen in patients recovering from COPD exacerbations in sub-study 2.5 Indeed, in sub study 2, we found that patients recovering from COPD exacerbations have very low walk times (mean of 3.8 minutes) during a 6MWT. Additional studies are needed to evaluate the accuracy of the top-performing pedometers over longer periods of observation for better characterization of these devices. Second, in sub-study 2 we only enrolled a small sample of patients recovering from COPD exacerbations following hospital discharge. Additional studies with larger numbers of patients recovering from COPD exacerbations are needed to confirm our results in more diverse populations.
The findings of our study have several implications. The high level of accuracy of the low-cost Fitbit Zip® ($60) we examined in sub-study 2 opens the door for the development and evaluation of home-based physical activity promotion programs with objective measures of physical activity in patients recovering from COPD exacerbations following hospital discharge. Such programs are needed as the majority of the studies to date have focused on physical activity promotion in individuals with stable COPD, highlighting the potential significance of our findings. The wireless connectivity feature of current devices, including the Fitbit Zip®, allows real-time monitoring of physical activity performance as part of a home-based exercise program. Newer generation pedometers now also include heart rate monitors and even pulse oximeters highlighting the potential for the development of even more sophisticated physical activity promotion programs that monitor heart rate and oxygen saturation.38,39 Additionally, the results of our study suggest that some accelerometers (Actigraph®) may have worse performance characteristics for assessing step counts than commercially available pedometers. Together, our findings highlight the importance of validating the performance of physical activity measures before they are deployed in specific patient populations.
In conclusion, commercially available pedometers have variable levels of accuracy. The Fitbit Zip® is a low-cost commercially available pedometer that outperformed a commonly used accelerometer when compared to direct observation in healthy volunteers. Moreover, the Fitbit Zip® performed exceedingly well compared to direct observation in patients recovering from COPD exacerbations. The results of this study could be used to inform the selection of devices for objective monitoring of physical activity in patients recovering from COPD exacerbation following hospital discharge.
Acknowledgements
The authors thank all study participants and the staff of the Breathe Chicago Center® at the University of Illinois at Chicago for their assistance in conducting this study.
Dr. Prieto-Centurion was supported by NIH T32 (2T32HL082547) institutional training grant.
Funding Support: Supported by the National Institutes of Health T32 (2T32HL082547) institutional training grant (V.P-C)
Abbreviations
- COPD
chronic obstructive pulmonary disease
- BMI
body mass index
- FEV1
forced expiratory volume in 1 second
- 6MWT
6-minute walk test
- SD
standard deviation
- CI
confidence interval
- mMRC
modified Medical Research Council
- VMU
vector magnitude units
Footnotes
Author contributions: V.P.-C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. V.P.-C. and J.A.K. contributed to the study conception, hypothesis delineation, data analysis and interpretation, writing, and revision of the manuscript. N.B., F.Z., and V.M. contributed to the acquisition and interpretation of the data and revision of the manuscript. L.N., A. A. M., D. B. C., C.S.R., and D. X. M. contributed to the revision of the manuscript.
Declaration of Interest
The other authors have no interests to declare.
References
- 1.Greening NJ, Harvey-Dunstan TC, Chaplin EJ, et al. Bedside assessment of quadriceps muscle using ultrasound following admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med. 2015;192(7):810–816. doi: 10.1164/rccm.201503-0535OC. doi: http://dx.doi.org/10.1164/rccm.201503-0535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med (Lond) 2006;6(2):190–196. doi: 10.7861/clinmedicine.6-2-190. doi: http://dx.doi.org/10.7861/clinmedicine.6-2-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest. 2012;142(2):338–346. doi: 10.1378/chest.11-2014. doi: http://dx.doi.org/10.1378/chest.11-2014. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen HQ, Chu L, Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):695–705. doi: 10.1513/AnnalsATS.201401-017OC. doi: http://dx.doi.org/10.1513/AnnalsATS.201401-017OC. [DOI] [PubMed] [Google Scholar]
- 5.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi: 10.1378/chest.129.3.536. doi: http://dx.doi.org/10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 6.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi: 10.1136/thoraxjnl-2013-204763. doi: http://dx.doi.org/10.1136/thoraxjnl-2013-204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962–969. doi: 10.1016/j.rmed.2012.03.016. doi: http://dx.doi.org/10.1016/j.rmed.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(5):446–452. doi: 10.1164/rccm.200408-1054OC. doi: http://dx.doi.org/10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 9.Zanoria SJ, ZuWallack R. Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(4):207–213. doi: 10.1177/1479972313505880. doi: http://dx.doi.org/10.1177/1479972313505880. [DOI] [PubMed] [Google Scholar]
- 10.Ehsan M, Khan R, Wakefield D, et al. A longitudinal study evaluating the effect of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(6):559–564. doi: 10.1513/AnnalsATS.201304-100OC. doi: http://dx.doi.org/10.1513/AnnalsATS.201304-100OC. [DOI] [PubMed] [Google Scholar]
- 11.Alahmari AD, Patel AR, Kowlessar BS, et al. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2014;14:98. doi: 10.1186/1471-2466-14-98. doi: http://dx.doi.org/10.1186/1471-2466-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305. doi: 10.1002/14651858.CD005305.pub3. doi: http://dx.doi.org/10.1002/14651858.cd005305.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Jones SE, Green SA, Clark AL, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69(2):181–182. doi: 10.1136/thoraxjnl-2013-204227. doi: http://dx.doi.org/10.1136/thoraxjnl-2013-204227. [DOI] [PubMed] [Google Scholar]
- 14.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. doi: http://dx.doi.org/10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 15.Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir Rev. 2014;45(2):347–354. doi: 10.1183/09031936.00084514. doi: http://dx.doi.org/10.1183/09031936.00084514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality of life domains and daily step counts in COPD: A randomized controlled trial. Chest. 2015;148(1):128–137. doi: 10.1378/chest.14-1466. doi: http://dx.doi.org/10.1378/chest.14-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141. doi: 10.1136/thorax.57.2.137. doi: http://dx.doi.org/10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007;131(3):696–704. doi: 10.1378/chest.06-1610. doi: http://dx.doi.org/10.1378/chest.06-1610. [DOI] [PubMed] [Google Scholar]
- 19.Annegarn J, Spruit MA, Savelberg HH, et al. Differences in walking pattern during 6-min walk test between patients with COPD and healthy subjects. PloS One. 2012;7(5):e37329. doi: 10.1371/journal.pone.0037329. doi: http://dx.doi.org/10.1371/journal.pone.0037329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Actigraph Corporation . Actigraph wGT3X-BT Monitor. Actigraph Corporation; [Accessed June 4, 2015]. website. http://www.actigraphcorp.com/products/wgt3x-bt-monitor/ [Google Scholar]
- 21.Moves mobile phone application. Moves-app; [Accessed June 4, 2015]. website. https://www.moves-app.com/ [Google Scholar]
- 22.Runtastic mobile phone apps. Runtastic; [Accessed June 4, 2015]. website. https://www.runtastic.com/ [Google Scholar]
- 23.Fitbit . Fitbit Zip Specifications. Fitbit Inc.; [Accessed January 19, 2015]. website. https://www.fitbit.com/zip/specs. [Google Scholar]
- 24.Fitbit . Fitbit Force Specifications. Fitbit Inc.; [Accessed June 4, 2015]. website. https://www.fitbit.com/ca/force/specs. [Google Scholar]
- 25.Behnke M, Taube C, Kirsten D, Lehnigk B, Jorres RA, Magnussen H. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med. 2000;94(12):1184–1191. doi: 10.1053/rmed.2000.0949. doi: http://dx.doi.org/10.1053/rmed.2000.0949. [DOI] [PubMed] [Google Scholar]
- 26.Kirsten DK, Taube C, Lehnigk B, Jorres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med. 1998;92(10):1191–1198. doi: 10.1016/s0954-6111(98)90420-6. doi: http://dx.doi.org/10.1016/S0954-6111(98)90420-6. [DOI] [PubMed] [Google Scholar]
- 27.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. doi: http://dx.doi.org/10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J,VB, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. doi: http://dx.doi.org/10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 29.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports. 1982;14(5):377–381. doi: http://dx.doi.org/10.1249/00005768-198205000-00012. [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. doi: http://dx.doi.org/10.1016/S0140-6736(86)90837-8. [PubMed] [Google Scholar]
- 31.Van Remoortel H, Raste Y, Louvaris Z, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PloS One. 2012;7(6):e39198. doi: 10.1371/journal.pone.0039198. doi: http://dx.doi.org/10.1371/journal.pone.0039198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takacs J, Pollock CL, Guenther JR, Bahar M, Napier C, Hunt MA. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014;17(5):496–500. doi: 10.1016/j.jsams.2013.10.241. [DOI] [PubMed] [Google Scholar]
- 33.Tully MA, McBride C, Heron L, Allen W, Hunter RF. The validation of Fibit ZipTM physical activity monitor as a measure of free-living physical activity. BMC Res Notes. 2014;7(1):952. doi: 10.1186/1756-0500-7-952. doi: http://dx.doi.org/10.1186/1756-0500-7-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vooijs M, Alpay LL, Snoeck-Stroband JB, et al. Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease. Interact J Med Res. 2014;3(4):e14. doi: 10.2196/ijmr.3056. doi: http://dx.doi.org/10.2196/ijmr.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergman RJ, Spellman JW, Hall ME, Bergman SM. Is there a valid app for that? Validity of a free pedometer iPhone application. J Phys Act Health. 2012;9(5):670–676. doi: 10.1123/jpah.9.5.670. [DOI] [PubMed] [Google Scholar]
- 36.Durheim MT, Smith PJ, Babyak MA, et al. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of global initiative for chronic obstructive lung disease 2011 group. Ann Am Thorac Soc. 2015;12(3):349–356. doi: 10.1513/AnnalsATS.201408-365OC. doi: http://dx.doi.org/10.1513/AnnalsATS.201408-365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moy ML, Teylan M, Weston NA, Gagnon DR, Danilack VA, Garshick E. Daily step count is associated with plasma c-reactive protein and il-6 in a us cohort with COPD. Chest. M2014;145(3):542–550. doi: 10.1378/chest.13-1052. doi: http://dx.doi.org/10.1378/chest.13-1052. [DOI] [PubMed] [Google Scholar]
- 38.Fitbit Charge HR Specifications. Fitbit, Inc.; [Accessed December 29, 2015]. website. https://www.fitbit.com/chargehr#specs. [Google Scholar]
- 39.Withings . Withings Pulse Ox, step into advanced activity tracking. Withings; [Accessed December 29, 2015]. website. http://www.withings.com/us/en/products/pulse. [Google Scholar]