Abstract
Preschool (age 3-5) is a phase of rapid development in both cognition and emotion, making this a period in which the neurodevelopment of each domain is particularly sensitive to that of the other. During this period, children rapidly learn how to flexibly shift their attention between competing demands and, at the same time, acquire critical emotion regulation skills to respond to negative affective challenges. The integration of cognitive flexibility and individual differences in irritability may be an important developmental process of early childhood maturation. However, at present it is unclear if they share common neural substrates in early childhood. Our main goal was to examine the neural correlates of cognitive flexibility in preschool children and test for associations with irritability. Forty-six preschool aged children completed a novel, child-appropriate, Stroop task while dorsolateral prefrontal cortex (DLPFC) activation was recorded using functional Near Infrared Spectroscopy (fNIRS). Parents rated their child's irritability. Results indicated that left DLPFC activation was associated with cognitive flexibility and positively correlated with irritability. Right DLPFC activation was also positively correlated with irritability. Results suggest the entwined nature of cognitive and emotional neurodevelopment during a developmental period of rapid and mutual acceleration.
Keywords: Cognitive flexibility, Irritability, fNIRS, Neurodevelopment, Early Childhood
The preschool years (ages 3 to 5) represent a period of acute acceleration in the development of executive functions, and the neural systems that underlie them (Carlson, 2005; Garon et al., 2008). The preschool period is also a time when children rapidly develop emotion regulation skills important for adapting to everyday challenges (Garner and Power, 1996), making this a period in which one of these neurodevelopmental processes may be particularly sensitive to the development of the other and highly influenced by the environment. The mastering of associated cognitive and emotional skills are hypothesized to interact, as the child reaches the more advanced stages of development, and to forecast social and academic outcomes at school age (Loeber and Hay, 1997; Tramontana et al., 1988). There have, however, been few studies testing how cognition and emotion are integrated in the preschool years, particularly at the neural level (Crone, 2009).
The integration of cognitive flexibility and emotion regulation in early childhood has been hypothesized as bi-directional (Zelazo and Cunningham, 2007). Researchers have postulated that emerging cognitive flexibility, defined as the ability to mentally switch between two or more demands (Scott, 1962), forms the basis of early emotion regulation strategies (Kopp, 1989; Zelazo and Cunningham, 2007). Relevant research has supported this hypothesis. For example, 5-6 year olds were able to flexibly change their thoughts and goals to reduce the intensity of negative affect (Davis et al., 2010). Relatedly, children who used a cognitive flexibility-based strategy, such as shifting their gaze or distracting themselves, delayed gratification longer than peers (Cole et al., 2011; Mischel and Mischel, 1983). Conversely, individual differences in emotion regulation may impact how executive functions like cognitive flexibility develop. It has been postulated that young children's ability to successfully implement executive functions invariably involves managing accompanying emotion (Metcalfe and Mischel, 1999). Consider a 4 year-old who has been promised ice cream after dinner, but is given a cookie when his parents realize they are out of ice cream. To respond adaptively to this situation, the child would need to have both the flexibility to shift expectations and attention towards the pleasant but unanticipated treat, but also the emotion regulation abilities to modulate negative affect brought on by the unexpected outcome. The potentially concordant development of these skills suggests that cognitive flexibility and emotion regulation may share common neural substrates and mutually affect maturational change.
Cognitive flexibility is associated with activation of the dorsolateral prefrontal cortex (DLPFC) in adults (Bunge and Crone, 2009), and these findings appear to extend to earlier developmental stages. Wood and colleagues (2009), using a numerical Stroop task, found that children ages 8–12 years, like adults, showed bilateral DLPFC activation when trying to sort numbers by numerical value instead of incongruent size. Morton and colleagues (2009) examined cognitive flexibility in children ages 11–13 years and adults who completed a modified version of the Dimensional Change Card Sort task (DCCS; Zelazo, 2006). Results showed increased DLPFC activation associated with switching dimensions in both children and adults. More recent advancements in functional Near Infrared Spectroscopy (fNIRS), a neuroimaging tool capable of measuring hemoglobin changes in the outer cortex non-invasively, has allowed researchers to measure neural activation in early childhood (Aslin and Mehler, 2005). Using fNIRS, Moriguchi and Hiraki (2009, 2011) recorded lateral PFC hemoglobin levels in preschool children and adults who completed a modified DCCS task. Results indicated that both preschool children and adults showed DLPFC activation while shifting mental sets. In addition, as children aged from 3 to 4 years, activation in this region increased as their performance improved.
Emotion regulation is defined as modulating the valence, intensity, or time-course of an emotional experience (Thompson, 1994). These emotional experiences can range across basic and complex emotions, but, in early childhood, the regulation of anger and frustration is particularly salient as children's goals are often blocked in the name of healthy diets, early bedtimes, or appropriate public behavior (Daniels et al., 2012). In following, irritability, or dispositional variability in anger/frustration regulation (Snaith and Taylor, 1985) represents a salient trait for understanding cognition-emotion interaction. Irritability is a dimensional phenotype present, at some level, in all children, that traverses the normal to abnormal spectrum (Wakschlag et al., 2015). While severe irritability is a symptom of psychopathology (Stringaris, 2011; Wakschlag et al., 2014), expressions of irritability such as temper tantrums and angry mood are common in early childhood (Daniels et al., 2012; Wakschlag et al., 2012). Thus, irritability within the normative range characterizes important individual differences in children's regulation of anger and frustration that likely interacts with the maturation of cognitive flexibility.
There is some evidence to suggest both a behavioral and neural interaction between cognitive flexibility and irritability in both typically developing and clinical populations. Adolescents receiving treatment for abnormally high levels of irritability show poorer cognitive flexibility (Dickstein et al., 2007) and reduced lateral PFC activation (Adleman et al., 2011). In typically developing preschool children, the neural circuitry of irritability shows similarities with that of cognitive flexibility. Perlman and colleagues (2014) found that DLPFC activation during frustration was positively associated with parent-rated irritability. This finding suggests that, in the non-impaired range of the irritability dimension, the DLPFC may be a neural mechanism by which relatively more irritable children control salient anger and frustration.
The present study had two main goals. First, our objective was to identify the neural correlates of cognitive flexibility in preschool. We tested 46 typically-developing 3-5 year old children who completed a novel and child-friendly Stroop task while prefrontal hemoglobin levels were recorded via fNIRS. We hypothesized a replication of the previous findings that children's cognitive flexibility would be associated with DLPFC activation. Second, we aimed to investigate the novel question of how cognitive flexibility-related neural activation would be associated with individual differences in children's irritability. We expected that preschoolers with relatively higher irritability would require greater DLPFC activation to perform at the level of their peers. Thus, we hypothesized that DLPFC activation during a cognitive flexibility task would be positively associated with irritability.
2. Materials and methods
This study was approved by the Institutional Review Board (IRB) of the University of Pittsburgh.
2.1. Participants
Forty-six 3- to 5-year-old children (24 boys; mean = 57.4 months, SD = 8.78, range = 38.0–71.0 months) were recruited from the local community through advertisements. Children were identified by their parent/guardian as 71.7% Caucasian and 28.3% African American (97.8% Non-Hispanic and 2.2% Hispanic). An additional 5 children were excluded from analyses because of poor quality data and/or technical error. Because our goal was to understand typical development, children were excluded if their parents reported they were seeking clinical services, had any current or past psychiatric diagnosis, or had a first degree relative with a severe psychiatric diagnosis.
2.2 Cognitive Task
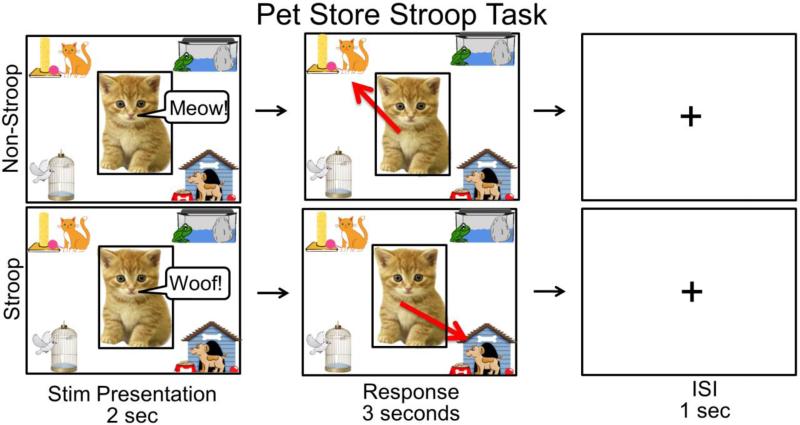
Children were seated at a child size desk in front of a touch-screen computer and completed the novel, child-friendly Pet Store Stroop Task (see Figure 1). The task was based on the classic Stroop paradigm (Stroop, 1935). First, children were told the story of a pet shop in which all of the animals had escaped from their cages. The child was instructed to put each animal, who appeared in the center of the screen while making a sound (2 seconds), back into the correct cage by touching one of four cages located in the corners of the screen (3 seconds). Children were told that sometimes the animals were “tricky” and liked to disguise themselves as other animals. In the Non-Stroop, sensory-motor control condition, animals made the sound of their species (e.g. cat says “meow”), but in the Stroop condition, animals made the sound of a different species (e.g. cat says “woof”). Thus, in the Stroop condition, children had to ignore a prepotent response to sort the animals based on their appearance and sort them based on the incongruent sound instead. The task comprised three Stroop and three Non-Stroop blocks, in alternating order. Each block comprised 6 trials. Children were presented with a fixation cross for one second between each trial and for 15 seconds between each block. The entire task lasted approximately 5 minutes. One experimenter worked with the child and explained the task while a second experimenter monitored fNIRS data acquisition.
Figure 1.
A depiction of one trial of the Pet Store Stroop Task. Children were instructed to put the animal in the correct cage based on the sound it made.
2.3 fNIRS Data Acquisition and Analysis
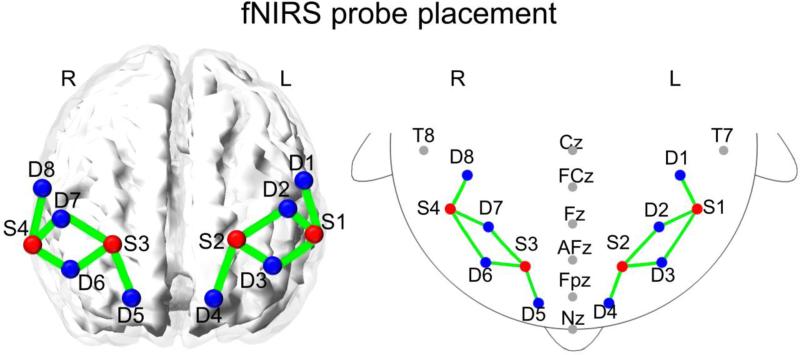
As previously described by Perlman and colleagues (Perlman et al., 2015a; Perlman et al., 2014), non-invasive optical imaging was performed with a CW6 real-time fNIRS system (Techen, Inc., Milford, MA, USA). The fNIRS probe comprised 4 light-source positions each containing 690nm (12mW) and 830nm (8mW) laser light, and 8 detectors, mounted within a child-friendly elastic cap. The average inter-optode distance was 3.2cm. The probe was positioned according to international 10-20 coordinates such that the interior medial corner of the probe was aligned with FpZ. Hair was manually parted under the optodes to improve signal detection. The probe extended over the dorsolateral prefrontal cortex, specifically Brodmann areas 10 and 46 on each hemisphere as shown in Figure 2. The probe was registered to an atlas brain image for display purposes based on the FpZ position relative to the probe. An LPT port was used to synchronize the stimulus timing from E-Prime (Psychology Software Tools, Sharpsburg, PA) with brain hemoglobin activation. The scanning rate was 20 Hz, which was later Nyquist filtered and down-sampled to 4Hz prior to analysis.
Figure 2.
Placement of the fNIRS probe on the prefrontal cortex, superimposed on a 3D mesh brain (left) and international 10–20 coordinates (right). Red dots, light- sources (S); blue dots, detectors (D); green lines, measurement channels.
As reviewed in Huppert and colleagues (2009), the fNIRS raw signals were first converted to optical density and hemoglobin concentration changes using the modified Beer-Lambert law (Delpy and Cope, 1997) using a differential path length term of 6 for both wavelengths (Strangman et al., 2003). For statistical analysis, we used an autoregressively whitened robust regression model (Barker et al., 2013). Specifically, this model allows robust estimation of fNIRS signal changes in the presence of motion-related artifacts. This model was also shown to have good control of type-I error (false-discovery rates; reviewed in Huppert, 2016). In brief, a linear model (e.g., y = Xβ + ε) was constructed, with X as the design matrix and β estimating the magnitude of brain activity in the evoked hemodynamic response. An iterative auto-regressive (AR) filtering was used to eliminate the serial correlations in the residual of the model by employing a linear whitening filter (S−1) on both sides of the equation (e.g., S−1·y = S−1· Xβ + S−1· ε). This AR model is estimated by Bayesian information criteria (BIC) search of AR model order to whiten the residual of the linear regression model. The solution to this model is then iteratively estimated by robust regression. The canonical design matrix (X) was calculated by the first-order convolution of the standard hemodynamic response function from SPM8 (Friston et al., 1994).
The estimated regression coefficients (β) for each condition, at each channel, and for each subject were exported to SPSS 22.0 (IBM Corp., Armonk, NY) for further statistical analysis. Cognitive flexibility was defined as the magnitude differences of brain activation between Stroop and Non-Stroop blocks (Δβ = β Stroop – β Non-Stroop). A single sample t-test was performed for all 12 channels at the group level to detect which channels were significantly activated during our cognitive flexibility task (significant difference between Δβ and 0). For visualization purposes, the optical probe in the study was registered to an age matched child's MRI brain template from a previous study (Perlman and Pelphrey, 2010, 2011).
2.4 Parent-rated Irritability
Parents rated their child's irritability using the Temper Loss subscale of the Multidimensional Assessment Profile for Disruptive Behavior (MAP-DB; Wakschlag et al, 2012). This scale was specifically developed to characterize the full dimension of normative to clinically salient irritability, and has shown good reliability and validity (Wakschlag et al., 2014; Wakschlag et al., 2012). The MAP-DB takes a dimensional approach to irritability from an epidemiological perspective and has been shown to predict children's brain activation following frustration (Perlman et al., 2015b; Perlman et al., 2014). The Temper Loss subscale comprised 22 items (e.g. “Act irritable”, “Stay angry for a long time”) rated on a 6-point Likert scale (0= “Never”, 5= “Many times each day”). Reliability of the scale was excellent (α = 0.96).
Parents reported a range of irritability scores (Mean = 19.42, SD = 15.78, Range 0–64; maximum possible score = 110). Based on scores of ≥ 43, representing 1.5 SD above the mean in the MAP-DB community sample (Wakschlag et al., 2012), 91% of the sample was in the normative range. Thus, irritability score distribution showed variability yet was consistent with a non-clinical, non-impaired sample.
2.5 Analysis Strategy
First, we tested for associations between Stroop performance, age, and irritability. Next, we contrasted hemoglobin changes during the Stroop and Non-Stroop conditions to identify specific areas of prefrontal activation associated with cognitive flexibility. Although it is not currently the standard in the field to correct for multiple comparison, as fNIRS is a region-of-interest and hypothesis driven method, we employed the False Discovery Rate (FDR) correction (Benjamini and Hochberg, 1995) within the contrasts of oxy- and deoxy-hemoglobin separately to provide a more conservative estimate of effects. Using correlation, we tested whether cognitive flexibility-related neural activation was association with age. Finally, using partial correlation, we tested whether neural activation during our cognitive flexibility task was associated with Stroop performance level, and level of irritability, when controlling for age.
3. Results
3.1 Stroop performance and its association with age and irritability
Mean reaction time was calculated from correct trials in the Stroop and Non-Stroop conditions. Paired-sample T tests were conducted to test whether accuracy and reaction time differed between conditions. As expected, children were less accurate (Stroop: M = 67%, SD = 0.20, Non-Stroop: M = 74.2%, SD = 0.19; t(44) = 5.56, p < 0.001) and slower (Stroop: M = 3.04 seconds, SD = 0.44, Non-Stroop: M = 2.72 seconds, SD = 0.19; t(44) = 2.83, p < 0.01) in the Stroop condition than in the Non-Stroop condition. Due to the rapid changes in the development of executive functions occurring during this period (Diamond, 2002), we tested for age related effects of Stroop performance. Age was significantly positively correlated with Stroop accuracy (r(44) = 0.43, p < 0.01) and negatively correlated with reaction time (r(44) = −0.54, p < 0.001). Stroop accuracy (r(46) = 0.014, p = 0.928) and reaction time (r(46) = 0.079, p = 0.602) was not associated with parent-rated irritability.
3.2 fNIRS Results
3.2.1 Neural activity contrast between Stroop and Non-Stroop conditions
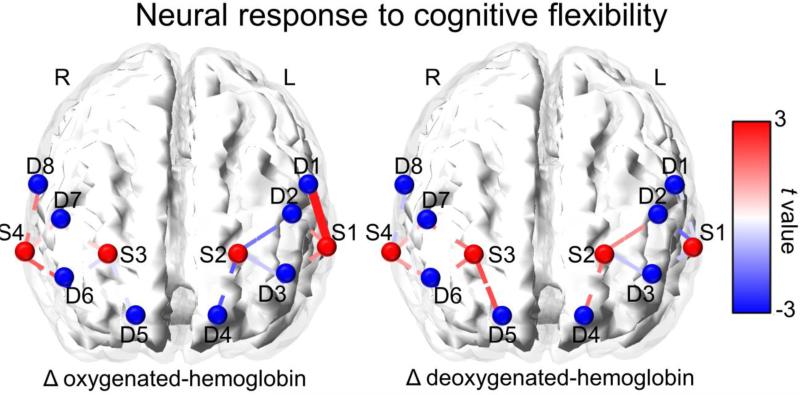
As shown in Figure 3, one channel in the left DLPFC showed significant increased oxy-hemoglobin concentration between Stroop versus Non-Stroop blocks (p < 0.01, uncorrected). Another channel in the right DLPFC showed marginally increased activation between Stroop and Non-Stroop conditions (p = 0.06, uncorrected). As shown in Figure 3, a single channel in the right DLPFC showed significantly (p < 0.05, uncorrected) increased deoxy-hemoglobin concentration between Stroop and Non-Stroop blocks. After FDR correction for multiple comparison, only the left DLPFC channel finding remained significant.
Figure 3.
Channel space maps showing oxygenated- and deoxygenated-hemoglobin differences between Stroop and Non-Stroop blocks. The color of each line indicates the t values associated with the change in β between Stroop and Non-Stroop conditions. Solid lines represent significant differences between conditions and dashed lines represent non-significant differences between conditions.
3.2.2 Individual differences in DLPFC activation
No correlation was found between age and oxy-hemoglobin changes during our cognitive flexibility task at any channel. However, because age was correlated with Stroop accuracy and reaction time, we controlled for age in subsequent analyses.
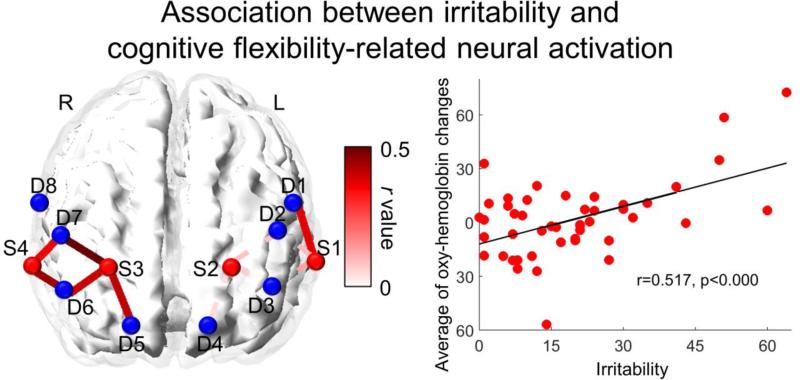
We examined the association between irritability and neural activation during our cognitive flexibility task. In the left hemisphere, results revealed a significant positive partial correlation (r(43) = 0.34, p < 0.05, uncorrected) between irritability and the same DLPFC channel that was significant in the Stroop vs. Non-Stroop contrast. Moreover, in the right hemisphere, irritability was positively partially correlated with oxy-hemoglobin levels between the Stroop and Non-Stroop condition in multiple DLPFC channels (r(43) ranges from 0.32 to 0.50, p ranges from 0.001 to 0.032, uncorrected, see Figure 4). To illustrate this trend, and for parsimony, a scatterplot between irritability and oxy-hemoglobin changes averaged across significant channels in the right DLPFC is shown in Figure 4.
Figure 4.
Channel space map showing associations between irritability and oxygenated-hemoglobin differences between Stroop and Non-Stroop conditions, controlling for age. Solid color lines represent significant correlations and dashed lines represent insignificant correlations between each source-detector pair and irritability. The color bar represents the intensity of the correlation coefficient, with a range of 0 to 0.5. Scatter plot showing the association between irritability and oxy-hemoglobin changes averaged across significant channels in the right DLPFC.
4. Discussion
In the present study, we found evidence that left DLPFC activation was associated with cognitive flexibility in typically developing preschool children. Irritability was unrelated to cognitive flexibility performance but was positively associated with cognitive flexibility-related neural activation. Children with relatively higher irritability showed greater activation in both the left and right DLPFC during a cognitive flexibility task. The present study fills in gaps in our understanding of how emotion regulation and the neural correlates of cognitive flexibility may be interwoven in early childhood. Previous studies have shown that, behaviorally, executive functions and emotion regulation are linked in early childhood. For example, Carlson and Wang (2007) found that 4 to 6 year olds’ ability to suppress negative and positive affect during emotional challenges was positively associated with performance on an inhibitory control battery . Other studies have found that children who can flexibly shift their thoughts, gaze, or attention were able to reduce subjective reports of negative affect or delay a reward longer than peers (Cole et al., 2011; Davis et al., 2010; Mischel and Mischel, 1983). The present study is, to our knowledge, the first to find that individual differences in irritability are associated with cognitive flexibility-related neural functioning. A positive association between irritability and cognitive flexibility, at the neural level, represents novel evidence that specific executive functions and emotion regulation may share some common neural circuitry. It may also represent an ability to employ a specific strategy, implemented through activation of the DLPFC, that is unique to the most irritable children. This finding, therefore, has novel implications for our understanding of how executive functions and emotion regulation develop and transact during the preschool years.
The present study used a novel, child-friendly, and fNIRS compatible Stroop task for preschool children. As such, our finding that the Pet Store Stroop Task elicited left DLPFC activation, in children as young as three years, makes several contributions to the cognitive development literature. While previous work has explored children's neuro-electrical activity during a cognitive flexibility task (see Wolfe & Bell, 2004, 2007), it is only recently, through non-fMRI techniques, that researchers have localized anatomical neural substrates of preschool cognitive flexibility. This small body of literature has largely focused on neural activation during the Dimensional Change Card Sort task (DCCS; Zelazo, 2006), a task with distinct differences from the Pet Store Stroop Task. For example, most 3 year olds fail the DCCS (are unable to shift from sorting stimuli along one dimension to sorting along a second dimension) while most 5 year olds pass (Zelazo, 2006). In the present study, while Stroop performance improved with age, 3 year olds were still able to understand and successfully complete the Pet Store Stroop Task (3 year olds were 51% accurate on Stroop trials and 62% accurate on Non-Stroop trials, with a 25% chance rate). In addition, two fNIRS studies by Moriguchi and Hiraki (2009, 2011) using the DCCS in 3 to 5 year-old children found significant pre-post switch hemoglobin increases only among children capable of passing the DCCS, not in the significant number of 3 year-olds who failed. In contrast, in the Pet Store Stroop Task, 3 year-olds showed significant DLPFC activation between Stroop and Non-Stroop conditions. This finding suggests that the neural substrates of cognitive flexibility might develop more continuously rather than in the stage-like (Piaget, 1971) format that has been previously measured by existing tasks. The DCCS and Pet Store Stroop Task thus represent complementary strategies for investigating early cognitive flexibility. The high difficulty level of the DCCS may be ideal for detecting age effects, whereas the low difficulty and repetitive trials of the Pet Store Stroop Task may be uniquely suited for detecting cognitive flexibility-related neural variability when behavioral abilities related to executive functions are in the early stages of emergence.
Some have suggested that certain emerging executive functions are strongly integrated with managing emotion while others are more removed from emotion (Zelazo and Carlson, 2012). This contention is largely based on studies showing that executive functions presumed to involve managing emotion, such as inhibitory control, and executive functions presumed to be distinct from emotion, such as cognitive flexibility, load onto separate factors (Kim et al., 2013; Willoughby et al., 2011). The Pet Store Stroop Task used in the present study had no reward associated with performance and was designed to be consistent with other, non-emotional, “cool”, measures of cognitive flexibility (Zelazo et al., 2005). Without the neural activation data collected in the present study, the non-association between irritability and Stroop performance might suggest that individual differences in children's response to frustration are unrelated to developing cognitive flexibility. Instead, we found evidence that in order to perform at the level of their peers, preschoolers with relatively higher but non-impairing levels of irritability showed greater DLPFC activation during our cognitive flexibility task. Given the importance of cognitive flexibility in the development of competent social and academic functioning (Blair and Razza, 2007; Clark et al., 2002), the field has seen an increase in interventions designed to directly strengthen cognitive flexibility in preschool children (e.g. Bodrova & Leong, 2007). The integration of irritability and cognitive flexibility in early childhood suggests that individual differences in irritability might predict preschool children's response to training programs designed to boost executive functions. Alternatively, early childhood interventions that target both emotion regulation and cognitive flexibility may be more efficacious.
As stated previously, the integration of cognitive flexibility and emotion regulation is hypothesized to be bi-directional (Zelazo and Cunningham, 2007). Although it is clear that emotion regulation develops rapidly during the preschool years (Eisenberg, 2000), evidence of how specific emotion regulation strategies emerge has been lacking. Many scholars contend that young children may utilize cognitive flexibility as an early emotion regulation strategy, but this hypothesized phenomenon has been under-researched (Cole et al., 2004; Gross, 1998; Kopp, 1989; Ochsner and Gross, 2008). Perlman and colleagues (2014) were the first to show that irritable yet non-impaired preschool children showed a greater DLPFC response to frustration than their less irritable peers. The association between irritability and DLPFC activation during a cognitive flexibility task suggest that relatively more irritable preschoolers may use well-developed cognitive flexibility, and underlying neural circuitry, to manage daily salient frustration. Shifting attention between competing demands may be one way irritable yet adaptively functional preschoolers manage emotional challenges.
As opposed to previous work on the neural correlates of childhood executive functions (see Moriguchi and Hiraki, 2011; Perlman et al., 2015a; Wood et al., 2009), our study is limited in that we did not find any age-related effects. This is perhaps because our Pet Store Stroop Task was specifically designed to be challenging, but easily attainable in order to investigate neural variation related to irritability rather than neural dissociations between those who could vs. could not complete the task. A second limitation of the study may be that fNIRS methodology is only able to detect hemoglobin changes in the outer cortex and is, thus, best suited for specific hypothesis testing rather than an investigation of widely distributed circuitry. In this study, we used a probe that focused on the DLPFC as a region of interest based on previous localization studies. Thus, other areas of the brain that underlie cognitive flexibility or contribute to irritability, such as the parietal cortex (Gottlieb, 2007; Gurd et al., 2002), anterior cingulate (Bush et al., 2000) or amygdala (Deveney et al., 2013; Leibenluft et al., 2003), could not be measured in the current study. A final limitation of this study that is poised to become a prime avenue for future direction concerns the specificity of our findings to a single dimension of negative affect (irritability). It is possible that DLPFC activation in relation to cognitive flexibility might correlate negatively with another dimension of negative affect, such as anxiety, or not at all, or that a relationship between this other dimensions might have a different neural substrate. Future studies employing fMRI will be better poised to examine these questions while investigating a more diffuse circuitry.
Although we did not employ a longitudinal design to test whether the association between irritability and cognitive flexibility indicates transactional or protective effects, our findings point toward this as a critical direction for future research. Longitudinal work with both typical and high-risk children has the potential to tease apart how irritability and the neural correlates of executive functions interact across development and forecast specific risk-related cut-off points. In conclusion, the present study is among the first to identify the neural correlates of cognitive flexibility in preschool children, and, to our knowledge, is the first to show that cognitive flexibility-related brain activation relates to individual differences in emotion regulation. The present study sets the stage for exciting future research exploring how the neurodevelopment of executive functions transacts with children's emotion regulation in the presence of family, peers, and community during additionally sensitive developmental periods.
Highlights.
The present study used fNIRS to investigate the neural correlates of cognitive flexibility in preschoolers (ages 3–5) and tested associations with level of irritability.
A novel, child-appropriate, Stroop task was used to assess preschoolers’ cognitive flexibility.
Cognitive flexibility was linked to increased oxygenated-hemoglobin in the left DLPFC.
Oxygenated-hemoglobin in the bilateral DLPFC during cognitive flexibility was positively correlated with irritability.
Acknowledgments
This work was supported by National Institutes of Health (K01 MH094467 PI: Susan Perlman, R21 MH100189 PI: Susan Perlman, and R01 MH107540 PI: Susan Perlman). Yanwei Li was sponsored by the China Scholarship Council. Adam Grabell received support from the National Institutes of Health (T32MH018951; PI: David Brent).
We thank Lisa Bemis, Caroline MacGillivray, Meghan Murphy, and Brianna Jones for their help in subject recruitment and data collection. FNIRS analytical methodology included in this manuscript is developed by Theodore Huppert and Jeffrey Barker and is made publicly available through the following link: https://bitbucket.org/huppertt/nirstoolbox/wiki/Home.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors of this work declare no conflicts of interest.
References
- Adleman NE, Kayser R, Dickstein D, Blair RJR, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:1173–1185. e1172. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN, Mehler J. Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. J. Biomed. Opt. 2005;10:011009–0110093. doi: 10.1117/1.1854672. [DOI] [PubMed] [Google Scholar]
- Barker JW, Aarabi A, Huppert TJ. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express. 2013;4:1366–1379. doi: 10.1364/BOE.4.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995:289–300. [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bodrova E, Leong DJ. Tools of the mind. Pearson Upper Saddle River, NJ.: 2007. [Google Scholar]
- Bunge SA, Crone EA. Neural correlates of the development of cognitive control. Neuroimaging Dev. Clin. Neurosci. 2009:22–37. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev. Neuropsychol. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cogn. Dev. 2007;22:489–510. [Google Scholar]
- Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J. Child Psychol. Psychiatry. 2002;43:785–796. doi: 10.1111/1469-7610.00084. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Dev. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Tan PZ, Hall SE, Zhang Y, Crnic KA, Blair CB, Li R. Developmental changes in anger expression and attention focus: learning to wait. Dev. Psychol. 2011;47:1078. doi: 10.1037/a0023813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA. Executive functions in adolescence: inferences from brain and behavior. Dev. Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Daniels E, Mandleco B, Luthy KE. Assessment, management, and prevention of childhood temper tantrums. J. Am. Acad. Nurse Pract. 2012;24:569–573. doi: 10.1111/j.1745-7599.2012.00755.x. [DOI] [PubMed] [Google Scholar]
- Davis EL, Levine LJ, Lench HC, Quas JA. Metacognitive emotion regulation: children's awareness that changing thoughts and goals can alleviate negative emotions. Emotion. 2010;10:498. doi: 10.1037/a0018428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D, Cope M. Quantification in tissue near–infrared spectroscopy. Philos. Trans. R. Soc. London B Biol. Sci. 1997;352:649–659. [Google Scholar]
- Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P, Pine DS, Leibenluft E. Neural mechanisms of frustration in chronically irritable children. Am. J. Psychiatry. 2013;170:1186–1194. doi: 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. Princ. Front. lobe Funct. 2002:466–503. [Google Scholar]
- Dickstein DP, Nelson EE, McCLURE EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annu. Rev. Psychol. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Garner PW, Power TG. Preschoolers' emotional control in the disappointment paradigm and its relation to temperament, emotional knowledge, and family expressiveness. Child Dev. 1996;67:1406–1419. [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 2008;134:31. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2:271. [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR. Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain. 2002;125:1024–1038. doi: 10.1093/brain/awf093. [DOI] [PubMed] [Google Scholar]
- Huppert TJ. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 2016;3:010401–010401. doi: 10.1117/1.NPh.3.1.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009;48:D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Nordling JK, Yoon JE, Boldt LJ, Kochanska G. Effortful control in “hot” and “cool” tasks differentially predicts children's behavior problems and academic performance. J. Abnorm. Child Psychol. 2013;41:43–56. doi: 10.1007/s10802-012-9661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Dev. Psychol. 1989;25:343. [Google Scholar]
- Leibenluft E, Blair RJR, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann. N. Y. Acad. Sci. 2003;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annu. Rev. Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol. Rev. 1999;106:3. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Mischel HN, Mischel W. The development of children's knowledge of self-control strategies. Child Dev. 1983;54:603–619. [Google Scholar]
- Moriguchi Y, Hiraki K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. 2009;106:6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Hiraki K. Longitudinal development of prefrontal function during early childhood. Dev. Cogn. Neurosci. 2011;1:153–162. doi: 10.1016/j.dcn.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JB, Bosma R, Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage. 2009;46:249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation insights from social cognitive and affective neuroscience. Curr. Dir. Psychol. Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Huppert TJ, Luna B. Functional Near-Infrared Spectroscopy Evidence for Development of Prefrontal Engagement in Working Memory in Early Through Middle Childhood. Cereb. Cortex. 2015a:bhv139. doi: 10.1093/cercor/bhv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, Phillips ML. Neural substrates of child irritability in typically developing and psychiatric populations. Dev. Cogn. Neurosci. 2015b;14:71–80. doi: 10.1016/j.dcn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Luna B, Hein TC, Huppert TJ. fNIRS evidence of prefrontal regulation of frustration in early childhood. Neuroimage. 2014;85:326–334. doi: 10.1016/j.neuroimage.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Regulatory brain development: balancing emotion and cognition. Soc. Neurosci. 2010;5:533–542. doi: 10.1080/17470911003683219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The theory of stages in cognitive development. In: Green DR, Ford MP, Flamer GB, editors. Measurement and Piaget McGrawHill. New York: 1971. pp. 1–11. [Google Scholar]
- Scott WA. Cognitive complexity and cognitive flexibility. Sociometry. 1962:405–414. [Google Scholar]
- Snaith R, Taylor C. Irritability: definition, assessment and associated factors. Br. J. Psychiatry. 1985;147:127–136. doi: 10.1192/bjp.147.2.127. [DOI] [PubMed] [Google Scholar]
- Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage. 2003;18:865–879. doi: 10.1016/s1053-8119(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Stringaris A. Irritability in children and adolescents: a challenge for DSM-5. Eur. Child Adolesc. Psychiatry. 2011;20:61–66. doi: 10.1007/s00787-010-0150-4. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643. [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monogr. Soc. Res. Child Dev. 1994;59:25–52. [PubMed] [Google Scholar]
- Tramontana MG, Hooper SR, Selzer SC. Research on the preschool prediction of later academic achievement: A review. Dev. Rev. 1988;8:89–146. [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi SW, Nichols SR, Kestler J, Burns JL, Carter AS, Henry D. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:82–96. e83. doi: 10.1016/j.jaac.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Choi SW, Carter AS, Hullsiek H, Burns J, McCarthy K, Leibenluft E, Briggs - Gowan MJ. Defining the developmental parameters of temper loss in early childhood: implications for developmental psychopathology. J. Child Psychol. Psychiatry. 2012;53:1099–1108. doi: 10.1111/j.1469-7610.2012.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, Voss JL, Pine DS, Leibenluft E, Briggs-Gowan ML. Clinical implications of a dimensional approach: the normal: abnormal spectrum of early irritability. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:626–634. doi: 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M, Kupersmidt J, Voegler-Lee M, Bryant D. Contributions of hot and cool self-regulation to preschool disruptive behavior and academic achievement. Dev. Neuropsychol. 2011;36:162–180. doi: 10.1080/87565641.2010.549980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. Working memory and inhibitory control in early childhood: Contributions from physiology, temperament, and language. Dev. Psychobiol. 2004;44:68–83. doi: 10.1002/dev.10152. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. Sources of variability in working memory in early childhood: A consideration of age, temperament, language, and brain electrical activity. Cogn. Dev. 2007;22:431–455. [Google Scholar]
- Wood G, Ischebeck A, Koppelstaetter F, Gotwald T, Kaufmann L. Developmental trajectories of magnitude processing and interference control: An fMRI study. Cereb. Cortex. 2009;19:2755–2765. doi: 10.1093/cercor/bhp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat. Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM. Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Dev. Perspect. 2012;6:354–360. [Google Scholar]
- Zelazo PD, Cunningham WA. Executive function: Mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. Guilford; New York: 2007. pp. 135–158. [Google Scholar]
- Zelazo PD, Qu L, Müller U, Schneider W, Schumann-Hengsteler R, Sodian B. Hot and cool aspects of executive function: Relations in early development. Young children's cognitive development: Interrelationships among executive functioning, working memory, verbal ability, and theory of mind. 2005:71–93. [Google Scholar]