Abstract
Amphetamine (Amp) increases exercise duration. It is thought to do so by masking fatigue, but there have been very few studies looking at the effect of amphetamine on VO2MAX and running economy. Furthermore, it is unknown if amphetamine’s effect on exercise duration occurs in a warm environment. We conducted separate experiments in male Sprague-Dawley rats testing the effect of amphetamine on maximal oxygen consumption (VO2MAX) (n=12), running economy (n=12), and exercise duration (n=24) in a warm environment. For VO2MAX and running economy, rats were randomized to either amphetamine at 1 mg/kg (Amp-1) or 2 mg/kg (Amp-2). Animals served as their own controls in a crossover design with the administration order counter-balanced. To study the effect of amphetamine on exercise duration, we conducted run-to-exhaustion treadmill testing on rats in a 32°C environment following administration of Amp-1, Amp-2, or Saline. Compared to control, Amp-2 increased VO2MAX (by 861 ± 184 ml/kg/hr, p=0.005) and the time to VO2MAX (by 2.5 ± 0.8 min, p=0.03). Amp-1 had no effect on VO2MAX but increased the time to VO2MAX (by 1.7 ± 0.5 min, p=0.03). Neither dose improved running economy. In the warm, only rats in the Amp-1 group (+9.4 min, p=0.02) had an increased time to exhaustion. Compared to control (41.6 ± 0.3°C), both amphetamine doses had higher temperatures at exhaustion: Amp-1 (42.0 ± 0.2°C) and Amp-2 (42.1 ± 0.2°C). Our results suggest that ergogenic effect of amphetamine occurs by masking fatigue but this effect may be offset in the warm with higher doses.
Keywords: Exhaustion, amphetamine, VO2Max, Running economy, Exertional heat stroke
INTRODUCTION
“The man who can drive himself farther once the effort gets painful will win” - Roger Bannister
While the media focuses predominantly on the illegal use of anabolic steroids and blood doping in sports, stimulants like amphetamines are still widely used in competition.1 In 2013, the World Anti-Doping Agency listed stimulants as the second most detected banned substance. Previous research has shown that amphetamine (Amp) improves exercise endurance.2–4How it does this is not known. It is largely thought to occur by masking the perception of fatigue.2–7 However, there have only been a few studies looking at peripheral mechanisms such as increasing oxygen utilization. In one study, a relatively small dose of Amp (~0.2 mg/kg) was given to six volunteers. While the time to exhaustion was increased, the VO2MAX was not affected.3 A similar study in two volunteers given a low dose of methamphetamine also showed no effect on VO2MAX but an increase in exercise endurance.7 Independent of exercise, amphetamines are known to increase body temperature by increasing heat production while simultaneously decreasing heat dissipation.8, 9 As body temperature is a key factor in limiting the duration of exercise,10 it is possible that any ergogenic advantages offered by amphetamines when used in a cool environment would be offset if used in the warm. Amphetamines effect on athletic performance in the warm has not however been evaluated.
Amphetamine is also known to improve exercise endurance in rats.5, 11, 12 There are no studies, however, in rats measuring the effect of higher doses of Amp on exercise-related VO2MAX or the effect of Amp on exercise endurance in the heat. Therefore, we sought to determine the effect of two doses of Amp on VO2MAX and running economy in rats at room temperature. Furthermore we tested if Amp increased running time in the heat and if so what affect this had on core body temperature.
METHODS
Animals
Male adult Sprague-Dawley rats (weight 300 ± 20 g; Harlan, Indianapolis, IN) were used in this study. All procedures were approved by the Indiana University Animal Care and Use Committee. Experiments were performed using single-housed rats that were maintained in a 12 h light/dark cycle and fed ad libitum. Experiments were conducted on fully conscious rats between 10:00 a.m. and 4:00 pm in a temperature-controlled room or in a specially designed environmental chamber.
Chemicals
d-Amphetamine was purchased from Sigma (Sigma-Aldrich, St. Louis, MO) and dissolved in sterile saline (0.9%) to a volume of 1 ml/kg of body weight.
Surgical preparation
Animals were anesthetized with 1.5–2% isoflurane in oxygen. For the measurement of core body temperature, TA-F40 telemetric transmitters (DSI, St. Paul, MN) were placed intraperitoneally (i.p.) via a 2 cm-long longitudinal medial skin incision and muscular wall incision at the linea alba. Following insertion of the transmitter into the abdominal cavity and surgical closure, rats were returned to their cage for at least 1 week before beginning treadmill familiarization.
Treadmill familiarization
Prior to experiments, rats were familiarized to running on a motorized rodent treadmill (Columbus Instruments, Columbus, OH) using one of two schedules, depending on the experiments to follow. To prepare rats for experiments that utilized an incremental treadmill protocol, such as for measurement of maximal rate of oxygen uptake (VO2Max), a 14-day familiarization protocol was used. Rats were run on the treadmill for 5 min/day with both the slope and speeds increasing daily; for example, on day one rats were run at a 5° incline at a maximum speed of 10 m/min and by day 15 at an incline of 20° and a maximum speed of 26 m/min. To prepare rats for experiments that utilized a fixed-speed protocol, such as those for measurement of time to exhaustion and running economy, a simpler 5-day familiarization program was used with a fixed incline at 5° and the speed increased progressively to a maximum of 18 m/min by day 5. Workloads of familiarization schedules accustom the rats to treadmill running, but are not sufficient to induce training adaptations.13 Mild electric stimulus at the back of the treadmill chamber promoted learned running behavior. Any animal that was unable to consistently run at the front of the treadmill by the end of the familiarization sessions was eliminated from the study. In all of the experiments a motorized fan on the front of the treadmill circulated fresh air into the chamber.
Temperature chamber
We constructed an environmental chamber to conduct experiments at elevated temperatures. The walls were made of ¼″ Plexiglas covered with ¾″ Styrofoam with the following internal dimensions 55′(w) × 35′(d) × 27′(h). A door in the front of the chamber was large enough to move the treadmill in and out. The motor of the treadmill outside the box and was connected to the lanes with a 1.5 m long shaft through the hole in the wall of chamber. To circulate air in the box we installed two 90×90 mm fans operated at 1800 rpm (low noise computer case fans). Heating was produced using a 600 W ceramic heater and the temperature was regulated with an electronic single-stage temperature controller (model111000-000, Ranco, Plain City, OH). The temperature controller thermistor was positioned next to the lane of the treadmill (or between lanes if two were present inside the environmental chamber), with the thermistor’s coating stripped off to increase its time constant of response. For experiments conducted at elevated temperature, the chamber temp was set at 32 ± 1°C and humidity <20%. For experiments conducted at room temperature the front door of the chamber was open. The room temperature was 25 ± 1°C and the humidity 40 ± 10%.
Effect of amphetamine on VO2MAX
To determine amphetamine’s effect on VO2MAX a repeated trials crossover design was utilized (n=6 per Amp dose). Rats that had been appropriately familiarized were randomized to one of two doses of amphetamine: Amp-1 (1 mg/kg) and Amp-2 (2 mg/kg). These doses were previously shown to increase the time to exhaustion in rats running at room temperature5. These doses are also in the doses shown to reduce hyperactivity in an rodent model of ADHD14, and are in the range of maximum doses taken by adults for ADHH (~ 60 mg/day)15.On the day of the experiment rats received either their designated dose of Amp or saline. On a subsequent day (at least 2 days after the first experiment) the animals received the other treatment (saline animals received Amp and vice versa). The order of administration was counter-balanced. Immediately after injection of drug or saline rats were placed in an enclosed metabolic treadmill. Measurements were obtained using an indirect open-circuit calorimetric system (Oxymax, Columbus Instruments, Columbus, OH). Gas analyzer calibrations were conducted before testing using standardized gas mixtures (Praxair, Danbury, CT). After a brief period of equilibration, baseline-resting measurements were collected until VO2 consumption stabilized (~3–5 min). VO2 was recorded every 30s and data are expressed relative to body weight. To determine VO2MAX, an incremental treadmill protocol in 3 min stages was utilized, modified from that previously described.16 The stepwise increase of treadmill speed and incline are as follows: stage I:10 m/min at 0°; stage II:10 m/min at 5°; and then an increase of 5 m/min and 5° for each consecutive stage. The test was terminated when VO2 plateaued despite increasing workload,14 or the rat was unable to maintain position on the belt and received three consecutive shocks without recovery. The highest VO2 achieved was recorded as VO2MAX. Following each test, rats were returned to their home cages for at least 2 days before the second trial of testing was performed.
Effect of amphetamine on running economy
In a separate group of rats we determined running economy. In a manner identical to the protocol used in VO2MAX testing, rats were randomized to receive saline or one of two dose of amphetamine (Amp-1 and Amp-2) in a repeated trials crossover design (n=6 per group). For determination of running economy, rats were placed on the treadmill after injection of saline or drug. After a brief period of equilibration, baseline-resting measurements were collected until VO2 consumption stabilized (~3–5 min). VO2 was recorded every 30s and data are expressed relative to body weight. To determine economy, speed and incline were adjusted to test three different workloads: 10 m/min at 0°, 15 m/min at 10°, and 18 m/min at 15°. These represented the estimated 70%, 80%, and 90% workloads based on previous VO2MAX testing. Each workload was tested for 6 min to achieve steady state energy expenditure with the final min VO2 recorded as the oxygen cost for that workload. Following each test, rats were returned to their home cages for at least 2 days before the second trial of testing was performed.
Effect of amphetamine on time to exhaustion in the heat
The rats were tested only once in experiments to determine whether Amp increases time to exhaustion and the core temperatures at which exhaustion occurs. After familiarization the rats were randomized into three groups (n=8/group): saline, Amp-1, or Amp-2. These doses were previously shown to increase the time to exhaustion in rats running at room temperature.5 On the day of testing, rats were injected i.p. with either saline or Amp and immediately placed on the treadmill within the heated environmental chamber. With the treadmill incline set to 5°, within a min the treadmill belt was brought up to a fixed speed of 18 m/min. Rats ran at this workload until exhaustion occurred. All the experiments were conducted by an investigator blinded to group allocation. Exhaustion was defined as the point at which a rat received three consecutive shocks without recovery (i.e., running again at the front of the treadmill). Core temperature was captured every minute by receiver plates (DSI, St. Paul, MN) placed on the top of the treadmill lane. Temperature was captured every minute using a At the end of the experiment rats were immediately removed from the heat and placed in their home cages at room temperature for recovery.
Data analysis and statistical procedures
Statistical analyses and graphing were performed using Prism (Graphpad Software, San Diego, CA) software. For analyses requiring between-trial comparisons (VO2Max, time to VO2max, and oxygen cost per workload), a paired student t-test compared the Amp trial (either Amp-1 or Amp-2) to its corresponding control trial (saline). Telemetric temperature data were measured every min. Analyses requiring between-group comparisons (time to exhaustion, and temperature at exhaustion) were performed using analysis of variance (ANOVA) with a least significant difference (LSD) post hoc analysis where indicated. Significance was defined as a p-value <0.05. Values are presented as means ±SEM.
RESULTS
VO2Max testing
Rats in the Amp-1 took 1.7 min longer to reach VO2MAX than in their saline trial (Fig 1A). The longer run time with Amp-1, however, was not accompanied by higher VO2Max values (Fig 1B). By comparison, Amp-2 animals took 2.5 min longer to reach VO2MAX and had higher VO2Max values (Figs 1A, 1B) compared to their saline trials.
Figure 1. Amphetamine increases aerobic capacity at room temperature.
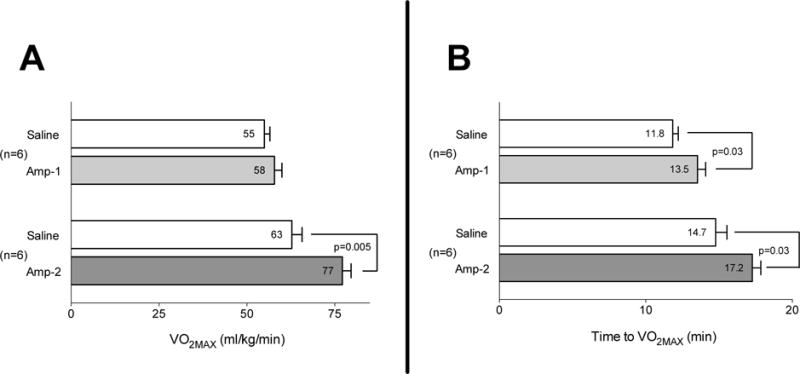
Amphetamine at a higher dose (2mg/kg) increased VO2Max (Fig 1A) and time to achieve VO2MAX (Fig 1B), while Amp at a lower dose 1 mg/kg) increased only time to VO2Max (Fig 1B). Rats received (i.p.) either saline or one of two doses of Amp; 5 min later they performed the incremental treadmill test at 24°C for assessment of VO2Max. Metabolic measurements were obtained via an indirect open-circuit calorimetric system with analysis of gases collected from an enclosed motorized treadmill chamber (Oxymax). In a crossover design, at least 2 days later, the animal groups were reversed and the trial repeated. Horizontal bars represent trial means ± SEM. n=6 per group.
Running economy
The effect of amphetamine on running efficiency is shown in Figs 2A and B. There was no difference in VO2 consumption at baseline or at a 70% workload in either group. At an 80% workload Amp-1 increased, and Amp-2 trended toward an increase, in VO2 consumption. Similar to the VO2Max testing (Fig 1), at a ~90% workload, VO2 consumption was increased in the Amp-2 but not Amp-1 group. Together this data suggests that while higher doses of Amp can increase the maximal aerobic capacity, this does not translate to an improved running efficiency.
Figure 2. Amphetamine does not improve running economy at room temperature.
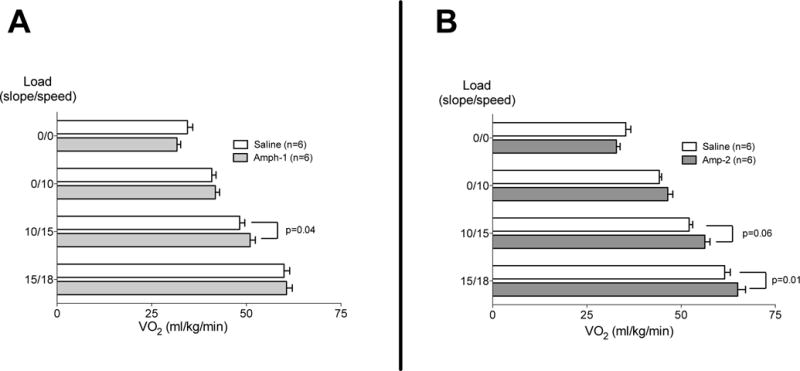
Rats received (i.p.) either saline or 1 mg/kg (Fig 2A) or 2 mg/kg (Fig 2B) of Amp (n=6 per group). Five min later, they were run at four different workloads on a motorized treadmill for 6 min/per load at 24°C. Running economy was assessed at each workload via analysis of expired gases for determination of steady-state O2 consumption (Oxymax). In a crossover design, at least 2 days later, the animal groups were reversed and the trial repeated. Horizontal bars represent trial means ± SEM.
The effect of amphetamine on body temperature and running endurance in the warm
The baseline temperatures between the control (37.4 ± 0.14°C), Amp-1 (37.3 ± 0.14°C), and Amp-2 (37.3 ± 0.1°C) groups were not different. Fig 3 shows the mean temperature curves for each group between t= −10 and t= 27 min. This times period was chosen since no animal in any of the groups had run to exhaustion before 27 min; this allows the reviewer to compare the temperature responses between groups with equal numbers of animals in each group. After 27 min various animals stopped running, with the Amp-1 animals able to run the longest. The squares to right of the curves represent the mean temperatures at exhaustion and the time to exhaustion for each corresponding group.
Figure 3. Temperature curves for rats running to exhaustion in the heat.
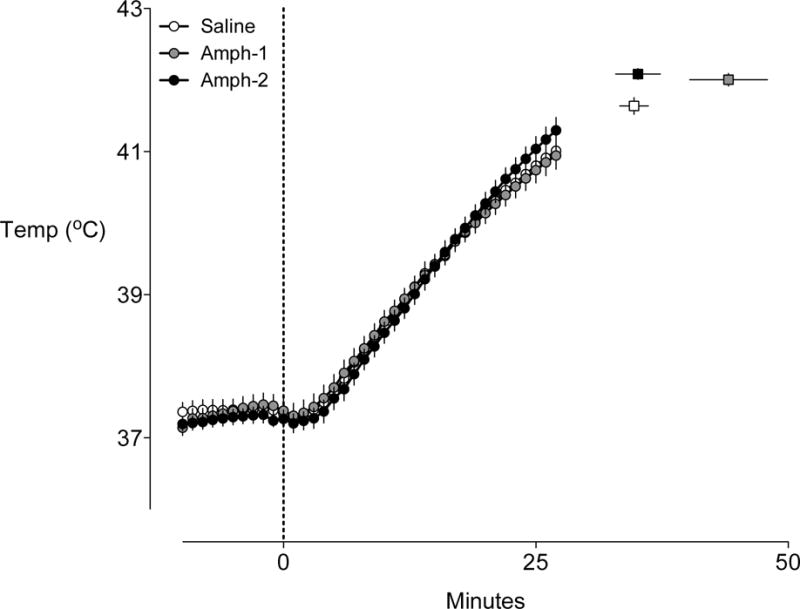
The mean temperature curves (generated between −10 and 27 min) for each group are shown. These times points were chosen as the represent the period in which no animals had yet achieved exhaustion. The corresponding squares to the right of the graph represent the grouped mean time to exhaustion and temperature at exhaustion. Error bars represent ± SEM, n=8 for each group
Compared to control, Amp-1 rats ran 9 min longer (p=0.02) before exhaustion occurred (Fig 4B). These animals also achieved higher core body temperatures (Fig 4A) (+ 0.4°C, p=0.01) compared to controls. The Amp-2 rats ran the same length of time as controls, but had higher temperatures at exhaustion (p=0.003)(Figs 4A, 4B).
Figure 4. Amphetamine increases the temperature at exhaustion.
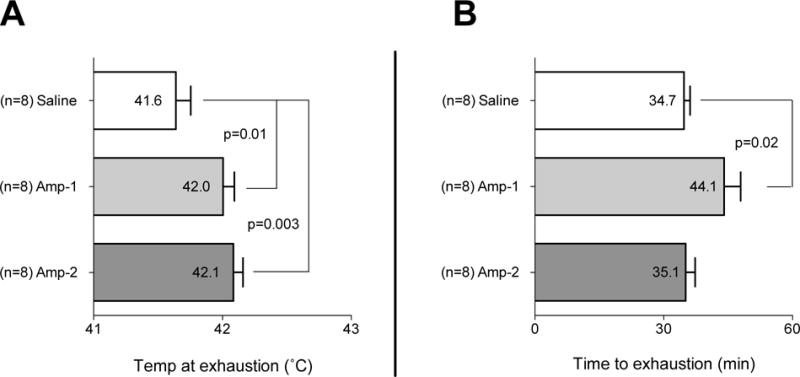
Rats received (i.p.) either saline or Amp (1 or 2 mg/kg). Five min later they were placed on a treadmill in a warm environment (32°C) and run at 18 m/min at a 5° incline. For rats running at this constant workload in the heat, low dose amphetamin (1 mg/kg) increased time to exhaustion (Fig 4A), and temperature at exhaustion (Fig 4B); Amp at higher dose (2 mg/kg) increased only the temperature at exhaustion (Fig 4B). Horizontal bars represent group means ± SEM, n=8 for each group.
DISCUSSION
Our conclusions showed that while both doses of Amp (1 and 2 mg/kg) increased VO2MAX testing time to exhaustion, only the higher dose increased VO2MAX. This increase however did not translate to improved running efficiency; to the contrary—running efficiency was worsened by Amp. Lastly, we showed that while lower dose of Amp increased running endurance and core body temperatures in the warm, higher doses only increased core body temperature at exhaustion. Collectively this data suggests that while Amp does confer an ergogenic advantage, by increasing the endurance time in rats, these effects are dose and environment dependent. Likely by increasing heat generation through non-work related means, any benefit conferred by higher doses of Amp are quickly offset in a warm environment with the animal achieving critical temperatures faster.
To our knowledge, the effect of amphetamine on VO2Max and running economy has never been studied in the rat. We showed that only the highest dose tested (Amp-2) increased VO2Max. By comparison, a small study in humans showed that a relatively low dose of Amp (0.2 mg/kg) had no effect on VO2Max,3 a similar study with methamphetamine showed the same.7 Like both of these human studies, we showed that Amp, at both doses tested, increased the time to VO2MAX. Since Amp did not improve running economy, its ergogenic advantage is likely due to masking central-mediated exhaustion.
In a study with nearly identical running conditions (speed 18.8 m/min at an 8° incline), Gerald showed that amphetamine at several doses, including 1.25 and 2.5 mg/kg, increased the time to exhaustion in rats at room temperature.5 In his study at the highest doses he tested (> 5 mg/kg), running duration was actually decreased. While the running times he reports for Amp-1 and Amp-2 are slight longer than those we report, animals in their study ran for 64 (Amp-1.2) and 76 (Amp-2.5) min and ours ran for 44 (Amp-1) and 35 (Amp-2), our experiments were conducted at 32°C. In experiments conducted at room temperature in our lab (data not shown), we have noted that untreated rats routinely run, at the same treadmill settings used in this study (18 m/min, 5°), for 60 min or longer. The difference in the time to exhaustion between these studies may be due, in part, to the slightly different doses, but more likely they reflect the importance of core temperature in mediating exhaustion.
This is the first investigation of the effect of amphetamine on exercise and exhaustion in the heat. This suggests that in a hot environment core temperature is the critical determinant of exhaustion. Studies in both rodents and humans likewise support that core temperature correlates with exhaustion and that manipulating body temperature, either through pre-cooling or pre-warming, affects the time to exhaustion.17–20
While both doses of Amp increased the temperature at exhaustion, only the 1 mg/kg dose increased exercise duration. By increasing non-work related heat production, higher doses of Amp may decrease the time it takes to reach the temperature at which exhaustion occurs. Activation of interscapular brown adipose tissue (iBAT) increases VO2 consumption, even in anesthetized animals,21 which may account for the VO2 consumption differences we saw with the higher dose of Amp. At the doses we studied, Amp is known to known activate thermogenesis in iBAT.9 Amphetamines have been shown to generate heat in skeletal muscle through non-shivering thermogenesis.22 Along with increasing heat production, amphetamines decrease heat dissipation by causing cutaneous vasoconstriction.8 Although done in anesthetized rats, these studies showed a dose response with higher dose of amphetamine causing greater vasoconstriction.8 Collectively, when used in a warm environment the ergogenic effect of masking exhaustion is likely offset with higher doses of amphetamine that would increase core temperature faster. By increasing the temperature at which exhaustion occurs, Amp may increase risk of developing heat stroke. While not formally studied in humans, there is anecdotal evidence supporting the link between Amp use and exertional heat stroke including several well-publicized deaths in professional athletes.23–25
The mechanism by which Amp is able to mask fatigue is not known. Drugs similar to Amp that increase central concentrations of dopamine and norepinephrine have also been shown to increase both the temperature at and time to exhaustion in humans. Men given bupropion or methylphenidate prior to a cycling challenge in the warm completed the task faster and had higher body temperatures at the time of exhaustion.26, 27 The brain areas responsible for amphetamines’ effects on exhaustion haven’t been identified. Previous research, however, has implicated the dorsomedial hypothalamus, the medial prefrontal cortex, and the ventral tegmental area (VTA) of the brain as possible sites. All of these brain regions have been shown to be involved in spontaneous locomotion evoked by amphetamines.28–30 Furthermore, the VTA has been implicated in exercise-related exhaustion: self-stimulation of the ventral tegmental area has been shown to increase time to exhaustion in rats.31 The VTA is a brain region involved in reward and motivation;32 contains large numbers of dopamine neurons whose firing rates are affected by Amp;33 sends projections to the prefrontal cortex;34 and receives them from the dorsomedial hypothalamus.35 Whether this putative circuit is involved in exercise induced exhaustion requires further study.
In conclusion, amphetamines mask fatigue conveying an ergogenic advantage in temperate conditions. In the warm however, higher doses of amphetamine may increase heat production causing critical temperatures to be achieved faster thereby negating any ergogenic advantage.
Acknowledgments
Grants
Research reported in this publication was supported by the National Institute on Drug Abuse of the NIH under award number R01DA026867. Furthermore, this work was conducted in a facility constructed with support from the National Center for Research Resources, of the NIH under award number C06 RR015481-010. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- Amp
amphetamine
- VO2Max
maximum oxygen consumption
- W
watt
- ANOVA
analysis of variance
- LSD
least squares difference
- iBAT
interscapular brown adipose tissue
- VTA
ventral tegmental area
Footnotes
Disclosures
There are no conflicts of interest, financial or otherwise, declared by the authors.
Contributor Information
Dmitry V Zaretsky, Email: dzaretsk@iupui.edu.
Mary Beth Brown, Email: brownmb@iu.edu.
Maria V Zaretskaia, Email: mazarets@iupui.edu.
Pamela J Durant, Email: pjdurant@iu.edu.
Daniel E Rusyniak, Email: drusynia@iu.edu.
References
- 1.WADA. 2012 anti-doping testing figures report. 2012. [Google Scholar]
- 2.Borg G, Edstrom CG, Linderholm H, Marklund G. Changes in physical performance induced by amphetamine and amobarbital. Psychopharmacologia. 1972;26:10–8. doi: 10.1007/BF00421914. [DOI] [PubMed] [Google Scholar]
- 3.Chandler JV, Blair SN. The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc. 1980;12:65–9. [PubMed] [Google Scholar]
- 4.Smith GM, Beecher HK. Amphetamine sulfate and athletic performance. I. Objective effects. Journal of the American Medical Association. 1959;170:542–57. doi: 10.1001/jama.1959.63010050001008. [DOI] [PubMed] [Google Scholar]
- 5.Gerald MC. Effects of (+)-Amphetamine on the treadmill endurance performance of rats. Neuropharmacology. 1978;17:703–4. doi: 10.1016/0028-3908(78)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Laties VG, Weiss B. The amphetamine margin in sports. Fed Proc. 1981;40:2689–92. [PubMed] [Google Scholar]
- 7.Wyndham CH, Rogers GG, Benade AJ, Strydom NB. Physiological effects of the amphetamines during exercise. S Afr Med J. 1971;45:247–52. [PubMed] [Google Scholar]
- 8.Borbely AA, Baumann IR, Waser PG. Amphetamine and Thermoregulation: Studies in the Unrestrained and Curarized Rat. Naunyn-Schmiedeberg’s Arch Pharmacol. 1974;281:327–40. doi: 10.1007/BF00499429. [DOI] [PubMed] [Google Scholar]
- 9.Wellman PJ. Influence of amphetamine on brown adipose thermogenesis. Res Commun Chem Pathol Pharmacol. 1983;41:173–6. [PubMed] [Google Scholar]
- 10.Noakes TD. Fatigue is a Brain-Derived Emotion that Regulates the Exercise Behavior to Ensure the Protection of Whole Body Homeostasis. Front Physiol. 2012;3:82. doi: 10.3389/fphys.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagat B, Wheeler N. Effect of amphetamine on the swimming endurance of rats. Neuropharmacology. 1973;12:711–3. doi: 10.1016/0028-3908(73)90124-x. [DOI] [PubMed] [Google Scholar]
- 12.Molinengo L, Orsetti M. Drug action on the “grasping” reflex and on swimming endurance; an attempt to characterize experimentally antidepressant drugs. Neuropharmacology. 1976;15:257–60. doi: 10.1016/0028-3908(76)90073-3. [DOI] [PubMed] [Google Scholar]
- 13.Lambert MI, Noakes TD. Dissociation of changes in VO2 max, muscle QO2, and performance with training in rats. J Appl Physiol (1985) 1989;66:1620–5. doi: 10.1152/jappl.1989.66.4.1620. [DOI] [PubMed] [Google Scholar]
- 14.Myers MM, Musty RE, Hendley ED. Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol. 1982;34:42–54. doi: 10.1016/s0163-1047(82)91397-8. [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Spencer TJ, Wilens TE, Weisler RH, Read SC, Tulloch SJ, group SLIs Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10:16–25. doi: 10.1017/s1092852900002406. [DOI] [PubMed] [Google Scholar]
- 16.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol Respir Environ Exerc Physiol. 1979;47:1278–83. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 17.Dugas J. Ice slurry ingestion increases running time in the heat. Clin J Sport Med. 2011;21:541–2. doi: 10.1097/01.jsm.0000407930.13102.42. [DOI] [PubMed] [Google Scholar]
- 18.Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol. 1998;84:877–83. doi: 10.1152/jappl.1998.84.3.877. [DOI] [PubMed] [Google Scholar]
- 19.González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;88:1032–9. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- 20.Walters TJ, Ryan KL, Tate LM, Mason PA. Exercise in heat is limited by a critical internal temperature. J Appl Physiol. 2000;89:799–806. doi: 10.1152/jappl.2000.89.2.799. [DOI] [PubMed] [Google Scholar]
- 21.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–40. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Sprague JE, Mallett NM, Rusyniak DE, Mills E. UCP3 and thyroid hormone involvement in methamphetamine-induced hyperthermia. Biochem Pharmacol. 2004;68:1339–43. doi: 10.1016/j.bcp.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Charatan F. Ephedra supplement may have contributed to sportsman’s death. BMJ. 2003;326:464. doi: 10.1136/bmj.326.7387.464/b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarkson PM, Thompson HS. Drugs and sport. Research findings and limitations. Sports Med. 1997;24:366–84. doi: 10.2165/00007256-199724060-00003. [DOI] [PubMed] [Google Scholar]
- 25.Rae DE, Knobel GJ, Mann T, Swart J, Tucker R, Noakes TD. Heatstroke during endurance exercise: Is there evidence for excessive endothermy? Med Sci Sports Exerc. 2008;40:1193–204. doi: 10.1249/MSS.0b013e31816a7155. [DOI] [PubMed] [Google Scholar]
- 26.Roelands B, Hasegawa H, Watson P, Piacentini MF, Buyse L, De Schutter G, Meeusen RR. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. 2008;40:879–85. doi: 10.1249/MSS.0b013e3181659c4d. [DOI] [PubMed] [Google Scholar]
- 27.Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. The Journal of physiology. 2005;565:873–83. doi: 10.1113/jphysiol.2004.079202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. Microinjection of muscimol into the dorsomedial hypothalamus suppresses MDMA-evoked sympathetic and behavioral responses. Brain Res. 2008;1226:116–23. doi: 10.1016/j.brainres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selken J, Nichols DE. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacol Biochem Behav. 2007;86:622–30. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaretsky DV, Zaretskaia MV, Durant PJ, Rusyniak DE. Inhibition of the dorsomedial hypothalamus, but not the medullary raphe pallidus, decreases hyperthermia and mortality from MDMA given in a warm environment. Pharmacol Res Perspect. 2014;2:e00031. doi: 10.1002/prp2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess ML, Davis JM, Borg TK, Buggy J. Intracranial self-stimulation motivates treadmill running in rats. J Appl Physiol. 1991;71:1593–7. doi: 10.1152/jappl.1991.71.4.1593. [DOI] [PubMed] [Google Scholar]
- 32.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang RY. Dopaminergic neurons in the rat ventral tegmental area. III. Effects of d-and l-amphetamine. Brain Res Rev. 1981;3:153–62. [Google Scholar]
- 34.Müller-Ribeiro FC, Zaretsky DV, Zaretskaia MV, Santos RA, DiMicco JA, Fontes MA. Contribution of infralimbic cortex in the cardiovascular response to acute stress. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303:R639–50. doi: 10.1152/ajpregu.00573.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–26. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


