Introduction
In humans as in other organisms, physiological needs induce powerful motivational states that organize behavior in order to fulfill the goals imposed by these states. Such states include hunger, thirst, pain, social contact, and others [3,5,13,18,49]. When multiple motivational states are present at the same time, they are thought to compete for priority, resulting in the inhibition of brain pathways that mediate low priority motivational states [1,18,20].
Pain can be thought of as a motivational drive state related to potential tissue damage, which motivates behavior to minimizing present and future injury [9,20,49]. Accordingly, the intensity and quality of pain is thought to be shaped by competing motivational states. Particularly, pain reduction due to competition among motivational states is linked to the activation of descending modulatory pathways and modulation at cerebral and spinal levels [20–22,26,31]. Such interactions of motivational states are described in the Motivation-Decision model of pain [20] and in more recent models [9,45]
These models provide a set of underlying principles that explain how competing motivational states shape pain experience and thus pain behavior observable in animals. Consistent with this model, animal research has shown that several motivational states reduce pain behaviors, including thirst [13] or the hedonic component of chocolate consumption [22]. In humans, motivation to obtain secondary reinforcers, i.e., monetary rewards, has been used to study motivational effects on pain perception. Gambles resulting in monetary gains or losses affected pain in opposite directions, with wins reducing, and losses increasing pain [4]. Rewarding participants for good task performance also reduces pain [46].
In addition to these direct effects on pain experience and pain behavior, motivational states can also induce changes in mood and emotions, especially when the strength of the competing state is increasing over time and the associated goal cannot be fulfilled, e.g., becoming more and more thirsty during a long day [9,45]. A number of studies have demonstrated that negative mood and emotions affects pain perception [25,34,36,37,51]. In those studies, negative mood enhanced pain and positive mood reduced it. Thus, competing motivational states associated with positive mood are expected to reduce pain due to both the effects of mood and the motivational demand itself, rendering it impossible to distinguish the two effects. By contrast, experimentally inducing aversive motivational states could help to disentangle the potentially opposing effects of mood and the motivational challenge, as they are expected to have opposite effects. In doing so, it is necessary to induce an aversive motivational state without concurrent cognitive demand (which is also expected to reduce pain [7,42]), and measure negative mood (which is expected to increase pain) as a potential suppressor of motivational challenge effects [29].
In this study, we manipulated thirst, a basic motivational state, by randomizing participants into a Thirst or a Control (no-thirst) group. We induced thirst via water deprivation and the consumption of salty food. Pain was tested at baseline, during the thirsty state, and after re-hydration along with measures of mood and thirst. According to the Motivation-Decision model, the thirst challenge should reduce pain [9,20,21]. Conversely, we expected the negative mood accompanying the challenge to increase pain [36,37]. Mediation analyses [28] were used to disentangle the direct motivational effect from effects mediated by mood.
Materials and Methods
Sample
Forty-two healthy participants (20 female) aged between 18 and 53 years of age (mean age: 27.2 years) participated in this study. Half of the participants were randomized into the Thirst group (12 female, mean age: 26.6 years) and the other half served as Control group (8 female, mean age: 27.9 years). We chose a sample size of at least 42 on the basis of expected effect sizes based on experience on pain modulatory studies from our lab. Sample sizes of about 42 provide approximately 80% power to detect an effect size (partial correlation) of 0.4 or larger (G*Power 3 [19]). Participants reported no history of neurological, psychiatric, or dermatologic conditions, and had not taken any medication during the 48 h prior to the experiment. Also, participants reporting acute and chronic pain conditions in an initial online screening were excluded from participation. We recruited participants from the university and local community via flyers and online ads. The Institutional Review Board of the University of Colorado Boulder approved the protocol.
Procedures
Participants first provided informed consent. Participants then rated their current thirst on a visual analogue scale (VAS), anchored at the extreme ends with “no thirst at all” and “extremely thirsty.” They also reported their current mood on three dimensions (pleasant-unpleasant, awake-sleepy, and calm-restless) using the Multidimensional Mood Questionnaire (MDMQ [43]). Participants then completed the first of three pain tests.
Each pain test included 16 heat pain trials, totaling 48 trials over the course of the experiment. Within each block, 8 different temperatures ranging from 45°C to 49.5°C in steps of 0.5°C were repeated 2 times each. Stimulus duration was 11.5 s (including 2 s ramp up and down to a 32°C baseline) followed by a delay of 5 – 7 s before the pain VAS appeared on the screen. During an inter-trial interval (ITI) of 12 – 16 s a fixation dot was presented centrally on the screen. The fixation dot remained unchanged on the screen for the duration of the cutaneous heat stimulation (Fig. 1b). A Peltier thermode (1.5 × 1.5 cm surface, PATHWAY ATS, Medoc, Inc., Israel) delivered heat to the left volar forearm of the participant. Thermode position was changed in each block to prevent sensitization. Participants were instructed how to use the VAS scale and how to interpret the anchors. The VAS had 3 anchors, “no pain”, “pain threshold”, and “unbearable pain” positioned at the 0, 25, and 100 marks of the scale, respectively. The order of temperatures was randomized across participants, but the same order was used across the three blocks for a single participant. Stimulus presentation and response logging were controlled by MATLAB (R2015a; The MathWorks Inc., Natick, MA) using the Psychophysics Toolbox v3 (http://psychtoolbox.org/).
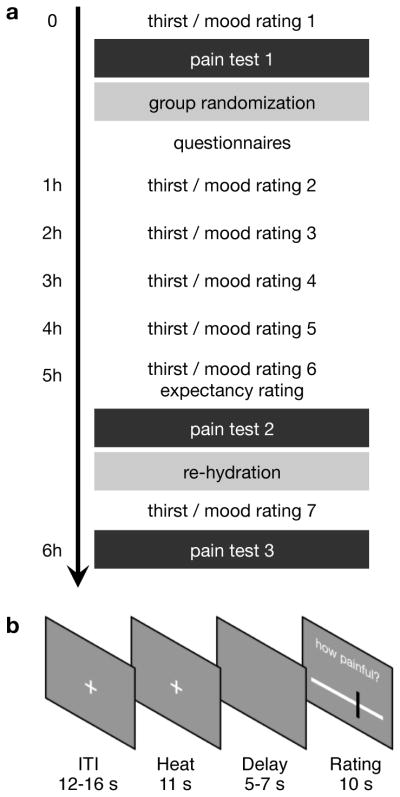
Figure 1.
Experimental design. (a) Timeline of the experiment. The experiment started with a thirst (VAS) and mood rating (MDMQ). This rating was repeated 7 times during the experiment. A baseline pain test was administered before participants were randomized into either thirst or control group. After a waiting period, a second pain test was completed. All participants were then provided with water ad libitum to eliminate their thirst. After 30 min a last pain test was completed. (b) Trial structure. Each trial started with a variable inter-trial-interval. A heat pain stimulus was then applied while the fixation crosshair remained unchanged on the screen. Following a variable delay, participants had 10 s to provide their pain rating on a visual analogue scale (VAS).
After completing of the first pain block, participants were randomized into either the Thirst or the Control group. Participants then moved to a separate room, where they spent a 4-hour waiting period. At the beginning of this waiting period, participants completed a set of questionnaires, including demographic information, the state-trait-anxiety inventory (STAI [41]), the pain catastrophizing scale (PCS [44]), the life orientation test (LOT-R [38]), the social desirability scale (SDS [10]), the fear of pain (FoP [30]), and the personal distress subscale of the interpersonal reactivity index (PD [12]). Every hour during the waiting period participants rated their thirst and completed the MDMQ using the same scales as detailed above.
All participants ate 1 package of salty pretzels (100 g) and 1 package of salty goldfish (75 g) while waiting. The Thirst group was not allowed to drink until the completion of the second pain test (Fig. 1a), whereas the control group was provided with water ad libitum during the waiting period.
Five hours after arrival, participants completed their sixth thirst and mood survey before undergoing the second pain block. Participants were then given as much water as they wanted to drink, regardless of which group that had been randomized into, and water intake was recorded. Thirty minutes later, participants completed the last thirst and mood questionnaire and a third pain block. Each pain block lasted 15 minutes and the entire procedure took about 6 hours per participant.
Analyses
VAS ratings were converted to numeric values ranging from 0 to 100 for both pain and thirst ratings. Pain ratings were averaged within each block for each participant for subsequent analyzes. We used the pleasant-unpleasant subscale of the MDMQ as a measure of negative mood in the following analyses. All results also hold for a summary score of the three MDMQ subscales. A 2 (control vs. thirst group) × 7 (time) mixed-ANOVA was conducted on the thirst ratings and pleasantness-unpleasantness MDMQ subscale. To explore the significant group effect in the thirst ratings, we conducted post-hoc two-sample t-tests for each time-point.
We hypothesized that the Thirst group would show a change in pain perception only when thirsty (Block 2), but not during baseline (Block 1) or after re-hydration (Block 3). We therefore computed an a priori contrast testing the effects of Thirst vs. Non-Thirst Blocks, i.e., comparing Block 2 (thirst) against the other conditions (pre- and post-thirst) using a multi-level general linear model (implemented in glmfit_multilevel.m [27]). This contrast assesses whether either of the two proposed mechanisms is strong enough to produce an overall change in mean pain ratings. Including participant sex as did not change the results and the sex effect was not significant (t39 = 0.76, p = 0.45). Stimulus intensity did not interact with group or the planned contrast of interest (all t39 < 1.22, all p > 0.22), suggesting stable effects across temperatures. Subsequent mediation analyses tested for effects controlling mood.
In order to disentangle opposing effects of the motivational challenge from the thirst and mood changes on pain, we used mediation analyses [28] implemented in the Multilevel Mediation and Moderation (M3) Toolbox for MATLAB (http://wagerlab.colorado.edu/tools; [50]). For the mediation analysis we focused on the second pain test, as this was the critical test condition when half of the participants were thirsty. We first tested whether the effect of the motivational challenge (Group) on pain was mediated by reported thirst. We then tested a second model to investigate whether the relationship between subjective thirst ratings and pain was in turn mediated by mood (note that higher values indicate more negative mood.)
Mediation analysis tests whether the covariance between two variables X and Y (group and pain) can be explained by a third variable M (thirst). The following equations capture the mediation model:
Here y, x, and m represent the outcome (y, the reported pain), the predictor (x, group), and data from a potential mediator (m, thirst or mood). ey, em, and e′y denote residual errors for the outcome and mediator controlling for x and the outcome controlling for x and m, respectively. Path a is the slope of m regressed onto x. Path b is the slope of the mediator-outcome relationship controlling for x. The paths c and c′ describe the linear relationship of x and y with and without the mediator, respectively. The mediation effect is obtained by the combined path ab. We report correlation coefficients and partial correlations as effect size measures for path coefficients a, b, and c′ [28]. For the indirect effect we report , in which the indirect effect is standardized by the standard deviations (SD) of X and Y [33]. This effect size indicates that Y increases by abcs standard deviations for every 1 SD increase in X indirectly via M [33]. As is common practice [40,50], we used bias-corrected, accelerated bootstrap tests [14] to assess statistical significance, using 50,000 bootstrap samples.
Results
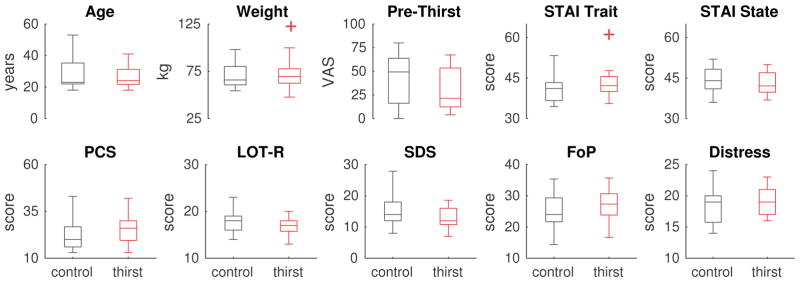
Thirst and Control groups did not differ in age (t40=0.53, p=0.59), male/female proportions (χ2 = 0.875, p=0.34), weight (t40=0.3, p=0.77), baseline thirst (t40=1.21, p=0.24), or psychological measures (all t40<1.8, all p>0.07; Fig. 2 and Table 1).
Figure 2.
Group comparisons. Thirst and Control groups did not differ in terms of age or psychological profile. None of the pairwise comparisons were significant at p<0.05, uncorrected. Box plots show medians within the box and boxes extend from 25th to 75th percentiles. Outliers more than 2 interquartile ranges outside the boxes are represented by plus-symbols. Pre-Thirst ratings were obtained at the start of the experiment before Block 1. STAI: State Trait Anxiety Inventory. PCS: Pain Catastrophizing Scale. LOT-R: Life Orientation Test – Revised. SDS: Social Desirability Scale. FoP: Fear of Pain. Distress: Personal Distress subscale.
Table 1.
Group Statistics
| Control group | Thirst group | |||
|---|---|---|---|---|
|
| ||||
| Mean | Std. | Mean | Std. | |
| Age (yrs) | 28.0 | 10.0 | 26.5 | 7.1 |
| Weight (kg) | 70.5 | 12.8 | 71.9 | 17.1 |
| STAI Trait | 40.9 | 5.0 | 42.8 | 5.3 |
| STAI State | 44.1 | 5.1 | 43.0 | 4.4 |
| PCS | 22.7 | 8.4 | 25.3 | 7.8 |
| LOT-R | 17.7 | 2.3 | 16.6 | 2.0 |
| SDS | 15.0 | 4.3 | 12.8 | 3.4 |
| FoP | 25.0 | 5.3 | 26.7 | 5.0 |
| Distress | 18.4 | 3.0 | 19.3 | 2.2 |
|
| ||||
| Thirst 1 | 39.5 | 26.9 | 30.4 | 21.8 |
| Thirst 2 | 29.0 | 21.7 | 42.1 | 22.9 |
| Thirst 3 | 20.6 | 18.6 | 49.8 | 21.7 |
| Thirst 4 | 17.6 | 18.7 | 54.9 | 21.8 |
| Thirst 5 | 12.3 | 10.3 | 57.8 | 24.8 |
| Thirst 6 | 10.5 | 10.6 | 64.3 | 24.4 |
| Thirst 7 | 9.1 | 11.2 | 17.9 | 17.7 |
|
| ||||
| Negative mood 1 | 12.7 | 3.8 | 11.6 | 2.8 |
| Negative mood 2 | 12.7 | 4.0 | 12.3 | 3.2 |
| Negative mood 3 | 13.0 | 3.6 | 13.0 | 3.3 |
| Negative mood 4 | 13.3 | 3.5 | 13.0 | 3.3 |
| Negative mood 5 | 13.2 | 3.3 | 14.4 | 3.8 |
| Negative mood 6 | 13.5 | 3.4 | 13.5 | 4.0 |
| Negative mood 7 | 14.0 | 4.0 | 13.0 | 3.2 |
Std.: standard deviation. STAI: State Trait Anxiety Inventory. PCS: Pain Catastrophizing Scale. LOT-R: Life Orientation Test – Revised. SDS: Social Desirability Scale. FoP: Fear of Pain. Distress: Personal Distress subscale.
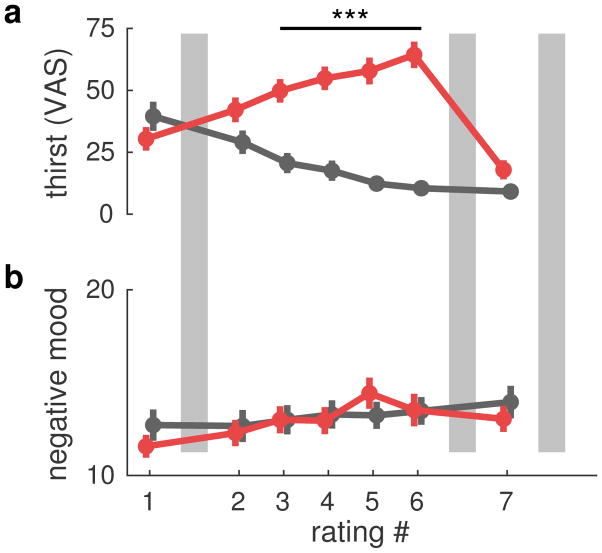
Testing the success of our thirst manipulation on the thirst ratings with an ANOVA revealed a significant main effect of Group (F1,40=26.0, p<0.001), a main effect of Time (F6,240= 17.7, p<0.001), and a significant Group × Time interaction (F6,240=30.9, p<0.001). Post-hoc t-tests revealed that the Thirst group was thirstier at time-points 3 to 6, that is from 2 h after the start of the experiment until the re-hydration period (all t40>18.8, all p<0.001, Fig. 3a). Furthermore, participants in the Thirst group drank more than participants in the Control group during the re-hydration period (MThirst = 667 ml, MControl = 205 ml, t40=4.64, p < 0.001). An ANOVA on the mood ratings revealed a significant main effect of Time (F6,240=15.2, p<0.001). No significant effects were observed for Group or the Group × Time interaction (all p>0.11). Plotting the mood ratings against time revealed an increase in negative mood over the course of the experiment (Fig. 3b).
Figure 3.
Thirst and mood ratings. (a) Thirst ratings over the course of the experiment (see Fig. 1a) for control (black) and thirst (red) groups. Thirst ratings differed significantly between groups at time-points 3 to 6 (all p<0.001). (b) Mood ratings (pleasantness-unpleasantness subscale) for both groups using the same color coding as in a). The ANOVA main of time was significant (p<0.001), but the group effect was not. Note that higher mood values indicate negative mood. Gray bars depict pain tests in both groups (see Fig. 1a).
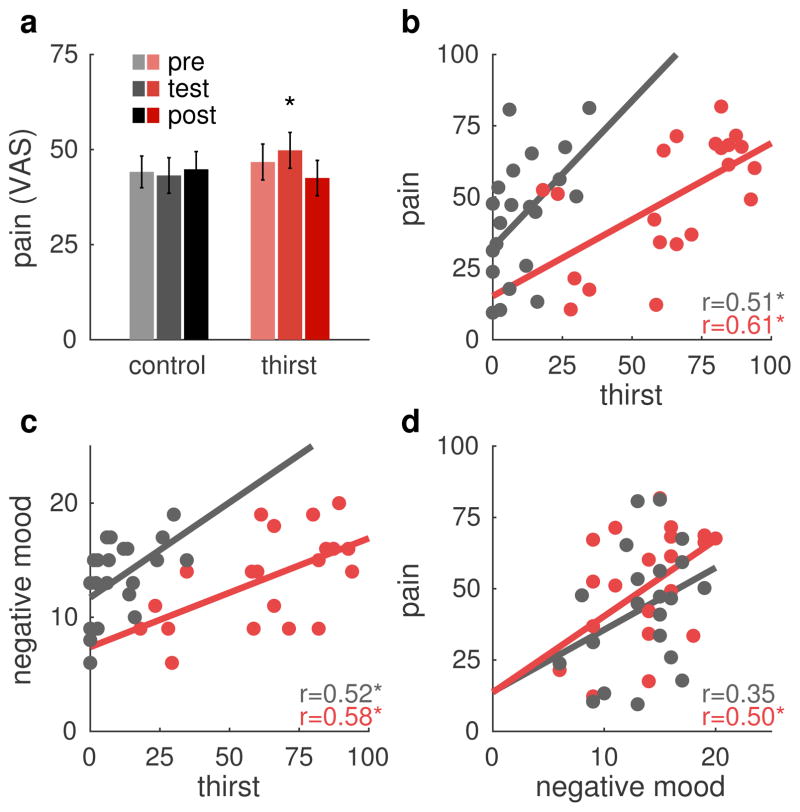
We next investigated group and thirst effects on pain perception. The planned contrast testing the Block × Group interaction was significant (b=1.08, Z=2.03, p=0.02) indicating that the pain increase during Block 2 was stronger in the Thirst group than in the Control group (Fig. 4a). If motivational competition was the only relevant processes here, we would expect a decrease in pain ratings at this time-point. Figure 4b–d shows pairwise relationships between the variables of interest: pain, mood, and thirst. Inspection of these scatterplots confirmed that no outliers were present.
Figure 4.
Pain ratings and bivariate relationships. (a) Mean pain ratings for control and thirst groups for the three pain tests. The a priori contrast testing for greater change at pain test 2 in the pain group than in the control group was significant (p=0.02). Errors bars are between participant standard error of the mean. (b) Mean pain ratings at pain test 2 plotted against thirst ratings immediately before the pain test. Thirst and pain correlate positively in both groups, but the intercept is larger in the control group than in the thirst group. (c) Negative mood ratings plotted against thirst at the same time as in b). Stronger thirst correlates with negative feelings. (d) Pain ratings from pain test 2 plotted against the negative mood ratings. Negative mood correlates positively with pain. * p<0.05.
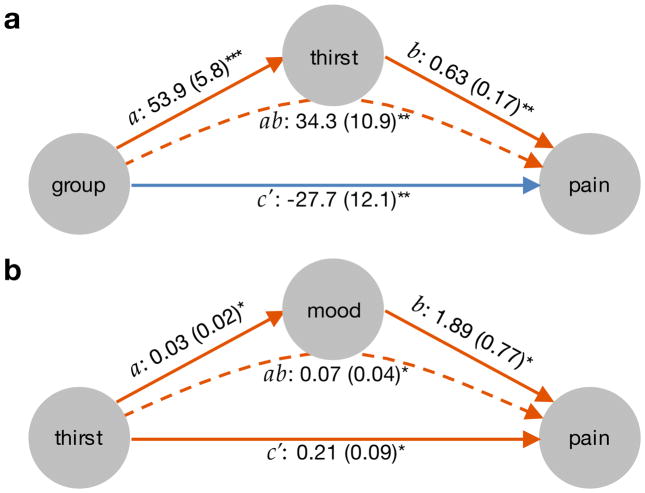
The first mediation model tested whether subjective thirst ratings (M) mediate effects of Group (X) on Pain (Y). All paths of the mediation model (Fig. 5a) were significant. As expected, Group had a negative direct effect on Pain, controlling for Thirst (path c′ = −27.7, p=0.002, rXY,M=−0.39). This demonstrates an analgesic effect of the Thirst challenge as predicted by the Motivation-Decision model [20]. Furthermore, participants in the Thirst group were thirstier than Control group participants as shown by the positive relationship between Group and Thirst (path a = 53.9, p<0.001, rXM=0.83). In addition, greater thirst predicted increased pain, controlling for Group (path b = 0.63, p=0.001, rMY,X=0.54). The indirect, mediated pathway from Group to Pain via Thirst was positive (path ab = 34.3, p=0.002, abcs = 0.81). The latter two path coefficients indicate a pain-enhancing effect of subjective thirst experiences, instead of a pain-reduction. The motivational challenge thus affected pain via two opposing processes, one direct analgesic effect (path c′), and another hyperalgesic process mediated by subjective thirst (path ab).
Figure 5.
Mediation analyses. (a) Mediation analysis of group onto pain perception by thirst. A significant mediation (path ab) by thirst was observed indicating that more thirst was related to more pain. The significant direct effect of group on pain (c′) controlling for thirst, showed that the motivational challenge reduced pain. (b) Mediation analysis of thirst on pain by mood. The effect of thirst on pain was partially mediated by reduction in mood (path ab). The significant path c′ indicates that mood does not explain the entire effect of thirst on pain. The same data as in Fig. 4 are used in the mediation analyses. Note that higher values of mood indicate more negative mood in order to ease interpretability of the mediation effect. All path coefficients are unstandardized, standard errors are shown in parentheses. Orange arrows depict positive paths; blue arrows represent negative path coefficients. * p<0.05; ** p<0.01; *** p<0.001.
In order to test whether the hyperalgesic thirst rating effect was related to pain modulation by negative Mood (M), we conducted a second mediation analysis. Again, all path coefficients were significant (Fig. 5b). A significant, direct effect of Thirst on Pain controlling for Mood (path c′ = 0.21, p=0.017, rXY,M=0.34), confirmed the positive relationship between Thirst and Pain that is partially independent of the mediator Mood. Path a demonstrated a positive relationship between Thirst and negative Mood (path a = 0.03, p=0.03, rXM=0.31). Negative Mood was in turn positively related to Pain (path b = 1.89, p=0.021, rMY,X=0.34) and negative Mood mediated effects of Thirst on Pain (path ab = 0.07, p=0.037, abcs = 0.1). The second mediation model demonstrated that negative Mood contributed positively to pain and that Mood mediated parts of the hyperalgesic Thirst effect.
In summary, the mediation models indicated that the motivational challenge (Group) had a direct analgesic effect, consistent with the Motivation-Decision model. This effect was opposed by a hyperalgesic effect of negative mood and subjective thirst.
To investigate personal differences contributing to pain, we explored relationships between personality traits and the pain. In this exploratory post-hoc analysis, only personal distress correlated with the increase in pain during thirst (r=0.47, p=0.001) across both groups.
Discussion
Using a primary motivational challenge in humans in combination with mediation analysis, we identified two opposing effects of basic motivational states on pain: first, a direct analgesic effect of the thirst challenge and, second, a hyperalgesic effect of subjective thirst mediated by mood. These results translate analgesic effects of primary motivational states previously observed in rodents to humans [13,20–22], and at the same time demonstrate the importance of opposing secondary processes, such as concurrent changes in mood.
Of these two component processes, the direct, analgesic effect of the motivational thirst challenge on pain supports theories of motivational competition [9,20,45]. This analgesic process is reflected by the lower intercept of the Thirst group in Fig. 4b and is evidenced by the significant direct path c′ from Group to Pain (Fig. 5a). Perceived pain is thus lower in the Thirst group than in the Control group after controlling for subjective thirst reports in both groups. As indicated by the partial mediation effect, the thirst ratings capture only part of the changes induced by the motivational challenge. However, the subjective ratings were positively related to pain whereas the motivational challenge (Group) was negatively related to pain.
Participants exposed to the motivational challenge were in greater need of re-hydration, a basic need. Current theories of pain processing suggest that information unrelated to the currently prioritized goal and underlying motivational state are suppressed, so pain should be reduced in this case [9,20], possibly via descending pain regulatory systems, which include the periaqueductal gray (PAG) and the rostral ventromedial medulla (RVM [6,21,26,31]). The degree to which one state is prioritized over the other may depend on personal pre-dispositions [2,8]. For instance, some people may tend to prioritize thirst processing over pain processing. However, as thirst was manipulated between groups and participants had no option to forgo painful stimulation, we reasoned that personal preference effects should be small compared to the group manipulation. Chronic pain patients, on the other hand, may prioritize pain over competing motivational states. Recent rat studies have reported reduced reward pursuit in experimental models of chronic pain. These motivational impairments have been linked to changes in the nucleus accumbens [35,39], long known to be a key player in motivational drives [5]. Our results suggest that a competing motivational state can reduce pain, if it is not accompanied by negative mood. Engaging in the pursuit of goals in situations that minimize frustration and negative mood could thus help pain patients to cope with their pain, and possibly even reduce pain via descending neuromodulation.
One alternative hypothesis would be that participants in the Thirst group shifted their attention away from pain towards the thirst as the water becomes more ‘salient’. Distraction and attentional shifts are known to reduce to reduce pain [7,32], in some cases via descending modulation [42]. Furthermore, attention and mood can act independently on pain perception [47,48], which we also observe here. However, we would expect distraction and attentional shifts to be stronger with more subjective thirst, resulting in less pain. Instead, we observed that higher subjective thirst ratings were associated with increased pain (see below). It is thus unlikely that the analgesic effect is purely attentional. Another explanation is based on research dissociating consciously accessible and pre-cognitive responses to threats and basic physiological challenges [23]. For example, a recent study found that subjective expectation ratings were positively related to higher pain ratings, after a cue was associated with a high pain condition [24]. Interestingly, in the same study, anticipatory skin conductance responses, as a measure of pre-cognitive processing, were negatively correlated with pain ratings, suggesting that pre-cognitive processes underlying autonomic threat responses can decrease pain. Pain perception is also susceptible to subliminally presented cues [23], suggesting that at least part of the pain modulatory system can operate without conscious processing. Further support for this idea comes from research on physiological responses in the anticipation of food and water consumption [16,52]. Here, anticipated motivationally relevant events, such as expecting water when thirsty, can induce compensatory reactions that oppose the normal responses to the anticipated event (e.g., insulin release in anticipation of food, which aids sugar clearance from the bloodstream). In addition, the opioidergic system, which is critical for endogenous pain regulation, seems to be particularly sensitive to exhibit such opposing, compensatory responses [15].
The second, hyperalgesic processes identified by the mediation analyses linked stronger thirst and more negative mood reports to increased pain. Choosing an aversive motivational state in this study ensured that the emotional reaction to this state would be negative, and in the present study an increase in negative mood led to increased pain ratings. This effect is in line with previous reports of thirst leading to negative mood [11,17] and of increased pain following negative mood [25,51]. For example, previous studies have reported increased pain perception when participants viewed unpleasant pictures [36,37]. Here, thirsty participants were eager to drink water, but we prevented them from reaching this goal until the re-hydration period after the second pain test. This goal interference induces negative affect [9]. Participants that felt most thirsty experienced this most strongly, leading to more negative effect, and consequently to increased pain. This finding is also in line with the idea that emotions provide valuable feedback about motivational states [2], thus helping humans to fulfill their needs. However, an interesting hypothesis for future studies suggested by Baumeister’s framework [2] is that motivational states may be able to affect perception, emotion, and cognition even in the absence of motivational competition. Although, we expected the thirst ratings to be related to the analgesic motivational process, their positive relationship with negative mood suggests that they are at least partially dissociable from the underlying motivational state.
Overall, the mood-related, hyperalgesic effect was stronger than the analgesic effect of the motivational challenge. The balance between these two processes may shift depending on the strength of the competing states. For example, water deprivation in rats led to an analgesic behavioral response [13]. In this case, the motivational state competition dominates the pain perception, whereas in our study the opposing (hyperalgesic) mood effect dominates pain experience. We have three explanations for this: First, the strength of the effects of competing states (e.g., thirst) may change with their survival-relevance. In rats, without any knowledge of the experimental context and in the absence of behavioral control, water deprivation represents a strong threat to survival. By contrast, participants in our study knew that they would receive water later. When participants know they are safe, the mood effect dominates pain ratings. Second, the change in mood may be specific for humans, and rats might simply not undergo a similar shift in mood following a thirst induction. Third, we measured perceived pain intensity, whereas rat studies measure pain-related changes in behavior. Although pain perception is thought to be reduced by competing states [20,21], pain behavior might be a more sensitive measure in the context of motivational demands [8].
A limitation of the current study is that we did not independently manipulate mood. This is clearly a logical next step. One potential problem is that such a mood manipulation would either work in the same direction as the naturally occurring mood changes due to the thirst, or the mood manipulation would have to be so strong as to overcome the mood effects of thirst. We thus decided to leverage the individual differences in mood in response to our thirst challenge rather than to attempt multiple simultaneous mood manipulations. Furthermore, measures of attention, stress, and choice behavior should be included in future studies to elucidate psychological processes related to the analgesic effect of the motivational challenge in more detail.
In summary, the present results demonstrate two important effects of motivational competition in human pain processing. A direct, analgesic effect occurs in response to a motivational challenge and an indirect, hyperalgesic process occurs at the same time that is mediated by the participants’ mood. The balance between these opposing processes will most likely depend on the strength of the competing motivational states. More broadly, studies of the effects of motivational states in humans must jointly consider the effects of the motivational challenge itself, and mood.
Acknowledgments
The authors declare no conflicts of interest. S.G. was supported by the DFG (GE 2774/1-1). T.D.W. was supported by NIH grants 2R01MH076136 and R01DA035484.
Footnotes
Author Contributions
S.G. and T.D.W. conceived the study. S.G. designed the paradigm. S.G. and J.T.C. performed experiments. S.G. performed the data analysis. S.G. drafted the manuscript, and J.T.C. and T.D.W. provided critical revisions. All authors approved the final version of the manuscript for submission.
References
- 1.Bahlmann J, Aarts E, D’Esposito M. Influence of Motivation on Control Hierarchy in the Human Frontal Cortex. J Neurosci. 2015;35:3207–3217. doi: 10.1523/JNEUROSCI.2389-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumeister RF. Toward a general theory of motivation: Problems, challenges, opportunities, and the big picture. Motiv Emot. 2016;40:1–10. [Google Scholar]
- 3.Bear T, Philipp M, Hill S, Mündel T. A preliminary study on how hypohydration affects pain perception. Psychophysiology. 2016;53:605–610. doi: 10.1111/psyp.12610. [DOI] [PubMed] [Google Scholar]
- 4.Becker S, Gandhi W, Elfassy NM, Schweinhardt P. The role of dopamine in the perceptual modulation of nociceptive stimuli by monetary wins or losses. Eur J Neurosci. 2013;38:3080–3088. doi: 10.1111/ejn.12303. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiol Bethesda. 2008;23:371–80. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- 7.Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149:19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claes N, Crombez G, Vlaeyen JWS. Pain-avoidance versus reward-seeking: an experimental investigation. Pain. 2015;156:1449–1457. doi: 10.1097/j.pain.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 9.Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: The next generation. Clin J Pain. 2012;28:475–483. doi: 10.1097/AJP.0b013e3182385392. [DOI] [PubMed] [Google Scholar]
- 10.Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- 11.D’anci KE, Vibhakar A, Kanter JH, Mahoney CR, Taylor HA. Voluntary dehydration and cognitive performance in trained college athletes. Percept Mot Skills. 2009;109:251–269. doi: 10.2466/PMS.109.1.251-269. [DOI] [PubMed] [Google Scholar]
- 12.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- 13.Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol Biochem Behav. 1984;21:259–266. doi: 10.1016/0091-3057(84)90224-7. [DOI] [PubMed] [Google Scholar]
- 14.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 15.Eikelboom R, Stewart J. Conditioned temperature effects using morphine as the unconditioned stimulus. Psychopharmacology (Berl) 1979;61:31–38. doi: 10.1007/BF00426807. [DOI] [PubMed] [Google Scholar]
- 16.Eikelboom R, Stewart J. Conditioning of drug-induced physiological responses. Psychol Rev. 1982;89:507–528. [PubMed] [Google Scholar]
- 17.Ely BR, Sollanek KJ, Cheuvront SN, Lieberman HR, Kenefick RW. Hypohydration and acute thermal stress affect mood state but not cognition or dynamic postural balance. Eur J Appl Physiol. 2013;113:1027–1034. doi: 10.1007/s00421-012-2506-6. [DOI] [PubMed] [Google Scholar]
- 18.Farmer MA, Leja A, Foxen-Craft E, Chan L, MacIntyre LC, Niaki T, Chen M, Mapplebeck JCS, Tabry V, Topham L, Sukosd M, Binik YM, Pfaus JG, Mogil JS. Pain Reduces Sexual Motivation in Female But Not Male Mice. J Neurosci. 2014;34:5747–5753. doi: 10.1523/JNEUROSCI.5337-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. n.d;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 20.Fields HL. A Motivation-Decision Model of Pain: The Role of Opioids. In: Flor H, Kalso E, Dostrovsky JO, editors. Proceedings of the 11th World Congress on Pain; Seattle: IASP Press; 2006. pp. 449–59. [Google Scholar]
- 21.Fields HL. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 22.Foo H, Mason P. Analgesia Accompanying Food Consumption Requires Ingestion of Hedonic Foods. J Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jepma M, Wager TD. Conceptual Conditioning: Mechanisms Mediating Conditioning Effects on Pain. Psychol Sci. 2015;26:1728–1739. doi: 10.1177/0956797615597658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology. 2005;42:559–67. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 26.Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: When relative relief renders pain pleasant. Pain. 2013;154:402–410. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist MA, Spicer J, Asllani I, Wager TD. Estimating and testing variance components in a multi-level GLM. NeuroImage. 2012;59:490–501. doi: 10.1016/j.neuroimage.2011.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation Analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the Mediation, Confounding and Suppression Effect. Prev Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil DW, Rainwater AJ. Development of the Fear of Pain Questionnaire-III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 31.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- 33.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 34.Reicherts P, Gerdes ABM, Pauli P, Wieser MJ. On the mutual effects of pain and emotion: Facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. PAIN. 2013;154:793–800. doi: 10.1016/j.pain.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 2016;19:220–222. doi: 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. [Google Scholar]
- 37.Roy M, Piché M, Chen J-I, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci. 2009;106:20900–20905. doi: 10.1073/pnas.0904706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 41.Spielberger C. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. (rev. ed) [Google Scholar]
- 42.Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. Attention Modulates Spinal Cord Responses to Pain. Curr Biol. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Steyer R, Schwenkmetzger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsfragebogen. Göttingen: Hogrefe; 1997. [Google Scholar]
- 44.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 45.Van Damme S, Crombez G, Eccleston C. Coping with pain: A motivational perspective. Pain. 2008;139:1–4. doi: 10.1016/j.pain.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DML, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: An experimental study. Pain. 2010;149:229–234. doi: 10.1016/j.pain.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Villemure C, Bushnell MC. Mood Influences Supraspinal Pain Processing Separately from Attention. J Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–8. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 49.Vlaeyen JWS. Learning to predict and control harmful events: chronic pain and conditioning. PAIN. 2015;156:S86–S93. doi: 10.1097/j.pain.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 50.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiech K, Tracey I. The influence of negative emotions on pain: Behavioral effects and neural mechanisms. NeuroImage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 52.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]