Abstract
The influence of routine guarana (Paullinia cupana) consumption on apparent tolerance to mercury intoxication has been proposed. The present study investigated this hypothesis in Caenorhabditis elegans, a suitable experimental model for studies in toxicology. Wild type (WT) and skn-1 (ok2315) worm strains were pretreated with guarana ethanolic extract (GEE) from larvae 1 (L1) to L4 stage and then exposed for 6 hours to methylmercury (MeHg). The analyses included evaluation of GEE’s effects on lethality, developmental delay, feeding, locomotion, gene expression (sod-3, gst-4, sir-2.1, hsf-1, snn-1, mtl-1, mtl-2, aat-1, aat-2 and aat-3) and antioxidant activity. GEE pre-treatment had no aberrant effects on WT worms exposed to MeHg, and protected skn-1 (ok2315) worms, which are more susceptible to environmental stresses. Protective effects of GEE might be dependent on modulation of genes other than those directly involved in antioxidant activity. GEE increased the expression of genes involved in metal transport (aat-2), metal detoxification (mtl-1 and mtl-2) and antioxidant responses (sir-2.1 and sod-3). Thus, routine consumption of guarana might be beneficial in protecting against MeHg-induced toxicity.
Keywords: mercury, natural products, neurological disorders, xanthines
Graphical Abstract
1. Introduction
Mercury (Hg) is a heavy metal present in the environment due to both natural and anthropogenic sources (1). A major route of mercury exposure in humans is the consumption of seafood containing methylmercury (MeHg) due to water contamination from agriculture and industries (2, 3). MeHg is more efficiently transported into the central nervous system (CNS) compared to inorganic mercurial, and has varying neurotoxic effects depending on age, dose and duration of exposure (2, 3). Although MeHg has demonstrated effects, including dopaminergic neurodegeneration, alterations in calcium homeostasis, oxidative stress induction and high affinity for cysteine, the specific molecular targets of MeHg are still unknown (4–7).
The Amazon region in Brazil has inherently high mercury levels in soil and water due to geological processes and gold mining (8), and the concentration of this metal in biological samples of the native population exceeds the recommended value established by the World Health Organization (WHO) (9). Therefore, the reported tolerance to mercury in this population might be secondary to nutritional factors as potential modifiers of mercury toxicity (10).
Paullinia cupana Mart var. Sorbilis, also referred to as guarana, is a native plant to the Amazon basin, especially in Brazil, with several reported effects including CNS stimulation (11), memory maintenance (11) and antioxidant activity (12). Previous studies also showed a protective effect against cadmium in adult rat testis (13, 14), and against rotenone in human dopaminergic neuroblastoma SH-SY5Y cells (15). Guarana seed extracts contain several methylxanthines, such as caffeine, theobromine and theophylline, and tannins, saponins, catechins, epicatechins, proanthocyanidols as well as other compounds in trace concentrations (16).
As the powder of guarana seeds is habitually consumed in the Amazon region (16), it might modulate mercury toxicity in this native population. The possible protective effects might contribute to the understanding of mechanisms of metal toxicity and to the development of new therapeutic strategies. The present study investigated this hypothesis using Caenorhabditis elegans, a suitable animal model for understanding organismal reactions to various environmental factors (17, 18) with high predictive value of toxicity to mammals (19–21). Thus, the aim of this study was to investigate whether guarana demonstrates protective effects against methylmercury-induced toxicity, as well as the mechanisms involved.
2. Materials and methods
2.1 C. elegans maintenance
The strains used in this study: Bristol N2 (wild-type; WT) and VC1772 (skn-1(ok2315) IV/nT1 [qIs51] (IV;V)), as well as the Escherichia coli OP50 were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, MN, USA.
Nematodes were maintained on nematode growth medium (NGM) agar plates carrying a lawn of E. coli OP50 (22). Synchronization of nematode cultures was achieved by bleaching treatment of gravid hermaphrodites (23) and eggs were allowed to hatch overnight in M9 buffer (42 mM Na2HPO4, 22 mM KH2PO4, 8.5 mM NaCl and 1 mM MgSO4).
2.2 Plant material and guarana extract preparation
The powder of toasted seeds of guarana was isolated and supplied by EMBRAPA Oriental (Agropecuary Research Brazilian Enterprise) located in the Western Amazon in Maués, Amazonas, Brazil. The hydro-alcoholic extract was obtained as previously described (24). Briefly, the extract was produced using 70% ethanol. After 24 hours, the resulting solution was filtered, the ethanol was removed and the extract was lyophilized. The predominant xanthines and catechins present in the guarana extract were analyzed by means of HPLC (25), showing the following concentrations: caffeine = 12.240 mg/g, theobromine = 6.733 mg/g and total catechins = 4.336 mg/g.
2.3 Guarana and MeHgCl treatments
Lyophilized guarana ethanolic extract (GEE) was dissolved in distilled autoclaved water and spread over NGM plates with E. coli as food source at final concentrations of 100, 500 and 1,000 μg/ml of agar. Plates were incubated at room temperature overnight to allow bacterial growth. Synchronized L1 larvae were transferred to treatment plates and cultured until L4 larvae stage at 20°C.
Methylmercury chloride (CH3HgCl, MeHgCl) exposures were performed for 6 hours in L4 worms (7) untreated and pretreated with GEE. Exposures were conducted in micro tubes by combining 500 L4 worms, 25 μl of concentrated E. coli OP50, 10 μl of MeHgCl dissolved in water at various concentrations, and M9 buffer to a volume of 500 μl. Following the exposure duration, animals were washed three times with M9 buffer by centrifugation and placed on OP50-containing NGM plates.
2.4 Lethality, developmental and behavioral parameters evaluation
A dose-response curve was generated to evaluate MeHg-induced lethality (0–150 μM). Following MeHg exposure and washing, approximately 100 worms per treatment were transferred to NGM plates seeded with E. coli OP50, without extract, and allowed to recover for 24 hours. Nematodes were then scored as dead or alive using a stereomicroscope. Animals that reacted to a mechanical stimulus with a platinum wire were scored as alive, and non-responding animals were considered to be dead (26). The assay was repeated four times.
The surviving worms were analyzed using a microscope and scored through the larval stages observing vulva development and presence of eggs in the uterus (27)
In order to evaluate behavioral parameters following MeHg exposure and washing, approximately 50 worms per treatment were transferred to NGM plates seeded with E. coli OP50 and analyzed immediately post MeHg exposure (0-hour post MeHg exposure) or 24-hour post exposure.
Pharyngeal pumping rate was assessed with a microscope by observing the number of pharyngeal contractions of worms during a 60 second interval in NGM plates seeded with E. coli OP50 (28).
Thrash frequency was selected for analysis of locomotion. Worms were individually picked and placed in a drop of M9 and allowed to adapt for 1 minute and then the number of thrashes were quantified with stereomicroscope during a 60 second interval. A thrash was defined as a change in the direction of bending at the middle of body (29).
Analyses were carried out in ten worms per group. The assays were repeated four times.
2.5 RNA isolation and real-time polymerase chain reaction (RT-qPCR)
Worms were analyzed 0- and 24-hour post the end of MeHg exposure for gene expression related to metal transport and response. For 24-hour post MeHg analyses, worms were transferred to plates containing 150 mM of FUDR (5-fluoro-2′-deoxyuridine) to inhibit reproduction. RNA from 20,000 worms per condition was isolated using Trizol followed by chloroform extraction, as previously described (30), 1 ug of input RNA was reverse transcribed to cDNA by Applied Biosystems’ high capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Expression analysis was performed by Custom TaqMan® Array Analysis utilizing the corresponding TaqMan® Gene Expression Assays for mitochondrial superoxide dismutase sod-3 (Ce02404515_g1), glutathione-S-transferase gst-4 (Ce02458730_g1), sirtuin sir-2.1 (Ce02459018_g1), heat shock factor hsf-1 (Ce02423758_m1), synapsin snn-1 (Ce02407220_m1), metallothioneins mtl-1 (Ce02551471_s1) and mtl-2 (Ce02551627_s1) and amino acid transporters aat-1 (Ce02458013_g1), aat-2 (Ce02479487_g1) and aat-3 (Ce02492836_g1) (Applied Biosystems). Target gene expression was normalized to the expression values of actin afd-1 (Ce02414573_m1). The relative quantification was determined with the 2−ΔΔCt method (31) and data were expressed as fold change in mRNA levels compared to control worms. This experiment was carried out in three independent worm preparations, each in triplicate.
2.6 Measurement of intracellular reactive oxygen species (ROS)
Reactive oxygen species (ROS) levels were measured in worms 24-hour post the termination of MeHg exposure with 2′,7′-dichlorofluorescein diacetate (DCFDA), following a previously described method (32) with minor modifications. Worms were collected from OP50-containing NGM plates, transferred to micro tubes and washed three times with M9. The samples were sonicated on ice and after centrifugation the supernatants (lysates) were collected. Aliquots were transferred to black 96-well plates containing M9 and incubated for 1 hour with 20 μM DCFDA (final concentration) at 20°C. ROS-associated fluorescence levels were measured in a microplate reader at 485 nm excitation and 520 nm emission wavelengths at room temperature. Data were normalized to protein content determined by the Bradford method (33). Analyses were carried in duplicate and the experiment was independently repeated four times.
2.7 Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA). The results were plotted as the mean ± SEM (standard error of the mean) of four individual experiments. Dose-response lethality curves and LD50 determination were generated using a sigmoidal dose-response model and analyzed with extra sum-of-squares F test method. Two-way ANOVA followed by Bonferroni’s Multiple Comparison Test was used to compare groups and One-way ANOVA followed by Bonferroni post hoc Test was used to compare concentrations in the same group. The statistical significance was set at p<0.05.
3. Results
3.1 MeHg-induced lethality and developmental delay
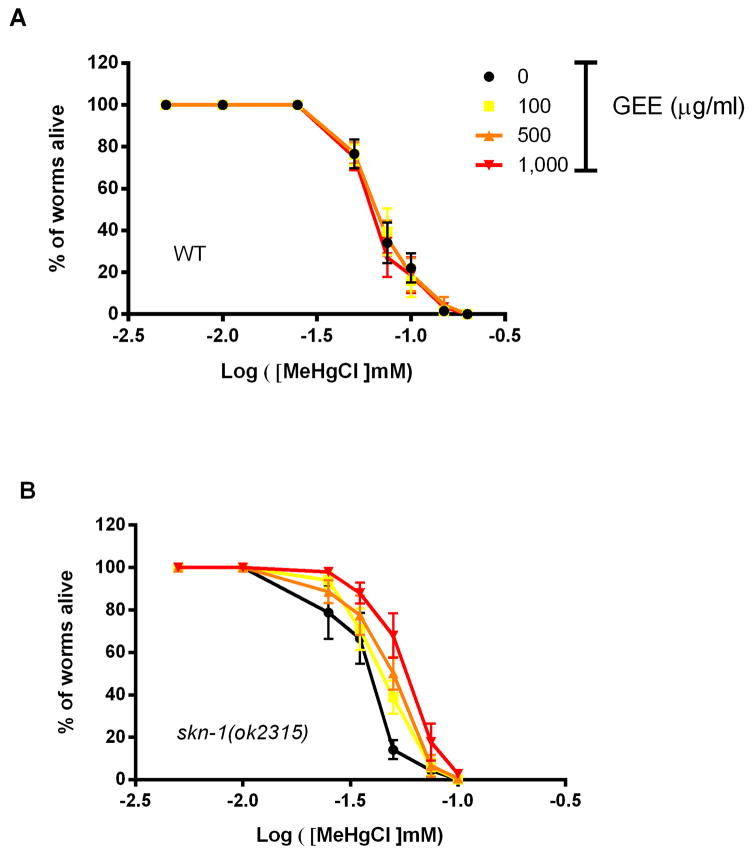
A dose-response curve was generated to evaluate MeHg toxicity in L4-larvae stage WT worms after a 6-hour exposure and 24-hour recovery (Fig 1A). The LD50 was 66 μM for MeHg and pre treatment with GEE 100, 500 and 1,000 μg/ml did not affect lethality. A more sensitive strain to MeHg, skn-1(ok2315), was also assessed, resulting in a LD50 of 38 μM of MeHg (Fig. 1B). GEE exerted a protective effect against MeHg-induced lethality in this strain. The extract at 100, 500 and 1,000 μg/ml significantly increased the dose required for LD50 in skn-1 (ok2315) worms to 45, 51 and 59 μM of MeHg, respectively. The highest GEE dose afforded a significant protection against MeHg-induced lethality compared to the other doses (Fig. 1B, p<0.05).
Fig. 1.
Dose-response curves of C. elegans lethality upon 6-hour MeHg (0 – 150μM) exposure and a 24-hour recovery period ± pre-treatment with guarana ethanolic extract (GEE). L4-larvae stage worms untreated and pretreated with GEE from L1 were exposed to methylmercury chloride (CH3HgCl, MeHgCl) for 6 hours. Following washing, worms were transferred to NGM plates seeded with E. coli OP50, allowed to recover for 24 hours and then scored as dead or alive using a stereomicroscope. GEE had no effect against MeHg-induced lethality on wild type (WT) worms (LD50≈66 μM) (A) but significantly increased survival in skn-1(ok2315) worms (LD50=38, 45, 51 and 59 μM to untreated and GEE pretreated worms at 100, 500 and 1,000 μg/ml, respectively) (B). Data were compared with extra sum-of-squares F test method, p<0.05, and are expressed as means ± SEM, n=4, approximately 100 worms analyzed per group in each assay
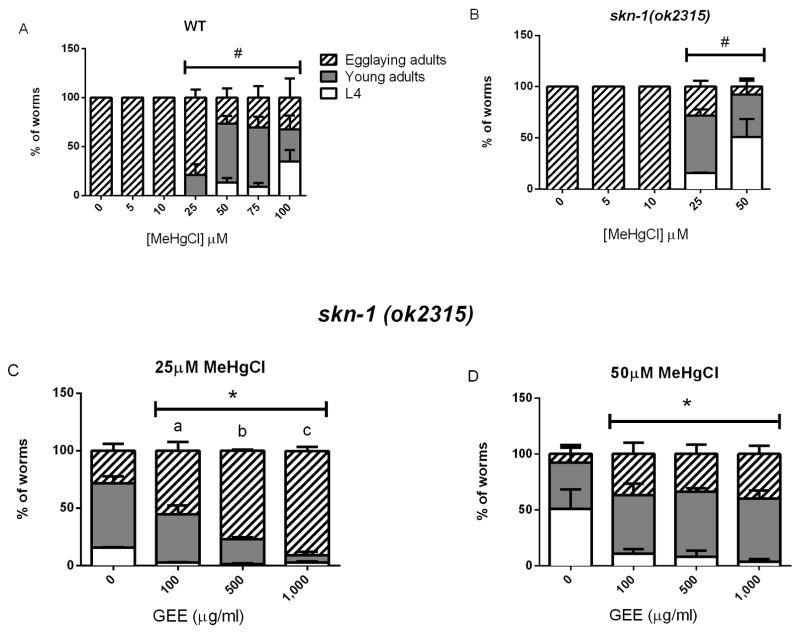
The living worms were also analyzed for developmental progress 24- and 48- hour post MeHg exposure. After 24 hours of recovery, WT and skn-1 (ok2315) control worms reached the egg laying adult-stage, while MeHg exposure induced a dose-dependent delay in worm development starting at 25 μM for both strains, with an accentuated effect in mutant worms (Fig 2A and B). Pre-treatment with GEE did not protect WT worms against MeHg-induced developmental delay at any of the tested doses (data not shown), however, GEE at doses between 100 and 1,000 μg/ml had protective effects in skn-1 (ok2315) worms exposed to 25 and 50 μM of MeHg, decreasing the developmental delay (Fig. 2C and D). After 48 hours of recovery, all worms from both strains reached the egg laying adult-stage (data not shown).
Fig. 2.
Developmental progress of wild type (A) and skn-1 (ok2315) (B) untreated and GEE-pretreated worms post 6-hour MeHg (0 – 50μM) exposure and 24-hour recovery. The surviving worms were analyzed using a microscope and scored through the larval stages observing vulva development and presence of eggs in the uterus. WT and skn-1 (ok2315) control worms reached the egg laying adult-stage, while MeHg exposure induced a dose-dependent delay in worm development starting at 25 μM for both strains, with an accentuated effect in mutant worms. Pretreatment with GEE significantly decreased the percentage of worms delayed only in the mutant strain at 25 (C) and 50 μM of MeHg (D). (#) represents a significantly difference between control and metal-exposed worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05) and (*) represents significant difference from untreated group and (a–b) represents significant difference between GEE doses by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05). Data are expressed as means ± SEM, n=4, at least 30 worms analyzed per group in each assay
3.2 MeHg-induced behavioral alterations
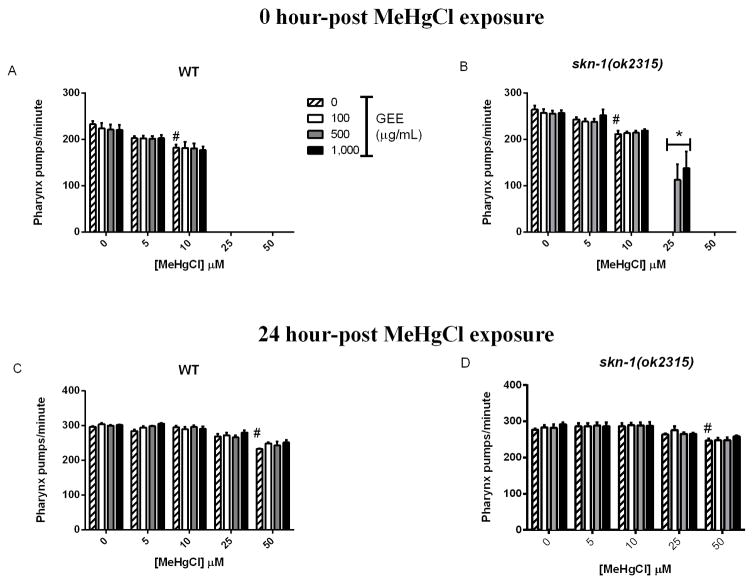
Pharyngeal pumping rates were significantly decreased immediately after 6-hour MeHg exposure in WT and skn-1 (ok2315) worms (Fig. 3A and B, p<0.05). WT control worms pumped at a rate of 232.8 ± 6.8 pumps per minute while worms exposed to 10 μM MeHg pumped at a rate of 182.8 ± 6.5 pumps per minute. Mutant skn-1 (ok2315) worms pumped at a rate of 264.7 ± 8.3 pumps per minute, while skn-1 (ok2315) worms exposed to 10 μM MeHg pumped at a rate of 211.8 ± 7.2 pumps per minute. Pharyngeal pumping was inhibited at 25 μM MeHg in both strains. Worms were also analyzed 24-hour post metal exposure, and MeHg-exposed worms showed a recovery in pharyngeal pumping rate to control levels, with the exception of the 50 μM MeHg group (Fig. 3C and D, p<0.05). Pre-treatment with GEE exerted difference only in skn-1 (ok2315) worms analyzed immediately after exposure to 25 μM MeHg, while pharyngeal pumping of untreated worms was inhibited (Fig. 3B p<0.05).
Fig 3.
Pharyngeal pumping rate in WT and skn-1 (ok2315) untreated and GEE-pretreated worms at 0 hours (A and B) and 24 hours (C and D) post 6-hour MeHg exposure. Number of pharyngeal contractions per minute was observed in worms in E.coli OP50-seeded NGM plates with a microscope. MeHg induced a dose-dependent decrease in pharyngeal pumping rates immediately post 6-hour exposure and GEE exerted protective effects only in skn-1 (ok2315) worms at 25 μM MeHg. Post 24 hours, worms showed a recovery in pharyngeal pumping rate to control levels, with the exception of the 50 μM MeHg group. (#) represents a significantly difference between control and metal exposed worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05) and (*) represents a significantly difference between untreated and GEE-pretreated worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05). Data are expressed as means ± SEM, n=4, 10 worms analyzed per group in each assay
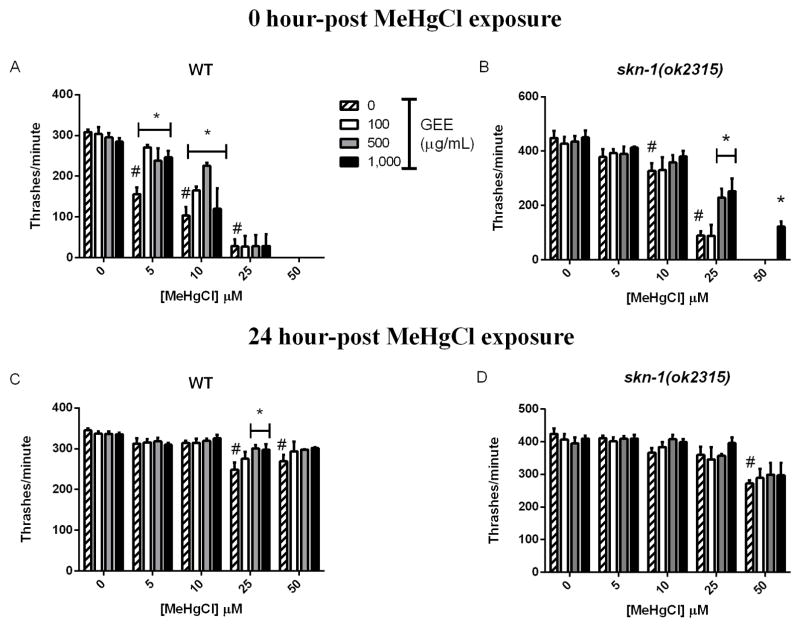
Thrash frequency was also altered in worms exposed to MeHg (Fig. 4). WT control worms showed a rate of 308.5 ± 6 thrashes per minute while MeHg induced a dose-dependent decrease in movements immediately post metal exposure starting at 5 μM (155.6 ± 6 thrashes per minute) and a total inhibition of movements at 50 μM. WT worms pre-treated with GEE showed a significantly higher thrash frequency of 251.9 ± 17 and 170.2 ± 21.9 thrashes per minute in comparison to GEE-untreated worms at 5 and 10 μM MeHg, respectively (Fig. 4A). The skn-1 (ok2315) control worms showed a rate of 448 ± 26 thrashes per minute, while MeHg induced a dose-dependent decrease in movements immediately post exposure starting at 10 μM (327.5 ± 28.7 thrashes per minute) and completely inhibited movements at 50 μM (Fig. 4B, p<0.05). Thrash frequency was significantly higher in skn-1 (ok2315) worms treated with GEE in comparison to untreated worms. At 24-hour post MeHg exposure, thrash frequency remained decreased in WT worms treated with 25 and 50 μM MeHg (248 ± 19.7 and 270 ± 15.8 thrashes per minute, respectively) in comparison to controls (345 ± 5.2 thrashes per minute). Worms pre-treated with GEE at 500 and 1,000 μg/ml showed a significantly higher thrash frequency with approximately 300 ± 11 thrashes per minute in comparison to GEE-untreated worms at 25 μM MeHg (Fig. 4C, p<0.05). Skn-1 (ok2315) worms showed MeHg-induced decrease in thrashes after a 24-hour recovery period only at 50 μM (272.4 ± 9.5 thrashes per minute) compared to controls (424.2 ± 17 thrashes per minute). Pre-treatment with GEE had no effect in this strain 24-hour post MeHg exposure at any of the tested doses (Fig. 4D).
Fig. 4.
Number of thrashes per minute in WT and skn-1(ok2315) untreated and GEE-pretreated worms 0 hours (A and B) and 24 hours (C and D) post 6-hour MeHg exposure. A thrash was defined as a change in the direction of bending at the middle of body and was assessed in worms individually placed in a drop of M9 with a stereomicroscope. MeHg induced a dose-dependent decrease in number of thrashes immediately post 6-hour exposure, and post 24 hours, worms partially recovered movements. (#) represents a significantly difference between control and metal exposed worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05) and (*) represents a significantly difference between untreated and GEE-pre treated worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05). Data are expressed as means ± SEM, n=4.
Only egg laying adults were analyzed for behaviors 24-hour post MeHg exposure to discard potential differences inherent to developmental delays.
3.3 MeHg-induced gene expression alterations
Gene expression was not altered by GEE pre-treatment in skn-1 (ok2315) worms immediately after MeHg exposure and thus was not analyzed in WT worms (data not shown).
Table 1 shows the fold change in mRNA expression compared to the control group in skn-1 worms 24-hour post MeHg exposure. Expression levels of mtl-1, mtl-2, aat-2, sod-3 and sir-2.1 were significantly increased in skn-1 (ok2315) worms pre-treated with GEE in comparison to GEE-untreated worms exposed to MeHg. Expression levels of snn-1, aat-1, aat-3 and hsf-1 after GEE pre treatment or MeHg exposure were not different from expression levels in control worms (data not shown). Gene expression levels that were altered in skn-1 (ok2315) post MeHg exposure were also investigated in WT worms (Table 2). Expression levels of aat-2 increased in WT worms after MeHg exposure, and were statistically lower in GEE-treated compared to -untreated worms. Expression levels of gst-4 were significantly increased in WT worms pretreated with GEE in comparison to untreated worms exposed to MeHg, while expression levels of mtl-1, aat-2 and sir-2.1 were significantly decreased in GEE-pretreated compared to -untreated worms exposed to the metal at 50 μM.
Table 1.
Fold change in mRNA expression of genes associated with metal transport, detoxyfication and antioxidant response in skn-1(ok2315) worms 24 hours post MeHg exposure
| MeHgCl | 0 μM | 5 μM | 25 μM | 50 μM | ||||
|---|---|---|---|---|---|---|---|---|
| GEE | 0 (μg/ml) | 1000 (μg/ml) | 0 (μg/ml) | 1000 (μg/ml) | 0 (μg/ml) | 1000 (μg/ml) | 0 (μg/ml) | 1000 (μg/ml) |
| Gene | ||||||||
| mtl-1 | +0.01e-2 (0.04) | +0.24 (0.13) | −0.15 (0.33) | +0.07 (0.31) | +1.16 (0.79) | +1.91 $ (0.60) | +0.32 (0.56) | +5.25 *$ (0.69) |
| mtl-2 | +0.06e-8 (0.03) | +0.31 (0.17) | −0.14 (0.20) | +1.36 $* (0.37) | −0.26 (0.10) | +0.01 (0.30) | −0.58 (0.08) | +0.08 (0.16) |
| aat-2 | +0.09e-8 (0.04) | +0.01 (0.14) | +0.26 (0.15) | +1.6 $* (0.47) | −0.29 (0.09) | +0.31 (0.17) | −0.27 (0.08) | −0.30 (0.04) |
| sod-3 | −0.01e-2 (0.03) | +0.24 (0.14) | −0.35 (0.04) | +1.11 $* (0.24) | −0.22 (0.08) | +0.48 * (0.26) | −0.86 # (0.02) | −0.63 $ (0.08) |
| sir-2.1 | +0.52e-9 (0.05) | +0.26 (0.08) | +0.02 (0.06) | +1.26 $* (0.21) | −0.10 (0.09) | +0.35* (0.01) | −0.27 (0.06) | −0.03 (0.08) |
means significant difference between worms exposed and non-exposed to MeHgCl,
means significant difference between GEE pre treated worms exposed and non exposed to MeHgCl and
means significant difference between GEE pre treated and untreated worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison test (p<0.05)
This experiment was assessed by RT-qPCR. and carried out in three independent worm preparations, each in triplicate. Data are expressed as means ±SEM in parentheses.
Table 2.
Fold change in mRNA expression of genes associated with metal transport, detoxyfication and antioxidant response in WT worms 24 hours post MeHg exposure
| MeHgCl | 0 μM | 5 μM | 25 μM | 50 μM | ||||
|---|---|---|---|---|---|---|---|---|
| GEE | 0 (μg/ml) | 1000 (μg/ml) | 0 (μg/ml) | 1000 (μg/ml) | 0 (μg/ml) | 100 (μg/ml) | 0 (μg/ml) | 1000 (μg/ml) |
| Gene | ||||||||
| mtl-1 | +0.02e-4 (0.07) | +0.23 (0.26) | −0.03 (0.29) | −0.43 (0.06) | +0.05 (0.30) | −0.17 (0.08) | −0.49 (0.13) |
−0.65
$ 0.06 |
| mtl-2 | −0.02 (0.03) | +0.18 (0.26) | +0.04 (0.25) | −0.37 (0.10) | −0.54 (0.18) | −0.41 (0.12) | −0.79 # (0.07) | −0.79 0.03 |
| aat-2 | +0.13 (0.04) | −0.07 (0.14) | +1.3 # (0.14) | +0.67 *$ (0.12) | +0.33 (0.19) | +0.11 (0.07) | +0.52 (0.10) | −0.16 * (0.11) |
| sir-2.1 | +0.01 (0.02) | −0.40 (0.02) | −0.27 0.15 |
−0.21 0.04 |
+0.02 (0.25) | −0.19 (0.05) | −0.58 (0.06) | −0.11 * (0,19) |
| gst-4 | +0.03e-2 (0.05) | +0.26 (0.29) | +0.82 (0.44) | +3.6 $* (0.54) | +0.40 (0.33) | +0.57 (0.44) | +0.32 (0.35) | +0.34 (0.45) |
means significant difference between worms exposed and non-exposed to MeHg,
means significant difference between GEE pre treated worms exposed and non exposed to MeHg and
means significant difference between GEE pre treated and untreated worms by two-way ANOVA followed by Bonferroni’s Multiple Comparison test (p<0.05).
This experiment was assessed by RT-qPCR carried out in three independent worm preparations, each in triplicate. Data are expressed as means ±SEM in parentheses.
3.4 MeHg-induced ROS levels alterations
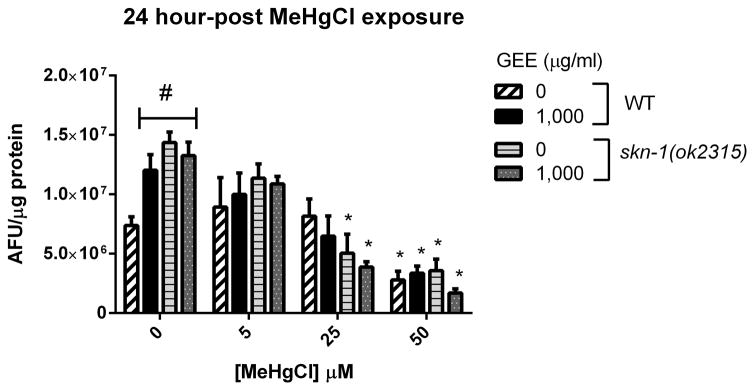
Figure 5 shows the ROS-associated fluorescence levels measured in WT and skn-1 (ok2315) worms untreated and pretreated with GEE 1,000 μg/ml after MeHg exposure and 24-hour recovery. Fluorescence levels were significantly increased in WT GEE-pretreated worms and in skn-1 (ok2315) GEE-untreated and -pretreated worms and non-exposed to MeHg in comparison to control worms (p<0,05). MeHg at 25 μM significantly decreased fluorescence levels in skn-1 (ok2315) worms and at 50 μM significantly decreased fluorescence levels in WT and skn-1 (ok2315) worms in comparison to MeHg-non-exposed worms of the same group (p<0.05). No significant differences were detected between GEE-untreated and -pretreated worms and between WT and skn-1 (ok2315) worms exposed to MeHg.
Fig. 5.
ROS-associated fluorescence levels in WT and skn-1 (ok2315) worms untreated and pretreated with GEE 1,000 μg/ml after MeHg exposure and 24-hour recovery. Aliquots of worm’s lysates were incubated for 1 hour with 20 μM DCFDA (final concentration) at 20°C and fluorescence were measured in duplicate in a microplate reader at 485 nm excitation and 520 nm emission wavelengths at room temperature. Data were normalized to protein content. Fluorescence levels were significantly increased in MeHg non-exposed groups in comparison to control and MeHg at 25 and 50 μM significantly decreased fluorescence levels in comparison to MeHg-non-exposed worms of the same group. No significantly differences were detected between untreated and GEE-pretreated worms and between WT and skn-1 (ok2315) worms exposed to MeHg. (#) represents a significantly difference between control and the other groups non-exposed to MeHg by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test (p<0.05) and (*) represents a significantly difference between MeHg-exposed and non-exposed worms of the same group by one-way ANOVA followed by Bonferroni post hoc test (p<0.05). Data are expressed as means of arbitrary fluorescence units (AFU) per μg of protein ± SEM, n=4
4. Discussion
The influence of routine guarana consumption on apparent tolerance to mercury intoxication has been proposed (9, 10, 34). The present study investigated the effects of pre-treatment with guarana ethanolic extract (GEE) on L4-larvae stage C. elegans exposed to MeHg for 6 hours. Our results show a protective effect of GEE against MeHg in skn-1 (ok2315) worms, but not in WT worms.
C. elegans is an suitable model for studying toxicity of many compounds, including metals (18, 21). Our data corroborated that 6-hour MeHg exposure induces lethality and behavior impairments in L4-larvae stage WT worms (7). However, herein, these effects were shown at lower concentrations (LD50 of 66 vs. 570 μM MeHg as previously demonstrated). This discrepancy may be due to differences in media density secondary to bacterial concentration, or a result of natural drifting in genetic variations in the worms’ population (35).
Skn-1 (ok2315) worms were more sensitive to MeHg than WT worms, as previously described (36, 37). SKN-1 is the ortholog of mammalian Nrf2, a leucine zipper class transcription factor that regulates a number of detoxifying enzymes, including glutathione-S-transferases, and is involved in cellular detoxification and stress response (38, 39). The skn-1 (ok2315) mutant strain is more susceptible to environmental stresses (36, 40). The LD50 of MeHg in WT worms treated with GEE were not significantly different compared to GEE-untreated worms. However, GEE afforded protection in skn-1 (ok2315) worms against MeHg toxicity, increasing the LD50 values in comparison to skn-1 (ok2315) GEE-untreated worms.
After embryogenesis, the worm undergoes to 6 stages of development (L1, L2, L3, L4, young adults and egglaying adults) at specific time points, dependent upon the temperature (Hope 1999). Normal development in C. elegans is arrested by numerous toxicants, returning to a normal pattern, once more favorable conditions are re-encountered (41, 42). Exposure of WT and skn-1 (ok2315) L4-larvae stage worms to MeHg induced a developmental delay in a dose-dependent manner, which was accentuated in mutant worms. GEE had no effect on the development of WT worms, however, it decreased the percentage of skn-1 (ok2315) worms with developmental delay after MeHg exposure in comparison to GEE-untreated worms.
GEE effectively protected skn-1 (ok2315) worms from MeHg-induced decrease in pharyngeal pumping and thrashes. Although GEE-untreated skn-1 (ok2315) worms also recovered their movements 24-hour post MeHg exposure, pre-treatment with the extract, accelerated the worms recovery. Thus, GEE might decrease MeHg toxicity by increasing antioxidant defenses and/or increasing MeHg excretion, as well as the rate of cellular repair in damaged worms (43, 44). However, GEE did not protect WT worms.
Analogous to behavior and development, gene expression was differentially affected by GEE exposure when compared to WT and skn-1 (ok2315) worms 24-hour post MeHg exposure. Expression of aat-2 and sir-2.1 was decreased in WT worms pre-treated with GEE, while expression of mtl-1, mtl-2, aat-2, sod-3 and sir-2.1 was increased in skn-1 (ok2315) worms pre-treated with GEE in comparison to untreated worms. Synthesis of metallothioneins, cysteine-rich metal biding proteins, are induced by metal exposure and other stressors, and they are involved in heavy metal detoxification and homeostasis (6, 45). C. elegans has 2 isoforms of metallothioneins, independently encoded by mtl-1 and mtl-2 (46), which were increased by GEE in skn-1 (ok2315) worms exposed to MeHg. In skn-1 (ok2315) worms, GEE also increased the expression of sirtuins, which lead to activation and expression of antioxidant genes (47), such as sod-3, which plays a critical role in the defense of cells against the toxic effects of oxygen radicals.
MeHg is lipid soluble and may distribute throughout the organism by diffusion, but it also has a high affinity for thiol groups (48). MeHg-L-cysteine conjugates are structurally similar to L-methionine, and thus by molecular mimicry enter cells through the L-type large neutral amino acid transporter 1 (LAT1) (49). C. elegans have nine genes encoding amino acid transporters and aat-1 through aat-3 have the highest homology to LAT1 (50). It was previously shown that knockdown of these three genes increased resistance of worms exposed to MeHg (51). However, the function and localization of these transporters in worms has yet to be characterized. Herein, aat-2 expression was increased in GEE-treated skn-1 (ok2315) worms exposed to MeHg, thus a potential corresponding increase in AAT-2 protein expression might hasten the extracellular transport of MeHg.
MeHg-induced impairments have been associated to increase in oxidative stress (1), and thus GEE could exert protective effects through its antioxidant activity (16). However, in this study, ROS levels were decreased post high doses of MeHg 6-hour exposure and 24-hour recovery with no significantly differences between GEE-pretreated and –untreated worms. Mitochondria produces ROS as part of aerobic respiration, which are also essential for normal cellular signaling (52). Decreased ROS levels may be a result of worm metabolism impairment and death. Thus, the protective effects of GEE in skn-1 (ok315) worms might be dependent on modulation of genes other than those directly involved in antioxidant activity.
Combined, our data suggest that GEE might exert protective effects against MeHg-induced toxicity by modulating genes related to metal transport, detoxification and antioxidant responses. In individuals exposed to pollutants (53), disease (54, 55) and nutritional deficiencies (56), routine consumption of guarana might be helpful in restoring cellular protection, such as is shown herein for MeHg-induced toxicity.
5. Conclusion
The effects of guarana ethanolic extract (GEE) on MeHg-induced toxicity were investigated in C. elegans. GEE afforded a protective effect in skn-1 (ok2315) worms, an effect likely modulated by upregulation of genes involved in metal transport, detoxification and antioxidant response. These mutant worms (skn-1 (ok2315)) are more susceptible to environmental stresses, thus mimicking physiological situations of unbalanced ROS production commonly inherent to human populations exposed to a myriad of xenobiotics. Therefore, routine consumption of guarana might be helpful in protecting against MeHg-induced toxicity in organisms subject to harmful environmental stress conditions.
Acknowledgments
We thank the Caenorhabditis Genetics Center (CGC) at the University of Minnesota for providing worm strains. This work was supported by the Brazilian National Council of Technological and Scientific Development (CNPq), “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES), “Programa de Apoio a Núcleos Emergentes” (PRONEM/FAPERGS/CNPq) and “Ministério da Ciência, Tecnologia e Inovação” MCTI/CNPq 472669/2011-7, 475896/2012-2. Michael Aschner was supported in part by NIH grant R01 ES10563.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Farina M, Avila DS, da Rocha JB, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62(5):575–94. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 4.Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. The American journal of physiology. 1992;262(5 Pt 2):R761–5. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- 5.Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. The Biochemical journal. 2002;367(Pt 1):239–46. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Finley EJ, Aschner M. Revelations from the Nematode Caenorhabditis elegans on the Complex Interplay of Metal Toxicological Mechanisms. J Toxicol. 2011;2011:895236. doi: 10.1155/2011/895236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmcke KJ, Syversen T, Miller DM, 3rd, Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicology and applied pharmacology. 2009;240(2):265–72. doi: 10.1016/j.taap.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman JC, Hacon S, Wasserman MA. Biogeochemistry of mercury in the Amazonian environment. Ambio. 2003;32(5):336–42. doi: 10.1579/0044-7447-32.5.336. [DOI] [PubMed] [Google Scholar]
- 9.de Castro NS, de Lima MO. Biomarkers of mercury exposure in the Amazon. Biomed Res Int. 2014;2014:867069. doi: 10.1155/2014/867069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman L, Chan HM. The influence of nutrition on methyl mercury intoxication. Environmental health perspectives. 2000;108(Suppl 1):29–56. doi: 10.1289/ehp.00108s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinola EB, Dias RF, Mattei R, Carlini EA. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. Journal of ethnopharmacology. 1997;55(3):223–9. doi: 10.1016/s0378-8741(96)01506-1. [DOI] [PubMed] [Google Scholar]
- 12.Mattei R, Dias RF, Espinola EB, Carlini EA, Barros SB. Guarana (Paullinia cupana): toxic behavioral effects in laboratory animals and antioxidants activity in vitro. Journal of ethnopharmacology. 1998;60(2):111–6. doi: 10.1016/s0378-8741(97)00141-4. [DOI] [PubMed] [Google Scholar]
- 13.Leite RP, Predes FS, Monteiro JC, Freitas KM, Wada RS, Dolder H. Advantage of Guarana (Paullinia cupana Mart.) supplementation on cadmium-induced damages in testis of adult Wistar rats. Toxicologic pathology. 2013;41(1):73–9. doi: 10.1177/0192623312447541. [DOI] [PubMed] [Google Scholar]
- 14.Leite RP, Wada RS, Monteiro JC, Predes FS, Dolder H. Protective effect of Guarana (Paullinia cupana var. sorbilis) pre-treatment on cadmium-induced damages in adult Wistar testis. Biological trace element research. 2011;141(1–3):262–74. doi: 10.1007/s12011-010-8729-7. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira DM, Barreto G, Galeano P, Romero JI, Holubiec MI, Badorrey MS, et al. Paullinia cupana Mart. var. Sorbilis protects human dopaminergic neuroblastoma SH-SY5Y cell line against rotenone-induced cytotoxicity. Human & experimental toxicology. 2011;30(9):1382–91. doi: 10.1177/0960327110389837. [DOI] [PubMed] [Google Scholar]
- 16.Schimpl FC, da Silva JF, Goncalves JF, Mazzafera P. Guarana: revisiting a highly caffeinated plant from the Amazon. J Ethnopharmacol. 2013;150(1):14–31. doi: 10.1016/j.jep.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Ye X, Linton JM, Schork NJ, Buck LB, Petrascheck M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging cell. 2014;13(2):206–15. doi: 10.1111/acel.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Martinez-Finley EJ, Bornhorst J, Chakraborty S, Aschner M. Metal-induced neurodegeneration in C. elegans. Front Aging Neurosci. 2013;5:18. doi: 10.3389/fnagi.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 2004;194(3):248–56. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106(1):5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams PL, Dusenbery DB. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicology and industrial health. 1988;4(4):469–78. doi: 10.1177/074823378800400406. [DOI] [PubMed] [Google Scholar]
- 22.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JA, Fleming JT. Basic culture methods. Methods Cell Biol. 1995;48:3–29. [PubMed] [Google Scholar]
- 24.Bittencourt LS, Machado DC, Machado MM, Dos Santos GF, Algarve TD, Marinowic DR, et al. The protective effects of guarana extract (Paullinia cupana) on fibroblast NIH-3T3 cells exposed to sodium nitroprusside. Food Chem Toxicol. 2013;53:119–25. doi: 10.1016/j.fct.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 25.Andrews KW, Schweitzer A, Zhao C, Holden JM, Roseland JM, Brandt M, et al. The caffeine contents of dietary supplements commonly purchased in the US: analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem. 2007;389(1):231–9. doi: 10.1007/s00216-007-1437-2. [DOI] [PubMed] [Google Scholar]
- 26.Bischof LJ, Huffman DL, Aroian RV. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods in molecular biology (Clifton, NJ) 2006;351:139–54. doi: 10.1385/1-59745-151-7:139. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49(1):200–19. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101(21):8084–9. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju J, Ruan Q, Li X, Liu R, Li Y, Pu Y, et al. Neurotoxicological evaluation of microcystin-LR exposure at environmental relevant concentrations on nematode Caenorhabditis elegans. Environ Sci Pollut Res Int. 2013;20(3):1823–30. doi: 10.1007/s11356-012-1151-2. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19(6):942–5. [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Smith JV, Luo Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis. 2003;5(4):287–300. doi: 10.3233/jad-2003-5404. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.da Krewer CC, Ribeiro EE, Ribeiro EA, Moresco RN, da Rocha MI, Montagner GF, et al. Habitual intake of guarana and metabolic morbidities: an epidemiological study of an elderly Amazonian population. Phytother Res. 2011;25(9):1367–74. doi: 10.1002/ptr.3437. [DOI] [PubMed] [Google Scholar]
- 35.Barriere A, Felix MA. Natural variation and population genetics of Caenorhabditis elegans. WormBook. 2005:1–19. doi: 10.1895/wormbook.1.43.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Finley EJ, Caito S, Slaughter JC, Aschner M. The Role of skn-1 in methylmercury-induced latent dopaminergic neurodegeneration. Neurochem Res. 2013;38(12):2650–60. doi: 10.1007/s11064-013-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanduyn N, Settivari R, Wong G, Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci. 2010;118(2):613–24. doi: 10.1093/toxsci/kfq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput Biol. 2008;4(8):e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Donohoe DR, Aamodt EJ, Osborn E, Dwyer DS. Antipsychotic drugs disrupt normal development in Caenorhabditis elegans via additional mechanisms besides dopamine and serotonin receptors. Pharmacol Res. 2006;54(5):361–72. doi: 10.1016/j.phrs.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruinsma JJ, Schneider DL, Davis DE, Kornfeld K. Identification of mutations in Caenorhabditis elegans that cause resistance to high levels of dietary zinc and analysis using a genomewide map of single nucleotide polymorphisms scored by pyrosequencing. Genetics. 2008;179(2):811–28. doi: 10.1534/genetics.107.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou T, Chen Y, Huang C, Chen G. Caffeine induction of sulfotransferases in rat liver and intestine. J Appl Toxicol. 2012;32(10):804–9. doi: 10.1002/jat.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pohanka M. The perspective of caffeine and caffeine derived compounds in therapy. Bratisl Lek Listy. 2015;116(9):520–30. doi: 10.4149/bll_2015_106. [DOI] [PubMed] [Google Scholar]
- 45.Aschner M, Syversen T, Souza DO, Rocha JB. Metallothioneins: mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp Biol Med (Maywood) 2006;231(9):1468–73. doi: 10.1177/153537020623100904. [DOI] [PubMed] [Google Scholar]
- 46.Freedman JH, Slice LW, Dixon D, Fire A, Rubin CS. The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J Biol Chem. 1993;268(4):2554–64. [PubMed] [Google Scholar]
- 47.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 48.Hughes WL. A physicochemical rationale for the biological activity of mercury and its compounds. Ann N Y Acad Sci. 1957;65(5):454–60. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- 49.Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462(1):31–9. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- 50.Veljkovic E, Stasiuk S, Skelly PJ, Shoemaker CB, Verrey F. Functional characterization of Caenorhabditis elegans heteromeric amino acid transporters. J Biol Chem. 2004;279(9):7655–62. doi: 10.1074/jbc.M309528200. [DOI] [PubMed] [Google Scholar]
- 51.Caito SW, Zhang Y, Aschner M. Involvement of AAT transporters in methylmercury toxicity in Caenorhabditis elegans. Biochem Biophys Res Commun. 2013;435(4):546–50. doi: 10.1016/j.bbrc.2013.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delmas-Beauvieux MC, Peuchant E, Dumon MF, Receveur MC, Le Bras M, Clerc M. Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin Biochem. 1995;28(2):163–9. doi: 10.1016/0009-9120(94)00071-3. [DOI] [PubMed] [Google Scholar]
- 55.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.John JH, Ziebland S, Yudkin P, Roe LS, Neil HA, Oxford F, et al. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. 2002;359(9322):1969–74. doi: 10.1016/s0140-6736(02)98858-6. [DOI] [PubMed] [Google Scholar]