Abstract
Objective
In observational epidemiologic studies, higher plasma high-density lipoprotein cholesterol (HDL-C) has been associated with increased risk of intracerebral hemorrhage (ICH). DNA sequence variants that decrease cholesteryl ester transfer protein (CETP) gene activity increase plasma HDL-C; as such, medicines that inhibit CETP and raise HDL-C are in clinical development. Here, we test the hypothesis that CETP DNA sequence variants associated with higher HDL-C also increase risk for ICH.
Methods
We performed two candidate-gene analyses of CETP. First, we tested individual CETP variants in a discovery cohort of 1149 ICH cases and 1238 controls from 3 studies, followed by replication in 1625 cases and 1845 controls from 5 studies. Second, we constructed a genetic risk score comprised of 7 independent variants at the CETP locus and tested this score for association with HDL-C as well as ICH risk.
Results
Twelve variants within CETP demonstrated nominal association with ICH, with the strongest association at the rs173539 locus (odds ratio (OR) 1.25, standard error (SE) 0.06, p=6.0×10−4) with no heterogeneity across studies (I2=0%). This association was replicated in patients of European ancestry (p=0.03). A genetic score of CETP variants found to increase HDL-C by ~2.85mg/dL in the Global Lipids Genetics Consortium was strongly associated with ICH risk (OR 1.86, SE 0.13, p=1.39×10−6).
Interpretation
Genetic variants in CETP associated with increased HDL-C raise the risk of ICH. Given ongoing therapeutic development in CETP inhibition and other HDL-raising strategies, further exploration of potential adverse cerebrovascular outcomes may be warranted.
INTRODUCTION
Serum levels of high density lipoprotein (HDL-C) are strongly and inversely associated with coronary artery disease (CAD) risk1. Of the many single nucleotide polymorphisms (SNPs) associated with HDL-C levels, those within cholesteryl ester transfer protein (CETP) have the strongest effect2-4. Inhibitory variants within CETP associated with increased HDL-C correlate with reduced risk of multiple cardiac risk factors, including metabolic syndrome and myocardial infarction (MI)5-8. Inhibitors of the CETP gene product, designed to raise HDL-C by limiting CETP-mediated exchange of cholesteryl esters and triglycerides between HDL and LDL/VLDL particles, are being investigated in ongoing Phase III trials as treatments to reduce CAD risk9,10.
In contrast, substantial data suggest that elevations in HDL-C may increase risk of spontaneous intracerebral hemorrhage (ICH)11,12.Furthermore, clinical trial data suggests an increased risk of ICH on statins despite a lack of significant differences in lipid levels13,14. Because of small sample sizes and confounding by environmental or medical exposures, prior studies have not been able to resolve this potentially paradoxical role of elevated HDL-C in ICH. While ICH comprises only 15-20% of all strokes, it accounts for 50% of all stroke-related mortality and 30% of total costs15,16. Blood pressure control remains the only available preventive strategy17. As HDL-C evolves as a cardiovascular treatment target and clinical trial data on therapeutic modifiers accrue, an improved mechanistic understanding of the pathways involved in hemorrhagic cerebrovascular disease could lead to alternative treatments and prevention strategies for ICH.
It is not known whether CETP inhibitors, which endeavor to produce a biological effect similar to known genetic variants in CETP, increase ICH risk. The objective of this study was to use genome-wide genotypes from individuals with and without ICH from the International Stroke Genetics Consortium to test genetic variants within CETP for association with ICH risk, under the hypothesis that the HDL-raising effects of inhibitory variants within CETP will result in increased ICH. CETP genetic variants that impact HDL-C are unconfounded by other exposures, remain constant throughout life, and may be more reflective of long-term levels than periodic lipid measurements18. Thus, examination of CETP genetic variation constitutes a valuable causal inference tool to help strengthen or disclaim prior observations of association between elevated HDL-C and ICH, and could provide additional clues about potential adverse effects of pharmacologic CETP inhibition.
METHODS
Study Design
We performed a two-stage (discovery and replication) case-control candidate-gene association study using both genome-wide data and direct genotyping. The discovery phase utilized data from 3 genome-wide association studies of ICH, sampling patients of European ancestry (Table 1)19. Replication involved direct genotyping of variants of interest from individuals recruited through 5 case-control studies of ICH, with no overlap between individuals from the discovery phase (Table 2). Detailed description of discovery and replication case and control recruitment architectures can be found in Supplementary Table S1.
Table 1.
Discovery populations
| Variable | GOCHA | ISGC ICH Study | GERFHS | |||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| n | 371 | 389 | 404 | 530 | 374 | 319 |
| Age, mean (SD) | 74 (10) | 72 (8) | 70 (13) | 66 (16) | 67 (15) | 67 (14) |
| Female, n (%) | 172 (46) | 195 (50) | 189 (47) | 266 (50) | 194 (52) | 172 (54) |
| HTN, n (%) | 274 (75) | 227 (58) | 278 (69) | 247 (47) | 241 (64) | 166 (52) |
| T2D, n (%) | 68 (18) | 35 (9) | 89 (22) | 68 (13) | 72 (19) | 42 (13) |
| HL, n (%) | 144 (39) | 195 (50) | 87 (22) | 48 (9) | 131 (35) | 133 (42) |
| Smoking, n (%) | 56 (15) | 15 (4) | 58 (14) | 74 (14) | 79 (21) | 46 (14) |
| Genotyping Platform |
Illumina 610 |
Illumina 610 |
Illumina 610 |
Illumina 610 |
Affymetrix 6.0 |
Affymetrix 6.0 |
| Lobar, n (%) | 205 (55) | - | 135 (33) | - | 156 (42) | - |
| Discovery totals | 2387 individuals (1149 cases, 1238 controls), 43% lobar ICH | |||||
Abbreviations: GERFHS = Genetic and Environmental Risk Factors for Hemorrhagic Stroke study; GOCHA = Genes and Outcomes of Cerebral Hemorrhage on Anticoagulation study; HL = Hyperlipidemia; HTN = Hypertension; ICH = Intracerebral hemorrhage; ISGC ICH study = International Stroke Genetics Consortium Intracerebral Hemorrhage Study; Lobar = Lobar ICH location; T2D = Type 2 Diabetes Mellitus
Table 2.
Replication populations
| Variable | MGH | ERICH | University of Brescia |
UMC Utrecht | University of Edinburgh |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Ctrl | Case | Ctrl | Case | Ctrl | Case | Ctrl | Case | Ctrl | |
| n | 240 | 458 | 920 | 826 | 198 | 185 | 157 | 160 | 110 | 216 |
| Age, n (SD) | 76 (10) |
69 (11) |
69 (14) |
68 (13) |
69 (13) |
63 (14) |
62 (13) |
56 (11) |
75 (9) | 76 (10) |
| Female, n (%) |
96 (40) |
206 (45) |
397 (43) |
371 (45) |
81 (41) |
85 (46) |
66 (42) |
67 (42) |
59 (54) |
118 (54) |
| Lobar , n (%) | 120 (48) |
- | 380 (41) |
- | 82 (41) |
- | 60 (38) |
- | 61 (55) |
- |
| Genotyping platform |
iPLEX | iPLEX | Taq- man |
Taq- man |
iPLEX | iPLEX | iPLEX | iPLEX | iPLEX | iPLEX |
| Replication Totals |
3470 individuals (1625 cases, 1845 controls), 42% lobar ICH | |||||||||
| Discovery + Replication Totals |
5625 individuals (2595 cases, 3030 controls), 45% lobar ICH | |||||||||
Abbreviations: Ctrl = Control; ERICH = Ethnic/Racial Variations of Intracerebral Hemorrhage; iPLEX = Sequenom MassARRAY iPLEX Platform; MGH = Massachusetts General Hospital; TaqMan = Applied Biosystems Taqman Genotyping Assay
All studies had approval from the local institutional review board or ethics committee at each participating institution. Informed consent was obtained from all patients, their legally authorized representatives, or was waived via protocol-specific allowance.
Cases
ICH was defined as a new and acute neurological deficit with compatible brain imaging. Enrolled patients were adult consenting primary acute ICH cases that presented to participating institutions with confirmation of primary ICH through computed tomography or magnetic resonance imaging. Exclusion criteria included trauma, brain tumor, hemorrhagic transformation of a cerebral infarction, vascular malformation, or any other cause of secondary ICH in all participating studies.
Case Populations
ICH cases w3ere recruited across multiple centers participating in the International Stroke Genetics Consortium from sites in the USA and Europe. For the purposes of reducing confounding by population stratification, only individuals of self-reported European (Caucasian) ancestry were included in the analysis. Likewise, several studies (GOCHA, ESS, LINCHPIN) recruited ICH patients with ICH in the presence of anticoagulation (typically warfarin) exposure. These individuals were excluded from analyses due to the etiopathological distinctness of warfarin-related primary ICH from other forms. Discovery case populations were enrolled according to methods previously described19. Replication cases were recruited from ISGC participating centers using similar criteria as discovery cases (Supplementary Table S2). Briefly, UMC Utrecht ICH study included additional screening for secondary ICH cases in follow-up. The Edinburgh Stroke Study recruited subjects aged > 55 years only, and specifically excluded individuals with antecedent illicit drug use or presentation > 1 week from onset of symptoms. The LINCHPIN study identified ICH cases aged > 16 with acute or chronic ICH from a prospective cohort of individuals living in the Lothian region of Scotland, UK.
Neuroimaging
Stroke neurologists and neuroradiologists at each participating site performed the neuroimaging assessment. Following known differences in underlying biology, ICH was classified as lobar or non-lobar according to location20. ICH originating in the cortico-subcortical junction (with or without involvement of subcortical white matter) was defined as lobar, whereas ICH selectively involving the thalamus, internal capsule, basal ganglia, brainstem or cerebellum was defined as non-lobar.
Controls
Controls were ICH-free individuals >18 years of age were enrolled from the same populations that gave rise to the cases. Controls were confirmed to have no history of previous ICH by interview and/or medical record review. Control population age restrictions were identical to case population age restrictions for all included studies.
Control Populations
ICH-free controls were recruited from the same populations that gave rise to the ICH cases, through inpatient recruitment, ambulatory centers in the local communities, blood donation centers serving the same population, and in the case of the Lothian Birth Cohort, a population cohort study (Supplementary Table S3). The Genetic and Environmental Risk Factors for Hemorrhagic Stroke (GERFHS) and Ethic/Racial Variations of Intracerebral Hemorrhage (ERICH) studies19,21 used random digit dialing, the Lothian Birth Cohort individuals were matched to case samples by local investigators22, and UMC Utrecht identified controls from the local blood donor population. The remainder of studies used random selection from ambulatory clinics or geographically-matched populations where cases were being recruited.
Exposure: common genetic variants within CETP
In the discovery phase, we ascertained variants within CETP by means of genome-wide genotyping followed by imputation using methods and quality control procedures previously described19. Briefly, DNA was isolated from fresh or frozen peripheral whole blood collected from cases and controls at each participating institution at the time of consent, quantified with a quantification kit (Qiagen, Valencia, CA, USA), and normalized to a concentration of 30 ng/μL. Cases and controls were plated together and genotyped on Illumina 610 or Affymetrix 6.0 platforms. Standard quality controls for genome-wide data were applied, and the resulting set of individuals and SNPs were carried forward to imputation, that was completed using IMPUTE2 with 1000 Genomes-based reference panels (version March 2012)23. Post imputation exclusion filters were minor allele frequency (MAF) <0.01 and information score <0.5. SNPs were extracted from the CETP gene region according to the human genome reference GRCh38.p2 annotated location (http://www.ncbi.nlm.nih.gov), +/− 50 kilobases.
Independent Replication
CETP variants exceeding Bonferroni-corrected significance and without significant heterogeneity (I2<40%) for association with ICH in the discovery phase were selected for replication24. Replication SNPs were chosen based on proxy status with index SNPs. Because replication of CETP variants was carried out as part of an ongoing GWAS of ICH, a constraint for the selection of replication SNPs was predicted genotyping success using both Sequenom iPLEX (Sequenom, San Diego, CA, USA) and Taqman (Applied Biosystems, Foster City, CA, USA) methodologies, which were employed at the MGH and University of Miami genotyping centers, respectively (Table 2). Ancestry informative markers were also genotyped to facilitate adjustment for population admixture.
Data Analysis
We present discrete variables as counts (percentage [%]) and continuous variables as mean (standard deviation [SD]) or median (interquartile range [IQR]), as appropriate.
Population Structure
Principal component analysis was implemented in both discovery and replication to account for population structure, using genome-wide data in discovery and pre-specified ancestry-informative markers in replication25,26. Caucasian population outliers were identified and removed by visual inspection of plots generated with principal components 1 and 2, and these principal components were included as covariates in regression models fitted for association testing. In GERFHS and ERICH samples, further refinement of population structure was achieved using the ADMIXTURE software tool to remove outliers27.
Association Testing
Prior to discovery association testing, SNPs within CETP were clumped into loci sharing linkage disequilibrium (LD) r2>0.5 using PLINK to allow discrimination of semi-independent loci across the gene. Association testing for SNPs within the CETP locus and ICH risk was completed separately for all ICH, as well as for lobar and non-lobar hemorrhages. Logistic regression models were fitted assuming independent additive genetic effects for dosage of the minor allele (1-degree-of-freedom additive trend test), and adjusting for age, gender, and principal components 1 and 2. A similar analytic approach was undertaken for analysis of replication data, using additive allele genotype data rather than dosage.
Meta-Analysis
Fixed effects, inverse variance weighted meta-analysis was used to pool effect estimates across studies, assessing heterogeneity by computing Cochrane’s Q (with corresponding p) and I2 (percent of effect size attributable to heterogeneity). Identical meta-analysis procedures were used for pooling of effects across studies in discovery, replication, and across all studies28.
Genetic Risk Score Analysis
Variants within the CETP locus with established association with HDL-C levels in the most recent Global Lipids Genetics Consortium (GLGC) analysis29 (Global Lipids Genetics Consortium, “Biological and clinical insights from exome array analysis of lipids in > 300,000 individuals”, under review) were extracted from the discovery dataset and tested for association with ICH using an additive multi-SNP genetic risk score approach using the GTX package (http://CRAN.R-project.org/package=gtx) in R (version 3.0). 10 variants surpassing exome array-wide significance (p<2.1×10−7) and demonstrating independence using a sequential forward selection model in the GLGC dataset were identified, of which 7 were available in our ICH discovery dataset30. These 7 variants, on average, were associated with a 0.19 standard deviation increase in HDL-C (~2.85mg/dL) in the GLGC population (p<1×10−200). This corresponds to a proportion of variance explained of 0.032. ICH risk was predicted from summary statistics, weighted according to the established HDL-C effect and oriented to the HDL-C increasing allele.
Statistical Testing and Software
We used a conservative Bonferroni-corrected threshold for statistical significance of p<0.004, adjusted for the number of semi-independent loci within CETP with r2<0.5 (12 tests in this analysis). Quality control procedures, genetic association testing for single variants, and score calculations were performed in SNPTest and PLINK v1.0726,31. Imputation was completed using IMPUTE223. All other statistical analyses were performed in SAS 9.2 (SAS Institute, Cary, NC USA).
RESULTS
Following relevant exclusions during quality control and principal component analysis, 1149 ICH cases and 1238 controls from 3 case-control studies of ICH were included in the discovery phase, 43% of which were of the lobar ICH subtype (Table 1).
CETP Genetic Variants
After imputation using 1000 genomes reference panels and application of genome-wide quality control filters, a total of 390 common variants of MAF > 0.01 were extracted from the CETP gene and 50 kilobase flanking regions (Supplementary Table S4)32. These 390 variants were present either via array-based ascertainment or imputation in all 3 of the discovery datasets, and were used for association testing.
Single-SNP Association Testing
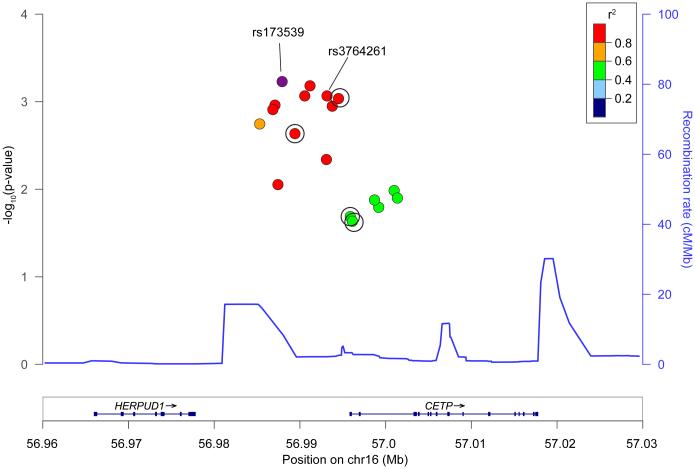
After testing all 390 SNPs within CETP clumped into regions sharing r2>0.5, 12 loci demonstrating nominal association with ICH (p<0.05) were identified (Supplementary Table S5). Three of these loci surpassed Bonferroni-correction (Table 3) with residual r2=0.25-0.39 between them. Among these, only rs173539 (odds ratio (OR) 1.25, standard error (SE) 0.06, p=6.00×10−4) met prespecified criteria for replication due to its homogeneity across discovery datasets (I2=0%). Of note, rs173539 was in high LD with rs3764261 (r2=0.98), the strongest associated SNP with HDL-C in published GWAS of lipid levels (Figure 1)33. Comparison of effects of the rs173539 locus on risk of lobar vs. non-lobar hemorrhage revealed no significant differences by ICH subtype (Supplementary Table S6).
Table 3.
Discovery CETP loci demonstrating Bonferroni-significant association with ICH
| Lead SNP | CHR | Tested allele |
MAF | Effect direction |
OR | SE | Discovery p |
I2 |
|---|---|---|---|---|---|---|---|---|
| rs173539 | 16 | T | 0.31 | +++ | 1.25 | 0.06 | 6.00E-4 | 0 |
| rs820299 | 16 | G | 0.38 | − − − | 0.81 | 0.06 | 7.50E-4 | 48 |
| rs158478 | 16 | A | 0.48 | +++ | 1.21 | 0.06 | 1.48E-3 | 56 |
CHR = chromosome, MAF = minor allele frequency, OR = odds ratio, SE = standard error, SNP = single nucleotide polymorphism, + = variant increases ICH risk, − = variant decreases ICH risk.
Figure 1. ICH-associated variants in the rs173539 locus in CETP.
Regional association plot of rs173539 and SNPs exhibiting r2>0.5 in association with ICH. SNPs available for replication are circled. Mean recombination rate across the locus is represented by the continuous blue line. The rs3764261 variant identified was the leading SNP in prior genome-wide association studies of HDL-C. Chr = chromosome, cMMb = centimorgans per megabase, Mb = megabase.
Replication and Meta-analysis of the rs173539 Locus
1625 ICH cases and 1845 controls of Caucasian ancestry were available for replication. Following application of predictive algorithms for SNP genotype ascertainment success using both genotyping methodologies employed, four SNPs in LD with rs173539 locus were selected for replication genotyping according to the constraints described (Tables 4 and 5). Both rs173539 and rs3764261 were predicted to fail in one or both replication pools. All four selected SNPs were successfully genotyped in all replication datasets. All replication results showed minimal heterogeneity and consistent directions of effect, and two variants replicated at p<0.05. In meta-analysis, all four SNPs within the rs173539 locus chosen for replication were strengthened by addition of the replication SNP data, with minimal heterogeneity in the final total sample size of 2595 ICH cases and 3030 controls (Table 5).
Table 4.
Discovery SNP rs173539 and local proxies in association with ICH risk
| SNP | CHR | Tested allele |
MAF | Effect direction |
OR | SE | Discovery p |
I2 |
|---|---|---|---|---|---|---|---|---|
| rs173539 | 16 | T | 0.31 | +++ | 1.25 | 0.06 | 6.00×10−4 | 0 |
| -- rs247617 (r2=0.99) | 16 | A | 0.31 | +++ | 1.24 | 0.06 | 8.74×10−4 | 0 |
| -- rs17231506 (r2=0.99) | 16 | T | 0.31 | +++ | 1.23 | 0.06 | 9.13×10−4 | 0 |
| -- rs711752 (r2=0.62) | 16 | A | 0.42 | ++− | 1.15 | 0.06 | 2.08×10−2 | 14 |
| -- rs708272 (r2=0.61) | 16 | A | 0.42 | ++− | 1.15 | 0.06 | 2.23×10−2 | 18 |
Association results for rs173539 in association with ICH risk, as well as four additional SNPs in linkage disequilibrium (LD) with rs173539 chosen for replication. CHR = chromosome, MAF = minor allele frequency, OR = odds ratio, SE = standard error, r2 = degree of LD with rs173539. SNP = single nucleotide polymorphism, + = variant increases ICH risk, − = variant decreases ICH risk.
Table 5.
Replication results for SNPs in LD with rs173539 and meta-analysis of all samples
| Replication | Discovery/Replication Meta-analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Effect | OR | SE | p | I2 | Effect | OR | SE | p | I2 |
| rs247617 | +++++ | 1.08 | 0.05 | 0.18 | 2 | +++/+++++ | 1.13 | 0.04 | 1.0×10−3 | 0 |
| rs17231506 | +++++ | 1.08 | 0.05 | 0.17 | 1 | +++/+++++ | 1.13 | 0.04 | 1.0×10−3 | 0 |
| rs711752 | ++++− | 1.12 | 0.05 | 0.03 | 7 | ++−/++++− | 1.13 | 0.04 | 1.0×10−3 | 0 |
| rs708272 | ++++− | 1.14 | 0.05 | 0.01 | 4 | ++−/++++− | 1.14 | 0.04 | 5.0×10−4 | 0 |
OR = odds ratio, SE = standard error, SNP = single nucleotide polymorphism, + = variant increases ICH risk, − = variant decreases ICH risk.
Genetic Risk Score Analysis
An additive multi-SNP genetic risk score was constructed using independent HDL-association data29. 10 variants were selected, of which 7 were present in the ICH discovery dataset (Table 6). 3 variants were unavailable in the ICH dataset due to differences in genotyping platforms (exome array vs. GWAS array) between the two studies. The genetic risk score of these 7 variants demonstrated association with ICH (OR 1.86, SE 0.13, p=1.39×10−6).
Table 6.
ICH association results for variants of known HDL-C effect used to compute genetic risk score
| SNP | Ref Allele |
MAF | ICH OR |
ICH Beta |
ICH SE |
ICH p |
HDL Effect Allele |
HDL Beta |
HDL SE |
Type |
|---|---|---|---|---|---|---|---|---|---|---|
| rs173539 | T | 0.31 | 1.25 | 0.222 | 0.065 | 0.0006 | T | 0.230 | 0.0028 | Intergenic |
| rs3764261 | A | 0.31 | 1.23 | 0.210 | 0.063 | 0.0009 | A | 0.239 | 0.0028 | Intergenic |
| rs247616 | T | 0.30 | 1.22 | 0.196 | 0.064 | 0.0023 | T | 0.242 | 0.0028 | Intergenic |
| rs9989419 | A | 0.40 | 0.92 | −0.079 | 0.059 | 0.1808 | G | 0.131 | 0.0026 | Intergenic |
| rs5880 | C | 0.04 | 1.22 | 0.202 | 0.151 | 0.1812 | G | 0.258 | 0.0067 | Nonsyn. |
| rs5882 | G | 0.32 | 1.06 | 0.057 | 0.065 | 0.3803 | G | 0.092 | 0.0028 | Nonsyn. |
| rs7499892 | T | 0.19 | 1.02 | 0.022 | 0.076 | 0.7758 | C | 0.230 | 0.0033 | Intronic |
CETP = cholesterol ester transfer protein, HDL = High Density Lipoprotein, ICH = intracerebral hemorrhage, MAF = minor allele frequency, Nonsyn. = nonsynonymous, OR = odds ratio, Ref = reference, SE = standard error
DISCUSSION
Our results demonstrate an association between CETP gene variants in the rs173539 locus and risk of ICH, opposite in direction to their effect on risk of CAD and metabolic syndrome5,7,8. Furthermore, an aggregated score of variants within CETP that raise HDL-C is strongly associated with increased ICH risk. These results suggest that there may be substantial differences in the roles of lipids in the progression of cerebrovascular and cardiometabolic diseases. Novel therapies targeting CETP along with other approaches to increase HDL-C are currently under active investigation in an effort to reduce the risk of CAD34. Because the cerebral small vessel diseases that lead to ICH are common in the aging population and frequently coincide with risk factors for cardiometabolic disease35,36, our observations supporting opposing effects of HDL-C on ICH and CAD underscore the need for a better understanding of which patients could be at increased risk of ICH on therapies aimed at increasing HDL-C.
Our findings support prior studies linking elevated HDL-C with increased risk of ICH. Unlike prior studies, however, our genetic approach limits confounding by dietary, environmental, or medication exposures. A recent meta-analysis of epidemiological studies examining associations between cholesterol levels and ICH found a dose-response relationship between HDL-C and ICH risk, with each 1mmol/L increase in HDL-C associated with a 17% increase in ICH risk11. This result was nullified when studies of subarachnoid hemorrhage patients were included, but strengthened by restriction to studies from the United States, highlighting the potential confounds of case misspecification and unmeasured environmental exposures in testing associations of this nature.
HDL-C appears to have a complex and context-dependent role in cerebrovascular disease. In contrast to ICH, elevated HDL-C is associated with reduced risk of ischemic stroke, particularly strokes caused by large artery atherosclerotic disease, consistent with the observed associations of HDL-C in CAD37. However, Mendelian Randomization (MR) studies of genetic variants predisposing to elevated HDL-C have not demonstrated association with either ischemic stroke or CAD, suggesting the observed relationships may not be causal38,39. Unfortunately, the limited sample size of genetics efforts in ICH coupled with acute changes in lipid values around the time of ICH currently preclude the use of this MR approach in our analyses40.
No study, including the present, has yet established a direct causal relationship between HDL-C and ICH risk. While associations between CETP genetic variants and ICH are almost certainly unidirectional due to the immutability of the genetic code, they still could impact an unseen risk factor that lies outside of the known HDL-C level determining effects of the gene. Even if causality can be ultimately established, the mechanism by which a CETP-mediated increase in HDL-C may worsen ICH risk remains unclear. Inhibition of CETP results in changes to HDL particle size and cholesterol efflux capacity in addition to the observed changes in HDL-C serum levels, and it may be through these accompanying changes in HDL function that ICH risk is conferred41. Furthermore, accumulating evidence suggests that HDL effects on endothelium are dynamic and modifiable, and can even become pro-inflammatory with the incorporation of serum amyloid A1, complement C3, and ceramides, resulting in altered immune regulation and reduced antioxidant effects42,43. It is therefore possible that elevated HDL-C provides a platform to further the vascular inflammatory processes that play a substantial role in the cerebral small vessel disease underlying ICH44. Further studies will be needed to dissect the pathways intersecting with HDL-C to clarify the foundational biology of its role in ICH.
Therapeutic development of small molecule and biologic compounds designed to raise HDL-C continue45. While the first wave of Phase III trials of CETP inhibitors were plagued by off-target effects and futility46, the REVEAL trial of anacetrapib was recently continued after unblinded interim review. Other HDL-raising strategies, including apolipoprotein-A1 (ApoA1)-rich reconstituted HDL particle infusions and ApoA1-mimetic peptides continue to be evaluated in preclinical and early-phase trials45. Given this pipeline of HDL-based therapeutic development, it is imperative that potential adverse clinical effects of such strategies be clarified. Early experiences with FDA-approved PCSK-9 inhibitors have led to predictions of widespread adoption of this new class of drugs and it is reasonable to expect that HDL-C targeted treatments would be no different, resulting in a potentially large population of aging individuals with pharmacologically-induced high HDL-C levels of uncertain long-term cerebrovascular risk47. The proportion of variance in HDL-C levels explained by our genetic risk score was 0.032. This is roughly commensurate with observed effects of statins, which in clinical trials raised HDL by 0.04-0.1048. With emerging HDL-C modifying strategies likely to exert more profound effects, the impact on ICH risk, if confirmed and verified to be causal, could be more substantial than indicated by our CETP genetic risk score.
As noted above, our study cannot determine whether the observed association between CETP and ICH risk is through HDL-C alone. While they exhibit their largest effect on HDL-C levels, CETP variants are also associated with low-density lipoprotein (LDL), triglycerides (TG), and total cholesterol (TC) levels3. While we cannot perform formal MR, the association between our HDL-C increasing genetic risk score at CETP and risk of ICH provides support for an HDL-specific effect. Even with this suggestion of HDL-C specificity, the composition of HDL particles can vary with respect to ratios of esterified to unesterified cholesterol as well as apolipoprotein content. Genetic variation that determines circulating HDL-C does not necessarily capture these secondary characteristics, which could have a substantial impact on biological effects.
An additional limitation of our study is the aggregation of case and control data across multiple sites, which could result in biases between cases and controls. We have attempted to control for study demographics and population structure in our regression analyses, and performed independent replication, but unmeasured confounding could still have impacted the observed associations. Related to this point, all analyses presented were in individuals of European ancestry due to small study populations, and therefore low statistical power, in individuals of other racial and ethnic backgrounds. As a result, our findings cannot be extended to minority populations at this time.
While our study utilized genomewide data for discovery and genetic risk score analyses, our approach was fundamentally a candidate gene study of CETP. Using GWAS data allowed for control of population stratification, which can be a major confounder in traditional candidate gene designs employing only direct genotyping. However, it was still based on an a priori hypothesis about CETP association with ICH. Therefore, the false discovery rate for association between variants at CETP and ICH risk, while stringently controlled using Bonferroni-correction at the CETP locus, may still be elevated in comparison with a standard GWAS. Due to the hypothesis-driven nature of our study, we by definition cannot provide novel results about lipid-related genetic loci that lie outside of the tested gene region.
Finally, the CETP gene contains several independent loci which have been associated with lipid levels and clinical endpoints3,5,7,33. This resulted in a more complex replication phase than would have been needed if the genetic architecture of the locus were centered about a single region of association. Coupled with the limitations of variant selection in our replication phase, we cannot distinguish a culprit variant to the exclusion of others. Although all variants chosen for replication demonstrated refined effect size estimates and greater statistical significance in meta-analysis with discovery data, replication was strongest for variants in slightly lower LD than the lead variant from discovery, and with slightly higher between-study heterogeneity. Whether this observation represents true heterogeneity of effect at the replicated variants will depend on future validation and extension studies.
We have demonstrated an association between genetic variants in CETP and risk of ICH, and have shown that CETP’s HDL-C raising effects could play a role in the pathogenesis of ICH. Further work will be needed to identify how the biological pathways impacted by HDL-C may impart increased risk of hemorrhage. These pathways may yield crucial novel targets for prevention of ICH and the cerebral small vessel diseases that lead to vessel rupture.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Miguel Hernan, from the Harvard T.H. Chan School of Public Health, for valuable counsel on research methods.
No funding entities had involvement in study design, data collection, analysis, and interpretation, writing of the manuscript and in the decision to submit for publication. This work has been supported by the NIH-NINDS through K23NS086873, R01NS059727 and P50NS061343, R01NS36695, U01NS069763, and R01NS30678 and the NIH-NIA through R01AG26484. Project support for the Global Lipids Genetics Consortium through Drs. Willer and Kathiresan is provided by NIH-NHLBI R01HL127564. Lund Stroke Register (LSR) has been supported by the Swedish Heart and Lung Foundation, Skåne University Hospital, Region Skåne, the Freemasons Lodge of Instruction EOS in Lund, King Gustaf V and Queen Victoria's Foundation, Lund University, and the Swedish Stroke Association, and Spain’s Ministry of Health (Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III FEDER, RD12/0042/0020). This report does not represent the official view of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), or any part of the US Federal Government. No official support or endorsement of this article by the NINDS or NIH is intended or should be inferred.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and Design of Study
CDA, GJF, CLP, FR, AB, GMP, SK, DW, JR
Acquisition and Analysis of Data
CDA, GJF, CLP, FR, HBB, TWKB, AB, GMP, DL, AMA, JNG, AV, SMG, MS, JFM, DLB, BBW, SLS, DLT, MLF, PK, JMJ, HS, BMH, JJC, EGS, RE, ECG, CS, KMvN, CJMK, KR, NS, RAS, CLS, IJD, AM, AP, JP, AU, AP, CE, BN, JM, IFC, PD, JR, AL, AS, RS, CSK, SJK, SPW, CDL, GA, CJW, SK, DW, JR
Drafting Manuscript and Figures
CDA, GJF, CLP, FR, AB, GMP, AMA, SK, JR
International Stroke Genetics Consortium Contributors
Please refer to Supplementary Table S6 for ISGC contributors and affiliations
POTENTIAL CONFLICTS OF INTEREST
Nothing to disclose.
REFERENCES
- 1.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014 Aug 16;384(9943):618–25. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 2.Asselbergs FW, Guo Y, van Iperen EP, et al. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. American journal of human genetics. 2012 Nov 2;91(5):823–38. doi: 10.1016/j.ajhg.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nature genetics. 2013 Nov;45(11):1274–83. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tada H, Won HH, Melander O, et al. Multiple associated variants increase the heritability explained for plasma lipids and coronary artery disease. Circulation Cardiovascular genetics. 2014 Oct;7(5):583–7. doi: 10.1161/CIRCGENETICS.113.000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraja AT, Vaidya D, Pankow JS, et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011 Apr;60(4):1329–39. doi: 10.2337/db10-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers JC, Elliott P, Zabaneh D, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nature genetics. 2008 Jun;40(6):716–8. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 7.Meiner V, Friedlander Y, Milo H, et al. Cholesteryl ester transfer protein (CETP) genetic variation and early onset of non-fatal myocardial infarction. Annals of human genetics. 2008 Nov;72:732–41. doi: 10.1111/j.1469-1809.2008.00464.x. Pt 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008 Jun 18;299(23):2777–88. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Rye KA. Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. Journal of lipid research. 2012 Sep;53(9):1755–66. doi: 10.1194/jlr.R024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastelein JJ, Besseling J, Shah S, et al. Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet. 2015 May 30;385(9983):2153–61. doi: 10.1016/S0140-6736(14)62115-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Dong Y, Qi X, et al. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke; a journal of cerebral circulation. 2013 Jul;44(7):1833–9. doi: 10.1161/STROKEAHA.113.001326. [DOI] [PubMed] [Google Scholar]
- 12.Raffeld MR, Biffi A, Battey TW, et al. APOE epsilon4 and lipid levels affect risk of recurrent nonlobar intracerebral hemorrhage. Neurology. 2015 Jul 28;85(4):349–56. doi: 10.1212/WNL.0000000000001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008 Jun 10;70(24):2364–70. doi: 10.1212/01.wnl.0000296277.63350.77. Pt 2. [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Armitage J, Parish S, et al. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004 Mar 6;363(9411):757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 15.Taylor TN, Davis PH, Torner JC, et al. Lifetime cost of stroke in the United States. Stroke; a journal of cerebral circulation. 1996 Sep;27(9):1459–66. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 16.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Current atherosclerosis reports. 2012 Aug;14(4):300–6. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passero S, Burgalassi L, D'Andrea P, et al. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 1995;26(7):1189–92. doi: 10.1161/01.str.26.7.1189. [DOI] [PubMed] [Google Scholar]
- 18.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. Journal of the American College of Cardiology. 2012 Dec 25;60(25):2631–9. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Woo D, Falcone GJ, Devan WJ, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. American journal of human genetics. 2014 Apr 3;94(4):511–21. doi: 10.1016/j.ajhg.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology. 2012 Dec 4;79(23):2275–82. doi: 10.1212/WNL.0b013e318276896f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke; a journal of cerebral circulation. 2013 Oct;44(10):e120–5. doi: 10.1161/STROKEAHA.113.002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deary IJ, Gow AJ, Pattie A, et al. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. International journal of epidemiology. 2012 Dec;41(6):1576–84. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- 23.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3 (Bethesda) 2011 Nov;1(6):457–70. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell; Chichester, England ; Hoboken, NJ: 2008. [Google Scholar]
- 25.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006 Aug;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome research. 2009 Sep;19(9):1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evangelou E, Ioannidis JP. Meta-analysis methods for genome-wide association studies and beyond. Nature reviews Genetics. 2013 Jun;14(6):379–89. doi: 10.1038/nrg3472. [DOI] [PubMed] [Google Scholar]
- 29.Global Lipids Genetics Consortium Biological and clinical insights from exome array analysis of lipids in > 300,000 individuals. 2016 In review. [Google Scholar]
- 30.Saeys Y, Inza I, Larranaga P. A review of feature selection techniques in bioinformatics. Bioinformatics. 2007 Oct 1;23(19):2507–17. doi: 10.1093/bioinformatics/btm344. [DOI] [PubMed] [Google Scholar]
- 31.Pei YF, Zhang L, Li J, et al. Analyses and comparison of imputation-based association methods. PloS one. 2010;5(5):e10827. doi: 10.1371/journal.pone.0010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JB, Cooke Bailey JN, Hoffman JD, et al. Estimating cumulative pathway effects on risk for age-related macular degeneration using mixed linear models. BMC bioinformatics. 2015;16:329. doi: 10.1186/s12859-015-0760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nature genetics. 2009 Jan;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinton EA, Kher U, Shah S, et al. Effects of anacetrapib on plasma lipids in specific patient subgroups in the DEFINE (Determining the Efficacy and Tolerability of CETP INhibition with AnacEtrapib) trial. J Clin Lipidol. 2015 Jan-Feb;9(1):65–71. doi: 10.1016/j.jacl.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Ding J, Sigurdsson S, Garcia M, et al. Risk Factors Associated With Incident Cerebral Microbleeds According to Location in Older People: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. JAMA neurology. 2015 Jun;72(6):682–8. doi: 10.1001/jamaneurol.2015.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palacio S, McClure LA, Benavente OR, et al. Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the secondary prevention of small subcortical strokes study. Stroke; a journal of cerebral circulation. 2014 Sep;45(9):2689–94. doi: 10.1161/STROKEAHA.114.005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amarenco P, Goldstein LB, Szarek M, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke; a journal of cerebral circulation. 2007 Dec;38(12):3198–204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- 38.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012 Aug 11;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pikula A, Beiser AS, Wang J, et al. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology. 2015 Feb 3;84(5):472–9. doi: 10.1212/WNL.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phuah CL, Raffeld MR, Ayres AM, et al. Subacute decline in serum lipids precedes the occurrence of primary intracerebral hemorrhage. Neurology. 2016 May 31;86(22):2034–41. doi: 10.1212/WNL.0000000000002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammadpour AH, Akhlaghi F. Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective. Clin Pharmacokinet. 2013 Aug;52(8):615–26. doi: 10.1007/s40262-013-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weichhart T, Kopecky C, Kubicek M, et al. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012 May;23(5):934–47. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papageorgiou N, Zacharia E, Androulakis E, et al. HDL as a prognostic biomarker for coronary atherosclerosis: the role of inflammation. Expert Opin Ther Targets. 2016 Mar 1;:1–15. doi: 10.1517/14728222.2016.1152264. [DOI] [PubMed] [Google Scholar]
- 44.Rouhl RP, Damoiseaux JG, Lodder J, et al. Vascular inflammation in cerebral small vessel disease. Neurobiology of aging. 2012 Aug;33(8):1800–6. doi: 10.1016/j.neurobiolaging.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011 May;8(5):266–77. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johns DG, Duffy J, Fisher T, et al. On- and off-target pharmacology of torcetrapib: current understanding and implications for the structure activity relationships (SAR), discovery and development of cholesteryl ester-transfer protein (CETP) inhibitors. Drugs. 2012 Mar 5;72(4):491–507. doi: 10.2165/11599310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Gutierrez R, Shah ND, Montori VM. Predicting the Overuse of PCSK-9 Inhibitors. JAMA. 2015 Nov 10;314(18):1909–10. doi: 10.1001/jama.2015.13414. [DOI] [PubMed] [Google Scholar]
- 48.McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008 Aug;22(4):321–38. doi: 10.1007/s10557-008-6113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.