Abstract
Background
Diabetes mellitus and its complications are a large and increasing burden for health care worldwide. Reduced pulmonary function has been observed in diabetes (both type 1 and type 2), and this reduction is thought to occur prior to diagnosis. Other measures of pulmonary health are associated with diabetes, including lower exercise tolerance, greater dyspnea, lower quality of life (as measured by the St. George’s Respiratory Questionaire [SGRQ]) and susceptibility to lung infection and these measures may also predate diabetes diagnosis.
Methods
We examined 7080 participants in the COPD Genetic Epidemiology (COPDGene) study who did not report diabetes at their baseline visit and who provided health status updates during 4.2 years of longitudinal follow-up (LFU). We used Cox proportional hazards modeling, censoring participants at final LFU contact, reported mortality or report of incident diabetes to model predictors of diabetes. These models were constructed using known risk factors as well as proposed markers related to pulmonary health, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, respiratory exacerbations (RE), 6-minute walk distance (6MWD), pulmonary associated quality of life (as measured by the SGRQ), corticosteroid use, chronic bronchitis and dyspnea.
Results
Over 21,519 person years of follow-up, 392 of 7080 participants reported incident diabetes which was associated with expected predictors; increased body mass index (BMI), high blood pressure, high cholesterol and current smoking status. Age, gender and accumulated smoking exposure were not associated with incident diabetes. Additionally, preserved ratio with impaired spirometry (PRISm) pattern pulmonary function, reduced 6MWD and any report of serious pulmonary events were associated with incident diabetes.
Conclusions
This cluster of pulmonary indicators may aid clinicians in identifying and treating patients with pre- or undiagnosed diabetes.
Keywords: chronic obstructive pulmonary disease, COPD, diabetes, pulmonary predictors
Background
Diabetes and its complications are a large and increasing burden for health care worldwide. Reduced pulmonary function has been observed in type 2 diabetes1–5 and this reduction is thought to occur prior to diagnosis.6–9 Other measures of pulmonary health are associated with diabetes, including lower exercise tolerance,10 greater dyspnea and lower pulmonary associated quality of life (as measured by the St. George’s Respiratory Questionaire [SGRQ])11 and these measures may also predate diabetes diagnosis. Hyperglycemia has been shown to increase glucose in airway surface liquid (ASL)12 promoting bacterial growth13 which may lead to increased pulmonary associated adverse events.14,15 These events can result in emergency room visits or hospitalizations and poor outcomes of those hospitalizations.16 Hyperglycemia can also reduce the effectiveness of the innate immune system of the lung by reducing surfactant protein-D17 which may be associated with lung infection and reduced diffusing lung capacity.18,19 Smoking has also been shown to be an independent predictor of type 2 diabetes20 and quitting smoking has been shown to reduce the risk of metabolic syndrome.21 Pulmonary risk factors beyond smoking such as reduced pulmonary function, reduced exercise capacity, increased dyspnea and increased propensity for lung infection may be associated with the development of diabetes.
This study used data collected at the baseline visit and during longitudinal follow-up of a large cohort of smokers (>10 pack years smoking history) from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study to investigate factors preceding diabetes diagnosis. Known diabetes risk factors include obesity, male gender, increased age, hypertension, high cholesterol and steroid use and potential diabetes risk factors include reduced measures of pulmonary function (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC] and their ratio), reduced 6-minute walk distance (6MWD), poor pulmonary associated quality of life measured by SGRQ, increased modified Medical Research Council (mMRC) dyspnea scale, chronic bronchitis, chronic obstructive pulmonary disease (COPD) and reported respiratory infection.
Methods
The COPDGene study has been described in detail elsewhere.22 Briefly, COPDGene is a case control study designed to identify genetic risk factors for COPD in a biracial population of non-Hispanic white (~2/3) and African American (~1/3) smokers (current and former) with at least 10 pack years history of cigarette use. COPDGene participants were between 45 and 80 years old at initial contact, spanning COPD disease severity as measured by the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria23 and include 10,192 participants from 21 clinical centers. COPDGene includes a longitudinal follow-up (LFU) component that collects data on participants once every 6 months using telephony and web-based surveys,24 this study uses data from the 21/October/2013 LFU dataset. This study presents results from the baseline visit and follow-up surveys capturing events and new comorbidities in 7080 participants who responded for each of the analysis variables and who did not report past physician diagnosis of diabetes or diabetes-associated medications (biguanides, thiazolidinediones and other drugs used to treat type 2 diabetes) at the baseline visit. Follow-up surveys in this group were queried for a new physician report of diabetes using the question “Have you been diagnosed with diabetes by a doctor?” and a new report of diabetes was considered an event (incident diabetes diagnosis) for this analysis.
Pulmonary function, FEV1, FVC and FEV1/FVC, were measured post-bronchodilator (albuterol) using the ndd EasyOne™ Spirometer and all tests were performed according to guidelines published by the American Thoracic Society.25 FEV1% predicted and FVC% predicted were used in models that did not include sex or ethnicity as these covariates are included in the sex and race-specific prediction equations.26 Quality of life was measured using the SGRQ which was obtained through participant self-report and overall and sub-scores were calculated using the appropriate standard protocol.27 The mMRC scale was obtained from the COPDGene questionnaire. Smoking exposure (current versus former), smoking intensity (pack years), medication use, high cholesterol and high blood pressure were obtained through participant self-report.
Respiratory exacerbations in COPD patients are referred to as acute exacerbation of COPD (AECOPD) and represents a worsening of symptoms of COPD that require alteration of medications or hospitalization. As AECOPD is specific to patients with COPD we will use the more general term respiratory exacerbations to refer to lung infections in participants without COPD as well as AECOPD in participants with COPD. Respiratory exacerbations were captured as part of the telephony interview using a series of questions capturing both changes in symptomatology and management change;
“Since we last talked have you had an episode of increased cough and phlegm or shortness of breath, which lasted 48 hours or more?
Did you receive a new antibiotic?
Did you receive any steroids either by pill or injection?
Did you go urgently to your doctor’s office?
Did you go to an emergency room?
Were you hospitalized?”
Respiratory exacerbation was recorded as Yes if the participant answered positively to receiving an antibiotic, steroid, urgent doctor’s visit, emergency room visit or hospitalization after reporting changes in symptomatology at any LFU contact for this analysis.
Statistical analyses were performed using the SAS system version 9.3 (Copyright (c) 2002–2008 by SAS Institute Inc., Cary, North Carolina). We analyzed categorical data using χ2 test for overall effects and by category using Kruskal-Wallis and continuous data using t tests (for normally distributed data) and transformed non-normally distributed data as appropriate and we considered a two-sided p value of α<0.05 to be statistically significant. Models were constructed using Cox Proportional Hazards models with time to event recorded as the time from the baseline visit until a physician diagnosis of diabetes, most recent contact via telephony or censoring due to all-cause mortality reported at the time of analysis. As a first step, a base model of incident diabetes diagnosis including known risk factors was fitted (sex, age, body mass index [BMI], smoking exposure, high cholesterol and high blood pressure) and these variables were retained in subsequent models. Additional pulmonary factors were added individually to this model to assess model improvement (FEV1%, FVC%, FEV1/FVC, SAE, chronic bronchitis, 6MWD, mMRC dyspnea score, corticosteroid use and GOLD category). Model fit was assessed comparing the reduction in −2 Log Likelihood with the addition of a pulmonary covariate distributed as chi-square with degrees of freedom contingent on the variable included.
Individuals who do not meet the GOLD criteria for COPD (FEV1/FVC<0.7) but who have impaired spirometry (FEV1<80%) are referred to as having preserved ratio with impaired spirometry (PRISm).28 All participants were placed into one of 3 categories; COPD (GOLD I–IV), PRISm or Control which includes all participants with neither COPD nor PRISm. FEV1% and FVC% are strongly collinear (Pearson Rho=0.79, p>=0.0001) and diabetes has been shown to have a stronger effect on FVC% than on FEV1%.5,11 We chose to exclude both FEV1% and FVC% from the stepwise selection that was used to assess the most parsimonious model of incident diabetes and instead undertook stepwise models including either FVC% or FEV1% separately.
Results
Incident diabetes was reported in 392 of 7080 participants during 21,519 person years of follow-up (mean follow-up time, 4.2 years). A total of 251 participants died and 15 participants’ died after a report of incident diabetes. These participants follow-up time was truncated at their report of diabetes diagnosis. At the baseline visit, participants’ who went on to develop diabetes were more likely to be current smokers (p=0.02), had higher BMI and increased high blood pressure and high cholesterol (all comparisons p<0.0001), but were not different by gender, pack years of smoking history or age [Table 1]. Participants classified as PRISm were observed to have higher incidence of diabetes diagnosis [Figure 1].
Table 1.
Characteristics of the COPDGene Cohort at Baseline Visit
| Incident Diabetes (n=392) | Controls (n=6688) | p | |
|---|---|---|---|
| Age (SD) | 60.2 ± 9.0 | 60.3 ± 9.0 | 0.9 |
| Pack Years Smoking (SD) | 46.0 ± 26.2 | 43.4 ± 23.8 | 0.05 |
| Current Smoking (%yes) | 0.54 | 0.47 | 0.02 |
| Gender (%m) | 0.49 | 0.50 | 0.8 |
| BMI | 32.0 ± 6.9 | 28.2 ± 5.9 | <0.0001 |
| High Blood Pressure (%yes) | 0.53 | 0.37 | <0.0002 |
| High Cholesterol (%yes) | 0.48 | 0.38 | <0.0003 |
Table 1 shows univariate comparisons of known risk factors for diabetes by incident diabetes status. BMI=body mass index; SD=standard deviation
Figure 1. Incident Diabetes by GOLD Classification of COPD.
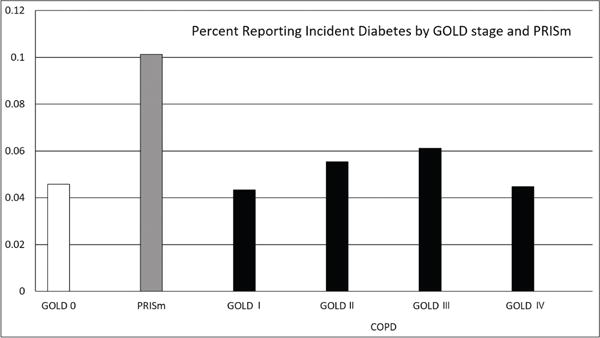
The Υ axis indicates the percentage of participants at each GOLD stage reporting incident diabetes. GOLD=Global initiative for chronic Obstructive Lung Disease; PRISm=preserved ratio with impaired spirometry
The base survival model of incident diabetes diagnosis included gender, age, smoking status, cumulative smoking exposure (pack years), high blood pressure, high cholesterol and BMI and this multivariable model agreed with the cross sectional results indicating that neither gender (p=0.5), cumulative smoking exposure (p=0.4) nor age (0.2) were significant predictors of diabetes [Table 2]. Subsequent models included these factors as potential confounders and precision variables. Significant predictors of incident diabetes diagnosis in the base model included current smoking status (hazard ratio [HR]=1.9, 95% confidence interval [CI] 1.5–2.4, p <0.0001), BMI (HR=1.09, 95% C.I. 1.07–1.10 for 1 unit difference), high blood pressure (HR=1.4, 95% CI 1.2–1.8, p=0.0009) and high cholesterol (HR=1.3, 95% CI 1.1–1.6, p=0.0081).
Table 2.
Base Survival Model of Incident Diabetes
| Hazard Ratio | 95% Confidence Limits | p | |
|---|---|---|---|
| Age (10 years) | 0.97 | 0.85 – 1.12 | 0.7 |
| ATS Pack Years (10 years) | 1.02 | 0.98 – 1.07 | 0.3 |
| Current Smoking (yes) | 1.91 | 1.52 – 2.39 | <0.0001 |
| Gender (m) | 1.07 | 0.87 – 1.31 | 0.5 |
| BMI (1 unit change) | 1.09 | 1.07 – 1.10 | <0.0001 |
| High Blood Pressure (yes) | 1.44 | 1.16 – 1.78 | 0.0009 |
| High Cholesterol (yes) | 1.33 | 1.08 – 1.64 | 0.008 |
Table 2 shows a Cox Proportional Hazards model including the known risk factors for incident diabetes collected by COPDGene. p-values <0.05 have been highlighted in yellow.
Both FEV1% and FVC% were significantly associated with incident diabetes when added to the base model after controlling for other known risk factors (p=0.0004 and <0.0001 respectively) though their ratio was not [Table 3]. Other pulmonary markers were also associated with incident diabetes; respiratory exacerbation during the follow-up period (HR 1.62, 95% CI 1.23–2.08), lower 6MWD (HR 0.93, 95% CI 0.91–0.96), corticosteroid use at baseline both oral (HR 1.80, 95% CI 1.03–3.15) and inhaled (HR 1.81, 95% CI 1.28–2.55), and PRISm pattern of lung function (HR 1.62, 95% CI 1.46–2.61). Neither chronic bronchitis (HR 1.10, 95% CI 0.85–1.42), nor COPD (HR 1.21, 95% CI 0.95–1.55) were associated with incident diabetes in these models.
Table 3.
Univariate Associations Between Proposed Pulmonary Markers and Incident Diabetes
| Estimate | Hazard Ratio | 95% CI | p | |
|---|---|---|---|---|
| FEV1% (10% change) | −0.008 | 0.92 | 0.88 – 0.97 | 0.0004 |
| FVC% (10% change) | −0.014 | 0.87 | 0.82 – 0.93 | <0.0001 |
| FEV1/FVC (0.1 unit change) | −0.32 | 0.97 | 0.90 – 1.04 | 0.4 |
| Serious Pulmonary Event | 0.48 | 1.62 | 1.23 – 2.08 | 0.0002 |
| Chronic Bronchitis | 0.09 | 1.10 | 0.85 – 1.42 | 0.48 |
| Distance Walked (100 feet) | −0.0007 | 0.93 | 0.91 – 0.96 | <0.0001 |
| mMRC Dyspnea Score (0 vs 1) | 0.26 | 1.30 | 0.95 – 1.79 | 0.0006 |
| mMRC Dyspnea Score (0 vs 2 | 0.43 | 1.53 | 1.11 – 2.11 | |
| mMRC Dyspnea Score (0 vs 3) | 0.48 | 1.61 | 1.20 – 2.15 | |
| mMRC Dyspnea Score (0 vs 4) | 0.67 | 1.96 | 1.40 – 2.75 | |
| Oral Corticosteroid Use | 0.059 | 1.80 | 1.03 – 3.15 | 0.04 |
| Inhaled Corticosteroid Use | 0.59 | 1.81 | 1.28 – 2.55 | 0.0008 |
| PRISm | 0.67 | 1.95 | 1.46 – 2.61 | <0.0001 |
| GOLD I–IV | 0.19 | 1.21 | 0.95 – 1.55 |
Table 3 shows 10 models that include all variables from Model 1 (Table 2) plus each pulmonary associated variable in a separate model. P reported is for the association between each marker and incident diabetes, p-values <0.05 have been highlighted in yellow. CI=confidence interval; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; mMRC=modified Medical Research Council; PRISm=preserved ratio with impaired spirometry; GOLD=Global initiative for chronic Obstructive Lung Disease
As this study represents only current and former smokers and over represents individuals with COPD compared to the general population, we explored respiratory exacerbation across GOLD stages and found that respiratory exacerbation occurred during the 4.2 years of follow-up at all levels of obstructive disease and also occurred in participants without COPD [Figure 2]. Participants with incident diabetes were at higher risk for respiratory exacerbation if they were PRISm (22% versus 10%, p<0.0001) or GOLD 0 (14% versus 5%, p=0.0006). Participants with COPD showed a significant increase in AECOPD only in GOLD III (45% versus 31%, p=0.04).
Figure 2. Serious Pulmonary Events by Diabetes Status.
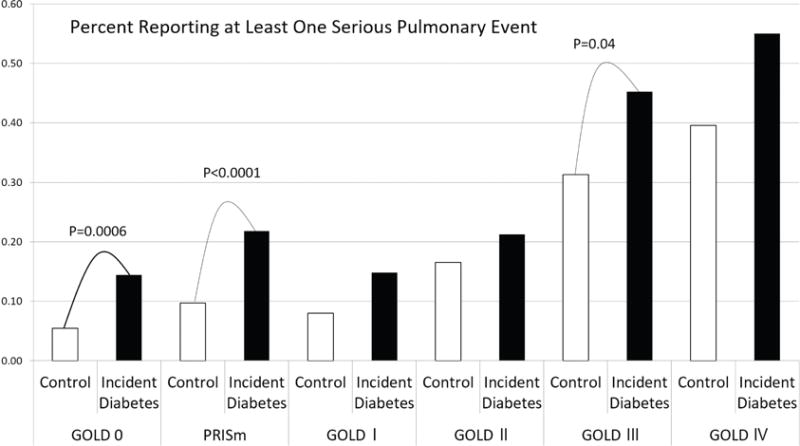
Figure 2 shows the percentage of participants reporting at least one serious pulmonary event (SPE)/acute exacerbation of COPD over the follow-up period (average 4.2 years) of the study.
The most parsimonious model of incident diabetes assessed using stepwise selection of all pulmonary variables is presented in Table 4. In addition to the base model of known risk factors, respiratory exacerbation (HR=1.5, 95% CI 1.2–2.0, p=0.0009), reduced 6MWD (−100 feet; HR=0.94, 95% CI 0.91–0.97, p<0.0001) and PRISm (PRISm compared to GOLD 0; HR=1.7, 95% CI 1.3–2.2, p=0.0003) were selected into the model. COPD (GOLD stage I–IV) was not significantly associated with incident diabetes (HR=0.95, 95% CI 0.7–1.2, p=0.7) nor were the other potential markers measured as part of the COPDGene study.
Table 4.
Final Survival Model of Incident Diabetes Including Pulmonary Measures
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| Age (10 years) | 0.97 | 0.8 – 1.1 | 0.7 |
| ATS Pack Years (10 years) | 1.0 | 0.96 – 1.05 | 0.8 |
| Current Smoking (yes) | 1.8 | 1.4 –2.3 | <0.0001 |
| Gender (m) | 1.2 | 0.9 – 1.5 | 0.07 |
| BMI (1 unit change) | 1.1 | 1.1 – 1.1 | <0.0001 |
| High Blood Pressure (yes) | 1.3 | 1.1 – 1.7 | 0.006 |
| High Cholesterol (yes) | 1.4 | 1.1 – 1.7 | 0.004 |
| Exacerbation (yes) | 1.5 | 1.2 – 2.0 | 0.0009 |
| 6 MWD (100 feet) | 0.94 | 0.91 – 0.97 | <0.0001 |
| PRISm (versus GOLD 0) | 1.7 | 1.3 – 2.2 | 0.0003 |
| GOLD I–IV(versus GOLD 0) | 0.95 | 0.7 – 1.2 | 0.7 |
Table 4 shows the results of stepwise selection including Model 1 and selecting from FEV1/FVC, SAE, chronic bronchitis, 6MWD, mMRC Dyspnea Score, corticosteroid use and GOLD classification. This selection was repeated adding FEV1 or FVC and the resulting models included identical variables. Neither FEV1 nor FVC were selected into this model, p-values <0.05 have been highlighted in yellow. ATS=American Thoracic Society; BMI=body mass index; 6MWD=6-minute walk distance; PRISm=preserved ratio with impaired spirometry; GOLD=Global initiative for chronic Obstructive Lung Disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; mMRC=modified Medical Research Council
Discussion
We observe, in this longitudinal study of current and former smokers, that markers of pulmonary health, beyond those measured using pulmonary function tests, are predictive of incident diabetes. Reduced 6MWD, respiratory exacerbation and PRISm were found to be associated with incident diabetes after controlling for known risk factors. While FEV1 and FVC have been shown to be associated with incident diabetes in other populations, these studies were not able to assess other measures of pulmonary health such as 6MWD, treatment for COPD with corticosteroids, dyspnea, respiratory exacerbation or specific types of lung disease such as PRISm or chronic bronchitis due to their study designs. We found, after accounting for the effects of these other important markers of pulmonary health, that neither FEV1, FVC, nor their ratio remained significant predictors of incident diabetes in this population. While the known risk markers for future diabetes (current smoking, BMI, hypertension and high cholesterol) all remained significant in the most parsimonious multivariable model, no direct measures of pulmonary function remained.
An indirect measure of pulmonary function, exercise tolerance measured by 6MWD, was a strong, independent predictor of new diabetes identification and may represent a better overall measure of integrative health status than measures based on spirometry. Respiratory exacerbation may represent a different aspect of pulmonary health: the lungs’ ability to defend against bacterial and viral infection which may be decreased with hyperglycemia and may be detected before diabetes diagnosis as respiratory exacerbation. PRISm pattern pulmonary function is associated with obesity and obesity is on the metabolic pathway leading to type 2 diabetes. This is likely reflected in the association between PRISm and incident diabetes even after controlling for BMI though it should be noted that including the PRISm comparison into the model with BMI does not alter the effect of BMI on incident diabetes. BMI is known to be a poor marker of total adiposity and body composition making its use difficult to interpret precisely as a predictor of incident diabetes.
The lack of association between COPD and incident diabetes may be partially explained by the strong effect of COPD on 6MWD. A potential effect of pre or undiagnosed diabetes on the lungs is through increased blood glucose and glucose non-enzymatically binding with collagen and elastin in lung parenchyma resulting in reduced lung elasticity and reduced 6MWD due to restrictions in airflow. Therefore, obstructive pattern lung disease may not be an important, independent predictor of future diabetes once the result of that obstruction, measured by reduced 6MWD, is accounted for. This observation would also help explain why dyspnea is a strong predictor of incident diabetes on its own but no longer predictive after controlling for PRISm pattern lung disease as dyspnea may measure a reaction to sub-optimal inflation in pre or undiagnosed diabetes. This would also hold true if increased glucose affected the innate immune system of the lung, increasing the risk of lung infection and subsequent functional degradation as hyperglycemia is known to be associated with other types of infection. Chronic bronchitis is not associated with incident diabetes but respiratory exacerbation is, after controlling for known risk factors, 6MWD and PRISm. This suggests that smokers without COPD may be at increased risk for viral or bacterial lung infection.
We observed a known pattern of factors predicting pre or undiagnosed diabetes in these adult current and former smokers where age, gender, current smoking status, BMI, high blood pressure and lipid abnormalities were predictive of diabetes diagnosis over our longitudinal follow-up and remained significant with the addition of novel pulmonary-associated markers.
We hypothesized that individuals with pre or unrecognized diabetes were at increased risk for lung infection and that in the context of current and former heavy smoking that risk could be recognized as exacerbations of COPD or respiratory exacerbation in participants who do not have COPD. Surfactant-D is a member of the innate immune system found in the lungs which has been shown to be reduced in obesity and type 2 diabetes.29 Surfactant-D has been shown to be reduced in smokers30 and serum surfactant-D has been shown to be associated with exacerbations of COPD potentially due to increased lung permeability.31 Recent studies have shown that high blood glucose also affects the innate immune system by reducing the activity of beta-defensins which act against viral infection but also act against bacterial and fungal infection and are observed playing a protective role in the lungs.32 Kiselar et al observed laboratory evidence that hyperglycemia increases the production of methylgloxal and glyoxal which irreversibly adduct with human β-defensin-2, reducing its antimicrobial function33 making infection more likely during hyperglycemia. Hamilton et al reported data from the Freemantle Heart Study showing that participants with type 2 diabetes were at double the risk for hospitalization due to any infection compared to controls without diabetes and that the most common reason for hospitalization was pneumonia (49.2%).34 A recent, large scale population based study using the Danish National Registry showed an association between antibiotic use and type 2 diabetes where antibiotic prescriptions were more likely to be filled in those with type 2 diabetes compared to controls (odds ratio [OR] 1.53, 95% CI 1.50–1.55) and this observation was found both 5 years prior to diagnosis and 5 years post diagnosis with diabetes.35 Our study found that serious pulmonary events (SPE)/AECOPD were significant predictors of incident diabetes lending weight to this biological pathway.
Potential weaknesses of this study include the use of baseline measurement of known predictors that could not be assessed during the LFU as this follow-up protocol included telephony or web contact only. Future follow-up visits in the COPDGene cohort will collect these predictors and assess their change over time. Diabetes diagnosis is self-reported in COPDGene and has not been evaluated using known biomarkers such as fasting glucose, oral glucose tolerance test or glycosylated hemoglobin (HbA1c). Baseline diabetes status was assessed using both self-report and recorded medication use and all participants reporting either were excluded from this incident study. Diabetes type was also not differentiated in COPDGene though the age structure of the study suggests that incident type 1 diabetes would be rare and all participants with existing diabetes were excluded from this incident study. Future studies of diabetes in COPDGene should assess diabetes status using gold standard biomarker measurement. The observed differences in SPE in participants without COPD (GOLD 0 and PRISm) may be due to a diabetes diagnosis that occurred because of health care contact resulting from the SPE itself. Evaluation of participant hospitalizations is not currently performed as a part of the COPDGene study.
The results of this study are of particular interest to clinicians as they identify new markers of pre or undiagnosed diabetes. SPEs, especially in current or former smokers without COPD (GOLD 0 and PRISm), may represent an early shift in a patient’s glycemic control that affects susceptibility to lung infection. We have shown that SPEs are independently associated with incident diabetes in participants with PRISm, those with no functional impairment (GOLD 0) and those with COPD [Figure 2]. This may represent a population of current and former smokers preferentially affected by reduced innate immunity in their lungs due to poor glycemic control.
Conclusions
SPEs, reduced exercise capacity as measured by 6MWD and the PRISm pattern lung function impairment are significant, previously unrecognized predictors of the development of diabetes among current and former heavy smokers. This cluster of indicators may aid clinicians in identifying and treating patients with pre- or undiagnosed diabetes. Future studies should ascertain whether treatment for hyperglycemia and insulin resistance can reduce the occurrence of lung infection in this population.
Acknowledgments
The authors would like to express their gratitude to the administrators and DCC of the COPDGene Study. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institutes or the National Institutes of Health.
Funding Support: The project described was supported by Award Number R01 HL089897, Award Number R01 HL089856 and Award Number K01 HL125858 from the National Heart, Lung, and Blood Institute. The COPDGene® project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
Abbreviations
- COPDGene
COPD Genetic Epidemiology
- LFU
longitudinal follow-up
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- RE
respiratory exacerbations
- 6MWD
6-minute walk distance
- BMI
body mass index
- PRISm
preserved ratio with impaired spirometry
- ASL
airway surface liquid
- mMRC
modified Medical Research Council
- AECOPD
acute exacerbation of COPD
- HR
hazard ratio
- CI
confidence interval
- OR
odds ratio
Footnotes
Declaration of Interest
Gregory L. Kinney, Oana L. Klein, Jennifer L. Black-Shinn, Emily S. Wan, Sharon M. Lutz, Elizabeth Regan, Russell P. Bowler, Kendra A. Young, Lindsey M. Duca, George R. Washko, James D. Crapo and John E. Hokanson have no conflicts to disclose. Emma H. Baker is joint holder of an MRC industry collaboration award with Astra Zeneca and principal investigator at St. George’s for CRN portfolio adopted studies for Sirocco (Astra Zeneca), Ideal (GlaxoSmithKline), Asthma survey (Boehringer Ingelheim) and Arietta (Roche). Barry Make has participated in research studies and/or served on medical advisory boards for AstraZeneca, Boehringer-Ingelheim, CSL Bering, GlaxoSmithKline, Forest, Novartis, Spiration, and Sunovion. In the past three years, Edwin K. Silverman received honoraria and consulting fees from Merck, grant support and consulting fees from GlaxoSmithKline and honoraria and travel support from Novartis.
References
- 1.Sandler M. Is the lung a ‘target organ’ in diabetes mellitus? Arch Int Med. 1990;150(7):1385–1388. doi: http://dx.doi.org/10.1001/archinte.1990.00390190051006. [PubMed] [Google Scholar]
- 2.Mirrakhimov A. Chronic obstructive pulmonary disease and glucose metabolism: a bitter sweet symphony. Cardiovasc Diabetol. 2012;11(1) doi: 10.1186/1475-2840-11-132. doi: http://dx.doi.org/10.1186/1475-2840-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler M, Bunn AE, Stewart RI. Cross-section study of pulmonary function in patients with insulin-dependent diabetes mellitus. Am Rev Respir Dis. 1987;135(1):223–229. doi: 10.1164/arrd.1987.135.1.223. [DOI] [PubMed] [Google Scholar]
- 4.Klein OL, Meltzer P, Carnethon M, Krishnan JA. Type II diabetes mellitus is associated with decreased measures of lung function in a clinical setting. Respir Med. 2011;105(7):1095–1098. doi: 10.1016/j.rmed.2011.03.010. doi: http://dx.doi.org/10.1016/j.rmed.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 5.van den Borst B, Gosker HR, Zeegers MP, Schols AMWJ. Pulmonary function in diabetes: A metaanalysis. Chest. 2010;138(2):393–406. doi: 10.1378/chest.09-2622. doi: http://dx.doi.org/10.1378/chest.09-2622. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Mannino DM. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care. 2004;27(12):2966–70. doi: 10.2337/diacare.27.12.2966. doi: http://dx.doi.org/10.2337/diacare.27.12.2966. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson KF, Lindgarde F. Poor physical fitness, and impaired early insulin response but late hyperinsulinaemia, as predictors of NIDDM in middle-aged Swedish men. Diabetologia. 1996;39(5):573–579. doi: 10.1007/BF00403304. doi: http://dx.doi.org/10.1007/BF00403304. [DOI] [PubMed] [Google Scholar]
- 8.Engstrom G, Janzon L. Risk of developing diabetes is inversely related to lung function: a population-based cohort study. Diabet Med. 2002;19(2):167–170. doi: 10.1046/j.1464-5491.2002.00652.x. doi: http://dx.doi.org/10.1046/j.1464-5491.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Frette C. NIDDM, impaired glucose tolerance, and pulmonary function in older adults. The Rancho Bernardo Study. Diabetes Care. 1996;19(12):1441–1444. doi: 10.2337/diacare.19.12.1441. doi: http://dx.doi.org/10.2337/diacare.19.12.1441. [DOI] [PubMed] [Google Scholar]
- 10.Bauer TA, Reusch JEB, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30(11):2880–2885. doi: 10.2337/dc07-0843. doi: http://dx.doi.org/10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 11.Kinney GL, Black-Shinn JL, Wan ES, et al. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care. 2014;37(2):389–395. doi: 10.2337/dc13-1435. doi: http://dx.doi.org/10.2337/dc13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalsi KK, Baker EH, Fraser O, et al. Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflügers Arch. 2009;457(5):1061–1070. doi: 10.1007/s00424-008-0576-4. doi: http://dx.doi.org/10.1007/s00424-008-0576-4. [DOI] [PubMed] [Google Scholar]
- 13.Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Phillips BJ. Hyperglycaemia and pulmonary infection. Proc Nutr Soc. 2006;65(3):227–35. doi: 10.1079/pns2006499. doi: http://dx.doi.org/10.1079/PNS2006499. [DOI] [PubMed] [Google Scholar]
- 14.Baker EH, Bell D. Blood glucose: of emerging importance in COPD exacerbations. Thorax. 2009;64(10):830–832. doi: 10.1136/thx.2009.118638. doi: http://dx.doi.org/10.1136/thx.2009.118638. [DOI] [PubMed] [Google Scholar]
- 15.Kornum JB, Thomsen RW, Riis A, Lervang H-H, Schǿnheyder HC, Srensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31(8):1541–1545. doi: 10.2337/dc08-0138. doi: http://dx.doi.org/10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker EH, Baines DL, Wood DM, Jones PW. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. doi: 10.1136/thx.2005.051029. doi: http://dx.doi.org/10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilyas R, Wallis R, Soilleux EJ. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216(1–2):126–131. doi: 10.1016/j.imbio.2010.06.002. doi: http://dx.doi.org/10.1016/j.imbio.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reading PC, Allison J, Crouch EC, Anders EM. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol. 1998;72(8):6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein OL, Smith W, Tipping M, Peng J, Williams MV. Reduced diffusion lung capacity in patients with type 2 diabetes mellitus predicts hospitalization for pneumonia. Diabetes Res Clin Pract. 2011;92(1):e12–15. doi: 10.1016/j.diabres.2010.12.012. doi: http://dx.doi.org/10.1016/j.diabres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Wannamethee SG, Shaper AG, Perry IJ. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001;24(9):1590–1595. doi: 10.2337/diacare.24.9.1590. doi: http://dx.doi.org/10.2337/diacare.24.9.1590. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: Effects of lifestyle changes. J Am Geriatr Soc. 2006;54(12):1909–1914. doi: 10.1111/j.1532-5415.2006.00974.x. doi: http://dx.doi.org/10.1111/j.1532-5415.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 22.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. doi: http://dx.doi.org/10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global initiative for chronic Obstructive Pulmonary Disease (GOLD) Global initiative for chronic Obstructive Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, updated November, 2006. GOLD website. http://wwwgoldcopdorg/uploads/users/files/GOLDReport2006_0122pdf. Published 2006. Accessed July 23, 2016.
- 24.Stewart JI, Moyle S, Crxiner GJ, et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010. doi: http://dx.doi.org/10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. doi: http://dx.doi.org/10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. doi: http://dx.doi.org/10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;14(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. doi: http://dx.doi.org/10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 28.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. doi: http://dx.doi.org/10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Real JM, Valdes S, Manco M, et al. Surfactant protein d, a marker of lung innate immunity, is positively associated with insulin sensitivity. Diabetes Care. 2010;33(4):847–53. doi: 10.2337/dc09-0542. doi: http://dx.doi.org/10.2337/dc09-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.More JM, et al. Smoking reduces surfactant protein D and phospholipids in patients with and without chronic obstructive pulmonary disease. BMC Pulm Med. 2010;10:53. doi: 10.1186/1471-2466-10-53. doi: http://dx.doi.org/10.1186/1471-2466-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakoori TA, et al. Serum surfactant protein D during acute exacerbations of chronic obstructive pulmonary disease. Dis Markers. 2009;27(6):287–294. doi: 10.3233/DMA-2009-0674. doi: http://dx.doi.org/10.1155/2009/759304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther. 2007;7(9):1449–61. doi: 10.1517/14712598.7.9.1449. doi: http://dx.doi.org/10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- 33.Kiselar JG, et al. Modification of beta-defensin-2 by dicarbonyls methylglyoxal and glyoxal inhibits antibacterial and chemotactic function in vitro. PLoS One. 2015;10(8):e0130533. doi: 10.1371/journal.pone.0130533. doi: http://dx.doi.org/10.1371/journal.pone.0130533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton EJ, Martin N, Makepeace A, Sillars BA, Davis WA, Davis TME. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the fremantle diabetes study. PLoS One. 2013;8(3):e60502. doi: 10.1371/journal.pone.0060502. doi: http://dx.doi.org/10.1371/journal.pone.0060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkelsen KH, Knop Fk, Frost M, Hallas J, Pottegård A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab. 2015:c20152696. doi: 10.1210/jc.2015-2696. doi: http://dx.doi.org/10.1210/jc.2015-2696. [DOI] [PMC free article] [PubMed]


