Abstract
Introduction
Dystrophinopathies are X-linked muscle degenerative disorders that result in progressive muscle weakness complicated by bone loss. This study's goal was to evaluate feasibility and tolerability of whole body low intensity vibration (WBLIV) and its potential effects on muscle and bone in patients with Duchenne or Becker muscular dystrophy.
Methods
This 12-month pilot study included 5 patients (age 5.9–21.7 years) who used a low-intensity Marodyne LivMD plate vibrating at 30–90 Hz for 10 minutes/day for the first 6 months. Timed motor function tests, myometry, and peripheral quantitative computed tomography were performed at baseline, 6, and 12 months.
Results
Motor function and lower extremity muscle strength remained either unchanged or improved during the intervention phase, followed by deterioration after WBLIV discontinuation. Indices of bone density and geometry remained stable in the tibia.
Discussion
WBLIV was well tolerated and appeared to have a stabilizing effect on lower extremity muscle function and bone measures.
Key terms: Duchenne muscular dystrophy, Becker muscular dystrophy, pQCT, vibration, myometry, timed function tests
INTRODUCTION
Dystrophinopathy (OMIM #310200) is a muscular dystrophy with a recessive X-linked inheritance mode, caused by loss-of-function mutations in the gene encoding dystrophin1. Clinical presentation ranges from more severe manifestations in Duchenne muscular dystrophy (DMD) to a milder allelic Becker muscular dystrophy (BMD)2,3. Dystrophin is critical for structural stability of myofibers in skeletal, diaphragm, and cardiac muscles4. In dystrophinopathy, its deficiency compromises functional integrity of muscle fibers and leads to progressive weakness manifesting as difficulty running and walking, rising from the floor, and taking stairs. Eventually loss of ambulation, respiratory insufficiency, and cardiomyopathy ensue and lead to death due to cardiorespiratory compromise. During the ambulatory stage of the disease, boys with dystrophinopathies walk less and at a much slower pace, are significantly less active than healthy peers, and are at risk of frequent falls.
A progressive decline in muscle function with age is accompanied by a decline in bone mineral density and bone quality, leading to increased bone fragility and long bone and vertebral fractures5–8. In many cases, a bone fracture becomes an inciting event that ends the ambulatory stage in these patients. Bone loss in dystrophinopathies is multifactorial due to reduced physical activity, immobility, chronic glucocorticoid treatment, hypogonadism, low vitamin D levels, inflammatory changes, and other metabolic abnormalities related to the disease process9,10.
The primary goal in treating boys with dystrophinopathies is to provide supportive measures through multidisciplinary care. The only accepted pharmacologic treatment thus far that slows disease progression is the use of glucocorticoids11. Unfortunately, chronic use of glucocorticoids is associated with significant multisystem side effects, including bone loss12. Physical and occupational therapy play a major role, including stretching exercises to prevent contractures and assistive devices to prolong ambulation and maintain independence13, but these interventions do little to help preserve bone health.
Mechanical vibration has gained considerable interest as a way to improve muscle function and serve as a bone-sparing strategy in children with disabling conditions, such as cerebral palsy14–16, osteogenesis imperfecta, or spinal dysraphism17. However, clinicians have been hesitant to use mechanical vibration in patients with dystrophinopathies due to concern about possible further damage to the diseased muscle18–22. A number of whole body vibration platforms are currently available, which vary in terms of vibration intensity (expressed in “g”, or g-force)23 and mode of vibration transmission (synchronous, meaning applied to both feet at the same time, or side-alternating). Low-intensity vibration devices deliver a force <1g (1g is earth’s gravitational field), while high-intensity vibration devices deliver acceleration intensity >1g (as high as 16.3g). In some cases this exceeds the safety Threshold Limit Values established by the International Standards Organization ISO-2631 depending on the vibration frequency and duration of exposure.23,24
In recent years, 3 pilot studies have been published that examine the safety of whole body vibration in patients with DMD using high-intensity side-alternating vibration platforms (Galileo training) for 4 weeks to 3 months, with varied target vibration frequencies of 15–24 Hz25–27. These studies concluded that the whole body vibration treatment was safe and tolerated by these patients, although its effect on bone and muscle was inconclusive.
None of the published studies examined the effects of a low-intensity vibrating platform in patients with DMD or BMD. Novotny et al. first investigated this approach in a preclinical study using the mdx mouse model for DMD28. The mdx mice were subjected to low magnitude whole body vibration at a frequency of 30 to 90 Hz, based on our previous work that identified 45 Hz as most effective at increasing the expression of osteogenic genes29. There was no evidence of loss of force generation or any other parameter of muscle contractility measured during 8 weeks of vibration training, which supported the notion that low magnitude whole body vibration is not deleterious to dystrophic muscles30 and has the potential to improve muscle contractility.
The primary aim of this pilot study was to test the feasibility and tolerability of low-intensity vibration as a therapeutic approach for patients with dystrophinopathies using a portable Marodyne LivMD plate that delivers WBLIV with an oscillating frequency of 30–90 Hz, at a maximum g-force of 0.4g, which is well within the ISO Threshold Limit Values.23,24 We hypothesized that this type of intervention would be feasible and well tolerated without adverse effects on the function of the lower extremity muscles with the greatest exposure to vibration. The secondary aim was to examine the effects of WBLIV on bone density, geometry, and strength of the tibia and radius. Since we know that transmissibility of vibrations is the highest to tissues closer to the source31, the hypothesis was that the intervention would stabilize bone measures of the tibia but not the radius during the interventional, vibration phase of the study.
METHODS
Participants
Participants were male patients (5.9 – 21.7 yo) with DMD or BMD confirmed through clinical examination and genetic testing, ambulatory (able to walk ≥75 meters unassisted), and not dependent on daytime ventilatory support. Exclusion criteria were treatment with human growth hormone or bisphosphonates, fracture within the last 4 weeks, or inability to stand for 10 minutes on flat feet. The participants were recruited from the Muscular Dystrophy Clinic at the University of Minnesota. Females who were manifesting carriers were not included due to minimal clinical manifestations. This protocol was approved by the Institutional Review Board at the University of Minnesota and was consistent with the requirements of the Declaration of Helsinki.
Study procedures
The study design included 2 baseline evaluations 2 weeks apart, followed by a 6-month intervention period and a subsequent 6-month follow-up period. At the initial visit each patient underwent a physical examination including anthropometry and Tanner staging of pubertal development based on testicular volume (by orchidometer) and pubic hair, with the higher of the 2 values being recorded. Baseline, 6-month, and 12-month evaluations included timed motor function tests, quantitative myometry, and peripheral quantitative computed tomography (pQCT) of the tibia and non-dominant radius.
Intervention
Each participant was given a portable Marodyne LivMD plate adjusted for lower weight (Marodyne Medical, Inc. Lakeland, FL) to be used at home while free standing for 10 consecutive minutes per day32, 7 days per week for 6 months. The Marodyne LivMD plate is designed to deliver low-magnitude (0.4g), high frequency vibration with an oscillating frequency of 30–90 Hz. Both participants and their caregivers were trained in the proper use of the vibratory plate. The first treatment was completed in the clinic after all baseline measurements were taken to demonstrate proper use of the plate. The second treatment took place at the subject’s home the next day. The study coordinator called the participants every 2 weeks to ensure compliance. The information about other interventions, including night-time splints, stretching, glucocorticoids, and novel drugs is provided in Table 1.
Table 1.
Patient characteristics at baseline
| Patients | 1 | 2 | 3 | 4* | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (years) | 5.9 | 7.5 | 12.4 | 9.8 | 21.7 | 16.4 |
| Tanner stage |
1 | 2 | 2 | 1 | 5 | 5 |
| Height Z-score |
−2.2 | −2.0 | −3.6 | −0.1 | 0.3 | −0.7 |
| BMI (kg/m2) |
14.8 | 18.2 | 21.0 | 14.7 | 29.2 | 23.7 |
| BMI Z-score |
−0.5 | 1.2 | 1.0 | −1.2 | 1.7 | 0.9 |
| Diagnosis | DMD | DMD | DMD | DMD | BMD | BMD |
| Gene mutation |
dup exon 49 OOF |
del 55 OOF |
exon 32 c.C4414T; p.Q1472X OOF |
dup exon 2 OOF |
del exon 3–7 IF |
del exon 3–7 IF |
| Interventions in addition to WBLIV |
DFZ, NTS, stretching |
DFZ, NTS, stretching |
DFZ, NTS, Ataluren stretching |
PRED, NTS, stretching |
stretching | stretching |
BMD, Becker muscular dystrophy; DFZ, deflazacort; DMD, Duchenne muscular dystrophy; del, deletion; dup, duplication; IF, in-frame; OOF, out-of-frame; WBLIV, whole body low intensity vibration; NTS, night time splint; PRED, prednisone;
dropped out of the study after 2 weeks due to worsening headaches.
Functional assessment
The 6-minute walk test (6MWT) was originally designed for adults, and for this reason it has been modified to fit the needs of children with muscular dystrophy33. This version has been shown to be a reliable and valid measure of ambulation as well as safe and easy to perform in patients with dystrophinopathies without severe cognitive or behavioral problems. It is a submaximal exercise test, and because most activities of daily living are performed at a submaximal level, 6MWT reflects function. Briefly, the 6MWT was assessed using a 25-meter taped line with 1 meter marked intervals and cones at each end of the tape to mark the ambulatory path. Arrows were placed at the end of the cones in a counter-clockwise direction to define the direction of ambulation. Continuous moderate verbal encouragement was allowed throughout the testing period. In addition, a “safety chaser” followed the child throughout the test to keep him on the correct path, to be in close proximity in case of a fall to help him to a standing position, or to tend to him quickly if an injury occurred. The following timed function tests (TFTs) were also performed: the time to run/walk test, measuring the time (in seconds) to walk or run, if possible, a distance of 10 meters; the time to climb 4 stairs test, measuring the time (in seconds) to ascend 4 standard stairs; and the supine-to-stand test, measuring the time (in seconds) for the child to assume a standing position from a supine starting position34,35.
Quantitative myometry
Strength testing included quantitative myometry using a handheld dynamometer (PowerTrack II commander, JTech Medical, Midvale, UT) to measure the maximum voluntary isometric force during muscle contraction of ankle plantar flexion, ankle dorsiflexion, hip flexion, knee flexion/extension, and elbow flexion/extension36. Values were recorded as Newtons of force. Participants were asked to perform each test bilaterally, and the average of the 2 forces produced was used in analyses.
Peripheral quantitative computed tomography (pQCT)
Measures of cortical and trabecular bone and estimated bone strength were obtained using pQCT (XCT-3000, Stratec Medizintechnik GmbH, Pforzheim, Germany). Images were taken at the distal 3% and 33% of the non-dominant radius with a scan speed of 20 mm/s. The distal 3% and 38% sites of the tibia were scanned at 25 mm/s. All scan sites had a section thickness of 2.4 mm and a voxel size of 0.4 mm. The reference line for both tibia and radius was placed at the proximal end of the distal growth plate using a scout view speed of 50 mm/s with a 1 mm step width. Image processing and calculation of bone parameters were completed using the manufacturer’s software (version 6.0). Phantom scanning was done daily for quality control.
Bone outcome measures37 at metaphyseal sites included trabecular volumetric bone mineral density (vBMD, mg/cm3), trabecular cross-sectional area (CSA, mm2), and total bone strength index (BSI, mg2/mm4) using contour mode 3 with a threshold of 169 mg/cm3 and peel mode 4 threshold 650 mg/cm3 with 10% concentric peel at the tibia and 15% at the radius. At diaphyseal sites, the measures included cortical vBMD, cortical CSA, cortical bone mineral content (BMC, mg/mm), cortical thickness (mm) using cort mode 2 with a threshold of 710 mg/cm3. Non-weighted polar section modulus (Zp, mm3) and strength strain index (SSI, mm3) were measured at 38% tibia site using cort mode 2 with a threshold of 480 mg/cm3.
Statistical analysis
For each outcome measure, the statistical analysis used a mixed linear model to account for correlation of multiple visits within person, with random effect person and fixed effect visit. In the analysis, the 2 baseline evaluations were included as separate visits; changes from baseline to 6 or 12 months were estimated and tested as contrasts in the visit fixed effect, specifically as the 6-month visit (or 12-month visit) minus the average of the 2 baseline visits. A measure's percent change was calculated from 0 to 6 months, from 6 to 12 months, and from 0 to 12 months. No adjustments were made for multiple testing because these results are preliminary and because the primary interest is in the broad pattern of results, not results for individual measures. All analyses were performed in JMP (v. 12 Pro, SAS Institute, Inc.).
RESULTS
Study participation
Six patients were enrolled in the study. One patient (Patient 4) with attention deficit hyperactivity disorder and prior history of headaches experienced worsening headaches during the first 2 weeks of intervention. While headaches were deemed unrelated to the study he found the exercise routine an excessive burden and dropped out of the study after 2 weeks. The remaining 5 patients completed the study and did not report any difficulty with using the platform, muscle pain, cramps, or any other adverse events. Table 1 shows characteristics of all 6 patients. None of the patients experienced fractures.
Six-minute walk test
Overall, 6MWT results showed no significant change in the distance walked over the course of this study. When analyzed for individual participants, results of 6MWT were consistent with natural history reported previously38 and predicted by baseline distance, age, or genetic mutation (out-of-frame vs in-frame). Patient 3 had an out-of-frame mutation and experienced a decline in the distance by 53 m over the course 12 months from 369 to 316 m, which is consistent with the expected decline because of his baseline distance. Another participant (Patient 2) declined by 28 m from 410.3 to 382 m. The results for the remaining 3 participants were essentially unchanged.
Timed function tests
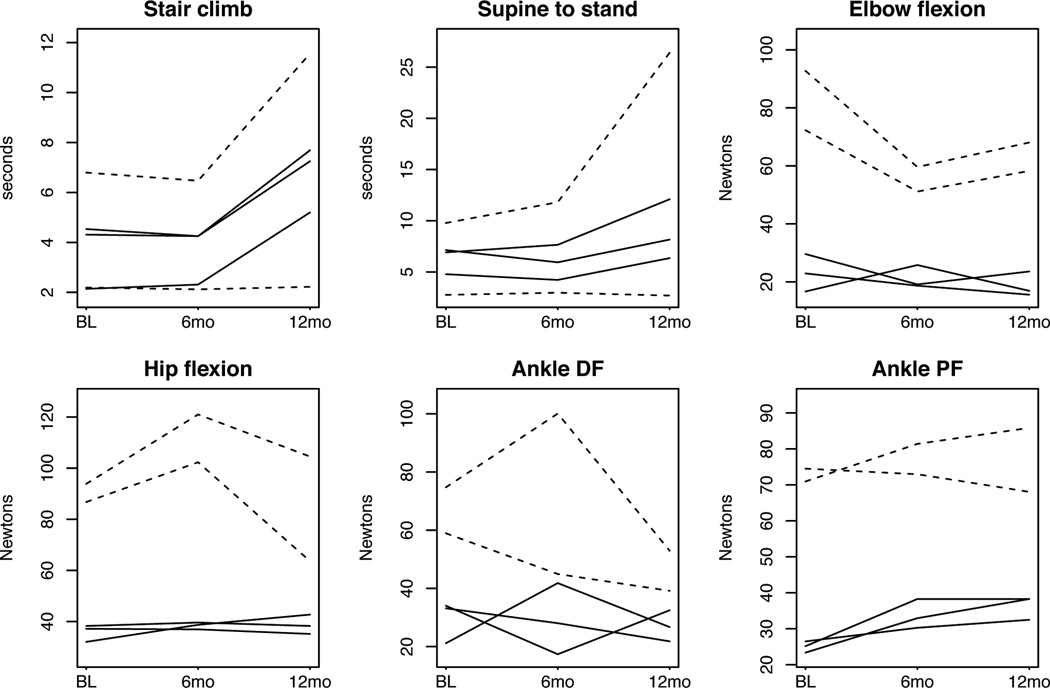
Motor function remained stable in all TFTs during the 6 months of intervention with WBLIV, followed by deterioration during the subsequent 6 months without WBLIV, which is consistent with the expected progressive deterioration in muscle function over time in dystrophinopathies. In the time to climb 4 stairs, the test showed 74.8% longer time at 12 months vs. 6 months. The time to stand test showed 70.9% longer time at 12 months vs. 6 months (Table 2 and Fig. 1). In both tests, 4 of 5 participants showed a significant (>10%) increase in time after WBLIV was discontinued at 6 months. Only 1 patient with BMD who had the best function in this group (Patient 6) remained unchanged. The changes from baseline to 12 months were also significant. Although performance in the 10-meter walk test did not change significantly during the intervention phase (0–6 months), the walking time was 23.6% longer at 12 months compared to baseline. In this test only 3 patients showed an increase in time >10%, and 2 remained unchanged.
Table 2.
Timed motor function tests before and after 6 months of vibration training as well as 6 months following discontinuation of vibration.
| Timepoints | Estimated change ± SE and % change | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 mo | 12 mo | 0–6 mo | % | 6–12 mo | % | 0–12 mo | % | |
| 6 min walk [m] |
412 | 395 | 396 | −16.8±12.0 | −4.1 | 0.8±13.9 | 0.2 | −16.0±12.0 | −3.9 |
| 10 m walk [s] |
5.6 | 6.0 | 7.0 | 0.4±0.5 | 6.4 | 1.0±0.6 | 16.2 | 1.3±0.5 | 23.6a |
| Stair climb [s] |
4.0 | 3.9 | 6.8 | −0.1±0.4 | −2.9 | 2.9±0.5c | 74.8c | 2.8±0.4c | 69.9c |
| Supine to stand [s] |
6.3 | 6.5 | 11.1 | 0.2±1.6 | 3.9 | 4.6±1.9a | 70.9a | 4.9±1.6b | 77.6b |
Data are shown as adjusted means, estimated change ± SE, and percent change;
P<0.05,
P<0.01,
P<0.001.
Figure 1. Plots of selected timed motor function tests and myometry for individual patients.
Dashed lines indicate patients with Becker muscular dystrophy, and solid lines indicate patients with Duchenne muscular dystrophy. DF, dorsiflexion; PF, plantar flexion.
Muscle strength testing
Muscle strength was measured by quantitative myometry. Elbow flexion strength decreased by 25.4% in the first 6 months compared to baseline and remained below baseline at 12 months (by 22.1%, Table 3) with changes more pronounced in patients with BMD (Fig. 1). There was no change in elbow extension strength (Table 3). Among lower extremity measures, ankle plantar flexion strength improved by 16% during the 6-month intervention period and remained above baseline at 12 months (19%). All participants showed an increase in ankle plantar flexion strength, ranging from 8 to 48% when compared to baseline. Ankle dorsiflexion strength remained stable during the intervention phase and decreased by 25.4% after discontinuation of vibration training, although this change was not statistically significant. There was also a trend toward increase in force in hip flexion during WBLIV (by 17.6%), followed by a 16% decrease toward baseline over the next 6 months (Table 3); these changes were also more apparent in patients with BMD. Overall, measures of upper extremity strength were either no different or worse over the 6-month intervention period, while measures of lower extremity strength were either stable or showed increased force after the 6-month intervention phase.
Table 3.
Myometry before and after 6 months of vibration training and 6 months following discontinuation of vibration.
| Timepoints | Estimated change ± SE and % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 mo | 12 mo | 0–6 mo | % | 6–12 mo | % | 0–12 mo | % | |
| Elbow flexion |
46.8 | 34.9 | 36.5 | −11.9±4.7a | −25.4a | 1.6±5.4 | 4.5 | −10.3±4.7a | −22.1a |
| Elbow extension |
33.2 | 36.3 | 32.5 | 3.2±2.8 | 9.5 | −3.8±3.2 | −10.4 | −0.6±2.8 | −1.9 |
| Hip flexion | 57.6 | 67.7 | 56.9 | 10.1±5.0 | 17.6 | −10.8±5.8 | −16.0 | −0.7±5.0 | −1.2 |
| Knee flexion |
50.0 | 53.2 | 56.3 | 3.2±4.0 | 6.4 | 3.0±4.6 | 5.7 | 6.3±4.0 | 12.5 |
| Knee extension |
60.0 | 59.6 | 63.2 | −0.4±5.5 | −0.7 | 3.6±6.4 | 6.0 | 3.2±5.5 | 5.2 |
| Ankle dorsiflexion |
44.4 | 46.4 | 34.6 | 2.0±5.7 | 4.5 | −11.8±6.5 | −25.4 | −9.8±5.7 | −22.0 |
| Ankle plantar flexion |
44.0 | 51.1 | 52.4 | 7.0±3.2a | 16.0a | 1.3±3.7 | 2.6 | 8.4±3.2a | 19.0a |
Data are shown as adjusted means, expressed in Newtons of force, estimated change ± SE, and percent change;
P<0.05.
pQCT bone measures
Measures of bone density and geometry of the radius are shown in Table 4. Downward trends were noted in most measures, with statistically significant decreases in the cortical cross-sectional area, cortical bone mineral content, cortical thickness, and bone strength index during the first 6 months followed by no significant changes from month 6 to 12.
Table 4.
pQCT bone measures for the radius before and after 6 months of vibration training and 6 months following discontinuation of vibration.
| Timepoints | Estimated change ± SE and % change | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 mo | 12 mo | 0–6 mo | % | 6–12 mo | % | 0–12 mo | % | |
| Trabecular vBMD |
224.1 | 211.0 | 203.0 | −13±12.9 | −5.8 | −7.6 ±13.6 | −3.6 | −20.6 ±21.1 | −9.2 |
| Trabecular CSA |
206.8 | 196.0 | 175.0 | −10.5±42.7 | −5.1 | −21.1±45.0 | −10.8 | −31.7±69.9 | −15.3 |
| Cortical vBMD |
1143.5 | 1146.0 | 1151.0 | 2.4±23.7 | 0.2 | 4.7±24.7 | 0.4 | 7.1±37.3 | 0.6 |
| Cortical CSA |
68.5 | 64.0 | 65.0 | −4.7±1.5a | −6.8a | 0.9±1.6 | 1.3 | −3.8±2.4 | −5.6 |
| Cortical BMC |
79.1 | 73.8 | 74.8 | −5.3±1.8a | −6.7a | 1.1±1.9 | 1.4 | −4.2±2.9 | −5.4 |
| Cortical thickness |
2.92 | 2.78 | 2.80 | −0.14±0.05a | −4.8a | 0.02±0.05 | 0.7 | −0.12±0.08 | −4.1 |
| BSI | 31.8 | 27.0 | 26.0 | −4.3±1.9a | −13.6a | −1.2±1.9 | −4.2 | −5.5±3.1 | −17.2 |
| Zp | 186.7 | 179.0 | 180.0 | −7.3±5.6 | −3.9 | 0.2±5.9 | 0.1 | −7.1±8.8 | −3.8 |
| SSI | 154.7 | 154.0 | 156.0 | −0.2±5.2 | −0.1 | 1.9±5.8 | 1.2 | 1.6±8.2 | 1.0 |
Data are shown as adjusted means for the radius length, estimated change ± SE, and percent change;
significant change, P<0.05. BMC, bone mineral content (mg/mm); BSI, bone strength index is expressed in mg2/mm4; cortical thickness is expressed in mm; CSA, cross-sectional area (mm2); vBMD, volumetric bone mineral density (mg/cm3); Zp, polar section modulus and SSI, strength strain index are expressed in mm3.
In the tibia, there was a trend toward an increase in trabecular cross-sectional area during the intervention phase (by 25% compared to baseline, Table 5). Other indices of bone geometry and strength did not change significantly.
Table 5.
pQCT bone measures for the tibia before and after 6 months of vibration training as well as 6 months following discontinuation of vibration.
| Timepoints | Estimated change ± SE and % change | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 mo | 12 mo | 0–6 mo | % | 6–12 mo | % | 0–12 mo | % | |
| Trabecular vBMD |
201.2 | 207.0 | 199.0 | 6.2±5.4 | 3.1 | −8.5±6.2 | −4.1 | −2.2±8.1 | −1.1 |
| Trabecular CSA |
544.0 | 680.0 | 627.0 | 136.1±66.7 | 25.0 | −52.8±75.8 | −7.8 | 83.3±99.9 | 15.3 |
| Cortical vBMD |
1111.6 | 1129.0 | 1134.0 | 17.0±25.1 | 1.5 | 5.7±28.3 | 0.5 | 22.7±35.4 | 2.0 |
| Cortical CSA |
178.8 | 175.0 | 171.0 | −3.6±7.2 | −2.0 | −4.0±8.1 | −2.3 | −7.6±10.2 | −4.2 |
| Cortical BMC |
202.2 | 198.5 | 195.8 | −3.7±7.4 | −1.8 | −2.7±8.4 | −1.4 | −6.4±10.5 | −3.2 |
| Cortical thickness |
3.99 | 4.01 | 3.97 | 0.03±0.1 | 0.8 | −0.04±0.11 | −1.0 | −0.02±0.14 | −0.5 |
| BSI | 60.8 | 60.0 | 58.0 | −1.0±1.5 | −1.6 | −1.6±1.7 | −2.7 | −2.6±2.2 | −4.3 |
| Zp | 1042.7 | 998.0 | 928.0 | −44.8±46.9 | −4.3 | −70.1±53.0 | −7.0 | −115.0±66.3 | −11.0 |
| SSI | 928.1 | 886.0 | 834.0 | −41.6±37.0 | −4.5 | −52.2±41.9 | −5.9 | −93.8±52.3 | −10.1 |
Data are shown as adjusted means for the tibia length, estimated change ± SE, and percent change. BMC, bone mineral content (mg/mm); BSI, bone strength index is expressed in mg2/mm4; cortical thickness is expressed in mm; CSA, cross-sectional area (mm2); vBMD, volumetric bone mineral density (mg/cm3); Zp, polar section modulus and SSI, strength strain index are expressed in mm3.
DISCUSSION
This pilot study, which examined 5 patients with dystrophinopathies, found that intervention with low-intensity vibration using a portable Marodyne LivMD plate was well tolerated by ambulatory patients with DMD or BMD, without adverse effects on the function of the lower extremity muscles with the greatest exposure to vibration. The patients did not experience increased fatigue, muscle soreness, cramps, falls, or fractures. The portability and the ease of use of a WBLIV plate made it suitable for use at home on a daily basis without a therapist.
Motor capacity measured by TFTs remained stable during the 6 months WBLIV intervention, followed by deterioration in the time to climb 4 stairs and the time to stand tests after discontinuation of vibration training, suggesting that WBLIV does not have deleterious effect and may have a stabilizing effect on muscle function. Lack of appreciable change in the 6MWT may be due to small sample size or variability in the natural history. Longitudinal studies have shown that boys less than age 7 years with DMD typically have an increase in 6MWT over a year, while boys older than 7 years may experience more of a plateau or decline in the performance of 6MWT.33,38 There is little to no information about the changes expected in 6MWT over 6 to 12 months in patients with BMD.
Measures of muscle strength by quantitative myometry in the lower extremities were either stable or showed a trend for improvement during the 6-month intervention phase, particularly in hip flexion and ankle plantar flexion strength. Although not statistically significant, an increase of 18% in hip flexion during the 6 months of the intervention is intriguing and suggests a positive trend. These results are corroborated by time to climb 4 stairs, which showed increase in time in 4 of 5 boys after discontinuation of intervention. Likewise, stabilization of ankle dorsiflexion after the intervention phase suggests a positive trend as compared to a 25% decrease in force after a 6-month period without intervention. In contrast, no trends for improvement were observed in muscle strength of the upper extremities. WBLIV appeared to have stabilizing effect on the pQCT measures of bone density and geometry of the tibia, and less so of the radius.
There is a suggestion of differential effect of WBLIV on upper vs. lower extremity muscle strength and bone indices with a trend for improvement in both muscle and bone measures in the lower extremities. This may be due to a positive effect of WBLIV on skeletal muscle tissue and bone in close proximity to the vibrating platform. This is consistent with other studies which showed that skeletal regions closest to the source of vibration have more robust responses39 compared to distal sites where transmission is diminished31. A decrease in biceps strength during the first 6 months of intervention was likely due to natural disease progression. In fact, the purpose of assessing the upper extremities in this study was to have an “internal” control for disease progression. This change is less likely to represent a deleterious effect of vibration because of its distance from the vibrating platform. However, this finding should be explored further in future studies.
In this study, we examined the effects of a low-intensity synchronous vibrating platform. Three previous studies performed on boys with DMD used high-intensity side-alternating vibration platform25–27. All 3 studies aimed primarily to examine safety and tolerability of vibration training, and all found whole body vibration to be feasible and safe, similar to our study and consistent with our preclinical observations28. Besides using a different platform, the previously published studies used a shorter duration of vibration training and a lower frequency of vibration than our study.
In the first study, from Sweden26, 6 ambulatory patients with DMD used whole body vibration (at 16–24 Hz) for up to 6 minutes 2–3 times weekly for 3 months. No changes in trabecular or cortical density of the tibia, muscle strength, motor function, or bone turnover markers were observed except for a trend toward an increase in a bone-specific alkaline phosphatase level and muscle density (by pQCT). The lack of a noticeable positive effect of vibration training on muscle and bone was thought to be due to small sample size, low vibration frequency, or short duration of training.
The second study, from Canada27, involved 4 ambulatory patients with DMD who participated in vibration therapy sessions (target frequency 20 Hz), each consisting of vibration 2 minutes on, 60 seconds off, and 2 minutes on, 3 times per week during a 4-week training period without any resultant major changes in functional mobility. The third study, from Germany25, included 14 ambulatory boys with DMD and 8 with spinal muscular atrophy. Galileo training (target frequency 15–18 Hz) was performed for 9 min twice a day, 5 days weekly for 8 weeks. Although no clear statistically significant effect of whole body vibration training was observed on muscle strength, function, or flexibility, some positive trends were noted. For example, patients with DMD had small improvements in 6MWT, stair climb test, muscle strength of the legs (knee myometry and ankle dorsiflexion), and ankle dorsiflexion angular degree of dorsiflexion. These trends suggested that perhaps a longer training period is needed to see a greater impact of vibration training. Overall, the effect of whole body vibration on bone and muscle using a side-alternating vibration platform for 4–18 min a day, 2–5 times weekly for 4 weeks to 3 months with target vibration frequencies of 15–24 Hz was deemed indeterminate.
In this study, we chose a 10-minute duration of low-intensity vibration training based on previous reports that low magnitude, high frequency whole body vibration treatment for 10 minutes per day resulted in increases in both bone and muscle mass in young women with low bone mineral density32. The vibration frequency delivered by Marodyne LivMD, 30–90 Hz, is similar to the frequency that was found to be safe, without deleterious effects on diseased muscle in an mdx mouse model of DMD28. Recent data show that, while high intensity training induces muscle damage in mdx mice, low intensity training reduces the level of protein carbonylation, a marker of oxidative damage, and rescues the mdx phenotype40. The duration of daily vibration therapy in our study was 6 months, which was the longest intervention so far and which could explain some of the promising positive effects on muscle function and strength. Our data also suggest that this intervention may need to continue long-term to maintain the improvements observed.
Other forms of physical training have also shown promise in delaying functional deterioration in patients with dystrophinopathies. For example, in a randomized controlled trial in 30 boys with DMD that involved assisted bicycle training for 24 weeks, the Motor Function Measure score remained stable in the intervention group, while it decreased significantly in the control group41. In the control group, 3 boys lost the ability to walk 10 m, and 2 boys lost the ability to rise from the floor. In the intervention group none of the boys lost the ability to walk 10 m, and only 1 boy lost the ability to rise from the floor during the training period. Range of motion remained relatively stable in the intervention group during the training period. Likewise, resistance training of upper and lower extremity muscles can increase muscle strength in muscular dystrophies, as shown in a study by Sveen et al.42 The latter study included 2 patients with BMD and 6 with limb-girdle muscular dystrophy. Elbow flexion and knee extension improved significantly after 6 months of low-intensity resistance training.
The limitations of this and other studies were small sample size, heterogeneous patient populations, and absence of matched controls. The analyses were necessarily exploratory and did not adjust for multiple comparisons. While this study demonstrated the feasibility and tolerability of this approach in patients with dystrophinopathies, any observable positive effects on muscle and bone, albeit encouraging, should be considered preliminary. They lay the groundwork for a larger, controlled trial to establish effective vibration parameters for a given type of platform, taking cost and portability into account. Since any biological effect of vibration is likely to decrease with increasing distance from the synchronous vibrating platform, a supplemental device that can be placed on the elbows16 or supplemental upper extremity exercise training may be needed.43 Vry et al.25 have shown that at least some of the clinically significant increase in joint flexibility could be attributed to mechanical stretching due to vibration. Thus, consideration should be given to supplementing traditional physiotherapy with whole body vibration as future rehabilitative approaches.
Acknowledgments
We would like to thank Dr. Jamie Marsh who performed assessments of muscle function and strength, Rebecca Hollister and Alise Thone for technical assistance with pQCT scanning, as well as patients and their families for their participation in the study. This study was supported by the Maslowski Foundation Grant to P.K., Muscular Dystrophy Association Research Grant Award #114071 to D.L., and the National Center for Advancing Translational Sciences of the NIH Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
LIST OF ABBREVIATIONS
- BMC
bone mineral content
- BMD
Becker muscular dystrophy
- BSI
bone strength index
- CSA
cross-sectional area
- DMD
Duchenne muscular dystrophy
- ISO
International Standards Organization
- 6MWT
6-minute walk test
- pQCT
peripheral quantitative computer tomography
- SSI
strength strain index
- TFTs
timed function tests
- vBMD
volumetric bone mineral density
- WBLIV
whole body low intensity vibration
- Zp
polar section modulus
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Wein N, Alfano L, Flanigan KM. Genetics and emerging treatments for Duchenne and Becker muscular dystrophy. Pediatric clinics of North America. 2015;62(3):723–742. doi: 10.1016/j.pcl.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Punnoose AR, Burke AE, Golub RM. JAMA patient page. Muscular dystrophy. Jama. 2011;306(22):2526. doi: 10.1001/jama.2011.1794. [DOI] [PubMed] [Google Scholar]
- 3.Flanigan KM. Duchenne and Becker muscular dystrophies. Neurologic clinics. 2014;32(3):671–688. doi: 10.1016/j.ncl.2014.05.002. viii. [DOI] [PubMed] [Google Scholar]
- 4.Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatric neurology. 2007;36(1):1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger M, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(11):2703–2711. doi: 10.1007/s00198-012-1911-3. [DOI] [PubMed] [Google Scholar]
- 6.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. Journal of pediatric orthopedics. 2000;20(1):71–74. [PubMed] [Google Scholar]
- 7.Houston C, Mathews K, Shibli-Rahhal A. Bone density and alendronate effects in Duchenne muscular dystrophy patients. Muscle & nerve. 2014;49(4):506–511. doi: 10.1002/mus.23948. [DOI] [PubMed] [Google Scholar]
- 8.McDonald DG, Kinali M, Gallagher AC, Mercuri E, Muntoni F, Roper H, et al. Fracture prevalence in Duchenne muscular dystrophy. Developmental medicine and child neurology. 2002;44(10):695–698. doi: 10.1017/s0012162201002778. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi ML, Mazzanti A, Galbiati E, Saraifoger S, Dubini A, Cornelio F, et al. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(9):761–767. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio LF, Jurkovic M, DeLullo J. Decreased bone density in ambulatory patients with duchenne muscular dystrophy. Journal of pediatric orthopedics. 2002;22(2):179–181. [PubMed] [Google Scholar]
- 11.Yilmaz O, Karaduman A, Topaloglu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2004;11(8):541–544. doi: 10.1111/j.1468-1331.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 12.King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68(19):1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 13.Ciafaloni E, Moxley RT. Treatment options for Duchenne muscular dystrophy. Current treatment options in neurology. 2008;10(2):86–93. doi: 10.1007/s11940-008-0010-4. [DOI] [PubMed] [Google Scholar]
- 14.Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: A randomized controlled pilot study. Journal of musculoskeletal & neuronal interactions. 2010;10(1):77–83. [PubMed] [Google Scholar]
- 15.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(3):360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 16.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(8):1759–1766. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 17.Semler O, Fricke O, Vezyroglou K, Stark C, Schoenau E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. Journal of musculoskeletal & neuronal interactions. 2007;7(1):77–81. [PubMed] [Google Scholar]
- 18.Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. Journal of muscle research and cell motility. 1993;14(4):446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- 19.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Necking LE, Lundstrom R, Dahlin LB, Lundborg G, Thornell LE, Friden J. Tissue displacement is a causative factor in vibration-induced muscle injury. J Hand Surg Br. 1996;21(6):753–757. doi: 10.1016/s0266-7681(96)80180-x. [DOI] [PubMed] [Google Scholar]
- 21.Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, et al. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol (1985) 2005;98(6):2376–2380. doi: 10.1152/japplphysiol.01135.2004. [DOI] [PubMed] [Google Scholar]
- 22.Necking LE, Lundstrom R, Lundborg G, Thornell LE, Friden J. Skeletal muscle changes after short term vibration. Scandinavian journal of plastic and reconstructive surgery and hand surgery / Nordisk plastikkirurgisk forening [and] Nordisk klubb for handkirurgi. 1996;30(2):99–103. doi: 10.3109/02844319609056390. [DOI] [PubMed] [Google Scholar]
- 23.Muir J, Kiel DP, Rubin CT. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. Journal of science and medicine in sport/Sports Medicine Australia. 2013;16(6):526–531. doi: 10.1016/j.jsams.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Standards EoHEtWBVGI, ISO-2631 OAfISOSt [Google Scholar]
- 25.Vry J, Schubert IJ, Semler O, Haug V, Schonau E, Kirschner J. Whole-body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2014;18(2):140–149. doi: 10.1016/j.ejpn.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Soderpalm AC, Kroksmark AK, Magnusson P, Karlsson J, Tulinius M, Swolin-Eide D. Whole body vibration therapy in patients with Duchenne muscular dystrophy--a prospective observational study. Journal of musculoskeletal & neuronal interactions. 2013;13(1):13–18. [PubMed] [Google Scholar]
- 27.Myers KA, Ramage B, Khan A, Mah JK. Vibration therapy tolerated in children with Duchenne muscular dystrophy: a pilot study. Pediatric neurology. 2014;51(1):126–129. doi: 10.1016/j.pediatrneurol.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Novotny SA, Mader TL, Greising AG, Lin AS, Guldberg RE, Warren GL, et al. Low intensity, high frequency vibration training to improve musculoskeletal function in a mouse model of Duchenne muscular dystrophy. PloS one. 2014;9(8):e104339. doi: 10.1371/journal.pone.0104339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novotny SA, Eckhoff MD, Eby BC, Call JA, Nuckley D, Lowe DA. Musculoskeletal response of dystrophic mice to short term, low intensity, high frequency vibration. Journal of musculoskeletal & neuronal interactions. 2013;13(4):418–429. [PMC free article] [PubMed] [Google Scholar]
- 30.McKeehen JN, Novotny SA, Baltgalvis KA, Call JA, Nuckley DJ, Lowe DA. Adaptations of mouse skeletal muscle to low-intensity vibration training. Medicine and science in sports and exercise. 2013;45(6):1051–1059. doi: 10.1249/MSS.0b013e3182811947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28(23):2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 32.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(9):1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 33.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle & nerve. 2010;41(4):500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 34.McDonald CM, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle & nerve. 2013;48(3):343–356. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzone E, Martinelli D, Berardinelli A, Messina S, D'Amico A, Vasco G, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscular disorders : NMD. 2010;20(11):712–716. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Hebert LJ, Maltais DB, Lepage C, Saulnier J, Crete M, Perron M. Isometric muscle strength in youth assessed by hand-held dynamometry: a feasibility, reliability, and validity study. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2011;23(3):289–299. doi: 10.1097/PEP.0b013e318227ccff. [DOI] [PubMed] [Google Scholar]
- 37.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2008;11(1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 38.McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, Gappmaier E, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle & nerve. 2013;48(3):357–368. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. Journal of biomechanics. 2007;40(6):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Hyzewicz J, Tanihata J, Kuraoka M, Ito N, Miyagoe-Suzuki Y, Takeda S. Low intensity training of mdx mice reduces carbonylation and increases expression levels of proteins involved in energy metabolism and muscle contraction. Free radical biology & medicine. 2015;82:122–136. doi: 10.1016/j.freeradbiomed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Jansen M, van Alfen N, Geurts AC, de Groot IJ. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial "no use is disuse". Neurorehabilitation and neural repair. 2013;27(9):816–827. doi: 10.1177/1545968313496326. [DOI] [PubMed] [Google Scholar]
- 42.Sveen ML, Andersen SP, Ingelsrud LH, Blichter S, Olsen NE, Jonck S, et al. Resistance training in patients with limb-girdle and becker muscular dystrophies. Muscle & nerve. 2013;47(2):163–169. doi: 10.1002/mus.23491. [DOI] [PubMed] [Google Scholar]
- 43.Alemdaroglu I, Karaduman A, Yilmaz OT, Topaloglu H. Different types of upper extremity exercise training in Duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle & nerve. 2015;51(5):697–705. doi: 10.1002/mus.24451. [DOI] [PubMed] [Google Scholar]