Abstract
Cue-induced drug-seeking in rodents progressively increases after withdrawal from operant self-administration of cocaine, heroin, methamphetamine, and alcohol, a phenomenon termed “incubation of drug craving.” Here, we used the opiate drug morphine and explored whether incubation of drug craving also occurs in a Pavlovian conditioned place preference (CPP) procedure in which rats learn to associate drug effects with a distinct environmental context. We also explored the role of amygdala ERK and CREB in this incubation. We found that the expression of morphine CPP progressively increases over the first 14 days after the last drug exposure in rats receiving 4 pairings of low-dose (1 or 3 mg/kg) but not high-dose (10 mg/kg) morphine with a distinct environment. The progressive increase in low-dose (3 mg/kg) morphine CPP was associated with increased ERK phosphorylation (a measure of ERK activity) and CREB (a downstream target of ERK) phosphorylation in central but not basolateral amygdala. Furthermore, inhibition of central but not basolateral amygdala ERK and CREB phosphorylation by U0126 decreased the enhanced (incubated) drug CPP after 14 days of withdrawal from morphine. Finally, stimulation of central amygdala ERK and CREB phosphorylation by NMDA enhanced drug CPP after 1 day of withdrawal from morphine, an effect reversed by U0126. These findings indicate that the rat’s response to environmental cues previously paired with morphine progressively increases or incubates over the first 14 days of withdrawal from low but not high morphine doses. Additionally, this “incubation of morphine craving” is mediated by acute activation of central amygdala ERK pathway.
Keywords: Amygdala, morphine, conditioned place preference, incubation, ERK, CREB, relapse
Introduction
Relapse to drug use in humans can occur after prolonged abstinence and is often precipitated by exposure to craving-provoking, drug-associated cues (O’Brien et al., 1992). Results from operant self-administration studies with cocaine (Neisewander et al., 2000; Grimm et al., 2001), heroin (Shalev et al., 2001), and methamphetamine (Shepard et al., 2004) demonstrate that the rat’s response to drug cues progressively increases over the first weeks after withdrawal from drugs. This “incubation” phenomenon was demonstrated in extinction (Lu et al., 2004; Lee et al., 2006), cue-induced reinstatement (Grimm et al., 2003), and acquisition of new response (Di Ciano and Everitt, 2004) procedures. These findings suggest that craving, a motivational state elicited by exposure to drug cues that often precedes and accompanies drug-seeking, incubates over time (Lu et al., 2004).
Here, we assessed whether “incubation of reward craving” is also manifested in a Pavlovian conditioned place preference (CPP) procedure where during the training phase one context is paired with drug injections, while another context is paired with vehicle injections. During subsequent drug-free CPP tests, rats choose between the drug- and vehicle-paired contexts. Increased preference for the drug context serves as measures of drug’s rewarding effects (Van der Kooy, 1987) and incentive motivational effects of drug cues (Mueller and Stewart, 2000).
To date, there is no evidence that the rat’s response to drug cues in the CPP procedure increases over time. Several investigators reported that CPP is maintained at similar magnitude for 2 to 12 weeks after withdrawal from morphine (Mucha and Iversen, 1984; Vezina et al., 1987; Mueller et al., 2002) or for 6 weeks after withdrawal from cocaine (Mueller and Stewart, 2000). In other studies, CPP became weaker over the first month after withdrawal from morphine or cocaine (Tzschentke and Schmidt, 1995; Lu et al., 2000b; Wang et al., 2000; Lu et al., 2001a). In these studies, the experimental procedures typically resulted in high drug CPP immediately after training. Thus in studies where CPP was maintained over time, a ceiling effect may have prevented the detection of further increases in drug CPP after prolonged withdrawal.
Here, we describe experimental parameters that led to progressive increases in drug CPP after withdrawal from morphine. This was achieved by assessing drug CPP 7 or 14 days after withdrawal from low (1–3 mg/kg) or high (10 mg/kg) morphine doses, which induce weak or strong CPP, respectively, after 1 withdrawal day. We found progressive increases in low- but not high-dose morphine CPP. Subsequently, we studied the role of amygdala extracellular signal-regulated kinase (ERK) in this incubation. The ERK pathway has been implicated in several behavioral effects of abused drugs (Lu et al., 2006; Girault et al., 2007). Previously, we found that central amygdala ERK activity mediates the incubation of cocaine craving in the operant self-administration procedure (Lu et al., 2005). We also assessed whether time-dependent increases in morphine are associated with activation of the transcription factor cAMP-response-element-binding protein (CREB). We studied whether CREB activity correlates with incubation of morphine craving because CREB is a downstream cellular target of ERK (Thomas and Huganir, 2004) that was implicated in morphine reward and dependence (Blendy and Maldonado, 1998; Carlezon et al., 2005).
Material and Methods
Subjects
Male Sprague-Dawley rats (weighing 220–240 g upon arrival) were housed in groups of five in a temperature-(23±2 degrees C) and humidity-(50±5%) controlled animal facility. The rats were maintained on a 12 h light/dark cycle with free access to food and water. The experimental procedures were performed in accordance with the National Institutes of Health guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Local Animal Care and Use Committee. A total of 435 rats were used in the experiments described below. This number includes 13 (8 in Expt. 2 and 5 in Expt. 5) naïve rats that did not participate in the behavioral experiments; the brains of these naïve rats were taken for assessing basal levels of ERK and CREB, which were used as a reference point for the graphical presentation of the molecular data (see figures).
Surgery
Rats (weighing 300–320 g when surgery began) were anesthetized with sodium pentobarbital anesthesia (60 mg/kg, i.p.). Guide cannulae (23 gauge; Plastics One, Roanoke, VA) were bilaterally implanted 1 mm above the basolateral and central amygdala. The respective coordinates (Paxinos and Watson, 2005) were: AP, −2.9 mm; ML, ± 5.0 mm; DV, −8.5 mm, and AP, −2.9 mm; ML, ± 4.2 mm; DV, −7.8 mm (Lu et al., 2005; Lu et al., 2007; Wang et al., 2008). The cannulae were anchored to the skull with stainless steel screws and dental cement. A stainless steel blocker was inserted into each cannula to keep them patent and prevent infection. The rats were allowed to recover from surgery for 5–7 days.
Intracranial injections
U0126 (100 ng/0.5 μl) or NMDA (250 ng/0.5 μl) was infused bilaterally into central or basolateral amygdala with Hamilton syringes connected to 30-gauge injectors (Plastics One, Roanoke, VA). NMDA (RBI, Natick, MA) was dissolved in sterile saline, and the pH of the drug solution was adjusted to 7.4 by adding NaOH. U0126 (a MEK inhibitor; Calbiochem, La Jolla, CA) was dissolved in 20% DMSO. A total volume of 0.5 μl was infused bilaterally over 1 min and the injector was kept in place for an additional 1 min to allow for diffusion. The doses of U0126 and NMDA were based on our previous study (Lu et al., 2005). We could not verify cannula placements by histological techniques because the brains of the rats were used for the Western blot assays. However, our success rates in previous studies were approximately 85–90% (Lu et al., 2007) and perhaps more importantly, the anatomical selectivity of our drug injections is verified by the demonstration of selective changes in phosphorylated ERK and CREB (pERK and pCREB, respectively) in the central but not basolateral injections and vice versa (see Figures).
Tissue sample preparation
The procedure is based on that used in our previous study (Lu et al., 2005). Most rats were decapitated without anesthesia 30 min after the end of the 15-min CPP test. Other rats were decapitated at different time points after the end of 7.5- or 15-min CPP tests (see Exp. 2 and Fig. 2). Rats in the different control conditions were decapitated just prior to the CPP tests. After decapitation the brains of all rats were rapidly extracted and frozen in −60 °C N-hexane. The brains were then transferred to a −80 °C freezer. We used a freezing cryostat (−20 °C) to make1-mm- thick coronal sections located approximately −2.5–3.0 mm from bregma. Bilateral tissue punches (16 gauge) of the central and basolateral amygdale were then taken. Tissue punches were homogenized (10–15 sec×3, 5 sec interval) with electrical disperser (Wiggenhauser, Sdn Bhd) after 30 min in RIPA lysis buffer (Beyotime Biotechnology, China; 20 mM Tris PH7.5, 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1% Na3VO4, 0.5 μg/ml leupeptin, 1 mM phenylmethanesulfonyl fluoride (PMSF)). Then, the tissue homogenates were subjected to 10,000 g centrifugation at 4°C for 20 min. The above procedures were carried out at 0 to 4 degrees C. The protein concentrations of all samples were determined using the bichinconinic acid assay (Beyotime Biotechnology, China). Samples were further diluted in RIPA lysis buffer to equalize the protein concentrations.
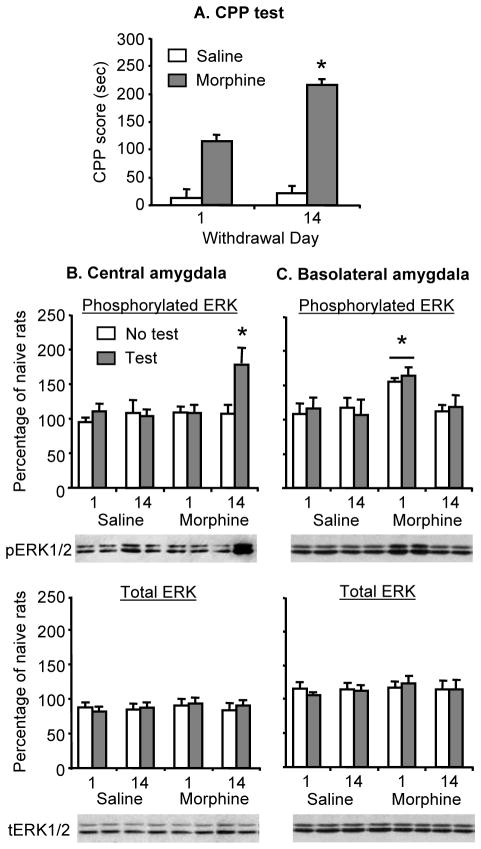
Figure 2. The progressive increases in morphine CPP is associated with increases in ERK phosphorylation in central amygdala.
(A) Progressive increases in morphine (3 mg/kg) CPP after withdrawal from the drug. Data are the mean ± SEM of preference score in sec during the CPP preference tests (4 groups). * Different from withdrawal day 1, p < 0.05. (B–C) Phosphorylated and total ERK in the central and basolateral amygdala; data are presented as a percentage (mean ± SEM) of phosphorylated and total ERK of naïve control rats (n=8). In the central amygdala, the morphine CPP test increased phosphorylated ERK after 14 days of withdrawal. In the basolateral amygdala, prior exposure to morphine increased phosphorylated ERK after 1 day of withdrawal; this effect was independent of the CPP test. * Different from the other experimental groups, p < 0.05 (n=7–8 per group). No group differences were observed for total ERK.
Western blot assays
The assay’s procedures were based on those used in our previous studies (Lu et al., 2003; Lu et al., 2005). Four x loading buffer (16% glycerol, 20%-mercaptoethanol, 2% SDS and 0.05% bromophenol blue) was added to each sample (3:1, sample: loading buffer) before boiling for 3 min. Samples were cooled and subjected to SDS-polyacrylamide gel electrophoresis (10% acrylamide/0.27% N, N′-methylenebisacryalamide resolving gel) for about 30 min at 80 V in stacking gel and about 1 h at 120V in resolving gel. For each electrophoresis, increasing amounts of protein pooled from all samples were electrophoresed to produce a standard curve. Proteins were transferred electrophoretically to Immobilon-P transfer membranes (Millipore Corp; Bedford, MA) at 0.25 A for 3 h. Membranes were washed with TBST (Tris-Buffered Saline plus 0.05% Tween-20, pH 7.4), and then dipped in blocking buffer (5% skimmed dry milk in TBST) overnight at 4°C. The next day, the membranes were incubated for 1 h at room temperature on orbital shaker with anti-phospho-ERK antibody (1:500; R&D System), anti-ERK antibody (1:500; R&D System), anti-phospho-CREB antibody (1:500; Upstate Biotechnology, Lake Placid, NY) or anti-CREB antibody (1:500; Upstate Biotechnology) in TBST plus 5% BSA and 0.05% sodium azide. After four 6-min washes in TBST buffer, the blots were incubated for 45 min at room temperature on shaker with horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit IgG; Santa Cruz PI-1000; Vector Labs) diluted 1:5000 in blocking buffer. The blots were then washed four 6-minutes in TBST and then incubated with a layer of mix of Super Signal Enhanced chemiluminescence (ECL) substrate (Detection Reagents 1 and 2 at a 1:1 ratio; Pierce Biotechnology) for 1 min at room temperature. Excess mix was dripped off before blots were wrapped with a clean piece of saran wrap (no bubbles between blot and wrap), and then exposed against X-ray film (Eastman Kodak Company) for 5–60 sec. Band intensities for ERK1 and ERK2, and pERK1 and pERK2 were quantified by two observers (blind to experimental groups) using Quantity One software (Version 4.4.0) from Biorad Corporation (Hercules, CA). Band intensities from each test sample were compared to the band intensities from the standard curves. The amount of protein of interest in the samples was interpolated from standard curves.
Conditioned place preference (CPP)
The CPP apparatus consisted of five identical three-chamber PVC boxes. Two large side chambers (27.9 cm long×21.0 cm wide×20.9 cm high) were separated by a smaller chamber (12.1 cm long ×21.0 cm wide ×20.9 cm high with smooth PVC floor). The two larger chambers differ in their floor texture (bar or grid) and provided the distinct contexts that were paired with morphine or saline injections. The three chambers were separated by manual guillotine doors and were equipped with infrared photobeams connected to a computer that recorded the rat’s location in the chambers.
In order to determine baseline place preference, the rats were initially placed in the middle chamber with the doors removed for 15 min (pre-conditioning test). Conditioning was performed using an unbiased, counterbalanced protocol, as described in our previous work (Wang et al., 2008). Twenty-one of the 435 rats that were tested for initial CPP preference showed strong unconditioned side preference (>540 s in any chamber) and therefore were excluded.
On the subsequent conditioning days, each rat was trained for 8 consecutive days with alternate injections of morphine (0, 1, 3 and 10 mg/kg, s.c.) and saline (1 ml/kg, s.c.). After each injection, the rats were confined to the morphine or saline conditioned chamber for 45 min before returning to their home cages. Subsequently, the expression of morphine CPP was assessed 1, 7 or 14 days after the last training session. The experimental conditions during the post-conditioning tests were the same as those described for the pre-conditioning test. The CPP score was defined as the time (sec) spent in the morphine-paired chamber minus the time spent in the saline-paired chamber.
Exp. 1: Effect of morphine training dose and withdrawal day on morphine CPP
Between-subjects assessment
We first assessed morphine CPP in 12 different groups of rats (n=9–10 per group) that were injected with saline or morphine (1, 3 or 10 mg/kg, sc) and tested for CPP 1, 7 or 14 days after the last training session. The experimental design included the between-subjects factors of Morphine Dose (0, 1, 3, and 10 mg/kg) and Withdrawal Day (1, 7, 14); the dependent measure was CPP score in sec.
Within-subjects assessment
We next assessed morphine CPP in 4 different groups of rats (n=9–10 per group) that were injected with saline or morphine (1, 3 or 10 mg/kg, sc) and were repeatedly tested for CPP 1, 7, and 14 days after the last training session. The experimental design included the between-subjects factor of Morphine Dose and the within-subjects factor of Withdrawal Day; the dependent measure was CPP score.
Exp. 2: Effect of morphine CPP testing after 1 or 14 withdrawal days on amygdala ERK and CREB
We initially assessed the effect of CPP testing on pERK and pCREB in central and basolateral amygdala in rats that underwent CPP training with 3 mg/kg of morphine or saline. For this purpose, we used 8 groups of rats (n=7–8 per group) in an experimental design that included the between-subjects factors of Morphine Dose (0, 3 mg/kg) and Withdrawal Day (1, 14), and CPP test (no, yes). The dependent measures were the CPP score (assessed in the 4 groups that underwent CPP testing), and pERK and pCREB levels in central and basolateral amygdala (assessed in all 8 groups). Thirty min after the end of the 15-min CPP test, all rats were decapitated and their brains were extracted for subsequent determination of pERK and pCREB in central and basolateral amygdala by western blotting.
We subsequently assessed in 8 groups of rats (n=8 per group) the time course of pERK and pCREB activation during and after the morphine CPP tests; these tests were performed on withdrawal day 14. The brains of rats from 2 groups were taken within 5 min or 30 min after a shorter 7.5-min CPP test, while the brains of rats from 5 other groups were taken within 5 min, or 15, 30, 60 or 180 min after a regular 15-min CPP test. The brains of rats from the 8th (control) group were taken immediately prior to the CPP test. The rats from all groups were trained for morphine (3 mg/kg) CPP as described above.
Exp. 3: Effect of inhibition of amygdala ERK and CREB on morphine CPP after 14 withdrawal days
To determine the functional role of amygdala ERK (and potentially CREB, see Discussion) in the progressive increases in morphine CPP, we inhibited central or basolateral amygdala ERK and CREB activity with the selective MEK antagonist U0126 (Davies et al., 2000) after 14 days of withdrawal from morphine. For this purpose, we used 4 groups of rats (n=7–8 per group) in an experimental design that included the between-subjects factors of U0126 Dose (0, 100 ng/side) and Amygdala Nucleus (central, basolateral); the dependent measures were the CPP score, and pERK and pCREB levels in central and basolateral amygdala. The morphine training dose was 3 mg/kg, and U0126 or its vehicle was injected 30 min before CPP testing. Thirty min after the end of the 15-min post-conditioning test, all rats were decapitated and their brains were extracted for subsequent determination of pERK and pCREB.
Exp. 4: Effect of activation of central amygdala ERK and CREB on morphine CPP after 1 withdrawal day
To further determine the role of central amygdala ERK and CREB in the progressive increase in morphine CPP after withdrawal, we first injected NMDA (which activates ERK and CREB) into the central amygdala on withdrawal day 1. We tested whether the induction of pERK and pCREB in the central amygdala would enhance morphine CPP during early withdrawal, when the conditioned response is weak. Because there are no selective agonists of the ERK pathway, we used NMDA to induce ERK and CREB phosphorylation (Fiore et al., 1993). For this purpose, we used 2 groups of rats (n=8 per group) in an experimental design that included the between-subjects factor of NMDA Dose (0, 250 ng/side); the dependent measures were the CPP score, and pERK and pCREB levels in central and basolateral amygdala. The morphine training dose was 3 mg/kg. NMDA or its vehicle was injected 5 min before CPP testing.
NMDA activates several intracellular signaling pathways in addition to the ERK pathway (Thomas and Huganir, 2004). Therefore, we subsequently determined whether the increase in morphine CPP on withdrawal day 1 by central amygdala injections of NMDA is dependent on ERK activation (i.e., reversed by U0126). For this purpose, we used 4 groups of rats (n=7–8 per group) in an experimental design that included the between-subjects factors of NMDA Dose (0, 250 ng/side) and U0126 Dose (0, 100 ng/side); the dependent measures were the CPP score and pERK and pCREB values in the central amygdala. U0126 or its vehicle was injected 30 min before CPP testing; NMDA or its vehicle was injected 5 min before testing.
Exp. 5: Effect of morphine CPP training on ERK and CREB activity in basolatoral amygdala
A surprising finding in Exp. 2 was that 4 injections of a low morphine dose (3 mg/kg) caused increased pERK and pCREB levels in the basolateral amygdala 1 day after the last drug injection; this effect occurred independent of CPP testing (Fig. 2–3). Therefore, In Exp. 5 we further explored this unexpected finding by determining whether this effect of morphine exposure is dependent on CPP training. For this purpose, we used 4 groups of rats (n=5 per group) in an experimental design that included the between-subjects factor of CPP training (no, yes) and Morphine Dose (0, 3 mg/kg); the dependent measures were pERK and pCREB levels in BLA. The rats that did not undergo CPP training were injected with morphine or vehicle in their home cage at the same time of the day as the rats that underwent the 8-day CPP training. One day after the last morphine injection, all rats were decapitated and their brains were extracted for subsequent determination of pERK and pCREB in the basolateral amygdala.
Figure 3. The progressive increase in morphine CPP is associated with increases in CREB phosphorylation in central amygdala.
(A–B) Phosphorylated and total CREB in the central and basolateral amygdala; data are presented as a percentage (mean ± SEM) of phosphorylated and total CREB of naïve control rats (n=8). In the central amygdala, the morphine CPP test increased phosphorylated CREB after 14 days of withdrawal. In the basolateral amygdala, prior exposure to morphine increased phosphorylated CREB after 1 day of withdrawal; this effect was independent of the CPP test. * Different from the other experimental groups, p < 0.05 (n=7–8 per group). No group differences were observed for total CREB. The morphine/saline CPP test data that correspond to the molecular data in Fig. 3 are presented in Fig. 2A.
Data analysis
Data are expressed as mean±SEM. Data were analyzed with ANOVAs using the appropriate between- and within-subjects factors for the different experiments (see Results). Significant main effects and interactions (p<0.05) from the factorial ANOVAs were followed by simple ANOVAs and Tukey’s post-hoc tests. The experimental manipulations had similar effects on ERK1 and ERK2 phosphorylation. Thus, these values were added and the analyses were performed on the combined values of phosphorylated ERK (Kelleher et al., 2004; Lu et al., 2005). Because our multifactorial ANOVAs yield multiple main effects and interaction effects, we only report significant effects that are critical for the interpretation of the data in the Results section. Additionally, for clarity purposes, post-hoc analyses are indicated in asterisks in the figures, but are not described in the Results section.
Results
Effect of morphine training dose and withdrawal day on morphine CPP
Morphine CPP progressively increased after withdrawal from the drug in rats previously exposed to low (1 or 3 mg/kg) but not high (10 mg/kg) morphine doses (Fig. 1). This effect was observed when different groups were tested at the different withdrawal days or when rats were repeatedly tested 1, 7 or 14 days after withdrawal from morphine.
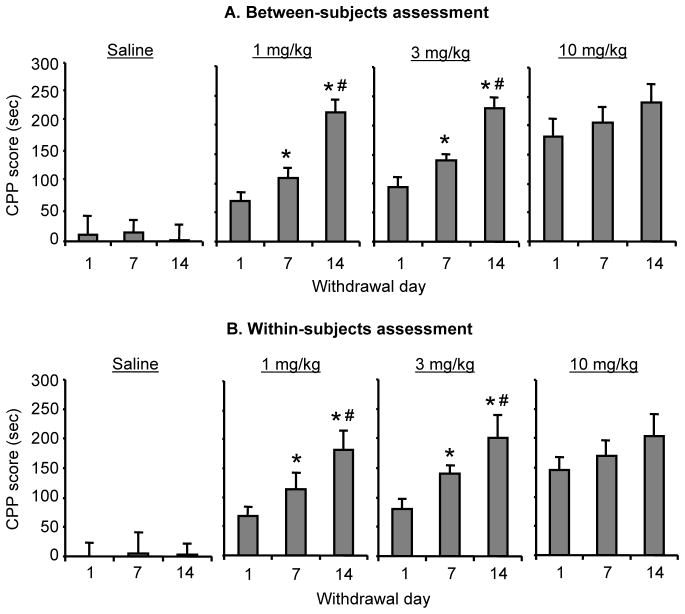
Figure 1. Time-dependent increases in drug CPP after withdrawal from low but not high doses of morphine.
Data are the mean ± SEM of preference score in sec (time in the morphine-paired chamber minus time in the saline-paired chamber) during the CPP preference tests performed 1, 7, or 14 days after withdrawal from morphine. (A) Between-subjects assessment using 12 groups of rats (n=9–10 per group), each group was tested at a single withdrawal day. (B) Within-subjects assessment using 4 groups of rats (n=9–10 per group) that were repeatedly tested after 1, 7, and 14 days of withdrawal from morphine. Different from withdrawal days 1 (*) and 7 (#) within each morphine training dose, p < 0.05.
Between-subjects assessment
We assessed morphine CPP in 12 different groups of rats that were injected with saline or morphine (1, 3 or 10 mg/kg) and tested for CPP 1, 7 or 14 days after the last training session. We analyzed the CPP score (time in the morphine-paired chamber minus time in the saline-paired chamber during testing) using ANOVA with the between-subjects factors of Morphine Dose and Withdrawal Day. This analysis revealed significant effects of Morphine Dose (F(3,108)=61.62; p<0.01), Withdrawal Day (F(2,108)=22.97; p<0.01), and an interaction between these two factors (F(6,108)=5.17; p<0.05). One-way ANOVA within each morphine dose revealed significant effects of withdrawal day for the training CPP doses of 1 and 3 mg/kg (F (2,27)=51.2, p<0.01, and F (2,27)=36.8, p<0.01), respectively, but not for the 10 mg/kg dose (p>0.05). Post-hoc group differences within each time point are indicated in Fig. 1a.
Within-subjects assessment
We assessed morphine CPP in 4 different groups of rats that were injected with saline or morphine (1, 3 or 10 mg/kg) and were repeatedly tested for CPP 1, 7 and 14 days after the last training session. We analyzed the CPP score using repeated-measures ANOVA with the between-subjects factors of Morphine Dose (0, 1, 3, and 10 mg/kg) and the within-subjects factor of Withdrawal Day (1, 7, 14). This analysis revealed significant effects of Morphine Dose (F (3,36)=24.3, p<0.01), Withdrawal Day (F (2,36)=32.5, p<0.01), and an interaction between these two factors (F (6,36)=3.8; p<0.05). One-way ANOVA within each morphine dose revealed significant effects of withdrawal day for the training CPP doses of 1 and 3 mg/kg (F (2,27)=12.5, p<0.01, and F (2,27)=8.9, p<0.01), respectively, but not for the 10 mg/kg dose (p>0.05). Post-hoc group differences within each time point are indicated in Fig. 1b.
Effect of morphine CPP testing after 1 or 14 withdrawal days on amygdala ERK and CREB
As in Exp. 1, in rats trained for morphine CPP with a low morphine dose (3 mg/kg), the CPP score during testing was higher after 14 withdrawal days than after 1 day (Fig. 2a). This progressive increase in morphine CPP after withdrawal from the drug was associated with increased pERK and pCREB in central but not basolateral amygdala (Fig. 2 and 3). The experimental manipulations had no effects on total ERK and CREB in the central or basolateral amygdala (Fig. 2 and 3) (statistical analyses not presented). Additionally, independent of the CPP testing, morphine exposure was associated with increased pERK and pCREB in the basolateral amygdala after 1 but not 14 withdrawal days.
Morphine CPP
We analyzed the morphine CPP score with ANOVA using the between-subjects factors of Morphine Dose (0, 3 mg/kg) and Withdrawal Day (1, 14). This analysis revealed significant effects of Morphine Dose (F (1,36)=8.1, p<0.01), Withdrawal Day (F (1,36)=38.7, p<0.01), and an interaction between these two factors (F (1,36)=8.36, p<0.01).
pERK and pCREB
Analyses were performed separately for central and basolateral amygdala, and included the between-subjects factors of Morphine Dose (0, 3 mg/kg), Withdrawal Day (1, 14), and CPP test (no, yes).
Central amygdala
The statistical analysis for both pERK and pCREB revealed significant interactions of Morphine Dose by Withdrawal Day by CPP test (F(1,56)=6.2, p<0.01 for pERK, and F(1,56)=4.3, p<0.05 for pCREB, respectively). These triple interactions are due to the fact that pERK and pCREB levels in morphine-exposed rats that underwent the CPP test after 14 withdrawal days were higher than those of the other 7 experimental groups (Fig. 2 and 3, p<0.01).
Basolateral amygdala
The statistical analysis for both pERK and pCREB revealed significant interactions of Morphine Dose by Withdrawal Day (F(1,56)=6.8, p<0.01 for pERK, and F(1,56)=10.7, p<0.01 for pCREB, respectively). The interactions between these two factors and CPP test were not significant. The second-order interaction between Morphine Dose and Withdrawal Day is due to the fact that, independent of CPP testing, pERK and pCREB levels were higher in day 1 morphine withdrawal rats compared to all other experimental groups (Fig. 2 and 3, p<0.01).
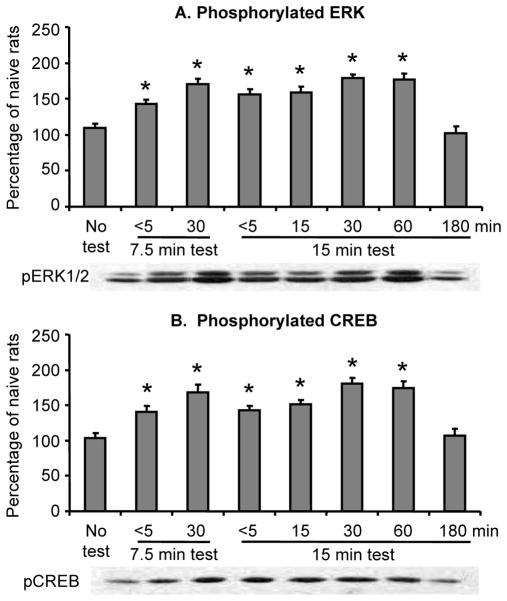
Finally, because the data described in Fig. 2 and 3 are of pERK and pCREB values of rats that their brains were taken 30 min after the end of the CPP tests, we also determined the time course pERK and pCREB during and after CPP tests that were conducted on withdrawal day 14. We found that pERK and pCREB values are rapidly increased during and immediately after the CPP test session; these values remained elevated for at least 60 min and returned to baseline (no test) levels after 180 min (Fig. 4). As mentioned above, the brains of rats from 2 groups were taken within 5 min or 30 min after a shorter 7.5-min CPP test, while the brains of rats from 5 other groups were taken within 5 min, or 15, 30, 60 or 180 min after a regular 15-min CPP test. The brains of rats from the 8th (control) group were taken immediately prior to the CPP test. The statistical analyses included the between-subjects factor of Group and revealed a significant effect of this factor for both pERK and pCREB (F (7, 56) =18.2, and F (7, 56) =16.7, p values <0.01, respectively). Post-hoc group differences are depicted in Fig. 4. The mean CPP score of the 5 groups of rats exposed to the 15 min test was 227.6±12.3 sec.
Figure 4. Time course of ERK and CREB activity during and after exposure to a CPP test on withdrawal day 14.
(A,B) Phosphorylated ERK and CREB in the central amygdala before (no test), during, and after morphine CPP testing. Data are presented as a percentage (mean ± SEM) of phosphorylated ERK and CREB of naïve control rats (n=8). * Different from the No Test group, p < 0.05.
Effect of inhibition of amygdala ERK and CREB on morphine CPP after 14 withdrawal days
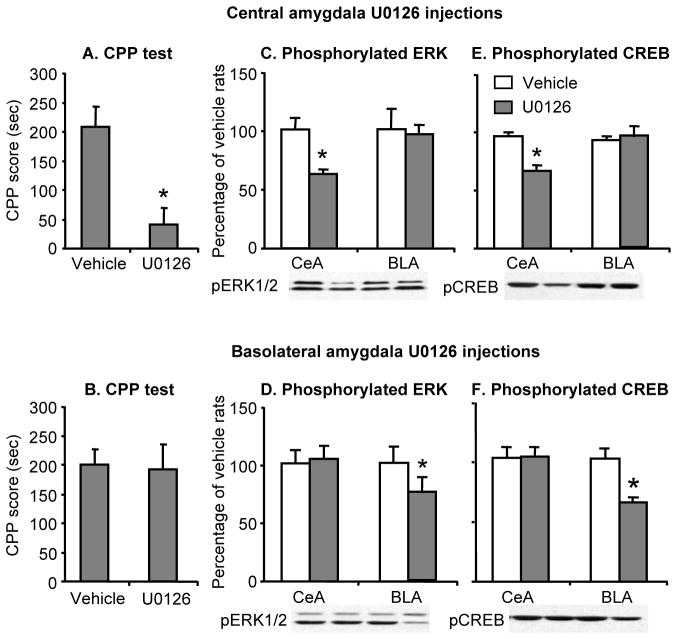
Inhibition of central amygdala but not basolateral amygdala pERK and pCREB decreased morphine (3 mg/kg) CPP after 14 withdrawal days (Fig. 5). The ANOVA for the CPP score included the between-subjects factors of U0126 Dose (0, 100 ng/side) and Amygdala Nucleus (central, basolateral). This analysis revealed a significant interaction between U0126 Dose and Amygdala Nucleus (F (1, 28)=8.9, p<0.01). The analysis of the data from the Western blot assays revealed that central amygdala infusions of U0126 decreased pERK and pCREB in the central (F(1,14)=12.6; p<0.01 and F(1,14)= 25.2, p<0.01, respectively) but not the basolateral (p>0.05) amygdala, while basolateral amygdala infusions of U0126 decreased pERK and pCREB in the basolateral (F(1,14)=6.1, p<0.01, and F(1,14)=16.2, p<0.01, respectively), but not the central (p>0.05) amygdala (Fig. 5).
Figure 5. Inhibition of ERK and CREB phosphorylation in the central amygdala decreased morphine CPP after 14 withdrawal days.
(A,B) Central but not basolateral amygdala injections of U0126 decreased morphine (3 mg/kg) CPP. Data are the mean ± SEM of preference score in sec during the CPP preference tests conducted 14 days after withdrawal from morphine. * Different from the vehicle condition, p < 0.05. (C,E) Central amygdala U026 injections decreased ERK and CREB phosphorylation in the central but not basolateral amygdala. (D,F) Basolateral amygdala U026 injections decreased ERK and CREB phosphorylation in the basolateral but not central amygdala. Data are presented as a percentage of the respective vehicle groups. * Different from the vehicle group within each amygdala sub-nucleus, p < 0.05 (n=7–8 per group).
Effect of activation of central amygdala ERK and CREB on morphine CPP after 1 withdrawal day
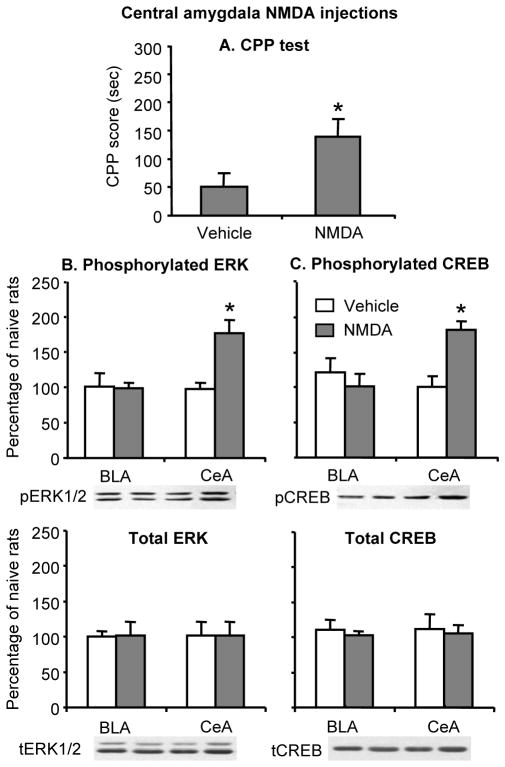
Activation of central amygdala pERK and pCREB increased morphine (3 mg/kg) CPP after 1 withdrawal day (Fig. 6). The simple ANOVA for the CPP score included the between-subjects factor of NMDA Dose (0, 250 ng/side). This analysis revealed a significant effect of NMDA Dose (F(1,12) = 30.3, p<0.01). The analysis of the data from the Western blot assays revealed that central amygdala infusions of NMDA increased pERK and pCREB in the central (F (1,12)=14.4, p<0.01, and F (1,12)=33.3, p<0.01, respectively) but not the basolateral (p>0.05) amygdala (Fig. 6).
Figure 6. NMDA-induced increases in ERK and CREB phosphorylation in the central amygdala enhanced morphine CPP after 1 withdrawal day.
(A) Central amygdala injections of NMDA increased morphine (3 mg/kg) CPP. Data are the mean ± SEM of preference score in sec during the CPP preference tests conducted 1 day after withdrawal from morphine. * Different from the vehicle condition, p < 0.05. (B,C) Central amygdala NMDA injections increased ERK and CREB phosphorylation in the central but not basolateral amygdala. The NMDA injections had no effect on total ERK and CREB. Data are presented as a percentage of the naive group. * Different from the vehicle group within each amygdala sub-nucleus, p < 0.05 (n=8 per group).
In a subsequent experiment we found that central amygdala U0126 injections reversed the effect of local NMDA injections on morphine CPP (Fig. 7a). Data were analyzed using the factors of NMDA Dose (0, 250 ng/side) and U0126 Dose (0, 100 ng/side). This analysis revealed a significant interaction between these two factors for CPP score (F (1,26)=15.7, p<0.01). Additionally, central amygdala U0126 injections decreased NMDA-induced increases in phosphorylated ERK and CREB. The analysis of the data from the Western blot assays revealed a significant interaction between NMDA Dose and U0126 Dose for both phosphorylated ERK and CREB (F(1,26)=19.6, p<0.01, and F(1,26)=16.7, p<0.01, respectively) (Fig. 7b,c). Post-hoc group differences are indicated in Fig. 7.
Figure 7. U0126 reversed the enhancement of morphine CPP and ERK and CREB phosphorylation in central amygdala by NMDA 1 day after withdrawal from the drug.
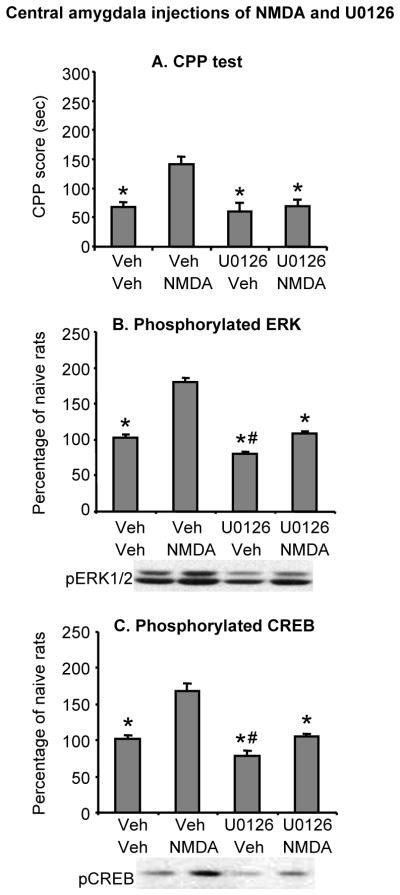
(A) Central amygdala injections of U0126 attenuated local NMDA-induced increases in morphine (3 mg/kg) CPP. Data are the mean ± SEM of preference score in sec during the CPP preference tests conducted 1 day after withdrawal from morphine. (B,C) Central amygdala injections of U0126 attenuated local NMDA-induced increases in ERK and CREB phosphonrylation. Data are presented as a percentage of the naive group. * Different from the vehicle-NMDA group, p < 0.05, # Different from the vehicle-vehicle group, p < 0.05 (n=7–8 per group).
Effect of morphine CPP training on ERK and CREB activity in basolatoral amygdala
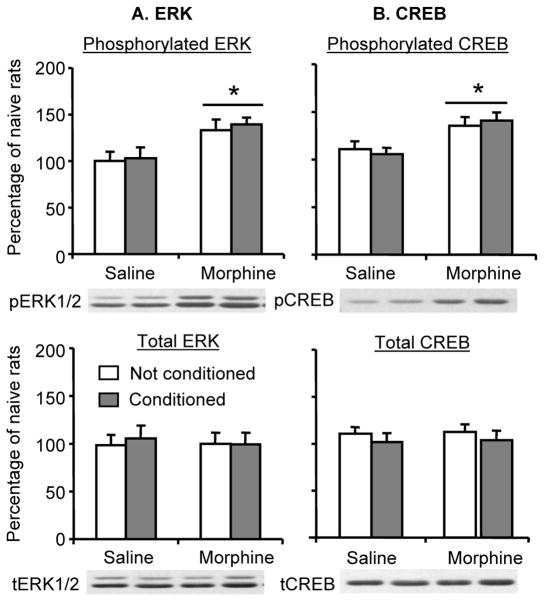
The aim of this experiment was to verify the unexpected finding that repeated exposure to low morphine dose (3 mg/kg) resulted in increased pERK and pCREB in basolateral amygdala after 1 withdrawal day, an effect that was independent of CPP testing. Here, we assessed whether this effect is dependent on training for morphine CPP. As can be seen in Fig. 8, increased pERK and pCREB in basolateral amygdala after 1 withdrawal day was observed both in rats conditioned to morphine in the CPP apparatus and in rats injected with morphine in their home cage. The statistical analysis included the between-subjects factor of CPP Training (yes, no) and Morphine Dose (0, 3 mg/kg). This analysis revealed a significant effect of Morphine Dose for pERK and pCREB (F (1,16)= 60.7, p<0.01, and F (1,16)=39.2, p<0.01, respectively), but not of CPP training or an interaction between CPP Training and Morphine Dose.
Figure 8. Repeated exposure to morphine increased ERK and CREB phosphorylation in the basolatoral amygdala on withdrawal day 1, an effect independent of CPP training.
The “conditioned” groups underwent morphine (3 mg/kg)-saline or saline-saline CPP training (4 pairings in each chamber over 8 days). The “unconditioned” rats were injected with morphine or saline in their home cage. ERK and CREB phosphorylation in the basolateral amygdala were assessed 1 day after the last morphine (or saline) injection (withdrawal day 1). Data are presented as a percentage (mean ± SEM) of phosphorylated and total ERK or CREB of naïve control rats (n=5). (A) Phosphorylated and total ERK in basolateral amygdala. (B) Phosphorylated and total CREB in basolateral amygdala. * Different from the saline-exposed rats, p < 0.05 (n=5 per group).
Discussion
We found that the expression of low-dose but not high-dose drug CPP progressively increased over the first 14 d after withdrawal from morphine. Our data indicate that this incubation of morphine craving is mediated by central amygdala ERK activity. Our data also indicate that incubation of morphine craving correlates with central amygdala CREB activity. Thus, enhanced morphine CPP after 14 withdrawal days was accompanied by enhanced ERK and CREB phosphorylation in central but not basolateral amygdala. Inhibition of central but not basolateral amygdala ERK and CREB phosphorylation by U0126 prevented the enhanced morphine CPP after 14 withdrawal days, while activation of central amygdala ERK and CREB phosphorylation by NMDA restored morphine CPP after 1 withdrawal day; NMDA-induced increases in morphine CPP was reversed by central amygdala U0126 injections. We also found that repeated exposure to morphine (either in home-cage or CPP apparatus) increased ERK and CREB phosphorylation in the basolateral amygdala after 1 withdrawal day.
Role of central amygdala ERK in incubation of morphine craving
Our results indicating a role of central amygdala ERK in incubation of morphine craving, as assessed in the CPP procedure, are in agreement with our previous work on the role of central amygdala ERK in incubation of cocaine craving, as assessed in the self-administration procedure (Lu et al., 2005). It is likely that central amygdala glutamate transmission mediates the ERK-dependent incubation of drug craving. We found that central amygdala NMDA injections increased ERK phosphorylation and enhanced morphine CPP on withdrawal day 1. Additionally, we previously found that these injections increased ERK phosphorylation and cue-induced cocaine seeking on withdrawal day 1; these effects were reversed by local U0126 injections (Lu et al., 2005). We also previously found that inhibition of local glutamate transmission by central amygdala injections of the NMDA receptor antagonist AP-5 or the mGluR2/3 agonist LY379268 (which decreases glutamate transmission) attenuated the enhanced cue-induced cocaine seeking after 3–4 weeks of withdrawal (Lu et al., 2005; Lu et al., 2007).
The downstream cellular mechanisms of central amygdala ERK activation that contribute to incubation of drug craving are unknown. Because of the timeframe of our behavioral assessments (minutes), we proposed that the expression of incubation of drug craving involves cue-triggered, ERK-mediated, rapidly-induced (within minutes, see Fig. 4) increases in neuronal excitability and synaptic transmission (Lu et al., 2006). These acute effects may be due to ERK-mediated inactivation of voltage-gated potassium channel subunit Kv4.2, leading to decreasing outward K+ current, resulting in increased membrane depolarization (Yuan et al., 2002). This depolarization would make neurons more responsive to external stimuli, such as drug cues. ERK’s role in incubation of reward craving may also involve cue-induced ERK-mediated potentiation of excitatory neurotransmission by AMPA receptor insertion into cell membranes (Qin et al., 2005).
Other investigators reported that systemic or accumbens, acute injections of U0126, or other inhibitors of ERK activity, decreased the expression of psychostimulant CPP (Mizoguchi et al., 2004; Miller and Marshall, 2005; Valjent et al., 2006a) and Pavlovian-to-Instrumental transfer of food cues (the potentiating effect of Pavlovian food cues on operant responding for the same food) (Shiflett et al., 2008). Thus, we propose that time-dependent increases in cue-induced drug seeking, as well as responses to reward cues in some behavioral procedures, involves ERK-mediated, acute, central effects on synaptic transmission (Lu et al., 2006). This mechanism is distinct from the more established ERK-related mechanism—long-term stable alterations in synaptic plasticity triggered by ERK-induced genomic activation via downstream cellular target—that contributes to diverse learning and memory processes (Sweatt, 2001; Adams and Sweatt, 2002), cocaine psychomotor sensitization (Valjent et al., 2006b; Boudreau et al., 2007), and consolidation and reconsolidation of memories for cocaine cues (Valjent et al., 2000; Miller and Marshall, 2005; Valjent et al., 2006a).
Role of central amygdala CREB in incubation of morphine craving
We studied whether CREB activity correlates with incubation of morphine craving because CREB is a downstream cellular target of ERK (Thomas and Huganir, 2004), and because CREB knockout mice do not acquire morphine CPP (Walters and Blendy, 2001). Additionally, cocaine CPP expression is associated with increased CREB phosphorylation in the accumbens and dorsal hippocampus (Miller and Marshall, 2005; Tropea et al., 2008), and nicotine CPP expression correlates with increased CREB activity in ventral tegmental area (Walters et al., 2005).
We extended these findings to demonstrate that morphine CPP expression correlates with increased central amygdala CREB phosphorylation. This effect is reversed by U0126 and thus ERK-dependent. These data extend previous findings indicating that drug- or cue-induced activation of accumbens CREB is ERK-dependent (Brami-Cherrier et al., 2005; Mattson et al., 2005; Miller and Marshall, 2005). Increased CREB phosphorylation occurred selectively in the central amygdala and was correlated with the time-dependent increases in drug CPP after withdrawal from morphine. Therefore, one interpretation of these data is that local CREB activity mediates the incubation of drug craving. However, activation of the transcription factor CREB is not likely to control conditioned responses within the timeframe of our behavioral assessment (15 min), because CREB-induced gene transcription typically requires longer time periods (Montminy et al., 1990; Nestler, 1993; Frank and Greenberg, 1994). Thus, the time-dependent increase in central amygdala CREB phosphorylation after 14 withdrawal days is probably a consequence, rather than a cause, of incubation of morphine craving. This issue can be addressed by using a manipulation that, unlike U0126, selectively prevents CREB phosphorylation without affecting ERK phosphorylation.
Generality of incubation of reward craving
Incubation of drug craving was inferred from the findings of time-dependent increases in cue-induced drug-seeking after withdrawal from cocaine (Grimm et al., 2001). This Incubation was demonstrated under different experimental conditions (Sorge and Stewart, 2005; Hollander and Carelli, 2007; Conrad et al., 2008; Freeman et al., 2008), and was also observed in rats trained to self-administer heroin (Shalev et al., 2001), methamphetamine (Shepard et al., 2004), and alcohol (Bienkowski et al., 2004). Incubation of reward craving is also evident with non-drug rewards. Seventy years ago Youtz (1938) demonstrated time-dependent increases in extinction responding in hungry rats previously trained to lever-press for food. More recently, Grimm et al. (2002; 2005) demonstrated time-dependent increases in both extinction responding and cue-induced reinstatement in rats previously trained to lever-press for sucrose.
Here we showed that incubation of reward craving can be detected in a Pavlovian CPP procedure that is widely used to assess the rewarding effects of abused drugs (Bardo and Bevins, 2000; Tzschentke, 2007). Under our experimental conditions, the critical factor is the drug training dose: increases in CPP over 14 days after last drug exposure were only observed in rats trained with low (1 or 3 mg/kg) but not high (10 mg/kg) doses. These data are different from those obtained in earlier studies by Lu et al. (2000a; 2001b) and B. Wang et al. (2000; 2002) who reported that CPP for morphine (10 mg/kg or 4 mg/kg, respectively) decreased over time. Potential reasons for these different results are the use of a 3-chamber CPP apparatus here versus a 2-chamber CPP apparatus in previous studies, and differences in the contextual cues used here versus those used in the previous studies of Lu and colleagues.
Our data on time-dependent increases in morphine CPP are also different from those of Mucha and Iversen (1984), Vezina and Stewart (1987), and Mueller and Stewart (2002) who reported that drug CPP is maintained at similar magnitude over weeks after withdrawal from morphine. The reasons for these different results are unknown but we would like to note that unlike these previous studies, in our study we systematically characterized the effect of morphine CPP training dose on CPP expression over time. Interestingly, data from Mueller and Stewart (2002) may be in agreement with our data. They trained rats for morphine CPP with 3 drug doses (1, 5 or 10 mg/kg) but assessed CPP expression data over time for all rats irrespective of their training dose (Fig. 2, p. 392). They also excluded rats that did not demonstrate day 1 morphine CPP. As can be seen in this figure, morphine CPP, which was strong after 1 day, was even stronger after 6 weeks; this effect, however, was not statistically significant. Perhaps, had the authors assessed morphine CPP expression within each training dose and not excluding rats demonstrating weak day 1 drug CPP, they might have obtained significant time-dependent increases in morphine CPP.
Concluding remarks
Our results indicate that central amygdala ERK activity mediates the incubation of morphine craving, as assessed in the CPP procedure. These results extend previous data on the role of central amygdala ERK in incubation of cocaine craving, as assessed in the self-administration procedure. This generality across drugs and procedures suggest that central amygdala ERK plays a general role in incubation of reward craving. We also found that central amygdala CREB activity is associated with incubation of morphine craving. Whether this activity is a cause or a consequence of incubation of drug craving is a subject for future research.
Acknowledgments
This work was supported in part by the National Basic Research Program of China (No: 2007CB512302 and 2009CB522004), the National High Technology Research and Development Program of China (863 Program, 2006AA02Z4D1), the Natural Science Foundation of China (No: 30670713 and 30725016) and the National Institute on Drug Abuse, Intramural Research Program. We thank Drs. Bruce Hope and Eisuke Koya for their helpful comments on earlier versions of the manuscript, and Drs. Julie Blendy and Bill Carlezon for helpful comments on issues related to CREB role in the behavioral effects of drugs.
Footnotes
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Maldonado R. Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med. 1998;76:104–110. doi: 10.1007/s001090050197. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Fiore RS, Murphy TH, Sanghera JS, Pelech SL, Baraban JM. Activation of p42 mitogen-activated protein kinase by glutamate receptor stimulation in rat primary cortical cultures. J Neurochem. 1993;61:1626–1633. doi: 10.1111/j.1471-4159.1993.tb09796.x. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Ceng X, Huang M. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport. 2000a;11:2373–2378. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol. 2001a;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000b;12:4398–4404. [PubMed] [Google Scholar]
- Lu L, Huang M, Ma L, Li J. Different role of cholecystokinin (CCK)-A and CCK-B receptors in relapse to morphine dependence in rats. Behav Brain Res. 2001b;120:105–110. doi: 10.1016/s0166-4328(00)00361-2. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol Pharmacol. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology. 1984;82:241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav Brain Res. 2002;136:389–397. doi: 10.1016/s0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Martini RP, Mauna JC, Foster RL, Peet E, Thiels E. Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. J Neurosci. 2008;28:1434–1443. doi: 10.1523/JNEUROSCI.2383-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl) 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine conditioned place preference behavior. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci Lett. 1995;193:37–40. doi: 10.1016/0304-3940(95)11662-g. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006a;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006b;7:1–11. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooy D. Place conditioning: A simple and effective method for assessing the motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 229–240. [Google Scholar]
- Vezina P, Kalivas PW, Stewart J. Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res. 1987;417:51–58. doi: 10.1016/0006-8993(87)90178-8. [DOI] [PubMed] [Google Scholar]
- Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci. 2001;21:9438–9444. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang B, Luo F, Zhang WT, Han JS. Stress or drug priming induces reinstatement of extinguished conditioned place preference. Neuroreport. 2000;11:2781–2784. doi: 10.1097/00001756-200008210-00034. [DOI] [PubMed] [Google Scholar]
- Wang B, Luo F, Ge XC, Fu AH, Han JS. Effects of lesions of various brain areas on drug priming or footshock-induced reactivation of extinguished conditioned place preference. Brain Res. 2002;950:1–9. doi: 10.1016/s0006-8993(02)02980-3. [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youtz REP. The change with time of a Thorndikian response in the rat. J Exp Psychol. 1938;23:128–140. [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]