Abstract
The CRISPR/Cas9 genome editing tool has increased the efficiency of creating genetically modified pigs for use as biomedical or agricultural models. The objectives were to determine if DNA editing resulted in a delay in development to the blastocyst stage or in a skewing of the sex ratio. Six DNA templates (gBlocks) that were designed to express guide RNAs that target the transmembrane protease, serine S1, member 2 (TMPRSS2) gene were in vitro transcribed. Pairs of CRISPR guide RNAs that flanked the start codon and polyadenylated Cas9 were co-injected into the cytoplasm of zygotes and cultured in vitro to the blastocyst stage. Blastocysts were collected as they formed on days 5, 6 or 7. PCR was performed to determine genotype and sex of each embryo. Separately, embryos were surgically transferred into recipient gilts on day 4 of estrus. The rate of blastocyst development was not significantly different between CRISPR injection embryos or the non-injected controls at day 5, 6 or 7 (p=0.36, 0.09, 0.63, respectively). Injection of three CRISPR sets of guides resulted in a detectable INDEL in 92% to 100% of the embryos analyzed. There was not a difference in the number of edits or sex ratio of male to female embryos when compared between days 5, 6 and 7 to the controls (p>0.22, p>0.85). There were 12 resulting piglets and all 12 had biallelic edits of TMRPSS2. Zygote injection with CRISPR/Cas9 continues to be a highly efficient tool to genetically modify pig embryos.
Keywords: CRISPR/Cas9, Zygote Injection, DNA editing, gBlock
Introduction
Pigs continue to provide the scientific community with an excellent biomedical model that is both similar in size and physiology to humans (Jensen et al. 2010; Renner et al. 2010; Rogers et al. 2008; Ross et al. 2012; Tsang et al. 2016). Genetically modified and DNA edited pigs are also powerful tools for agricultural (Lillico et al. 2016; Prather et al. 2013; Whitworth et al. 2014; Whitworth et al. 2016) as well as for xenotransplantation research (Kolber-Simonds et al., 2004; Lai et al., 2002; Zeyland et al., 2014; (Niemann and Petersen 2016). Using the CRISPR/Cas9 system as a genome editing tool (Cong et al. 2013; Cong and Zhang 2015) has greatly increased the efficiency for providing scientific models to study basic biology. In species like pigs that lack authentic embryonic stem cell lines, this technology has revolutionized the ability to create DNA edited and genetically modified animals.
In the past three years there have been numerous reports of production of modified pig embryos either by direct zygote injection of CRISPR/Cas9 RNA or by somatic cell nuclear transfer with CRISPR/Cas9 modified cells. For example, the CRISPR/Cas9 system was used to create pigs with the human albumin cDNA replacing the pig locus resulting in pigs with human serum albumin that could be used to alleviate the shortage and risks associated with human blood (Peng et al. 2015). CRISPR/Cas9 was also used to target the von Willebrand factor (vWF) gene in pig zygotes to model the human von Willebrand disease (vWD) (Hai et al. 2014). Additionally, the CRISPR/Cas9 system was used to efficiently manipulate a cell line for a xenotransplantation model. Three genes, GGTA1, CMAH and putative iGb3S were all edited in a cell line that was used for somatic cell nuclear transfer and resulted in live edited piglets (Li et al. 2015). DNA editing by CRISPR/Cas9 recently made a significant impact on the swine industry when the CD163 gene was edited to create a line of pigs that are resistant to the devastating porcine reproductive and respiratory syndrome virus (PRRSV) (Whitworth et al. 2016).
In our laboratory, zygotes for CRISPR/Cas9 RNA injection are produced by in vitro maturation of oocytes and subsequent in vitro fertilization and embryo culture until embryo transfer into the recipient gilt. Several studies have shown that CRISPR/Cas9 RNA injection had very little effect on overall blastocyst formation when compared to water injected controls (Hai et al. 2014; Wang et al. 2015). In a previous study, CRISPR/Cas9 RNA injection of pig zygotes resulted in 100% of the piglets having biallielic DNA edits of the targeted CD163 or CD1D gene (Whitworth et al. 2014). Interestingly, 7 of the 8 offspring from this study were male. There is very little information published about whether DNA editing by CRISPR/Cas9 affects embryo development and/or the sex of the resulting offspring. The objectives of this study were to measure the effects of CRISPR/Cas9 guide RNA injection on the rate of embryo development and to determine if CRISPR/Cas9 RNA injection altered the sex ratio of the resulting blastocyst-stage embryos. The secondary objective of this experiment was to create pigs with a DNA edit in the TMPRSS2 gene for use as a biomedical model of pigs that may be resistant to certain types of influenza viruses (Hatesuer et al. 2013) (Sakai et al. 2014) (Tarnow et al. 2014).
Materials and Methods
Chemical and Reagents
Unless otherwise stated, all of the chemicals used in this study were purchased from Sigma, St. Louis, MO.
Animal and Recombinant DNA Usage
The use of animals was approved by University of Missouri Animal Care and Use Committee. The use of recombinant DNA was approved by the Institutional Biosafety Committee.
Design of gRNAs to build specific CRISPRs
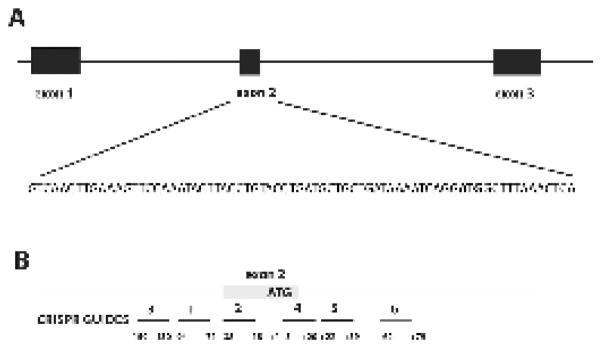
Guide RNAs were designed to be used in pairs to remove the start codon from exon 2 of the TMPRSS2 gene. Six 18–20 bp guides were designed to target sequence located adjacent to an S. pyogenes (Spy) protospacer adjacent motif (PAM) (Ran et al. 2015). The targets were selected by the following method. Repeat Masker (Smit and Green 1996) (“Pig” repeat library) was used to identify any repetitive elements in the TMPRSS2 genomic sequence and these areas were not used as potential targets. Specificity of each potential guide was then confirmed by searching for similar porcine sequences in GenBank. If guides and the adjacent PAM sequence had similarity to other areas of the genome, they were removed from subsequent analysis. Lastly, structural analysis of the 20 bp guide with the CRISPR RNA (crRNA) and the trans-activating crRNA (tracrRNA) (Hsu et al. 2013) was evaluated for potential distruption of gRNA structure by mFold (http://unafold.rna.albany.edu/). If potential guides were predicted to form an appropriate “handle” to interact with Cas9 and were not predicted to form a tight hairpin that could potentially prevent interaction with the genome, then they were added to the finalized list of potential guides. Six guides were chosen for the experiment based on the criteria listed above. Three guides were located upstream of the start codon in exon 2, and three guides were located downstream of the start codon in exon 2 (Figure 1). The six guides and the PAM (bold) include TMPRSS2 Guide 1, AGACTGTAAAATTTCCATACCGG, TMPRSS2 Guide 2, ATCAGGTACAGGTAAGTATTTGG, TMPRSS2 Guide 3, CCCTCACCCAGAGAGCCTTCTGG, TMPRSS2 Guide 4, GGCTTTAAACTCAGTAGGTGG, TMPRSS2 Guide 5, GTTAATTATTACCTCCCTGG, TMPRSS2 Guide 6, GTGCCTTCTGTTAGTTCCAGCGG. The distances between the guides in each pair were 39 bp between 2+4, 127 bp between 1+5 and 222 bp between 3+6 when measured from N to N in the NGG PAM sequence. Proximity to the start codon are shown in Figure 1.
Figure 1.
A: Genomic locus of targeted exon 2. B: Location of guides flanking exon 2 of the TMPRSS2 gene. +1 represents the A in the start codon ATG. Guides 1+5, 2+4 and 3+6 were mixed and coinjected. A designed deletion would result in the removal of exon 2 and the start codon.
In vitro transcription of single guide RNAs for the CRISPR/Cas9 system
Template guide DNA was first synthesized by Integrated DNA Technologies in the form of a gBlock (Supplemental Table 1). A T7 promoter sequence was added upstream of the guide for in vitro transcription. Each gBlock was diluted to final concentration 0.1 ng/μl and PCR amplified with a gBlock F (ACTGGCACCTATGCGGGACGAC) and a gBlock R primer (AAAAGCACCGACTCGGTGCCAC) with Q5 (New England Biolabs, Ipswich, MA) following standard protocol. PCR conditions consisted of an initial denaturation of 98°C for 1 min followed by 35 cycles of 98°C (10 sec), 68°C (30 sec) and 72°C (30 sec). Each PCR amplified gBlock was purified by using a QIAGEN (Valencia, CA) PCR purification kit following standard protocol. Purified gBlock amplicons were then used as template for in vitro transcription by standard protocol with the MEGAshortscript (Ambion, Thermofisher, Grand Island, NY). Quality of the synthesized RNAs were visualized on a 2.0% RNA-free agarose gel and concentrations 260:280 ratios were determined via Nanodrop spectrophotometry. Capped and polyadenylated Cas9 mRNA was purchased from Sigma (St. Louis, MO). Single guide RNA (sgRNA) and Cas9 mRNA were diluted in nuclease-free water and combined at a final concentration of 20 ng/μL and 20 ng/μL, respectively. sgRNA guides 1 and 5, sgRNA guides 2 and 4 and sgRNA guides 3 and 6 were each mixed together with Cas9. RNA aliquots were stored at −80°C until zygote injection.
IVF In vitro fertilization (IVF)
For IVF, ovaries from pre-pubertal gilts were obtained from an abattoir (Smithfield-Farmland., Milan, MO). Immature oocytes were aspirated from medium size (3–6 mm) follicles by using an 18-gauge hypodermic needle attached to a 10 ml syringe. Oocytes with evenly dark cytoplasm and intact surrounding cumulus cells were then selected for maturation. Between 200–250 cumulus oocyte complexes were placed in a 35mM petri dish (BD 35-1008) containing 2.0ml of maturation medium, TCM 199 (Invitrogen, Grand Island, NY) with 3.05 mM glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 10 ng/ml epidermal growth factor (EGF), 0.5 μg/ml luteinizing hormone (LH), 0.5 μg/ml follicle stimulating hormone (FSH), 10 ng/ml gentamicin (APP Pharm, Schaumburg, IL), and 0.1% polyvinyl alcohol (PVA) for 42–44 h at 38.5°C, 5% CO2, in humidified air. At the end of the maturation, the surrounding cumulus cells were removed from the oocytes by vortexing for 3 min in the presence of 0.1% hyaluronidase. Then in vitro matured oocytes were placed in 50 μL droplets of IVF medium (modified Tris-buffered medium with 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2, 11 mM glucose, 20 mM Tris, 2 mM caffeine, 5 mM sodium pyruvate, and 2 mg/ml BSA) in groups of 25–30 oocytes. One 100 μl frozen semen pellet was thawed in 3 ml of DPBS supplemented with 0.1% BSA. Either frozen wild type was washed in 45% percoll for 20 min at 550 × g and in MTBM for 10 min by centrifugation. The semen pellet was then re-suspended with IVF medium to 0.5×106 cells/ml. Fifty microliter of the semen suspension was introduced into the droplets with oocytes. The gametes were co-incubated for 5 h at 38.5 °C in an atmosphere of 5% CO2 in air. After fertilization, the embryos were incubated in MU2 ((Bauer et al. 2010; Yoshioka et al. 2002) at 38.5 °C, 5% CO2 in air atmosphere.
Zygote injections
Guide pair mixes 1+5, 2+4 and 3+6 and Cas9 RNA (Sigma, St. Louis, MO) were co-injected as individual treatments into the cytoplasm of presumptive zygotes at 14 hours post-fertilization (presumptive zygotes) by using a FemtoJet microinjector (Eppendorf; Hamburg, Germany). Glass pipettes with an outer diameter (OD) of 1.0 mm and an inner diameter of 0.78 mm were pulled to a fine point of < 1.0 microns (Sutter Instrument, Navato, CA, USA). Microinjection was performed in manipulation medium (TCM199 with 0.6 mM NaHCO3, 2.9 mM Hepes, 30 mM NaCl, 10 ng/ml gentamicin, and 3 mg/ml [BSA; and osmolarity of 305) on the heated stage of a Nikon inverted microscope (Nikon Corporation; Tokyo, Japan) with an injection pressure ranging from 150–200 hPa. Injected zygotes were then transferred into the MU1 with 5 ng/ml PS48 for culture to the blastocyst stage. There were three replicates of zygote injections performed for each experiment.
Blastocyst Collection on days 5, 6 or 7
Culture plates were monitored daily for blastocyst formation. On days 5, 6 or 7, embryos that formed blastocysts were collected for subsequent sex determination and evaluation for DNA editing. The zona pellucidae were removed by treatment with a physiological saline at pH 1.79 and rinsed in DEPC treated PBS. An absent zona pellucida will prevent the wild type genome from attached sperm from interfering with genotyping assays. Individual embryos were transferred to 0.5 ml microcentrifuge tubes and snap frozen.
Embryo Transfer
Embryos generated to produce TMPRSS2 edited pigs (RRID:NSRRC:0060) were surgically transferred into surrogate gilts on day 4 after standing estrus. Zygotes were cultured in MU2 (MU1 supplemented with the phosphopeptide mimetic that triggers PDK1 phosphorylation, PS48 (5 ng/ml) (Stemgent, Inc, Cambridge, MA) until embryo transfer. MU1 and MU2 have been described previously (Redel et al. 2015) (Spate et al. 2015). The embryos, 60 into one recipient and 75 into a second recipient, were surgically transferred into the ampullary-isthmic junction of the oviduct of the surrogate (Lee et al. 2013).
PCR screening for INDELS
Three assays were designed to assess the presence of INDELS in the resulting embryos and offspring including a small deletions assay with an amplicon size of 544 bp, a medium range assay with an amplicon size of 2181 and a long range assay with an amplicon size of 4013. Primer sequences are listed in Supplemental Table 2.
Small INDEL Assay
Small INDELs were determined by PCR amplification of the TMPRSS2 gene in a region flanking the projected cutting site introduced by the CRISPR/Cas9 system with primers TMPRSS2 F1 and TMPRSS2 R2. PCR conditions of the small INDELs assays consisted of an initial denaturation of 95°C for 2 min followed by 35 cycles of 94°C (30 sec), 56°C (30 sec) and 72°C (1 min). Insertions and deletions (INDELs) were identified by separating PCR amplicons by agarose gel electrophoresis. The resulting PCR products were also Sanger DNA sequenced with primer TMPRSS2 F1 at the University of Missouri DNA Core. Chromatographs were analyzed for the presence of INDELS in Finch TV (Perkin Elmer Waltham, Massachusetts).
Medium Range Assay
Medium sized INDELs were evaluated by PCR amplification of the TMPRSS2 gene with primers TMPRSS2 2156F and TMPRSS2 6149 R. PCR conditions of the medium INDEL assays consisted of an initial denaturation of 94°C for 1 min followed by 35 cycles of 94°C (30 sec), 50°C (30 sec) and 68°C (5 min). Insertions and deletions (INDELs) were identified by separating PCR amplicons by agarose gel electrophoresis. The medium range assay was not further evaluated by Sanger Sequencing.
Long Range Assay
Large INDELs were evaluated by PCR amplification of TMPRSS2 gene with primers TMPRSS2 3968 F and TMPRSS2 6149 R. PCR conditions of the large INDEL assays consisted of an initial denaturation of 94°C for 2 min followed by 35 cycles of 94°C (30 sec), 50°C (30 sec) and 68°C (5 min). INDELs were identified by separating PCR amplicons by agarose gel electrophoresis. The resulting PCR products were also Sanger DNA sequenced with primer TMPRSS2 3968F at the University of Missouri DNA Core. Chromatographs were analyzed for the presence of INDELS in Finch TV.
Genotyping of TMPRSS2 edited offspring
PCR amplicons from each piglet from the small INDEL assay and the long range assay were TOPO cloned using the TOPO TA and TOPO XL kit, respectively (Thermo Scientific) by following standard protocol. Clones were propagated on Luria-Bertani (LB) agarose plates containing 50 μg/ml kanamycin and resistant recombinants were selected. Plasmids containing the TMPRSS2 amplicon were identified by either EcoRI or EagI digestion, and subsequent DNA agarose gel electrophoresis. Plasmids that contained a TMPRSS2 amplicon were DNA sequenced by the University of Missouri DNA core by using the TMPRSS2 F1 or TMPRSS2 3968F oligonucleotide, respectively. Sequences were aligned to the wild type (WT) TMPRSS2 gene in ApE (http://biologylabs.utah.edu/jorgensen/wayned/ape/) and INDELS were examined.
Breeding of TMPRSS2 edited pigs to create F1 offspring
One founder boar (37-1) was bred to three founder females (3-2, 3-4 and 37-5) by artificial insemination.
PCR Screening for Sex Determination
DNA lysate (1 μl) from each blastocyst stage embryo was used to determine the sex by using a PCR based assay described previously (Hao et al. 2006; Whitworth et al. 2010). Briefly, PCR was performed by using GoTaq Green Master Mix (Promega, Madison, WI) with primers specific for sex determining region Y (SRY, GenBank NM_214452, Y chromosome-specific) and nuclear receptor subfamily 0, group B, member 1 (NR0B1, GenBank AF035816, X-chromosome-specific) loci. Oligonucleotides were purchased from Integrated DNA Technologies (IDT, Coralville, IA) and the reaction conditions were 94°C for 5 min followed by 35 cycles of 94°C (30 sec), 58°C (30 sec), 72°C (30 sec) with a final elongation step at 72°C for 3 min. Female blastocysts had a single NR0B1 band at 179 bp while male blastocysts had both the SRY (131 bp) and NR0B1 bands. Genomic DNA from a known male and female pig was used as positive controls for sexing and water was used as the nontemplate control.
Statistical Analysis
Differences in blastocyst development at days 5, 6 or 7 were determined by using PROC GLM of SAS 9.4 (Cary, NC) with a p-value of <0.05 being significantly different. Embryos that developed to the blastocyst stage were classified as 1 and embryos that did not reach the blastocyst stage were classified as 0. Data are presented as mean percent to blastocyst stage ± SEM. Significant differences in sex ratios and modified vs non modified blastocyst stage embryos were determined by chi square using PROC FREQ.
Results
Blastocyst Rates
The overall blastocyst rates for CRISPR guide pairs 1+5, 2+4, 3+6 injected embryos and the controls were 15.9%, 19.2%, 15.9% and 23.9%, respectively. There was no significant difference in development between any of the injected CRISPR pairs and the non-injected controls (P>0.34). The rate of blastocyst development between CRISPR injected embryos and non-injected controls was also not significantly different at day 5 or 6, but was approaching significance by day 7 of embryo culture (p=0.35, 0.08, 0.06, n=566, 863, 859, 3 replicates, respectively).
Comparison of the modification rates between the injected CRISPR pairs
Genotypes were determined on 62.5% of single embryos collected (90/144 embryos). Any embryo that did not result in an amplicon by PCR was removed from the analysis and not sequenced. Injection of CRISPR guides 1+5, 2+4 and 3+6 resulted in a detectable INDEL by either DNA gel electrophoresis or Sanger Sequencing in 92%, 100% and 97.1% of the embryos analyzed (Table 1). There was no significant difference between modification rate between the CRISPR pairs. All three pairs had a significantly higher rate of modification than the non-injected controls which were all not modified (0%, P<0.0001). The rate of biallelic vs. monoallelic modification was not evaluated for embryos.
Table 1.
Comparison of the DNA editing rates between the injected CRISPR pairs 1+5, 2+4 and 3+6
| CRISPR Guide Pairs | Edited Embryos | Total Number of Embryos (n) | Percent |
|---|---|---|---|
| 1+5 | 23 | 25 | 92.0a |
| 2+4 | 31 | 31 | 100.0a |
| 3+6 | 34 | 35 | 97.1a |
| Control | 0 | 11 | 0.0b |
Comparison of modification rates at days 5, 6 or 7 of culture
There was not a significant difference in the number of modifications when compared between days 5, 6 and 7 (P>0.21) with a 100%, 98.2% and 88.9%, respectively (Table 2).
Table 2.
Comparison of DNA editing rates at days 5, 6 or 7 of culture
| Day of Culture | Edited Embryos | Total Number of Embryos (n) | Percent |
|---|---|---|---|
| 5 | 6 | 6 | 100.0a |
| 6 | 54 | 55 | 98.2a |
| 7 | 16 | 18 | 88.9a |
Comparison of sex ratio between modified and unmodified embryos at days 5, 6 and 7 of embryos culture Gender was successfully determined in 56.3% of the embryos (81/144 embryos). Again, any embryo that did not result in an amplicon by PCR was removed from the analysis and gender was not determined. The sex ratio of male and female embryos was not significantly different at the blastocyst stage between day 5, 6 and 7 of development in CRISPR injected embryos or the controls (P>0.84) (Table 3).
Table 3.
Sex Ratio of blastocyst-stage embryos at days 5, 6 and 7 of culture
| Day of Culture | Male Blastocyst Stage Embryos | Female Blastocyst Stage Embryos | Total | Percent Male | Percent Female |
|---|---|---|---|---|---|
| 5 | 5 | 4 | 9 | 55.6a | 44.4a |
| 6 | 25 | 26 | 51 | 49.0a | 51.0a |
| 7 | 9 | 12 | 21 | 42.9a | 57.1a |
Birth of live piglets
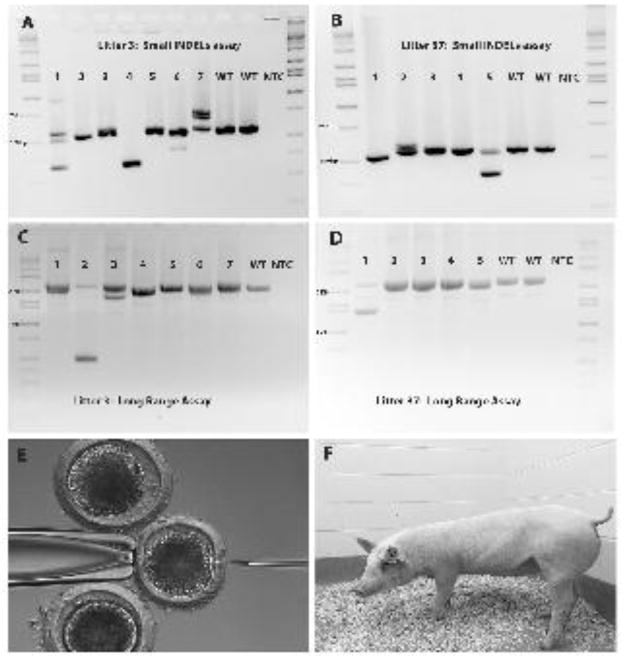
Both of the recipient gilts became pregnant and farrowed healthy piglets. There were 5 piglets in the first litter (litter 37) and 7 piglets in the second litter (litter 3). Litter 37 had 4 male piglets and 1 female piglet. Litter 3 had 6 female piglets and 1 male piglet resulting in a sex ratio of 41.6% males and 58.3% female between the two litters. All 12 piglets were biallelically edited and the details of the edits are listed in Table 4. The corresponding DNA gel electrophoresis for the small deletions assay and the long range assay are shown in Figure 2A–B. The rate of mosaicism was not determined in the embryo studies, but 5 of the 12 (41.7%) piglets were mosaic.
Table 4.
Genotypes of TMPRSS2 edited piglets
| Piglet ID | Sex | Edit Type | Allele 1 | Allele 2 | Description-Allele 1 | Description-Allele 2 |
|---|---|---|---|---|---|---|
| 37-1 | Male | Biallelic/Mosaic | 875 bp deletion | 23 bp deletion | Edit removes exon 2 and all CRISPR binding sites | Intronic edit in CRISPR 6 |
| 37-2 | Male | Biallelic/Mosaic | 1 bp addition | 6 bp deletion | Intronic edit in CRISPR 6 | Intronic edit in CRISPR 6 |
| 37-3 | Male | Biallelic | 4 bp deletion | 1 bp deletion | Intronic edit in CRISPR 4 | Intronic edit in CRISPR 4 |
| 37-4 | Male | Biallelic | 4 bp deletion | 3 bp deletion | Intronic edit in CRISPR 4 | Intronic edit in CRISPR 4 |
| 37-5 | Female | Biallelic | 131 bp deletion | 3 bp deletion | Exon 2 deleted between CRISPR 1 and 5 | Intronic edit in CRISPR 5 |
| 3-1 | Female | Biallelic/Mosaic | 207 bp deletion | 18 bp insertion | Exon2 deleted between CRISPR 3 and 6 | Intronic edit in CRISPR 6 |
| 3-2 | Female | Biallelic | 1739 bp deletion+7 bp | 19 bp deletion | Edit removes exon 2 and all CRISPR binding sites | Intronic edit in CRISPR 5 |
| 3-3 | Female | Biallelic/Mosaic | 3 bp deletion | 1 bp addition | Intronic edit in CRISPR 5 | Intronic edit in CRISPR 5 |
| 3-4 | Female | Biallelic | 211 bp deletion+11 bp | 210 bp deletion+17 bp | Exon2 deleted between CRISPR 3 and 6 | Exon2 deleted between CRISPR 3 and 6 |
| 3-5 | Female | Biallelic | 1 bp addition | 3 bp deletion | Intronic edit in CRISPR 4 | Intronic edit in CRISPR 4 |
| 3-6 | Female | Biallelic | 3 bp deletion | 118 bp deletion | Intronic edit in CRISPR 4 | Exon 2 upstream of CRISPR 4 |
| 3-7 | Male | Biallelic/Mosaic | 1 bp deletion | 14 bp adddition | Intronic edit in CRISPR 4 | Intronic edit 59 bp downstream of CRISPR 4 |
Figure 2.
Genotyping results for the small deletions assay and long range assay for TMPRSS2 DNA edited piglets in litter 3 (A,C) and 37 (B,D). Panel E represents an example of a zygote being injected with CRISPR/Cas9 RNA. Panel F is a healthy TMPRSS2 DNA edited pig.
Birth of TMPRSS2 edited F1 offspring
All three gilts became pregnant and had normal gestations resulting in three litters of F1 offspring. Pigs 3-2, 3-4 and 37-5 had litter sizes of 11, 4 and 9 respectively. All of the piglets inherited an edited TMPRSS2 allele from the founder resulting in a healthy knock-out phenotype.
Discussion
The objectives of this study was to determine if CRISPR/Cas9 edited embryos developed at the same rate as non-modified embryos and to determine if sex ratios were affected. The authors had observed a tendency for male offspring in a previous experiment that utilized CRISPR/Cas9 zygote injection and wanted to confirm that female embryos were not more sensitive to potential adverse effects of modification by this technique (Whitworth et al. 2014). In this set of experiments, there was no significant effect of zygote injection of CRISPR/Cas9 RNA on rate of development to blastocyst stage. Additionally the sex ratio was not different in edited zygotes on day 5, 6 or 7 of embryo culture. DNA editing by CRISPR/Cas9 is a highly embryo culture dependent technique and despite this necessity, very little research has been done to determine how modification of the DNA by this method affects the resulting embryos. It has been shown previously that overall blastocyst rates are not adversely affected by injection (Hai et al. 2014; Wang et al. 2015). One group did utilized the CRISPR/Cas9 system to knock out the pluripotency marker POU5F1 and showed that early blastocyst formation was not affected, but POUF1 was necessary for proper inner cell mass formation (Kwon et al. 2015). The lack of measurable effect on the rate of embryo development and sex ratio further validates that this technique does not have a toxic effect on the resulting embryos. The authors acknowledge that the sample size was somewhat low and this set of RNA guides were particularly efficient in creating INDELS.
A skewing of the sex ratio at the blastocyst stage was not observed in this series of experiments, but it has been shown previously that male and female embryos do respond differently to in vitro culture. Male and female bovine embryos have different metabolic requirements and male embryos have been shown to reach first-cleavage stages earlier than female embryos (Lonergan et al. 1999). In vitro cultured human male embryos also have higher cell numbers at day 6 when compared to their female counterparts (Ray et al. 1995). In the mouse, in vitro cultured male embryos have increased cell numbers by day 3 of culture when compared to female embryos. Interestingly the same study showed that in vivo derived female mouse embryos compact earlier than male embryos, further illustrating the differential effect of culture on the sex ratios of embryos (Peippo and Bredbacka 1995).
In the pig, embryos that cleaved before 30 hours tended to be males while later cleaving embryo shifted to female in a NCSU23 medium modified to be pyruvate-lactase based (Petters and Wells 1993; Torner et al. 2013). The embryos used in this experiment were cultured in an arginine optimized pyruvate, lactate, glutamine based PZM3 medium, MU1 (Redel et al. 2015; Yoshioka et al. 2002) and cultured in MU2 (Spate et al. 2015). Embryos that formed morula and blastocysts on day 5 of culture were transferred to the recipient gilts. The first litter resulted in 4 males and 1 female. The subsequent litter resulted in 6 females and 1 male with an overall percent of male offspring being 41.7%. In the previous study, 87.5% of the resulting piglets were male in two litters (Whitworth et al. 2014). Importantly, both the CRISPR guide RNAs and the source of Cas9 were different between the two experiments and therefore not directly comparable, but the overall sex ratio of blastocysts in the present experiment paralleled what was observed in the resulting offspring.
Zygote injection with CRISPR/Cas9 guide RNA was used to efficiently (100%) create pigs with a biallelic edit of the TMPRSS2 gene for use as a biomedical model (Figure 2 E,F). In 6 of the pigs, exon 2 was successfully deleted on at least one allele and would be expected to have a knock-out phenotype. It appeared that CRISPR guides 4, 5 and 6 were very efficient in producing INDELS by non-homologous end join repair (NHEJ) (Table 4), but these guides were located in the intron preceding the targeted exon 2. If RNA splicing was not disrupted by these edits, the pigs would be expected to have normal expression of TMPRSS2 and no phenotype. As detailed in Figure 1, the RNA guide pairs were spaced at 3 different distances from each other, 39 bp between 2+4, 127 bp between 1+5 and 222 bp between 3+6. The experiment was originally designed to test the efficiency of designed deletions with CRISPR guides at 3 different proximal locations. RNA guides 4, 5 and 6 clearly functioned at a higher efficiency as most INDELs were located in proximity to these guides. A comparison of distance between guides could not be systematically evaluated unless all of the guides functioned at the same efficiency. However, of the four observed designed deletions, pair 3+6 (222bp) was most effective and resulted in 3 designed deletions.
A TMPRSS2 biallelic knock-out model was successfully produced by this method. The offspring will be an important tool to address the role of the swine TMPRSS2 protease in swine influenza pathogenesis. Since cleavage of the influenza hemagglutinin by host proteases is essential for the infectivity of influenza viruses (Hatesuer et al. 2013; Tarnow et al. 2014), a TMPRSS2 biallelic knock-out pig should be resistant to various influenza viruses. As observed so far, the knock-out pigs do not show a distinct phenotype, suggesting functional redundancy in the pig. The production of swine resistant to swine influenza viruses (SIVs) would not only benefit the pork industry which suffers significant economic losses associated with SIV infections, but also public health, since many SIVs have zoonotic potential and swine are considered the “mixing vessel” for the making of novel reassortant influenza viruses (Ma et al. 2009). In addition, since TMPRSS2 plays a role in the activation of the fusion proteins of influenza B viruses, parainfluenza viruses, human metapneumoviruses, and coronaviruses (Tarnow et al., 2014), a TMPRSS2 knock-out pig might be a useful model to study the role of host proteases in the pathogenesis of these respiratory infections.
In summary, edited embryos in this set of experiments reached the blastocyst stage at day 5, 6 or 7 of culture at the same rate as non-edited control embryos. The sex ratio measured in the resulting blastocysts and piglets was not significantly affected by in vitro culture or CRISPR/Cas9 RNA injection. The results of this study further illustrate the effectiveness of DNA editing by CRISPR/Cas9 system with no measurable adverse effects on embryo development.
Supplementary Material
Supplemental Table 1. Template for gBlocks
Supplemental Table 2. Oligonucleotides used for the three PCR based assays to determine DNA editing after CRISPR/Cas9 RNA injection
Acknowledgments
This project was supported by the National Swine Resource and Research Center via funding from the National Institutes of Health (U42 OD011140 to RSP) and Food for the 21st Century at the University of Missouri, National Institutes of Health under contract number HHSN266200700005C (JAR). The authors were like to acknowledge Mykel Anderson for her assistance with genotyping and Jason Dowell for coordinating recipient gilts and surgical assistance. All Sanger DNA sequencing was promptly performed at the University of Missouri DNA core which was greatly appreciated by the authors.
References
- Bauer BK, et al. Transcriptional Profiling by Deep Sequencing Identifies Differences in mRNA Transcript Abundance in In Vivo-Derived Versus In Vitro-Cultured Porcine Blastocyst Stage Embryos. Biol Reprod. 2010 doi: 10.1095/biolreprod.110.085936. biolreprod.110.085936 [pii] [DOI] [PubMed] [Google Scholar]
- Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. doi: 10.1007/978-1-4939-1862-1_10. [DOI] [PubMed] [Google Scholar]
- Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell research. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YH, et al. Production of endothelial nitric oxide synthase (eNOS) over-expressing piglets. Transgenic research. 2006;15:739–750. doi: 10.1007/s11248-006-9020-8. [DOI] [PubMed] [Google Scholar]
- Hatesuer B, Bertram S, Mehnert N, Bahgat MM, Nelson PS, Pohlmann S, Schughart K. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS pathogens. 2013;9:e1003774. doi: 10.1371/journal.ppat.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TW, Mazur MJ, Pettigew JE, Perez-Mendoza VG, Zachary J, Schook LB. A cloned pig model for examining atherosclerosis induced by high fat, high cholesterol diets. Animal biotechnology. 2010;21:179–187. doi: 10.1080/10495398.2010.490693. [DOI] [PubMed] [Google Scholar]
- Kwon J, Namgoong S, Kim NH. CRISPR/Cas9 as tool for functional study of genes involved in preimplantation embryo development. PLoS One. 2015;10:e0120501. doi: 10.1371/journal.pone.0120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, et al. Piglets produced from cloned blastocysts cultured in vitro with GM-CSF. Mol Reprod Dev. 2013;80:145–154. doi: 10.1002/mrd.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2015;22:20–31. doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]
- Lillico SG, et al. Mammalian interspecies substitution of immune modulatory alleles by genome editing. Scientific reports. 2016;6:21645. doi: 10.1038/srep21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P, Khatir H, Piumi F, Rieger D, Humblot P, Boland MP. Effect of time interval from insemination to first cleavage on the developmental characteristics, sex ratio and pregnancy rate after transfer of bovine embryos. J Reprod Fertil. 1999;117:159–167. doi: 10.1530/jrf.0.1170159. [DOI] [PubMed] [Google Scholar]
- Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The role of swine in the generation of novel influenza viruses. Zoonoses and public health. 2009;56:326–337. doi: 10.1111/j.1863-2378.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Niemann H, Petersen B. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 2016 doi: 10.1007/s11248-016-9934-8. [DOI] [PubMed] [Google Scholar]
- Peippo J, Bredbacka P. Sex-related growth rate differences in mouse preimplantation embryos in vivo and in vitro. Mol Reprod Dev. 1995;40:56–61. doi: 10.1002/mrd.1080400108. [DOI] [PubMed] [Google Scholar]
- Peng J, et al. Production of Human Albumin in Pigs Through CRISPR/Cas9-Mediated Knockin of Human cDNA into Swine Albumin Locus in the Zygotes. Scientific reports. 2015;5:16705. doi: 10.1038/srep16705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl. 1993;48:61–73. [PubMed] [Google Scholar]
- Prather RS, et al. An intact sialoadhesin (Sn/SIGLEC1/CD169) is not required for attachment/internalization of the porcine reproductive and respiratory syndrome virus. Journal of virology. 2013;87:9538–9546. doi: 10.1128/JVI.00177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil. 1995;104:165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- Redel BK, Tessanne KJ, Spate LD, Murphy CN, Prather RS. Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase?dimethylarginine dimethylaminohydrolase?nitric oxide axis. Reprod Fertil Dev. 2015 doi: 10.1071/RD14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner S, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JW, et al. Generation of an inbred miniature pig model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012;53:501–507. doi: 10.1167/iovs.11-8784. iovs.11-8784 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, et al. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J Virol. 2014;88:5608–5616. doi: 10.1128/JVI.03677-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AF, Green P. RepeatMasker. 1996 Published on the web at http://www.repeatmasker.org.
- Spate LD, Brown A, Redel BK, Whitworth KM, Prather RS. PS48 can replace bovine serum albumin in pig embryo culture medium, and improve in vitro embryo development by phosphorylating AKT. Mol Reprod Dev. 2015;82:315–320. doi: 10.1002/mrd.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnow C, et al. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J Virol. 2014;88:4744–4751. doi: 10.1128/JVI.03799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner E, Bussalleu E, Briz MD, Yeste M, Bonet S. Energy substrate influences the effect of the timing of the first embryonic cleavage on the development of in vitro-produced porcine embryos in a sex-related manner. Mol Reprod Dev. 2013;80:924–935. doi: 10.1002/mrd.22229. [DOI] [PubMed] [Google Scholar]
- Tsang HG, Rashdan NA, Whitelaw CB, Corcoran BM, Summers KM, MacRae VE. Large animal models of cardiovascular disease. Cell biochemistry and function. 2016 doi: 10.1002/cbf.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Efficient CRISPR/Cas9-mediated biallelic gene disruption and site-specific knockin after rapid selection of highly active sgRNAs in pigs. Scientific reports. 2015;5:13348. doi: 10.1038/srep13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KM, et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biology of reproduction. 2014;91:78. doi: 10.1095/biolreprod.114.121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KM, et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat Biotechnol. 2016;34:20–22. doi: 10.1038/nbt.3434. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, Spate LD, Li R, Rieke A, Sutovsky P, Green JA, Prather RS. Activation method does not alter abnormal placental gene expression and development in cloned pigs. Mol Reprod Dev. 2010;77:1016–1030. doi: 10.1002/mrd.21235. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biology of reproduction. 2002;66:112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Template for gBlocks
Supplemental Table 2. Oligonucleotides used for the three PCR based assays to determine DNA editing after CRISPR/Cas9 RNA injection