Abstract
Emerging experimental evidence suggests that both networks and their component neurons respond to similar inputs differently depending on the state of network activity. The network state is determined by the intrinsic dynamical structure of the network and may change as a function of neuromodulation, the balance or stochasticity of synaptic inputs to the network and the history of network activity. Much of the knowledge on state-dependent effects comes from comparisons of awake and sleep states of the mammalian brain. Yet, the mechanisms underlying these states are difficult to unravel. Several vertebrate and invertebrate studies have elucidated cellular and synaptic mechanisms of state-dependence resulting from neuromodulation, sensory input, and experience. Recent studies have combined modeling and experiments to examine the computational principles that emerge when network state is taken into account; these studies are highlighted in this article. We discuss these principles in a variety of systems (mammalian, crustacean, and mollusk) to demonstrate the unifying theme of state-dependence of network output.
Keywords: Network, Neuromodulation, Noise, Sensory Neurons, Motor Control, Olfactory, Stomatogastric, Aplysia, Computer
Classical studies of processing in the central nervous system have typically assumed that its networks have relatively fixed input-output relationships. Many experimental studies now indicate, however, that neuronal networks respond to inputs differently depending on their state. The network state is often defined as the state of intrinsic activity of the network at any time, determined by the structure of the network's dynamics and not driven by any specific stimulus (Fontanini and Katz, 2008). Changes in the network state can have major effects on the input-output relations of the network's individual neurons and, as a consequence, of the whole neuronal ensemble.
Within the network, the dynamics that produce the network state are determined by the intrinsic cellular properties of the neurons and their synaptic connections. These can change as a result of the network activity on many different time scales, from milliseconds (e.g. with short-term synaptic plasticity) to days or months (e.g. with long-term potentiation or depression, changes in receptor expression). Globally, the properties can be modified by external neuromodulatory inputs and by higher-order factors such as behavioral state, attention, and learning (Brunel, 2000; Destexhe and Marder, 2004; MacLean et al., 2005; Karmarkar and Dan, 2006).
Due to the dynamic nature of the network state, it is often unclear how to examine its consequences on neuronal processing. There are simply too many interacting components to be able to manipulate or even to identify the network state purely experimentally. A number of computational studies, however, have demonstrated the importance of the network state in determining the neuronal response (Chance et al., 2002; Fellous et al., 2003; Prescott and De Koninck, 2003; Destexhe and Contreras, 2006). Here we highlight four recent examples in which a computational approach has been combined with experiments to further explore the mechanisms and functional roles of the network state.
Neuronal responsiveness during stochastic network states
In the awake state, the mammalian thalamus and cerebral cortex display highly stochastic neuronal activity. This stochasticity can be observed through various measurement techniques such as intracellular recordings, local field potentials, electroencephalograms and optical imaging. For instance, in vivo recordings show that the membrane potential of individual cortical neurons exhibits considerable fluctuations (“noise”) due to the activity of thousands of synapses that converge on the neuron. Recent results show that such stochastic activity affects information transfer by neurons and networks.
Traditionally, cellular neurophysiologists have studied the integrative properties of single neurons by considering them as isolated entities. It is only relatively recently that it was realized that the input-output transfer function of neurons is greatly affected by the activity of the surrounding network. An elegant approach to investigate this is to use the dynamic-clamp technique to artificially reproduce different levels of stochastic synaptic activity (presumably representing different network states) by injecting the corresponding computer-generated conductance into a living neuron (Destexhe et al., 2001; Chance et al., 2002; Prescott and De Koninck, 2003; Shu et al., 2003). This approach was first applied to cortical neurons, and revealed an important effect of the stochastic synaptic activity the on neuron's responsiveness, similar to computational model predictions (Ho and Destexhe, 2000). Models also predicted that such synaptic activity may affect the local integrative properties of dendrites (Rudolph and Destexhe, 2003), but this prediction remains to be tested.
More recently, the same technique was applied to neurons that display dominant intrinsic properties, such as the burst-generating thalamic neurons. The transfer function is particularly important in this case, because thalamic neurons relay sensory information to the cortex. Here too, dramatic effects of stochastic synaptic activity on responsiveness were found. But, in this case, the intrinsic properties combine with the stochastic synaptic activity to yield a specific global responsiveness to inputs (Wolfart et al., 2005) which is highly dependent on the burst-generating properties of the thalamic neurons. The bursting occurs more frequently at hyperpolarized potentials. Remarkably, counting the total number of spikes (occurring both individually and in bursts) per unit time shows that responsiveness in stochastic conditions is approximately independent of the membrane potential. The insensitivity to baseline membrane potentials depends on the presence of the T-type calcium current which results in bursting in response to hyperpolarization, thus compensating for the expected reduction in spike rate. This is only possible in the presence of synaptic noise and therefore only occurs in states of intense network activity. This example shows that the global responsiveness of neurons is fundamentally activity-dependent and must be understood through a combination of intrinsic properties and stochastic synaptic activity.
At the network level too, experiments have shown that responsiveness is strongly dependent on network state. Early studies indicated different types of state-dependent processing according to the wake/sleep cycle or the level of attention (reviewed in (Steriade, 2003; Destexhe and Contreras, 2006)). More recently, theoretical studies have found strong effects of added noise on the information processing capabilities of neuronal networks. Evidence that the activity of the cerebral cortex shares statistical properties with stochastic processes such as Poisson-like spike discharges (Softky and Koch, 1993; Bedard et al., 2006) provides a biological context to this effect of added noise, implying that neurons in activated brain states (such as wakefulness) are indeed embedded in a very noisy environment, and such network-level effects of noise may actually be more realistic than previously thought.
In biological networks noise is mostly internally generated. Recent theoretical studies have begun to investigate how networks of neurons can generate noisy states of activity leading to stochastic firing patterns in the form of asynchronous irregular (AI) states. It is unclear whether such AI states present any advantage for information processing. A complication is that small model networks can generate AI states which are not in a realistic conductance state (due to the limited amount of connectivity synapses need to be unrealistically strong) and this excessive conductance has detrimental effects on information propagation (El Boustani et al., 2007). More recently it was shown that AI states displaying the correct global conductance state require large networks with diluted connectivity, with many synapses per neuron, but of small weight (El Boustani et al., 2007; Kumar et al., 2008b). Such network configurations seem to be better candidates to study information processing and indeed such large networks were recently found to sustain synfire chain propagation (Kumar et al., 2008a). Because simulation of such large networks requires substantial computational resources, these networks are more readily implemented on parallel computers. With the recent advances in measuring the activity of cortical neurons in awake animals, e.g., (Gobel et al., 2007; Greenberg et al., 2008), we anticipate that such network-level studies will become essential to our understanding of information processing in stochastic network states in the awake and attentive brain.
Neuromodulation of network state
Neuromodulatory substances modify the properties of neurons and synapses, thereby reconfiguring networks and altering their activity (Marder and Thirumalai, 2002). Network activity is often conditional upon the presence of neuromodulators, which are typically released as circulating hormones or by modulatory projection neurons. An extreme example of neuromodulator-dependent changes in network state is reflected in the different states of the brain during wakefulness and different stages of sleep (Giocomo and Hasselmo, 2007). In particular, it has been suggested that REM sleep and wakefulness are functionally equivalent in activity patterns but differ in aminergic and cholinergic modulatory states (Llinas and Ribary, 1993; Kahn et al., 1997).
Much of our understanding of the network-level effects of neuromodulation comes from the studies of invertebrate networks, in particular central pattern generators (CPGs) underlying rhythmic movements (Dickinson, 2006). Here we give an example of how neuromodulation can affect the state of a CPG network and, as a result, the mechanisms underlying pattern generation in the network. Recent studies in an invertebrate CPG, the crustacean gastric mill network, show that the neuromodulatory state of the network can change the mechanism through which network oscillations are produced without affecting the characteristics of the oscillations such as cycle frequency and the relative phases of the component neurons. As a consequence, in these different network states, network activity remains similar yet the locus of control of the network activity is different.
The activation of the gastric mill CPG in the crab stomatogastric ganglion (STG) is controlled by activity of descending projections to the STG. Identified projection neurons release neuromodulatory substances and interact with the target circuit neurons to elicit the rhythmic oscillations (Nusbaum et al., 2001; Nusbaum and Beenhakker, 2002). At the heart of the gastric mill CPG is a half-center oscillator comprised of two reciprocally inhibitory neurons, LG and Int1. In the absence of the rhythm, the LG neuron is silent while Int1 is active. Previous experimental and modeling work has shown that the gastric mill rhythm elicited by the projection neuron MCN1 depends crucially on presynaptic inhibition of the MCN1 axon terminals by the LG neuron (Coleman et al., 1995; Nadim et al., 1998). Without the presynaptic inhibition, oscillations break down. Bath application of the modulatory neuropeptide pyrokinin (PK) elicits a similar gastric mill oscillation but without MCN1 participation (Saideman et al., 2007a; Saideman et al., 2007b). PK is not released by MCN1, and it is the first known neuromodulator to elicit a gastric mill oscillation when bath applied to the crab STG. Although the mechanism that underlies the PK-elicited oscillation is unknown, a recent modeling analysis of the gastric mill network suggests three distinct ionic mechanisms, all depending on the modulation of the LG neuron by PK (Kintos et al., 2008). All three mechanisms result in the ability of the LG neuron to produce plateau potentials and, using phase plane analysis, it is possible to show that plateau potentials in the LG neuron result in oscillations that are qualitatively equivalent to the MCN1-elicited gastric mill oscillation.
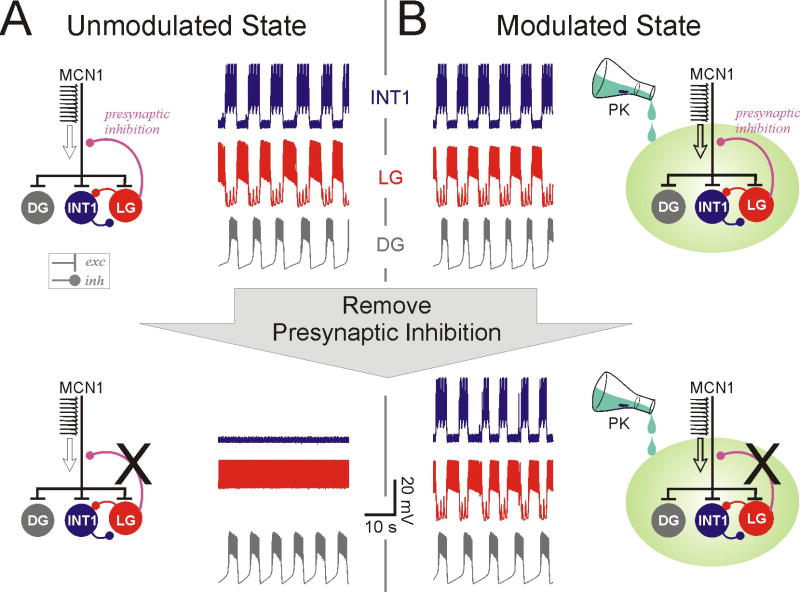
The similarity between the PK- and MCN1-elicited oscillations raises the question as to the importance of the presynaptic inhibition in the presence of PK. Recent experiments show that PK can modulate the MCN1-elicited oscillations at low concentrations where it cannot elicit a rhythm by itself (M.P. Nusbaum, personal communication). Mathematical analysis and computational modeling can be used to examine the role of the presynaptic inhibition in the presence of the neuromodulator PK. The results indicate that, depending on the strength of the modulation by PK, the MCN1-elicited oscillations can switch between modes that depend on the presynaptic inhibition (Fig. 1A) or are independent of it (Fig 1B). At intermediate strengths, oscillations persist in the absence of either PK or the presynaptic inhibition but not both.
Figure 1.
Neuromodulation of the crustacean gastric mill CPG network states. Computational modeling shows that neuromodulation (by the peptide pyrokinin: PK) of the gastric mill rhythm elicited by the projection neuron MCN1 can result in little qualitative change in network output (top traces). Yet, in contrast to the unmodulated or weakly modulated state (A), the generation of network oscillations in the strongly modulated state (B) does not depend on presynaptic inhibition of the axon terminals of the projection neuron MCN1 (bottom traces). Models modified from Kintos et al (2008).
These interactions provide an interesting example of network states in which the generation and control of oscillations can switch between distinct mechanisms depending on the level of the neuromodulator's actions (e.g., depending on its concentration) yet the characteristics of the oscillations remain unchanged. As a result, sensory, coordinating or additional modulatory inputs to the network (Beenhakker et al., 2005; Blitz et al., 2008) may have distinct actions on these two seemingly undistinguishable states.
Network states and sensory perception
Sensory perception can be significantly altered by behavioral states including attention, hunger and social status. These behavioral states have been shown in many instances to affect network states in early sensory processing via central feedback and neuromodulatory inputs from other brain areas (McClurkin et al., 1994; Worgotter et al., 2002; Alitto and Usrey, 2003; Kiselycznyk et al., 2006; Gilbert and Sigman, 2007). The olfactory bulb (OB), a cortical sensory area receiving direct input from sensory neurons without relay through the thalamus, lends itself particularly well to investigations of the relationship between network and behavioral states and the resulting changes in sensory perception (Cleland and Linster, 2005; Kiselycznyk et al., 2006). A recent study by Beshel et al (2007), for example, showed for the first time that behavioral task demands can modulate oscillatory processes in the OB, strengthening the idea that oscillations and the underlying neural circuitry may reflect network states that directly affect odor perception (Cleland and Linster, 2002; Kay, 2003; Cleland and Linster, 2005). These results are in good agreement with previous work in honeybees and mice showing that perceptual changes accompany changes in oscillatory dynamics in the olfactory system (Stopfer et al., 1997; Laurent et al., 2001; Kay and Stopfer, 2006).
Recent computational modeling illustrates the mechanisms through which network state is altered and how this alteration affects perception. Models of the honeybee antennal lobe, the analogue to the mammalian OB, show that oscillatory dynamics and accompanying changes in spike synchrony among antennal lobe output neurons alone, independent of changes in overall spike rate, can modulate odor discrimination when paired with a hebbian learning rule (Linster and Cleland, 2001). Furthermore, modulation of synchrony alone can replicate behaviorally observed changes in odor discrimination at different stimulus intensities (Cleland and Linster, 2002). These computational results in this system clearly show a correlation between odor discrimination and network state. The dynamic state of the network is regulated by extrinsic factors such as stimulus concentration, amounts of dopamine conveyed by neuromodulatory neurons, and intrinsic factors such as synaptic efficacy, voltage-dependent intrinsic currents and short-term plasticity, as has been shown experimentally (Stopfer et al., 1997; Stopfer and Laurent, 1999; Friedrich and Stopfer, 2001; Stopfer et al., 2003; Brown et al., 2005).
In the rodent OB, network state is influenced by sensory inputs from the nose, centrifugal inputs from other brain areas, as well as short- and long-term synaptic plasticity and changes in neuronal survival affecting bulbar processing (reviewed in (Cleland and Linster, 2005; Kay and Stopfer, 2006)). All these changes have been shown to directly affect odor perception in rodents. First, perceptual similarities of odor pairs are highly correlated with similarities among activity patterns evoked in the OB input layer (Linster and Hasselmo, 1999; Linster et al., 2001b; Rubin and Katz, 2001; Cleland et al., 2002; Youngentob et al., 2006). Second, modulation of these patterns by cholinergic inputs to the OB in a computational model predicts perceptual changes confirmed by behavioral lesion and pharmacological experiments (Linster et al., 2001a; Linster and Cleland, 2002; Mandairon et al., 2006d). Third, recent experiments have shown that manipulations that change the state of the bulbar network, for example, direct local infusion of NMDA, significantly affect odor perception (Mandairon et al., 2006b). Fourth, olfactory experience has been shown to modulate the survival of newborn cells in the OB. Given that these cells are inhibitory interneurons known to be involved in regulation of response curves and dynamics, these results also establish a clear relationship between network state and perception (Rochefort et al., 2002; Lledo and Saghatelyan, 2005).
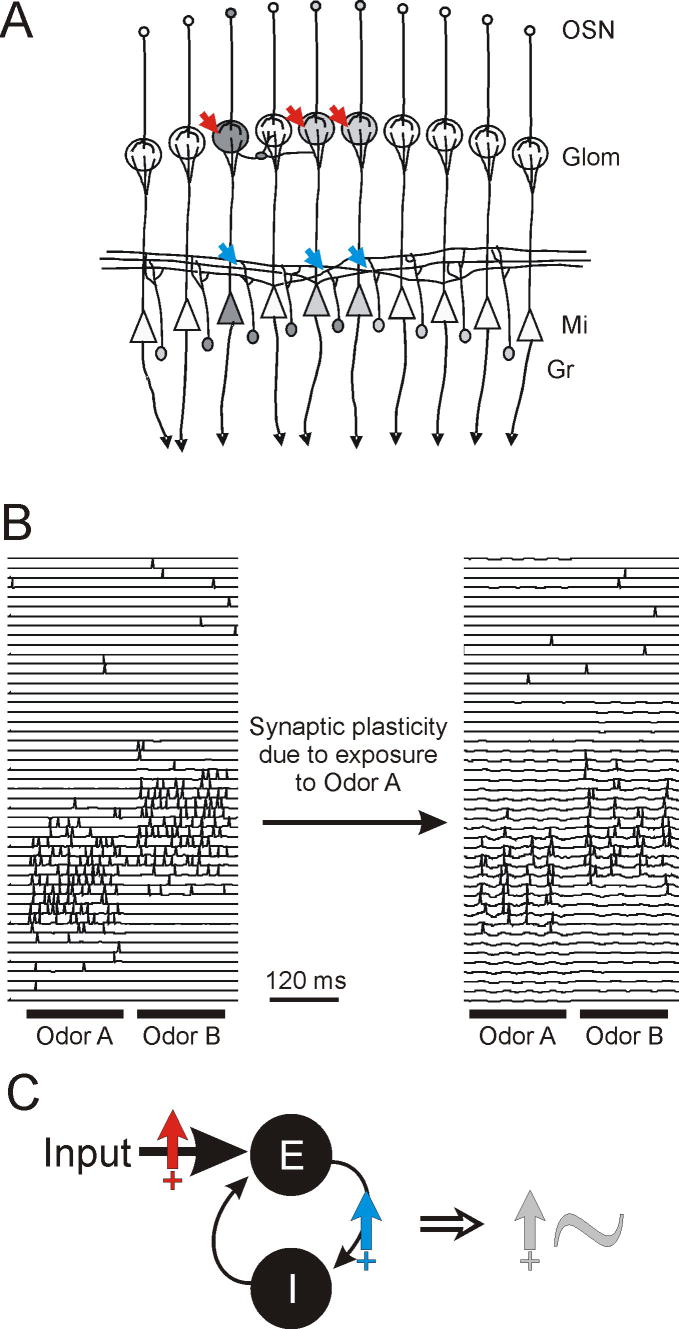
Recent experiments show that daily experience with odorants significantly affects the rat's ability to discriminate between chemically and perceptually very similar odorants (Mandairon et al., 2006c; Mandairon et al., 2006a; Escanilla et al., 2008). Interestingly, similar effects can be obtained by manipulation of OB processing by replacing experience with odorants by daily injections of NMDA, thought to mimic activation of OB neurons by odorants (Mandairon et al., 2006b). Furthermore, activation of NMDA receptors is necessary for perceptual changes to occur with experience with odorants and responsiveness of local interneurons, as measured with immediate-early gene mapping, is significantly increased in animals with daily odor experience as compared to control animals (Mandairon et al., 2006b; Mandairon et al., 2008). Computational modeling shows that NMDA-dependent plasticity, implemented on olfactory sensory neuron-to-mitral and mitral-to-granule cell synapses (Ennis et al., 1998; Satou et al., 2005; Satou et al., 2006) during odor exposure times results in increased activation of mitral cells as well as increased interneuron responsiveness to odorants (Fig. 2A, B). The resulting increased inhibition changes the network state and its response to sensory inputs in two ways. First, mitral cell odor responses become sparse and the overlap between neurons responsive to chemically similar odorants is reduced. Second, oscillatory dynamics and the accompanying synchrony among olfactory neurons is increased due to the increased efficacy of the excitatory-inhibitory feedback loop between mitral and granule cells (Fig. 2C). Both effects potentially contribute to the enhanced perceptual discrimination measured behaviorally. These studies show how experience with odorants can affect network state, sensory processing and perception on long time scales (up to three weeks). OB network states can also be affected on a short time scales by cholinergic, noradrenergic and other centrifugal inputs, leading to changes in odor perception and learning.
Figure 2.
Large scale modeling of olfactory bulb network state. A, Schematic illustration of the model. Olfactory sensory neurons (OSN), responding to odor input in a distributed manner, each project to one glomerulus (Glom), in which they make excitatory synapses with olfactory bulb output neurons (mitral cells, Mi) and diverse local interneurons (not shown). Each mitral cell receives sensory input from a single class of OSNs within a given glomerulus. Mitral cells have extensive lateral dendrites through which they form reciprocal synaptic connections with inhibitory interneurons (granule cells, Gr). Mitral cell axons project onto a large number of secondary olfactory structures. Based on data from brain slice experiments, synaptic plasticity was implemented on excitatory synapses between OSNs and mitral cells as well as between mitral and granule cells (arrows). B, Simulation results showing the membrane potential and action potentials of a subset of mitral cells in response to stimulation with two odors A and B, before and after simulated exposure to odor A. Note the sparsening of mitral cell responses to odorants accompanied by increased oscillatory dynamics and synchrony among individual mitral cells. C, Synaptic plasticity in the model leads to stronger inputs to mitral cells (red arrow) due to increased synaptic efficacy as well as a stronger feedback loop (blue arrow) between mitral and granule cells. Together, these changes enhance the oscillatory power (grey arrow) of the excitatory-inhibitory feedback loop formed by the mitral/granule cell reciprocal synapse.
History-dependence of the network state
In the most interesting cases, the network state is strongly driven by its own internal dynamics and, consequently, the present state can depend strongly on its own history. Inputs to the network do not produce outputs in any simple manner, but are better thought of as perturbing the trajectory of the network state, which produces the outputs, thereby “interpreting” the inputs depending on the history (Beer, 1995, 1997). Concepts of this kind are applicable in sensory processing (Fontanini and Katz, 2008), perception (Engel et al., 2001), attention (Deco and Rolls, 2005), and motor control, where controllers are often viewed as incorporating internal models that, for example, continually evaluate the error between the predicted and the actual sensory stimuli that are received as feedback as the behavior is executed (Kawato, 1999; Bays and Wolpert, 2007). Most such systems, however, are complex and many important components are unknown. For our final example, we describe a simple system in which the basic principles can already be seen, studied at a variety of levels both experimentally and computationally, and readily connected to the functional behavior of the animal.
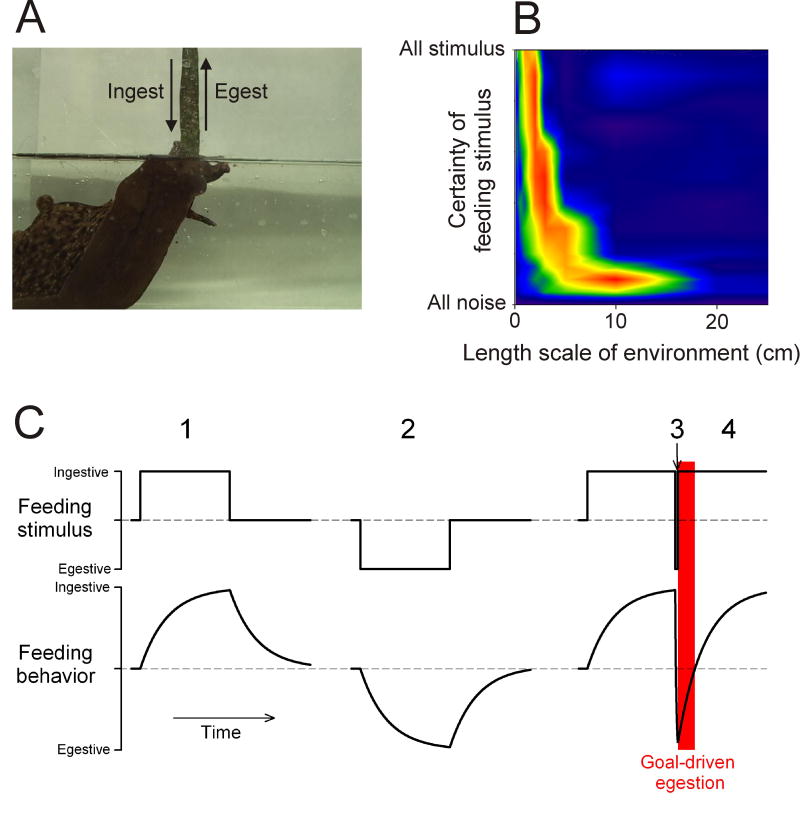
The sea slug Aplysia eats seaweed, often in the form of long fronds or strips, in a cyclical, rhythmic fashion, incrementally swallowing one short segment of the strip after another (Kupfermann, 1974; Morton and Chiel, 1993; Lum et al., 2005). At the core of the feeding system is a multitasking CPG that produces motor programs that underlie this ingestive behavior as well as an egestive behavior: a reversal of the phasing of the feeding movements so that seaweed or other material that has been swallowed but is then judged inedible is incrementally expelled again (Fig. 3A). The CPG does not cycle spontaneously: each cycle must be triggered by sensory stimuli. Because these stimuli are signals that seaweed is present or, conversely, that inedible material has been swallowed, one might think that they would trigger either an ingestive or egestive motor program in response. However, such a direct response is not produced. Rather, as first observed by Proekt et al (2004), the state of the CPG network mediates between the stimulus and the response. The sensory stimuli act through the dynamics of the CPG to influence the evolution of the network state, but it is the state of the network at any moment that determines the type of motor program that is produced.
Figure 3.
Feeding CPG dynamics and behavior in Aplysia. A, Aplysia in a feeding posture. The ingestive and egestive arrows indicate movement of the seaweed strip into and out of the mouth of the animal, respectively. Adapted from Lum et al (2005). B, Functional performance of a computational model of the feeding CPG dynamics shown in C in a simulated feeding task modeled on the behavior in A, plotted over a range of two basic parameters of a simulated feeding environment: the length scale of the environment (the average length of the seaweed strips present in the environment) and the certainty with which the feeding stimuli in the environment can actually be detected. Warm colors indicate good performance, cool colors poor performance. C, Cartoon of the dynamics of the feeding CPG as quantified by Proekt et al (2004). Discrete motor programs are not represented; rather, the underlying evolution of their ingestive-egestive character is indicated by a time-continuous variable, the “feeding behavior.” The red rectangle indicates a period during which, in the behavioral simulations described in the text, the model would perform goal-driven egestion, that is, egestion despite an ingestive stimulus.
The dynamics of the CPG are for the most part slow (Proekt et al., 2004; Zhurov et al., 2005). They integrate the incoming stimuli over multiple cycles so that the character of the motor programs progressively evolves in the ingestive direction with repeated ingestive stimuli, and in the egestive direction with repeated egestive stimuli (Fig. 3C, 1 and 2). Furthermore, after a switch from egestive to ingestive stimuli, the programs exhibit inertia: they remain egestive for some time (Fig. 3C, 4). After the converse switch from ingestive to egestive stimuli, however, there is no inertia: the programs become egestive immediately (Fig. 3C, 3). This one component of fast dynamics in the system thus creates an asymmetry in the response depending on the order of the ingestive and egestive stimuli—an asymmetry that plays an important role in the behavior (see below). Within the CPG, the dynamics are created by such mechanisms as activity-dependent plasticity at synapses between neurons that represent, and in some cases are specifically recruited by, the type of stimulus and/or response (Proekt et al., 2004; Proekt et al., 2007; Sasaki et al., 2008). Activity-dependent release of slowly acting modulatory neuropeptide cotransmitters from many of the CPG neurons probably also plays an important role, so that the network state may in part be due to its modulatory state (Morgan et al., 2002; Jing et al., 2007).
Similar mechanisms operate simultaneously in the peripheral musculature of the feeding system. When the motor neurons that drive the feeding muscles become active, they also release modulatory cotransmitters that modify the muscle contractions (Weiss et al., 1992; Brezina et al., 1996, 2000). These modulatory actions, too, have both slow and fast components. From extensive biophysical and physiological data available in this system, these dynamics have been captured in realistic models (Brezina et al., 2003a, b). Computational simulations and theoretical analysis of these models under various behavioral circumstances (Brezina et al., 2005) suggests that the modulatory dynamics likewise constitute a network, albeit a non-neural one, with its own state, its own memory of the history of the feeding system, and its own dynamical computational capabilities, in conjunction with which those of the CPG must act in order to execute functional feeding movements.
What behavioral functions are in fact performed by the dynamics in this system? In the field of computational neuroethology, it has been argued (e.g., by Chiel and Beer (1997)) that adaptive behavior is ultimately a product of the entire dynamical system of the nervous system, the non-neural structures of the body, and the environment. This suggests the computational approach of running a model embodying the observed CPG and neuromuscular dynamics—in effect, an autonomous Aplysia agent—in various simulated feeding environments and analyzing the performance of the entire system in a realistic feeding task. The results show that the slow integrative CPG dynamics, which conservatively produce motor programs similar to preceding ones, allow efficient ingestion of long seaweed strips even in the face of considerable noise from sensory inputs and other uncertainties resulting from the true state of the environment. If, however, even a brief egestive stimulus is received, signaling that the ingested seaweed strip may be inedible, the fast component of the CPG dynamics triggers a rapid switch to a different mode, in which the system transiently ignores the incoming stimuli and follows an internal “goal,” emergent from the dynamics, to egest the entire strip again (see red rectangle in Fig. 3C). This goal-driven egestion has the properties of a reflex or fixed-action pattern, and generally resembles vertebrate regurgitation or vomiting behavior, which may also be driven by a CPG-like neural network (Hornby, 2001; Horn, 2008). The time scales of the dynamics are tuned for optimal performance over a range of environmental parameters (Fig. 3B) that corresponds well to those that Aplysia encounter in the wild and in which the observed CPG and neuromuscular interactions have presumably evolved.
The network state of the Aplysia feeding CPG, together with that of the peripheral musculature, is thus able to interpret incoming sensory information in the light of past experience and the current functional goals of the animal to produce the optimal behavioral output. Operationally, the state of Aplysia feeding CPG has been described as a “predictive,” “expectational,” or “intentional” internal state (Proekt et al., 2004). If performed by the mammalian CNS, these would be understood as cognitive functions. Yet here they are performed by a simple motor network. Numerous parallels can indeed be drawn between mammalian cortical circuits and CPGs (Yuste et al., 2005), and it may furthermore be that the implementation of high-level cognitive functions even in mammals is to some degree distributed throughout the nervous system, including its low-level circuits, and even non-neural structures that are involved in behavior (see (Clark, 1997, 2000)).
Concluding remarks
As our four examples suggest, the network state can be considered from a bottom-up or a top-down perspective, from the viewpoint of mechanism or of function. Often, the two go hand-in-hand. In three of our examples, mechanisms that change the network state (stochastic or oscillatory neuronal activity, synaptic plasticity, neuromodulation) are at least partially identified and the changes in network state are easily recognized. At the same time, it is possible to observe some of the functional consequences for the input-output processing performed by the network. In the crab STG example, however, the internal configuration of the network, and therefore the network state, changes with neuromodulation, yet the observed activity of the network remains unchanged. Although this may represent redundancy in network output, it is more likely that the change in network state may be read out in other parameters, or alter the effect of additional (e.g. sensory or coordinating) inputs to the network. Conversely, in the Aplysia feeding example, the functional approach predominates. Then similar dynamics and input-output transformations can suggest a functional similarity of networks, even non-neural networks, which may have very different internal mechanisms.
Acknowledgments
Supported by NIH-MH60605 (FN), NIH-NS41497 (VB). AD is supported by CNRS, ANR and European Community (FACETS).
Contributor Information
Farzan Nadim, New Jersey Institute of Technology & Rutgers University, Newark, NJ.
Vladimir Brezina, Mount Sinai School of Medicine, New York, NY.
Alain Destexhe, CNRS, Gif sur Yvette, France.
Christiane Linster, Cornell University, Ithaca, NY.
References
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol. 2003;13:440–445. doi: 10.1016/s0959-4388(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Bays PM, Wolpert DM. Computational principles of sensorimotor control that minimize uncertainty and variability. J Physiol. 2007;578:387–396. doi: 10.1113/jphysiol.2006.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard C, Kroger H, Destexhe A. Does the 1/f frequency scaling of brain signals reflect self-organized critical states? Phys Rev Lett. 2006;97:118102. doi: 10.1103/PhysRevLett.97.118102. [DOI] [PubMed] [Google Scholar]
- Beenhakker MP, DeLong ND, Saideman SR, Nadim F, Nusbaum MP. Proprioceptor regulation of motor circuit activity by presynaptic inhibition of a modulatory projection neuron. J Neurosci. 2005;25:8794–8806. doi: 10.1523/JNEUROSCI.2663-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RD. A dynamical systems perspective on agent-environment interactoin. Artif Intel. 1995;72:173–215. [Google Scholar]
- Beer RD. The dynamics of adaptive behavior: a research program. Robot Auton Syst. 1997;20:257–289. [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Functional uncoupling of linked neurotransmitter effects by combinatorial convergence. Science. 1996;273:806–810. doi: 10.1126/science.273.5276.806. [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Optimization of rhythmic behaviors by modulation of the neuromuscular transform. J Neurophysiol. 2000;83:260–279. doi: 10.1152/jn.2000.83.1.260. [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Neuromuscular modulation in Aplysia. II. Modulation of the neuromuscular transform in behavior. J Neurophysiol. 2003a;90:2613–2628. doi: 10.1152/jn.01093.2002. [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Neuromuscular modulation in Aplysia. I. Dynamic model. J Neurophysiol. 2003b;90:2592–2612. doi: 10.1152/jn.01091.2002. [DOI] [PubMed] [Google Scholar]
- Brezina V, Horn CC, Weiss KR. Modeling neuromuscular modulation in Aplysia. III. Interaction of central motor commands and peripheral modulatory state for optimal behavior. J Neurophysiol. 2005;93:1523–1556. doi: 10.1152/jn.00475.2004. [DOI] [PubMed] [Google Scholar]
- Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat Neurosci. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- Brunel N. Dynamics of networks of randomly connected excitatory and inhibitory spiking neurons. J Physiol Paris. 2000;94:445–463. doi: 10.1016/s0928-4257(00)01084-6. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chiel HJ, Beer RD. The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- Clark A. Being there: putting brain, body, and world together again. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Clark A. Mindware: an introduction to the philosophy of cognitive science. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Cleland TA, Linster C. How synchronization properties among second-order sensory neurons can mediate stimulus salience. Behav Neurosci. 2002;116:212–221. doi: 10.1037//0735-7044.116.2.212. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Computation in the olfactory system. Chem Senses. 2005;30:801–813. doi: 10.1093/chemse/bji072. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature. 1995;378:502–505. doi: 10.1038/378502a0. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET. Attention, short-term memory, and action selection: a unifying theory. Prog Neurobiol. 2005;76:236–256. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D. Neuronal computations with stochastic network states. Science. 2006;314:85–90. doi: 10.1126/science.1127241. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24. doi: 10.1016/s0306-4522(01)00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol. 2006;16:604–614. doi: 10.1016/j.conb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- El Boustani S, Pospischil M, Rudolph-Lilith M, Destexhe A. Activated cortical states: experiments, analyses and models. J Physiol Paris. 2007;101:99–109. doi: 10.1016/j.jphysparis.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ennis M, Linster C, Aroniadou-Anderjaska V, Ciombor K, Shipley MT. Glutamate and synaptic plasticity at mammalian primary olfactory synapses. Ann N Y Acad Sci. 1998;855:457–466. doi: 10.1111/j.1749-6632.1998.tb10606.x. [DOI] [PubMed] [Google Scholar]
- Escanilla O, Mandairon N, Linster C. Odor-reward learning and enrichment have similar effects on odor perception. Physiol Behav. 2008;94:621–626. doi: 10.1016/j.physbeh.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Rudolph M, Destexhe A, Sejnowski TJ. Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience. 2003;122:811–829. doi: 10.1016/j.neuroscience.2003.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. Behavioral states, network states and sensory response variability. J Neurophysiol. 2008 doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Stopfer M. Recent dynamics in olfactory population coding. Curr Opin Neurobiol. 2001;11:468–474. doi: 10.1016/s0959-4388(00)00236-1. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Gobel W, Kampa BM, Helmchen F. Imaging cellular network dynamics in three dimensions using fast 3D laser scanning. Nat Methods. 2007;4:73–79. doi: 10.1038/nmeth989. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- Ho N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- Horn CC. Why is the neurobiology of nausea and vomiting so important? Appetite. 2008;50:430–434. doi: 10.1016/j.appet.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111 8A:106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn D, Pace-Schott EF, Hobson JA. Consciousness in waking and dreaming: the roles of neuronal oscillation and neuromodulation in determining similarities and differences. Neuroscience. 1997;78:13–38. doi: 10.1016/s0306-4522(96)00550-7. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–585. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- Kay LM, Stopfer M. Information processing in the olfactory systems of insects and vertebrates. Semin Cell Dev Biol. 2006;17:433–442. doi: 10.1016/j.semcdb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Kintos N, Nusbaum MP, Nadim F. A modeling comparison of projection neuron- and neuromodulator-elicited oscillations in a central pattern generating network. J Comput Neurosci. 2008;24:374–397. doi: 10.1007/s10827-007-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem. 2006;13:575–579. doi: 10.1101/lm.285706. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rotter S, Aertsen A. Conditions for propagating synchronous spiking and asynchronous firing rates in a cortical network model. J Neurosci. 2008a;28:5268–5280. doi: 10.1523/JNEUROSCI.2542-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Schrader S, Aertsen A, Rotter S. The high-conductance state of cortical networks. Neural Comput. 2008b;20:1–43. doi: 10.1162/neco.2008.20.1.1. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol. 1974;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66:497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. How spike synchronization among olfactory neurons can contribute to sensory discrimination. J Comput Neurosci. 2001;10:187–193. doi: 10.1023/a:1011221131212. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 2002;15:709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001a;115:826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001b;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum CS, Zhurov Y, Cropper EC, Weiss KR, Brezina V. Variability of swallowing performance in intact, freely feeding aplysia. J Neurophysiol. 2005;94:2427–2446. doi: 10.1152/jn.00280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Linster C. Olfactory enrichment improves the recognition of individual components in mixtures. Physiol Behav. 2006a;89:379–384. doi: 10.1016/j.physbeh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Didier A, Linster C. Odor enrichment increases interneurons responsiveness in spatially defined regions of the olfactory bulb correlated with perception. Neurobiol Learn Mem. 2008;90:178–184. doi: 10.1016/j.nlm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci U S A. 2006b;103:13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Enrichment to odors improves olfactory discrimination in adult rats. Behav Neurosci. 2006c;120:173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006d;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- McClurkin JW, Optican LM, Richmond BJ. Cortical feedback increases visual information transmitted by monkey parvocellular lateral geniculate nucleus neurons. Vis Neurosci. 1994;11:601–617. doi: 10.1017/s0952523800002492. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol. 2002;87:49–61. doi: 10.1152/jn.00438.2001. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol [A] 1993;172:17–32. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- Nadim F, Manor Y, Nusbaum MP, Marder E. Frequency regulation of a slow rhythm by a fast periodic input. J Neurosci. 1998;18:5053–5067. doi: 10.1523/JNEUROSCI.18-13-05053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc Natl Acad Sci U S A. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci U S A. 2004;101:9447–9452. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the aplysia feeding network. J Neurophysiol. 2007;97:3046–3056. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Spatial coding of enantiomers in the rat olfactory bulb. Nat Neurosci. 2001;4:355–356. doi: 10.1038/85997. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Destexhe A. A fast-conducting, stochastic integrative mode for neocortical neurons in vivo. J Neurosci. 2003;23:2466–2476. doi: 10.1523/JNEUROSCI.23-06-02466.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. J Neurosci. 2007a;27:6664–6674. doi: 10.1523/JNEUROSCI.0315-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman SR, Ma M, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007b;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Jing J, Due MR, Weiss KR. An input-representing interneuron regulates spike timing and thereby phase switching in a motor network. J Neurosci. 2008;28:1916–1928. doi: 10.1523/JNEUROSCI.4755-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou M, Anzai S, Huruno M. Long-term potentiation and olfactory memory formation in the carp (Cyprinus carpio L.) olfactory bulb. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:421–434. doi: 10.1007/s00359-005-0600-5. [DOI] [PubMed] [Google Scholar]
- Satou M, Hoshikawa R, Sato Y, Okawa K. An in vitro study of long-term potentiation in the carp (Cyprinus carpio L.) olfactory bulb. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:135–150. doi: 10.1007/s00359-005-0056-7. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Neuronal substrates of sleep and epilepsy. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Brezina V, Cropper EC, Hooper SL, Miller MW, Probst WC, Vilim FS, Kupfermann I. Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia. 1992;48:456–463. doi: 10.1007/BF01928164. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Debay D, Le Masson G, Destexhe A, Bal T. Synaptic background activity controls spike transfer from thalamus to cortex. Nat Neurosci. 2005;8:1760–1767. doi: 10.1038/nn1591. [DOI] [PubMed] [Google Scholar]
- Worgotter F, Eyding D, Macklis JD, Funke K. The influence of the corticothalamic projection on responses in thalamus and cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1823–1834. doi: 10.1098/rstb.2002.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, MacLean JN, Smith J, Lansner A. The cortex as a central pattern generator. Nat Rev Neurosci. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- Zhurov Y, Proekt A, Weiss KR, Brezina V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. J Neurosci. 2005;25:1268–1280. doi: 10.1523/JNEUROSCI.3361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]