Abstract
The zebrafish is a highly relevant model organism for understanding the cellular and molecular mechanisms involved in neurogenesis and brain regeneration in vertebrates. However, an in-depth analysis of the molecular mechanisms underlying zebrafish adult neurogenesis has been limited due to the lack of a reliable protocol for isolating and culturing neural adult stem/progenitor cells. Here we provide a reproducible method to examine adult neurogenesis using a neurosphere assay derived from zebrafish whole brain or from the telencephalon, tectum and cerebellum regions of the adult zebrafish brain. The protocol involves, first the microdissection of zebrafish adult brain, then single cell dissociation and isolation of self-renewing multipotent neural stem/progenitor cells. The entire procedure takes eight days. Additionally, we describe how to manipulate gene expression in zebrafish neurospheres, which will be particularly useful to test the role of specific signaling pathways during adult neural stem/progenitor cell proliferation and differentiation in zebrafish.
Keywords: Developmental Biology, Issue 108, Zebrafish, neurogenesis, neural adult stem/progenitor cells, neurosphere assay, miRNA, genetic manipulation
Introduction
Mammalian neural stem cells (NSCs) have been characterized in vitro by their ability to grow in free-floating cultures as clusters of dividing cells termed neurospheres1. In the presence of epidermal growth factor (EGF) and fibroblast growth factor (FGF), NSCs divide either symmetrically to generate self-renewing NSCs, or asymmetrically to generate two different daughter cells, i.e., a differentiating progenitor cell and a novel NSC. Neurosphere cultures are therefore a mixture of neural stem/progenitor cells and more differentiated neural cells2–4. NSCs can, however, be distinguished from other neurosphere cell-types by two specific properties: they display long-term self-renewal in free-floating cultures and they can differentiate into all neural cell lineages (i.e., neurons, astrocytes, and oligodendrocytes) following withdrawal of growth factors and adhesion to extracellular matrix substrates. In mammals, the neurosphere culture system was the first in vitro system used to demonstrate the presence of NSCs in the adult brain and remains the most commonly used tool to analyze proliferation, self-renewal capacity and multipotency of neural stem and progenitor cells. Therefore, even though sphere-forming assays suffer from some disadvantages and limitations4, this culture system is valuable for evaluating the potential of a cell to behave as a stem cell when removed from its in vivo niche4 and has been instrumental in identifying key regulators of NSC self-renewal and cell fate determination5–7.
In contrast to mammals who have limited adult neurogenesis, zebrafish constitutively produce new neurons along the whole brain axis throughout their life. The zebrafish adult brain displays multiple neurogenic niches harboring neural stem/progenitor cells making zebrafish a powerful model organism for understanding the stem cell activity in the brain as well as the molecular programs required for central nervous system regeneration. Over the past 17 years, several research groups developed methodologies for isolating and culturing zebrafish neural cells8,9. These studies were aimed at producing embryonic neuronal and glial cells in vitro, but not at maintaining NSCs and investigating their properties. Although neurospheres have been generated in the adult Apteronotus leptorhynchus (Brown Ghost Knifefish)10, a neurosphere-forming assay in the zebrafish remained to be established.
Here we describe a neurosphere-forming assay to demonstrate the role of miR-107 during zebrafish neurogenesis11. The protocol enables: 1) the collection of adult neural stem/progenitor cells either from zebrafish whole brain or from several dissected brain regions such as the telencephalon, the tectum, and the cerebellum; 2) the generation of floating and self-renewing neurospheres from adult neural stem/progenitor cells; 3) the down- and up-regulation of the expression of coding genes or small non-coding RNAs11 in neurospheres, in order to investigate their roles in the proliferation and differentiation of neural stem/progenitor cells.
Protocol
Zebrafish of the WTCF strain were raised and maintained according to protocols approved by the Yale University Institutional Animal Care and Use Committee (IACUC protocol number 2012–11473). All experiments should first be approved by all relevant governmental and institutional ethics regulating bodies regarding the use of animals for research purposes.
1. Preparations
Prepare 10 ml of dissection medium, add 200 μl of 100x penicillin-streptomycin into 9.8 ml of DMEM/F12.
Prepare L-Cysteine solution: to 10 ml of water for tissue culture, add 120 mg of L-cysteine. Store the L-cysteine solution at 4 °C for up to 2 weeks, or in aliquots at −20 °C for up to 1 year.
Prepare DNase I solution: to 1 ml of water for tissue culture, add 10 mg of DNase I. Store the DNase I solution at 4 °C for up to 2 months.
Prepare papain solution: to prepare papain solution, add 100 μl papain (approximately 140 units), 100 μl DNase I (1%) and 200 μl L-cysteine (12 mg/ml) into 5 mL of DMEM/F12. Freshly prepare the papain solution every time and sterilize with a 0.22 μm pore size filter before use.
Prepare washing solution: to prepare 100 ml of washing solution, add 650 μl glucose 45%, 500 μL HEPES 1 M and 5 ml FBS into 93.85 ml DPBS 1x. Sterilize the washing solution with a 0.22 μm pore size filter before use. Store the washing solution at 4 °C for up to 2 months.
Prepare insulin solution: to prepare 2 ml of insulin solution, add a 25 μl drop of 10 N NaOH and 100 mg insulin into 2 ml of water for tissue culture.
Prepare EGF and FGF solutions: Dissolve both mitogens in DMEM/F12 at 100 μg/ml concentration. Store as 10 μl aliquots at −20 °C.
Prepare B-27 and N-2 media: store B-27 and N-2 supplements at −20 °C as 500 μl and 1 ml aliquots, respectively.
Prepare Z-differentiation condition medium: to prepare 100 ml of Z-differentiation condition medium, add 40 μl insulin (50 mg/ml), 500 μl B-27, 1 ml N-2, 650 μl glucose 45% and 1 ml of 100 x penicillin-streptomycin into 97.81 ml of DMEM/F12. Sterilize the Z-differentiation condition medium with a 0.22 μm pore size filter before use. Store the Z-differentiation condition medium at 4 °C for up to 1 week.
Prepare Z-condition medium: to prepare 50 ml of Z-condition media, add 10 μl of EGF and 10 μl of FGF into 50 ml of sterile Z-differentiation condition medium (20 ng/ml). Store the Z-condition medium at 4 °C for up to 1 week.
Prepare coating solution for differentiation culture: for 5 ml extracellular coating solution, add 100 μl of the extracellular matrix solution (e.g., Matrigel) into 4.9 ml of DMEM/F12. Thaw extracellular matrix solution at 4 °C on ice. Once defrosted, keep at 4 °C for up to 2 weeks.
2. Dissection of the Adult Zebrafish Brain
Prepare a dissection bed by filling a 100 mm × 15 mm Petri dish with gel ice packs. Then place the lid on the petri dish and incubate at −20 °C until the gel freezes. On top of the lid place a square of clean filter paper and wrap both the filter paper and petri dish with plastic film.
Clean and sterilize all the microdissection instruments by 70% ethanol or heat before each use. Place all sterilized dissection instruments near the dissecting microscope and, right before euthanasia, place the dissection bed under the microscope with optical fiber illumination.
Collect 2 adult zebrafish for a whole brain neurosphere preparation; and 3 to 4 zebrafish to generate neurospheres from dissected brain regions.
-
Euthanize adult zebrafish (8–12 months old) using a protocol approved by the Institutional Animal Care and Use Committee. Next, immerse the fish in 75% ethanol for 5–10 sec and quickly place in the dissection bed followed by decapitation at the level of the gills using a surgical blade.
To euthanize animals, administer an overdose (300 mg/L) of tricaine methanesulfonate until the animal’s heart-beat gradually slows down and circulation stops, then immerse in iced water.
Turn the head dorsal side down, and using the scissors make a longitudinal cut from the cut side to the mouth. Using the forceps expose the base of the skull and remove all the adjacent tissue. Cut the lateral walls of the skull from the beginning of the spinal cord towards the tectum.
Using the scissors, cut and remove the optic nerves and then remove both sides of the most lateral part of the skull at the level of the tectum. Turn the head ventral side up. Using forceps, peel off the rest of the most apical part of the skull.
Transfer the brain along with the remaining part of the skull into a new dish with the dissection medium (1.1). Clean the brain tissue in the dissection medium using the micro knife’s plastic handle, keeping all brain structures intact.
-
From this point, continue the protocol using the whole zebrafish brain.
Alternatively adapt this protocol to specific brain regions to generate neurospheres from whole zebrafish brain or from the telencephalon, tectum/diencephalon or cerebellum dissected with a fresh scalpel. Use a neural specific fluorescent zebrafish neural transgenic line to dissect the brain region of interest according to Figure 3 and reference 11.
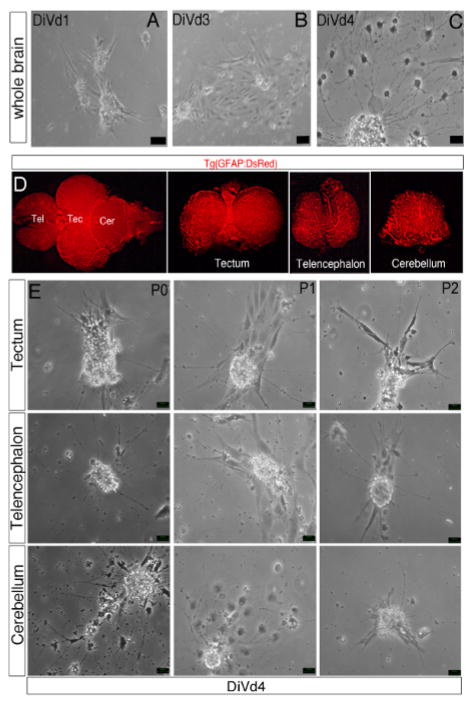
Figure 3. Differentiation of Zebrafish Brain-derived Neurospheres.
(A–C, E) Phase contrast images of whole brain-derived neurospheres cultured in the Z-differentiation medium during 1 (A, DiVd1), 3 (B, DiVd3) and 4 (C and D, DiVd4) days. (E) Dorsal view of the whole brain of a 12 month old Tg(GFAP:DsRed) zebrafish. The telencephalon (Tel), tectum (Tec) and cerebellum (Cer) were dissected and collected as shown. (D) Images of DiVd4 neurospheres derived from the tectum, telencephalon and cerebellum at Passage 0 (P0), 1 (P1) and 2 (P2). Scale bar: 25 μm.
3. Single Cell Dissociation of Adult Brain
Perform all subsequent work in a biological safety cabinet. Transfer the brain tissue into a 1.5 ml tube containing 800 μl of dissection medium (prepared in step 1.1). Remove the dissection medium by pipetting, without touching the brain tissue.
Add 500 μl of papain solution (step 1.4) and digest the brain tissue at 37 °C for 10 min. Do not incubate longer than 15 min as it could decrease cell viability.
After incubation, transfer the brain tissue with 500 μl of the papain solution into a 15 ml conical tube using a 1,000 μl pipet tip cut at ~0.25 inches from the bottom. Gently dissociate the brain tissue by slowly pipetting up and down 10 times with the same tip. Do not pipet up and down more than 15 times and do not generate air bubbles during this step, as it could alter cell viability.
Incubate the brain tissue again at 37 °C for 10 min followed by pipetting up and down 10–13 times using an uncut 1,000 μl regular tip. Do not pipet up and down more than 15 times, to avoid cell death.
Stop the enzymatic digestion by adding 2 ml of washing solution (step 1.5), and centrifuge the cell suspension at 800 x g for 5 min at room temperature (RT). Carefully decant the supernatant and resuspend the pellet in the remaining solution by tapping the tube vigorously with a finger and then by pipetting up and down with a 1,000 μl regular tip. Next, add 2 ml of washing solution.
At this stage, check under the microscope that a single cell suspension has been obtained. Centrifuge the cell suspension again at 800 x g for 5 min at RT. Remove the supernatant and re-suspend the pellet using 1 ml of fresh Z-condition medium (step 1.10).
Stain cells by using trypan blue 12 and count the living cells using a hemocytometer by excluding blue dead cells. Prepare a 24-well plate with 200 μl of cell suspension and 300 μl of fresh Z-condition medium in each well. Seed cells at a density of ~500 cells/μl. Maintain cultured cells in an incubator at 30 °C in 5% CO2, since zebrafish brain-derived neurospheres do not grow well at 37 °C.
4. Generation of Primary Neurospheres
After 1 day in vitro (DiV1), observe the single cell suspension obtained from the adult whole brain under the microscope and note whether debris has accumulated in the center of the well. Carefully remove any debris by pipetting approximately 100 μl of medium (less if possible). Next add 100 μl of fresh Z-condition medium. Single cell suspensions should be observed at this time (Figure 2A DiV1).
After 2 days in vitro (DiV2), expand the cultured cells. Using a 1,000 μl pipette with the tip cut, collect and transfer 250 μl of cell suspension from each well into a new well. Add 250 μl of fresh Z-condition medium into all wells. Before expanding the cultured cells, homogenize the suspension very gently by pipetting up and down throughout the well.
-
Repeat steps 4.1 and 4.2 for the following 4 days in vitro (DiV4). A progressive increase in size of neurospheres should be visible at the center and edges of the well at DiV3 and DiV4 (Figure 2B and C).
Note: Primary neurospheres can be processed for passage or differentiation to assess multipotency. Alternatively, they can be processed for nucleofection or lipo-transfection11 to characterize the role of specific genes during the differentiation process.
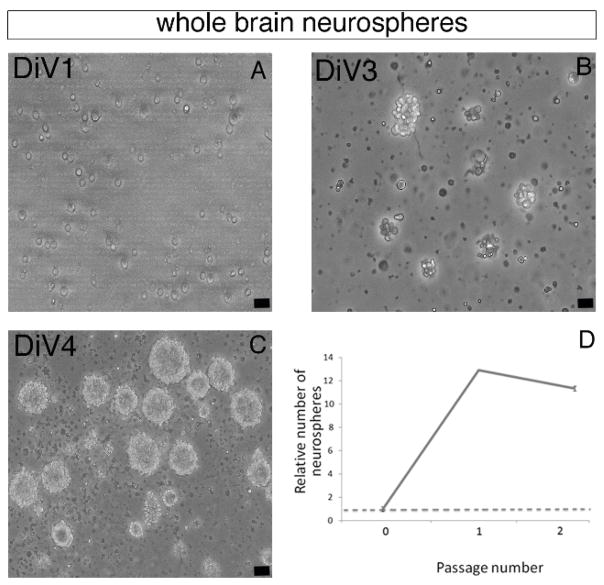
Figure 2. Forming Zebrafish Brain-derived Neurospheres.
(A–C) Representative phase contrast images of whole brain-derived primary floating neurospheres observed at day 1 (DiV1, A), day 3 (DiV3, B) and day 4 (DiV4, C). (D) Chart representing the relative number of neurospheres at Passages 1 and 2 compared to the neurosphere number at Passage 0. After Passage 2, neurospheres formation was drastically decreased. Scale bar: 25 μm.
5. Passaging of Primary Neurospheres
Remove 250 μl of tissue culture from each well and mechanically dissociate DiV4 neurospheres with a 1 ml pipette. Do not pipet up and down too rapidly as air bubble formation may increased cell death.
Count the cells with a hemocytometer and distribute 800 cells/μl in 250 μl of primary culture supernatant into each well of a 24-well plate.
Add 250 μl of Z-condition medium to each well.
After 2 days in vitro (DiV2), expand the cultured cells as reported in step 4.2 and 4.3.
6. Differentiation of Primary Neurospheres
Sterilize cover glasses by immersion in 70% ethanol for 10 min, then dry cover glasses at RT by placing them into each well of a 12-well plate.
Add 300 μl of extracellular coating solution (step 1.11) at the center of the cover glass in such a way that it can expand widely and cover the entire cover glass. Then, incubate at 37 °C for 1 h (using the humidified cell culture incubator).
Remove the extracellular coating solution after incubation, and dry the cover glass for 2 hr at 37 °C.
Remove 250 μl of tissue culture from each well. Mechanically dissociate all the DiV4 primary neurospheres per well with a 1 ml pipette (with wider-end tip) by pipetting up and down.
Seed dissociated neurosphere cell suspensions at a cell density of 4 × 103 cells/ml on previously prepared extracellular matrix-coated coverglasses (step 6.1–6.3) and keep for at least 30 min, at 30 °C in 5% CO2, until attached to the substrate. Then, remove the culture medium and rapidly add 1 ml of pre-warmed fresh Z-differentiation condition medium (step 1.9) before culturing the cells for 24 hr at 30 °C in 5% CO2.
Every two days, replace half of the Z-differentiation condition medium during the time of cell differentation.
7. Gene Manipulation of Primary Neurospheres
Collect DiV3-4 primary neurospheres (step 4.3) by centrifuging for 8 min at 80 x g at 4 °C. Prepare for liposome transfection of commercial oligonucleoatides as previouly described11 or nucleo-transfection of DNA plasmid vectors.
Resuspend neurosphere pellet at a cell density of 4 × 103 cells/μl in 100 μl of a reaction mixture (82 μl nucleofactor solution and 18 μl of supplement).
Mix the neurosphere suspension with 5 μl of the DNA plasmid vectors of choice (plasmid stocks at 1 μg/μl). Transfer the neurosphere/DNA mixture into a nucleofector cuvette for nucleotransfection and select nucleofector program according to the manufacturer’s instructions provided by the nucleofector kit.
Immediately after nucleofection, add a volume of 500 ml of pre-warmed Z-condition media (1.10) to the cells and plate the transfected neurospheres for studying their cell renewal and/or differentiation properties, as described above.
Representative Results
General Scheme of the Adult Zebrafish Neurosphere Culture
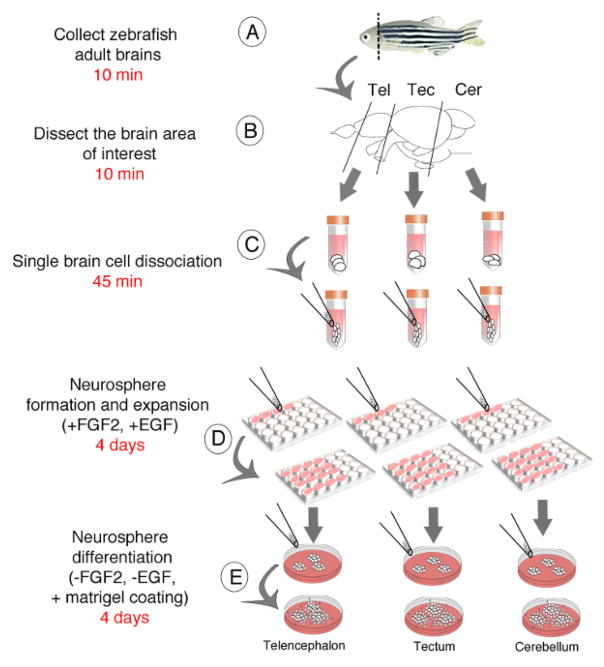
Here we describe all the steps of the protocol of a neurosphere-forming assay performed from the adult zebrafish brain. First, adult neural stem/progenitor cells have been collected either from zebrafish whole brain or from several dissected brain regions such as the telencephalon, the tectum and the cerebellum (Figures 1A–C). Single cell suspension of adult neural stem/progenitor cells have then been used to generate floating and self-renewing neurospheres (Figures 1D, E). Finally, neurospheres have been instrumental in studying the down- and up-regulation of the expression of coding genes or small non-coding RNAs11 in order to investigate their roles in the proliferation and differentiation of zebrafish neural stem/progenitor cells.
Figure 1. Schematic Representation of Protocol Steps.
The procedures and associated timing include the dissection of either the whole brain or the telencephalic, tectal and cerebellar regions of the adult zebrafish brain (A, B), the obtention of a single cell suspension (C), the generation of floating neurospheres (D) and the differentiation of neurospheres (E).
Neurosphere Passaging
Zebrafish primary neurospheres can self-renew following several passages. To generate neurospheres at Passage 1 and 2, steps 5.1–5.4 were repeated twice, in a total of 6–8 days. From DiV2-4, the size of the neurospheres increased up to around 50 μm in diameter. Secondary and tertiary neurospheres were also obtained after Passage 1 and 2, respectively (Figure 2D). At Passage 3, zebrafish neurospheres were however unable to grow up at the critical size of 50 μm diameter and failed to self-renew, suggesting that our culture condition rather selects a pool of stem/progenitor cells than a pure population of neural stem cells4.
Neurosphere Differentiation
Primary, secondary or tertiary neurospheres derived from either the whole brain or from the telencephalic, cerebellar and tectal area of adult zebrafish were tested for their differentiation potentialities. Between 1 and 3 days in vitro in differentiation conditions (DiVd1-DiVd3), a monolayer of adherent cells was observed (Figures 3A, B). As illustrated in Figure 3C, at DiVd4, axonal like projections as well as glia cells were visible and distinguishable by immunohistological or gene expression analyses11, assessing that neural stem/progenitor cells had differentiated. Similarly, neurons and glial cells differentiated from neural stem/progenitor cells isolated from different brain territories (Figures 3D, E).
Gene Expression Manipulation in Zebrafish Neurospheres
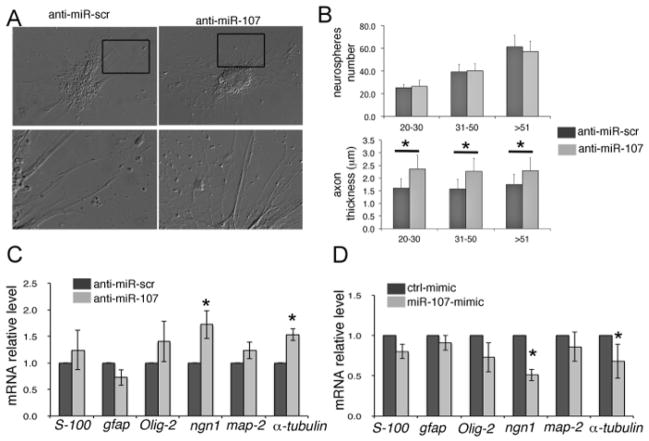
We have tested the role of miR-107 on the neuronal and glial differentiation of whole zebrafish brain-derived neural stem/progenitor cells (Figure 4). We showed that the down regulation of miR-107 by anti-miR-107 did not affect neurosphere formation but altered neuronal differentiation, as indicated by the abnormal growth of axonal processes (Figures 4A, B). RT-PCR gene expression analyses confirmed that inhibition of miR-107 leads to an increased expression of both the neuroblast marker Neurogenin-1 (Ngn-1) and axon-specific molecules, such as Map-2 and α-tubulin, without affecting glial cell marker expression (S-100, GFAP, Olig2) (Figure 4C). Accordingly, the gain of miR-107 expression by miR-107-mimic induced a decrease of neuroblast- and axon-specific marker expression (Figure 4D), assessing that, in vitro, miR-107 acts as a neuronal differentiation regulator during zebrafish neurogenesis11.
Figure 4. Analysis of Neurospheres Formed following MiR107 Manipulation.
(A) Phase contrast images showing Div4d neurospheres treated at Div4 with the indicated oligonucleotides. Black boxes in the top panels indicate the area magnified in the bottom panels. (B) Top chart represents the quantification of the number of neurospheres formed with, or without, miR107. Neurospheres are subgrouped by their size (20–30 μm, 31–50 μm, >51 μm in diameter). Bottom chart indicates the quantification of the axonal projections from neurospheres treated and sub grouped as above. (C, D) qRT-PCR expression analysis of indicated genes by Div4d neurospheres previously treated with indicated oligonucleotides at Div4. Data represent the mean ± SEM, * p <0.05, n = 3. Scr: scramble. Scale bar: 25 μm.
Discussion
The main aim of this protocol is to isolate and culture adult zebrafish brain-derived neurospheres for studying the cellular and molecular features of neural stem/progenitor cells. Here, we report how to select multipotent neural cells and generate the three neural cell types, i.e., astrocytes, neurons and oligodendrocytes, from the adult zebrafish brain. The protocol is highly significant since a reproducible neurosphere-forming assay had not been established in the zebrafish so far.
A few critical steps in the protocol need to be respected and have been recapitutaled in the troubleshooting advice (Table 1). First, cell death occurs during both the dissection of the adult zebrafish brain and the single cell suspension procedure. To limit the extend of cell death, it is essential to use clean and sharp tools as well as to strictly respect the timing and temperature of incubation recommended in the present protocol. Secondly, neurosphere culture should be performed with freshly prepared media and with a careful pipetting technique. Third, cell density recommendations should be followed to obtain reliable renewal or differentiation of neurosphere cells. With this in mind, anyone skilled in cell-culture techniques should be able to use the protocol and carry out the isolation, culture and gene manipulation of adult zebrafish brain-derived neurospheres.
Table 1.
Troubleshooting Advice Listing, for Each Step of the Protocol, the Possible Issues and the Solutions to Overcome Them.
| Problem | Possible reason | Solution |
|---|---|---|
| Recovery of too many dead cells | Time delay in preparing the brain before enzymatic treatment | Make sure the dissection set up and media are ready before sacrificing the fish (also check sterility, temperature) |
| Stringent papain treatment | Mechanical dissociation needs to be delicate enough to generate a live single cell suspension, but strong enough to avoid leaving behind too many clumps of tissue. Respect the recommended times and temperatures for incubation/centrifugation periods | |
| Neurospheres do not appear or grow poorly | Incorrect temperature | Check that incubator set at 30 °C is providing the correct temperature. |
| Growing spheres removed with debris | For each well, the aspiration of debris has to be performed well on top of the medium. Avoid entering into the medium with your pipet | |
| The integrity of some reagents (B27 supplement, EGF, FGF) is weakened | This integrity constitutes limiting factors in cell growth. Check batch number, and the way samples were preserved. Avoid thaw/refreeze cycles of any sampled reagent used in culture | |
| Presence of single cells | Mode of transfer/expansion of neurospheres is too harsh | This step is an expansion/dilution step because too many spheres are formed in the well. Avoid harsh sphere collection during transfer to prevent neurosphere dissociation into single cells |
| Absence of cells on matrigel | Matrigel was not properly thawed/diluted | Matrigel from −20 °C needs at least 30 min at 4 °C to thaw and remains viscous Pipet carefully |
| No adhesion on matrigel | The volume of cell suspension has to be small enough to allow cell deposition on matrigel coat | |
| Allow more time for cell deposition (up to 2 hr if larger volumes are used) | ||
| Fresh differentiation medium too cold | When replacing the cell-attachment medium, the differentiation medium needs to be warmed up at 30 °C to prevent thermal shock on the deposited cells | |
| Poor nucleofection | Dead cells | Make sure that you do not generate air bubbles while performing nucleofection. Use high quality DNA vectors by using Maxi-preparations. At least 1 – 2 hr after nucleofection, replace culture medium using either fresh Z-differentiation condition medium or Z-condition medium. |
| Few positive neurospheres | Neurospheres of varying sizes can lead to poor nucleofection. Using neurospheres at DiV2 and DiV3 are ideal for nucleofection. Density of cells should not exceed 4 × 103 neurospheres ml−1 in a 100 ml reaction. |
The sphere-forming assays in the zebrafish is suffering from the same limitations previously described in mammalian species4: 1) the behavior of neural stem/progenitor cells is altered following their isolation from their natural brain niche; 2) neurospheres are not a homogeneous population of stem cells, but include stem cells, progenitor cells as well as post-mitotic differentiated cells. It is moreover worth noting that our protocol generates aclonal neurosphere cultures. The cell density of cultures is a critical parameter in sphere-forming assays since it determines clonality, i.e., whether the culture is clonal, semi-clonal or aclonal. Clonal conditions allow to accurately characterize and quantify the different pools of stem and progenitor cells present in culture. We have not been able to perform cell clonality assays so far and our culture conditions still need more optimization before we can discriminate neural stem cells from other progenitor cell pools.
Zebrafish neurosphere cultures can be used for a large range of experiments. They allow gene expression analysis as well as manipulation of gene expression during neurogenesis as illustrated by our study assessing miR-107 function in the tissue specific determination of neural cell fate (Figure 4 and 11). Additional applications include genome editing strategies, such as the CRISPR/Cas9 system, in order to test the role of neural stem/progenitor genes in neurogenesis and brain repair13. Finally, our protocol shows that zebrafish neurospheres can be derived from different brain regions opening the possibility to study region-specific features of neurons and glia cells and to explore the cellular and molecular basis of neural cell heterogeneity in different ventricular domains of the zebrafish brain14.
Acknowledgments
The authors thank Guillermina Hill-Teran and Marie-Elise Schwartz for assistance. This work was supported by grants from the National Institutes of Health (5R00HL105791 to S.N.) and from the Alzheimer (NIRP 12-259162). This work was also supported by Institut National de la Santé et de la Recherche Médicale (CFC and JLT), Agence Nationale de la Recherche (13-BSV4-0002-01 (JLT), NIH (1R01EB016629-01A1 (JLT), Connecticut Stem Cell Research Fund (13-SCA-Yale-04 (JLT).
Footnotes
Video Link
The video component of this article can be found at http://www.jove.com/video/53617/
Disclosures
The authors declare no competing financial interests.
References
- 1.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Patzlaff NE, Jobe EM, Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat Protoc. 2012;7:2005–2012. doi: 10.1038/nprot.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter M, et al. Vertebrate neural stem cell segmentation, tracking and lineaging with validation and editing. Nat Protoc. 2011;6:1942–1952. doi: 10.1038/nprot.2011.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh C, Liu Y, Ma C, Collodi P. Cell cultures derived from early zebrafish embryos differentiate in vitro into neurons and astrocytes. Cytotechnology. 1997;23:221–230. doi: 10.1023/A:1007915618413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, et al. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio) PloS one. 2013;8:e57539. doi: 10.1371/journal.pone.0057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinsch K, Zupanc GK. Generation and long-term persistence of new neurons in the adult zebrafish brain: a quantitative analysis. Neuroscience. 2007;146:679–696. doi: 10.1016/j.neuroscience.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Ristori E, et al. A dicer-miR-107 interaction regulates biogenesis of specific miRNAs crucial for neurogenesis. Dev Cell. 2015;32:546–560. doi: 10.1016/j.devcel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis SK, Slegel AC. Cell Viability Analysis Using Trypan Blue: Manual and Automated Methods. Methods Mol Bio. 2011;740 doi: 10.1007/978-1-61779-108-6_2. [DOI] [PubMed] [Google Scholar]
- 13.Harrison MM, Jenkins BV, O’Connor-Giles KM, Wildonger J. A CRISPR view of development. Genes dev. 2014;28:1859–1872. doi: 10.1101/gad.248252.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marz M, et al. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia. 2010;58:870–888. doi: 10.1002/glia.20971. [DOI] [PubMed] [Google Scholar]