Abstract
Background
MET gene amplification and Met protein overexpression may be associated with poor prognosis. MET/Met status is typically determined with fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC), respectively. Targeted proteomics uses mass spectrometry-based selected-reaction-monitoring (SRM) to accurately quantitate Met expression. FISH, IHC, and SRM analyses were compared to characterize the prognostic value of MET/Met in gastroesophageal adenocarcinoma (GEC).
Methods
Samples from 447 GEC patients were analyzed for MET gene amplification (FISH) and Met protein expression (IHC and SRM). Cox proportional hazards models and Kaplan-Meier estimates were applied to explore relationships between Met, overall survival (OS) and clinical/pathologic characteristics. Spearman’s rank coefficient assessed the correlation between parameters.
Results
Patients with MET-amplified tumors had worse OS (MET/CEP7 FISH ratio ≥2 (hazard ratio [HR] 3.13; 95% CI 1.84-5.33), MET gene copy number ≥5 (HR 2.51; 95% CI 1.45-4.34), or ≥10% of cells ≥15 copies (HR 4.28; 95% CI 2.18-8.39). Similar observations were made with Met protein overexpression, by IHC (≥25% tumor cell membrane, ≥1+ intensity) (HR 1.39; 95% CI 1.04-1.86) or SRM (≥400 amol/μg) (HR 1.76; 95% CI 1.06-2.90). Significant correlation was observed between MET FISH/Met IHC, MET FISH/Met SRM, and Met IHC/Met SRM; only MET FISH and Met SRM were independent negative prognostic biomarkers in multivariate analyses.
Conclusions
MET amplification/overexpression, assessed by multiple methods, were associated with worse prognosis in univariate analyses. However, only MET amplification by FISH and Met expression by SRM were independent prognostic biomarkers. Compared with IHC, SRM may provide added benefit towards informed decisions about Met-targeted therapy.
Introduction
The Met receptor tyrosine kinase (c-Met or hepatocyte growth factor (HGF) receptor), is a single-pass transmembrane receptor that undergoes homodimerization and activation upon binding of HGF, its only known ligand.1 Numerous signaling pathways are activated by interaction of HGF/Met2; thus, Met plays a critical role in many biological functions ranging from embryogenesis to wound healing. Mechanisms for Met-induced oncogenesis include constitutive activation of the kinase domain and/or dysregulated paracrine and/or autocrine signaling.1,3,4 The underlying activating mechanism typically involves MET gene amplification, Met and/or HGF protein overexpression, or, rarely, domain-specific sequence mutations/translocations,2,3 including the more recent observation of Met exon 14 skipping mutations in non-small cell lung cancer.5,6
Aberrant Met activity has been ubiquitously reported across cancers.1,2,7,8 For GEC, MET amplification and Met protein overexpression range between 4-10% and between 25-70% of GEC cases, respectively, depending on the definition of positive.9-16 As with other malignancy types,17,18 patients with Met-positive GEC (as defined by various criteria) have reportedly worse prognoses.10,12,15,16,19-21 Given the clinical impact and relevance of Met, diagnostic determination of its expression and/or mutational/amplification status can provide physicians with critical information to understand patient prognosis, identify clinical trials for which the patient may be a candidate and, importantly, to make a determination as to whether the patient may benefit from Met-targeted therapies.
This study sought to further evaluate Met’s prognostic potential from a large multi-institutional, clinically-linked cohort of GEC patients and to compare various diagnostic platforms for identifying Met-positive tumors. Fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) were utilized to examine the frequency of MET gene amplification and Met protein overexpression, respectively. Due to the inherent limitations with FISH (time-consuming, labor-intensive, subjective scoring) and IHC (variability in tissue staining, lack of intra-sample reproducibility over time, subjective scoring), mass spectrometry-based selected reaction monitoring (SRM)11,22 was also used to objectively quantitate Met protein expression.11 The relationship between MET/Met status and OS was assessed, using all three approaches in both univariate and multivariate analyses adjusting for known prognostic covariates of GEC. Additionally, the data from MET FISH, Met IHC, and Met SRM were compared for inter-method agreement.
Materials & Methods
Patients and samples
Formalin-fixed, paraffin-embedded (FFPE) gastroesophageal adenocarcinoma samples were obtained from the University of Chicago (Illinois) or the Università di Urbino (Urbino, Italy) from 2000 to 2015. Samples were collected/annotated under IRB approval protocols. Median follow-up time was 113.0 months (80% CI 99.3-126.3); median follow-up times for patients with and without curative intent surgery were 115.3 months (80% CI 99.3-129.0) and 31.6 months (80% CI 14.5-31.6), respectively. Clinical/pathologic characteristics of the samples are in Supplemental Tables 1 and 2.
MET Analysis
Dual-color MET FISH assay was conducted using the MET IQFISH Probe with centromere enumeration probe for chromosome 7 (CEP7) (Agilent Technologies, Santa Clara, CA) and Histology FISH Accessory Kit (DAKO North America, Carpinteria, CA), scoring as previously described.11,23,24 Average MET gene copy number ≥5, ratio (MET/CEP7) ≥2, and ≥10% of counted tumor nuclei with ≥15 gene copies were three amplification scoring criteria considered positive.24,25
Met IHC was performed using HRP-labeled dextrose-based polymer complex bound to secondary antibody (DAKO North America) as previously described.21,26 Intensity of cell membrane staining scored by experienced pathologist (0 none, 1 low, 2 intermediate, or 3 high), along with the percentage of tumor cells (extensity) for each sample were documented.11,21 The “Membrane Max Intensity” parameter of a given tumor was obtained from the highest IHC score that had a non-zero percentage of staining. For example, if a tumor had a score breakdown of 10%/65%/25%/0% (for scores of 0/1/2/3, respectively), the Membrane Max Intensity was “2”. The “Predominant Score” parameter was obtained by the highest percentage positive score; in the provided example, this was “1”. IHC was defined as positive if ≥25% of tumor cell membranes stained ≥1+ intensity; a second analysis was also performed at a higher extensity of ≥50% of cells ≥1+.21,26 Pathologists conducting FISH and IHC were blinded to results of previously performed assays and clinical outcomes.
For Met protein quantification by mass spectrometry-based SRM, tissue sections (10 μm) were prepared as previously described.11,22,27 This tumor tissue was solubilized using Liquid Tissue® and the resulting tryptic peptides were analyzed using a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with a nanoAcquityLC system (Waters, Milford, MA). The SRM conditions have been described.11,22
Statistical Methods
Spearman’s rank correlation coefficient was used to assess correlations between parameters. Cox proportional hazards models and Kaplan-Meier estimates were applied to explore relationships between Met, overall survival, and other clinical and pathologic characteristics. Multivariate analysis was conducted on Met biomarker status (amplification by FISH, expression by IHC, expression by Met-SRM) and outcome after adjusting for baseline covariates including: age, sex, race, histology, biopsy, diagnosis, stage, lesion status, curative intent surgery, and perioperative therapy.
Results
Of the 447 samples available, 344 (77.0%) were evaluable by MET FISH, 332 (74.3%) by Met IHC and 282 (63.1%) by Met SRM (Table 1). Inter-method correlations were performed using tumors evaluable by more than one approach (discussed in further detail below). Comparison of single-method subsets by patient demographic and clinical characteristics indicated a balanced representation of each subset (Supplemental Tables 1 and 2).
Table 1. Number of GEC tumor samples evaluated for MET/MET, by method of assessment.
Samples were analyzed for MET amplification by FISH and/or for Met protein expression by IHC or SRM; quantities for each individual subgroup as well as paired evaluation subgroups are represented in the middle column. The quantity of each individual subgroup with corresponding outcomes data is represented in the far right column.
| Methods | Samples | Samples with Outcome data |
|---|---|---|
| FISH | 344 | 338 |
| FISH+IHC | 304 | |
| FISH+SRM | 255 | |
| IHC | 332 | 324 |
| IHC+SRM | 229 | |
| SRM | 282 | 281 |
When MET gene amplification was defined as a ratio of MET/CEP7 per cell ≥2,11 16/344 (4.7%) tumors were MET amplified (Supplemental Table 3). When scored using the percentages of tumor cells with mean MET gene copy number [GCN] ≥5,12 18 (5.2%) tumors were amplified. When defined as having ≥10% of tumor cells with at least 15 copies of MET, 9 (2.6%) tumors were amplified. These results were similar to previously published rates of MET gene amplification in GEC.9-11,13,26
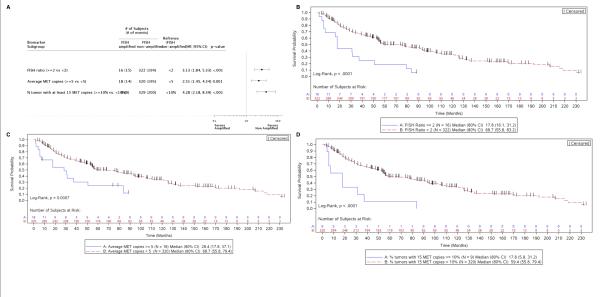
Regardless of the definition of amplification, MET FISH was an indicator of poor prognosis in univariate analyses. Amplification-positive patients exhibited increased risk of death (Figure 1A), and the median OS of non-amplified patients was much longer than that of patients with MET/CEP7 ≥2 (68.7 months vs. 17.8 months, p<0.0001; Figure 1B), mean MET GCN ≥5 (68.7 months vs. 28.4 months, p<0.0007; Figure 1C), or having ≥10% of tumors with ≥15 copies of MET (59.4 months vs. 17.8 months, p<0.0001; Figure 1D). Using the MET/CEP7 ratio and adjusting for numerous baseline covariates (age, sex, race, histology, biopsy, diagnosis, stage, lesion status, curative intent surgery, and perioperative therapy), MET amplification (FISH ratio ≥2) was independently prognostic of OS (HR 2.55, 95% CI 1.27-5.09, p<0.008) when compared to a ratio <2 (Figure 2).
Figure 1. Patient risk and overall survival as assessed by MET FISH status.
A, Cox proportional hazards model evaluation of FISH classification on overall survival. Hazard ratios are depicted (with 95% CI) for MET-amplified tumors compared to non-amplified tumors, as characterized by 3 separate criteria. B-D, Kaplan-Meier curves depicting overall survival of subjects with MET-amplified tumors vs. non-amplified tumors, as characterized by MET:CEP7 ratio (B), average gene copy number (C), and percentage of tumor cells containing at least 15 copies of MET (D).
Figure 2. MET amplification is an independent indicator of overall survival.
Cox proportional hazards model evaluation of FISH classification on overall survival, using multivariate analysis and MET:CEP7 ratio as the FISH characterization approach. Hazard ratios for each baseline covariate are depicted (with 95% CI).
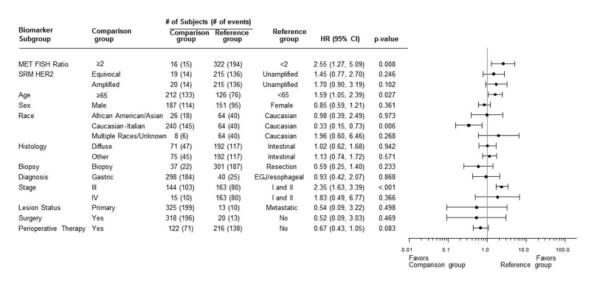
By IHC, 273/332 (82.2%) samples exhibited Met expression at any intensity (intensity score of ≥1+) and any extensity (percentage of tumor cells positive) level; Supplemental Table 4). When Met IHC positivity (combined percentages of cells that were positive for Met expression at 1+, 2+, and 3+) was slightly more restrictively defined as any staining intensity ≥1+ in either ≥25% or ≥50% of tumor cells11,21,26, 117 (35.2%) and 43 (13.0%) samples scored positive, respectively.
Increased expression of Met protein by IHC was also indicative of poor prognosis in GEC in univariate analysis. Met IHC-positive patients exhibited worse prognosis, and there was a general upward trend in HR with increasing IHC extensity staining (Figure 3A). Patients whose tumors had ≥25% positive membrane staining at ≥1+ intensity (predefined cut-offs) experienced shorter OS than patients with lower staining levels (54.2 months vs. 78.4 months; HR 1.39; 95% CI 1.04-1.86, p=0.025). The survival difference between patients at the ≥50% positive membrane staining extensity criterion was slightly greater (43.2 months vs. 78.2 months HR 1.62; 95% CI 1.10-2.28, p=0.013) (Figures 3B and 3C). However, multivariate analysis adjusting for known prognostic covariates indicated Met IHC, by these two definitions of positivity, was not an independent marker of prognosis (p=0.36; Figure 3D).
Figure 3. Patient risk and overall survival as assessed by Met IHC expression.
A, Cox proportional hazards model evaluation of IHC classification on overall survival. Hazard ratios (with 95% CI) comparing Met-positive to Met-negative patients at each cut-off value are depicted. B, C, Kaplan-Meier curves depicting overall survival of subjects by Met IHC status, using cut-offs of 25% (B) and 50% (C). D, Cox proportional hazards model evaluation of IHC status on overall survival, using multivariate analysis and 25% staining as the Met-positive cut-off. Hazard ratios for each baseline covariate are depicted (with 95% CI).
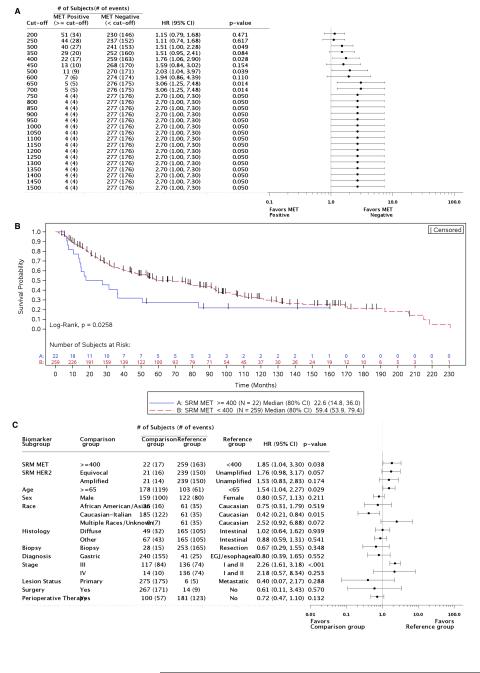
Using mass spectrometry-based SRM, 231/282 samples (81.9%) had Met levels below the lower limit of quantitation of 200 amol/μg (Supplemental Table 5). Protein concentrations in the remaining 51 samples (18.1%) ranged from 200-3245.5 amol/μg.11,27 Using previous established expression levels in an independent cohort,11 and minimum p-value and hazard modeling, 400 amol/μg was tested and established as the cut-off for ‘Met-positive’ expression, as this value was determined to have the most significant effect on OS (Figure 4A). As such, 22 tumors (7.8%) were classified as Met-positive in this study. The median OS for patients above this cut-off was significantly shorter than for their Met-negative counterparts (22.6 months vs 59.4 months, p=0.0258; Figure 4B). After adjusting for covariates, patients with Met expression ≥400 amol/μg exhibited significantly higher risk for death (HR 1.85; 95% CI 1.04-3.30) (Figure 4C).
Figure 4. Patient risk and overall survival as assessed by Met SRM expression.
A, Cox proportional hazards model evaluation of Met SRM quantitation on overall survival. Hazard ratios (with 95% CI) comparing Met-positive to Met-negative patients at each cut-off value are depicted. The number of MET-amplified tumors present within each Met-positive cut-off group is represented. B, Kaplan-Meier curves depicting overall survival of subjects by Met SRM expression, using 400 amol/μg as the Met-positive cut-off. C, Cox proportional hazards model evaluation of Met SRM expression on overall survival, using multivariate analysis and 400 amol/μg as the Met-positive cut-off. Hazard ratios for each baseline covariate are depicted (with 95% CI).
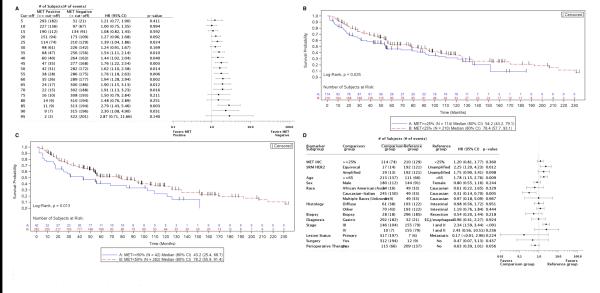
FISH and SRM results were assessed for correlation with the clinicopathologic variables listed in Supplementary Table 1. FISH amplification was associated with esophageal primary location (16.3%) compared to distal gastric (3.7%), and Met-SRM >400amol/ug was associated with higher tumor stage (III/IV) (Supplementary File 1). There were no other statistically significant correlations observed.
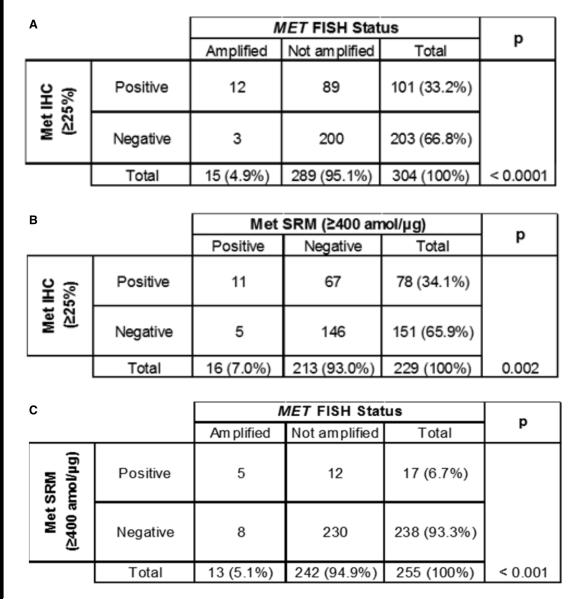
FISH, IHC, and SRM results were also assessed for cross-method sensitivity and specificity, using subsets of tumor samples that were analyzed by multiple methods (Table 1). Using MET:CEP7 ≥2 as the reference, Met IHC at the ≥25% staining cut-off was 80% sensitive (12/15 FISH-positive samples identified) and 69.2% specific (200/289 negative samples identified) for MET amplification by FISH (Figure 5A), with a significant correlation (p <0.0001). Using SRM at ≥400 amol/μg as the reference, Met IHC exhibited 68.8% sensitivity (11/16 Met SRM-positive samples identified) with 68.5% specificity (146/213 Met SRM-negative samples) (p = 0.002; Figure 5B). Finally, when compared to MET:CEP7 ≥2, SRM (≥400amol/μg) identified 5/13 MET-amplified tumors (38.5% sensitivity) and 230/242 non-amplified tumors (95.0% specificity), with a significant correlation between the 2 methods (p <0.001; Figure 5C). Thus, although both Met IHC and Met SRM correlated with MET FISH, they were relatively insensitive to discern MET FISH amplification status in this study. Additionally, although in a very limited subset, applying a previously-defined cut-off of ≥1500 amol/μg11 resulted in a Met SRM specificity of 100% for identifying MET amplification (no negative FISH samples being identified within this high Met-positive SRM cut-off).
Figure 5. Correlation of MET/MET analytical approaches.
MET gene amplification results were compared to Met IHC (A) and SRM (C) cut-off-derived protein expression; the correlation between Met IHC and Met SRM was also examined (B).
Discussion
This study evaluated Met as a prognostic biomarker for GEC, contrasting technical methods of FISH, IHC, and SRM, and assessing various criteria for defining genomic or proteomic positivity. Moreover, an association of each of these methods/definitions with clinical outcome was evaluated, as univariate and covariate adjusted analyses. The findings are consistent with some smaller studies demonstrating similar concordance patterns between Met SRM and IHC and/or FISH,11 and comparable adverse prognosis in univariate analysis when the Met biomarker (FISH, IHC, or SRM) was positive.10,12,15,21 Notably, the findings are unique to demonstrate independently worse prognosis of amplification as determined by FISH ratio and overexpression by SRM, after adjusted multivariate analysis; in contrast, IHC using currently-applied positivity criteria was not an independent prognostic biomarker.26
Numerous complexities contribute to determining the strength of Met as a predictive and/or prognostic biomarker; these can often be associated with lack of consensus of a diagnostic assay (FISH, Next-Generation Sequencing (NGS), IHC, and SRM), and varying reported incidence rates of, and thresholds for, Met positivity (Supplemental Table 6). In this study we assessed the prognostic value of MET amplification by FISH and Met expression by IHC and SRM, without consideration of their predictive value for benefit of anti-Met therapy, or of other emerging prognostic/predictive Met biomarkers including MET mutations and/or exon 14 skipping, HGF aberrations, or Met aberrations within circulating tumor cells or circulating tumor DNA.5,6,12,28-33
Generally, MET amplification denotes increased GCN, is considered a genomic driver of the tumor, and results in extreme overexpression of the Met protein.11,14 However, with respect to amplification and consequent overexpression, the degree of amplification is important to consider – low level amplification (e.g., MET:CEP7, 3:1.5 = 2) generally does not correlate with extreme overexpression of the protein (Supplemental Table 6). This was previously demonstrated with FISH ratio and SRM expression evaluated both as linear variables, compared to a binary FISH ratio of ≥2.11 This is also quite analogous to HER2 amplification, as recently described.27 Therefore, the ease of categorizing to binary subsets (≥ or < 2 ratio) should be weighed against the weaker observed correlation with expression when combining higher and lower FISH ratios within one category. This may have implications regarding the strength of FISH as a prognostic and predictive biomarker. Ultimately, targeted therapies inhibit proteins, therefore excluding patients with gene amplification (low-level) that lacks resulting overexpression, may enrich for the cohort most likely to benefit. Regardless, the rates of MET amplification in this study by FISH ratio ≥2 (4.7%), FISH GCN ≥5 (5.2%), and FISH GCN ≥15 in >10% of cells (2.6%) were consistent with rates previously reported,9-11,13,15 and were the poorest prognostic factors amongst the MET biomarkers evaluated. This is intuitive given that when amplified, MET is the driver oncogene portending significant metastatic and aggressive tumor behavior. In this setting Met is exceptionally overexpressed, several-fold higher than Met-expressing tumors in the absence of gene amplification.11 Although a lower cut-off by SRM of ≥400 amol/μg (set to define ‘overexpression’, not amplification) had low accuracy in discerning MET amplification by FISH, the predefined level of ≥1500 amol/μg did show 100% specificity (in a small cohort analyzed both by FISH and SRM analysis). This level of ≥1500 amol/μg has consistently demonstrated excellent accuracy in identifying truly cluster MET-amplified tumors.11 However, when heterogeneous MET amplification is present within a sample, FISH identified the amplified areas, while SRM represented the aggregate Met expression level of the entire tumor, resulting in lower-than-expected expression levels of a homogenously-amplified tumor (Supplemental Figure 1).11
In contrast to amplification, Met protein overexpression determined by IHC or SRM includes the small subset with overexpression due to gene amplification, but consists mainly of expression without amplification. The incidence of Met overexpression depends on the lower limit set for positivity. Indeed, the rate reportedly varies widely from 24-70%, in part due to variability of this lower limit, along with other analytic variables associated with IHC, the assay typically used for Met expression. Previously described, Met SRM was more objective compared to IHC, and correlated better with FISH ratio and GCN values.11 This was confirmed in this study; Met overexpression, as determined by SRM, accounted for 18.1% of samples at any level above the lower limit of detection (≥200 amol/μg), and 7.8% of samples at the predefined threshold of ≥400 amol/μg. In contrast, 82.2% of samples in this study demonstrated any positivity by IHC, and 35.2% or 13% by any staining intensity (≥1+ intensity in ≥25% or ≥50% of tumor cells, respectively). Interestingly, a recent phase III trial, RILOMET-1, reported a Met positivity rate of 81%, compared to the phase II rate of 64%, with the DAKO IHC criteria (≥1+ intensity in ≥ 25% extensity), suggesting inter-study variability even when using the same IHC antibody and scoring.21,26 In addition to observed variability between the two rilotumumab trials using the DAKO antibody, it is also possible that the rate of Met positivity differs between locally-advanced disease and metastatic disease, as well as primary tumor versus metastatic biopsies.
In this study, although IHC was associated with poor prognosis in univariate analysis, this did not persist after adjusting for other prognostic covariates. Low-level expression of Met may be associated with these poor prognostic covariates, yet not independently associated with outcome upon adjusting. In contrast, extreme overexpression as a consequence of MET amplification or by SRM (determined here ≥400 amol/μg) in the absence of MET amplification remained prognostic after adjusting for other variables.
Finally, Met may be a negative predictive biomarker for standard cytotoxic therapies,15,34 and a positive predictive biomarker for Met-directed therapies, particularly when selecting for MET amplification.13-15,35,36 However, all trials to date selecting for MET amplification have been open-label, single-arm trials – undoubtedly due to the low incidence of MET amplification, aggressive tumors with quickly progressing clinical course, and difficulty accruing towards proper randomization. Given the putative negative prognosis rendered by MET amplification, single-arm trials may underestimate the benefit of anti-Met therapies if this overall poor baseline prognosis is not considered. On the other hand, despite early evidence of predictive value of anti-Met therapy for tumors that overexpress Met (irrespective of GCN/amplification status),21,23 two recent phase III trials, RILOMET-1 (with rilotumumab anti-HGF antibody) and METGastric (with onartuzumab anti-Met antibody), both failed to meet their primary endpoints in “Met-positive” patients, as determined by two different IHC methods.26,37 Although it is quite plausible that Met inhibition, in the setting of Met overexpression lacking amplification, is not sufficient to improve outcome (i.e., a negative prognostic biomarker is not by default also a positive predictive biomarker to a targeted therapy), there is also potential that the positivity criteria was too lenient. The phase I patient that responded to onartuzumab monotherapy, 23 was later found to have expression at 526.93 amol/ug.11 Indeed the positivity rate in RILOMET-1 [≥1+ intensity, ≥25% extensity] was 81%, with only 21% of patients (71 patients with rilotumumab versus 57 patients with placebo) having higher level expression [≥2+ intensity, ≥50% extensity].26 In fact, in the METGastric trial, a predefined subset analysis (IHC ≥2+ intensity, ≥50% extensity) suggested improved overall survival with HR 0.64 (p=0.06) with 105 and 109 patients in the onartuzumab versus control arms, respectively, or 38% of all enrolled patients. Given that the trial closed early at only 70% of the intended accrual (562/800), the intended power to detect a HR of 0.49 in the higher-expressing subset was compromised; moreover, if the more likely true HR is 0.6-0.8, the trial was significantly underpowered to detect this true benefit. Unfortunately, given that METGastric, and even more so RILOMET-1, did not have sufficient power to evaluate the benefit in those samples/patients with higher expression cut-offs, they do not provide guidance for this select patient population. Coupling these results with inherent limitations associated with IHC (antigen dependency, variability in tissue staining, lack of temporal reproducibility, subjective scoring of positive staining) suggests the technique may not be a definitive method for identifying patients likely to respond to Met-targeted therapy. Regardless, it is clear that low-level Met expression, or worse, treatment of all-comers with no selection, does not predict benefit to anti-Met agents.26,37,38
In summary, MET FISH and Met SRM were independently associated with poor prognosis in this large cohort of GEC patients, while Met IHC at currently applied positivity cut-offs was not independently associated with outcome. SRM is a novel technology demonstrating clinical utility with Met and Her2,11,27,39 additionally having the advantage of multiplex peptide analysis,22 analogous to NGS for genomic aberrations. The results of this study suggest that amplification by FISH and/or overexpression by SRM should be incorporated into multivariate models when assessing the prognostic significance of other novel covariates. Moreover, amplification and overexpression as determined by FISH and SRM may better direct treatment, and in particular Met-targeted therapies for GEC, principally in the setting of next-generation clinical trial designs.40
Supplementary Material
Condensed Abstract.
In a large study, MET gene amplification and/or protein overexpression, as assessed by various assays, were associated with poor prognosis in univariate analyses. However, only MET amplification by FISH and Met expression by Selected Reaction Monitoring (SRM) Mass Spectrometry were independent prognostic biomarkers; compared with immunohistochemistry, SRM may provide added benefit towards informed decisions about Met-targeted therapy.
Acknowledgements
The authors wish to acknowledge Ellen Wertheimer and Christopher Parsons for their assistance in the preparation and review of this manuscript.
DVTC would like to thank the LLK and Sal Ferrara Funds for which this work would not be possible.
Funding: This work was supported by NIH K12 award (CA139160-01A), NIH K23 award (CA178203-01A1), UCCCC (University of Chicago Comprehensive Cancer Center) Award in Precision Oncology- CCSG (Cancer Center Support Grant) (P30 CA014599), Cancer Research Foundation Young Investigator Award, ALLIANCE for Clinical Trials in Oncology Foundation Young Investigator Award, Amgen Collaboartive Research Agreement, Oncoplex Dx Collaborative Research Agreement, LLK (Live Like Katie) Foundation Award, and the Sal Ferrara II Fund for PANGEA (to D.V.T.C).
Footnotes
This study has been presented in parts at poster sessions of the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL and the 2015 European Cancer Congress (ESMO) held in Vienna, Austria.
Author Contributions:
Conceived and designed study: DVTC, AA, TH, FG
Clinical trial and translational statistical design: DVTC, JS.
Sample collection, assay performance, and data management: DVTC, AA, WLL, EO, PX, AR
Data interpretation: DVTC, AA, RDL, TH, FG
Manuscript writing and editing: DVTC, AA, WLL, JS, EO, PX, RDL, FC, TH, AR, FG
Disclosures/Conflicts of Interest:
DVTC: Received honoraria for advisory boards/consulting from Amgen, Genentech Inc./Roche, Lilly Oncology, OncoplexDx/Nantomics and research funding from Amgen, and Genentech and OncoplexDx/Nantomics.
AA, JS, RDL: Amgen employees
WLL, FC, TH: OncoplexDx/Nantomics employees
References
- 1.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 2.Petrini I. Biology of MET: a double life between normal tissue repair and tumor progression. Ann Transl Med. 2015;3:82. doi: 10.3978/j.issn.2305-5839.2015.03.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:553–72. doi: 10.1517/14728222.2012.680957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 5.Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol. 2016;34:721–30. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 6.Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5:842–9. doi: 10.1158/2159-8290.CD-14-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piguet AC, Medova M, Keogh A, et al. Impact of MET targeting on tumor-associated angiogenesis and growth of MET mutations-driven models of liver cancer. Genes Cancer. 2015;6:317–27. doi: 10.18632/genesandcancer.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco JJ, Clague MJ. Dysregulation of the Met pathway in non-small cell lung cancer: implications for drug targeting and resistance. Transl Lung Cancer Res. 2015;4:242–52. doi: 10.3978/j.issn.2218-6751.2015.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass AJ, Thorsson V, Shmulevich I, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catenacci DV, Cervantes G, Yala S, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011;12:9–46. doi: 10.4161/cbt.12.1.15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catenacci DV, Liao WL, Thyparambil S, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One. 2014;9:e100586. doi: 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graziano F, Galluccio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789–95. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
- 13.Jardim DL, Tang C, Gagliato Dde M, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I Clinic. Clin Cancer Res. 2014;20:6336–45. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 14.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–10. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger ML, Behrens HM, Boger C, et al. MET in gastric cancer - discarding a 10% cutoff rule. Histopathology. 2016;68:241–53. doi: 10.1111/his.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–8. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghav KP, Wang W, Liu S, et al. cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res. 2012;18:2269–77. doi: 10.1158/1078-0432.CCR-11-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hack SP, Bruey JM, Koeppen H. HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget. 2014;5:2866–80. doi: 10.18632/oncotarget.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S, Yu Y, Zhao N, et al. C-Met as a prognostic marker in gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e79137. doi: 10.1371/journal.pone.0079137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–18. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 22.Hembrough T, Thyparambil S, Liao WL, et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn. 2013;15:454–65. doi: 10.1016/j.jmoldx.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Catenacci DV, Henderson L, Xiao SY, et al. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1:573–9. doi: 10.1158/2159-8290.CD-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catenacci DVT, Tang R, Oliner KS, et al. MET as a prognostic biomarker of survival in a large cohort of patients with gastroesophageal cancer (GEC). ASCO Annual Meeting Proceedings; 2015.p. 4034. [Google Scholar]
- 25.Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–74. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. ASCO Annual Meeting Proceedings; 2015.p. 4000. [Google Scholar]
- 27.Catenacci DV, Liao WL, Zhao L, et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: comparison to IHC and FISH. Gastric Cancer. 2015 doi: 10.1007/s10120-015-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asaoka Y, Tada M, Ikenoue T, et al. Gastric cancer cell line Hs746T harbors a splice site mutation of c-Met causing juxtamembrane domain deletion. Biochem Biophys Res Commun. 2010;394:1042–6. doi: 10.1016/j.bbrc.2010.03.120. [DOI] [PubMed] [Google Scholar]
- 29.Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–9. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 30.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–53. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 32.Penuel E, Li C, Parab V, et al. HGF as a circulating biomarker of onartuzumab treatment in patients with advanced solid tumors. Mol Cancer Ther. 2013;12:1122–30. doi: 10.1158/1535-7163.MCT-13-0015. [DOI] [PubMed] [Google Scholar]
- 33.Wallenius V, Hisaoka M, Helou K, et al. Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors. Am J Pathol. 2000;156:821–9. doi: 10.1016/S0002-9440(10)64950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Kang Y-K, LoRusso P, Salgia R, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). ASCO Annual Meeting Proceedings; 2015.p. 167. [Google Scholar]
- 36.Kwak EL, LoRusso P, Hamid O, et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. ASCO Annual Meeting Proceedings; 2015.p. 1. [Google Scholar]
- 37.Shah MA, Bang Y-J, Lordick F, et al. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2−) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). ASCO Annual Meeting Proceedings; 2015.p. 4012. [Google Scholar]
- 38.Shah MA, Wainberg ZA, Catenacci DV, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PloS one. 2013;8:e54014. doi: 10.1371/journal.pone.0054014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuciforo P, Thyparambil S, Aura C, et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol. 2016;10:138–47. doi: 10.1016/j.molonc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catenacci DV. Next-generation clinical trials: Novel strategies to address the challenge of tumor molecular heterogeneity. Molecular oncology. 2015;9:967–996. doi: 10.1016/j.molonc.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–73. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, McCleland M, Stawiski EW, et al. Integrated exome and transcriptome sequencing reveals ZAK isoform usage in gastric cancer. Nat Commun. 2014;5:3830. doi: 10.1038/ncomms4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi J, Lee HE, Kim MA, et al. Analysis of MET mRNA expression in gastric cancers using RNA in situ hybridization assay: its clinical implication and comparison with immunohistochemistry and silver in situ hybridization. PLoS One. 2014;9:e111658. doi: 10.1371/journal.pone.0111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.