Abstract
Adaptive thermogenesis is an important component of energy expenditure. Brown adipocytes are best known for their ability to convert chemical energy into heat. Beige cells are brown-like adipocytes that arise in white adipose tissue in response to certain environmental cues to dissipate heat and improve metabolic homeostasis. A large body of intrinsic factors and external signals are critical for the function of beige adipocytes. In this review, we discuss recent advances in our understanding of neuronal, hormonal, and metabolic regulation of the development and activation of beige adipocytes, with a focus on the regulation of beige adipocytes by other organs, tissues, and cells. Understanding the cellular and molecular mechanisms of inter-organ regulation of adipose tissue browning may provide an avenue for combating obesity and associated diseases.
Keywords: Thermogenesis, beige adipocytes, browning, inter-organ crosstalk, obesity
Introduction
A major type of thermogenic tissue in mammals is brown adipose tissue (BAT). In mice, BAT is located constitutively in the anterior subcutaneous region including the interscapular, axillary and cervical fat (Diaz et al., 2014). Brown adipocytes are characterized by multilocular lipid droplets and a high density of iron-containing mitochondria that give the eponymous appearance (Cinti, 2001). Uncoupling protein 1 (UCP1) is a key thermogenic factor in brown adipocytes. When activated by long-chain fatty acids, UCP1 catalyzes a proton leak across the inner membrane, thus bypasses ATP synthase, dissipates the electrochemical gradient, and generates heat (Bartelt et al., 2011; Krauss et al., 2005). Moreover, high vascularization of BAT facilitates sufficient nutrition and oxygen supply as well as efficient heat output to the whole body through circulation.
Recent studies have uncovered another type of thermogenic adipocytes known as beige/brite (brown in white) adipocytes. Beige/brite cells share morphological and functional similarities with brown adipocytes. Beige/brite cells express a key set of brown fat-specific genes including UCP1 and also undergo thermogenesis via uncoupling of oxidative phosphorylation from ATP production (Wu et al., 2015). Although it seems interchangeable between the terms “beige” and “brite” adipocytes, the beige originally refers to those thermogenic adipocytes isolated from subcutaneous WAT depots like inguinal WAT and the brite are from their visceral counterparts such as epididymal and mesenteric depots (Petrovic et al., 2010; Wu et al., 2012). Typically, the inguinal WAT is more prone to browning/beiging (Vitali et al., 2012), but the epididymal WAT also possess bipotent adipogenic precursor cells that can differentiate into both white and UCP1+ adipocytes under different conditions (Lee et al., 2012). Hereinafter, all these inducible thermogenic adipocytes will be referred to as beige adipocytes.
The recruitment of beige adipocytes, i.e. the browning process, is potently activated by cold exposure. Chronic cold acclimation of mice induces a substantial amount of beige adipocytes in posterior subcutaneous, retroperitoneal and perigonadal fat depots (Vitali et al., 2012; Young et al., 1984). Moreover, cold-induced formation of beige adipocytes in mice can be reversed within 5 weeks of warm adaptation, suggesting that browning is a reversible and dynamic process (Rosenwald et al., 2013).
Distinct from brown adipocytes, beige adipocytes have certain unique features. A majority of brown adipocytes arise from multipotent cells of the dermomyotome (Kajimura et al., 2015; Seale et al., 2008), whereas beige adipocytes are thought to originate from mesenchymal precursors that also give rise to smooth muscle or smooth muscle-like cells (Lee et al., 2012; Long et al., 2014). It has been further demonstrated that RhoA signaling controls the fate of mesenchyme stem cells (MSCs) to an adipogenic versus smooth muscle-like lineage (McDonald et al., 2015). In addition, it is generally believed that most cold-induced beige adipocytes originate from de novo differentiated adipocytes (Wang et al., 2013). However, beige adipocytes may also derive from the conversion of mature white adipocytes in response to cold or β3-adrenergic stimuli (Himms-Hagen et al., 2000; Lee et al., 2012; Long et al., 2014; Rosenwald et al., 2013; Wang et al., 2013; Wu et al., 2012). The latter is corroborated by a recent study showing that most UCP1+ adipocytes in inguinal WAT upon cold exposure stem from pre-existing mature adipocytes (Lee et al., 2015b).
So far, many intrinsic transcription factors and cofactors have been identified that robustly trigger beige adipocyte biogenesis (Emont et al., 2015; Kajimura et al., 2015; Wu et al., 2013). One of these regulators is PRDM16 that is highly expressed in inguinal white fat relative to other white fat depots in mice and plays a key role in regulating the determination and activation of beige adipocytes (Chi and Cohen, 2016; Kajimura et al., 2008; Ohno et al., 2012; Seale et al., 2011). Moreover, PRDM16-binding partners such as PPARγ coactivator 1α (PGC1α), C/EBP-β, Euchromatic histone-lysine N-methyltransferase 1 (EHMT1), and Zfp516 serve as powerful transcriptional activators (Dempersmier et al., 2015; Kleiner et al., 2012; Ohno et al., 2013).
Human thermogenic fat
It has long been known that classical brown adipocytes exist in the interscapular regions of human infants and diminish with age (Aherne and Hull, 1966; Heaton, 1972). The existence of brown adipocytes has been detected in adult human samples from both the periadrenal region of benign adrenal tumor patients and the supraclavicular region of healthy individuals (Lidell et al., 2013).
Although brown adipocytes have been identified in multiple BAT depots of adult humans ranging from supraclavicular area, posterior mediastinum, retroperitoneal and intraabdominal regions to mesenteric depots, their identity is still controversial. Several reports suggest that these brown adipocytes in adult humans resemble murine beige adipocytes (Sharp et al., 2012; Wu et al., 2012). This view is further supported by the analysis of molecular signatures of clonally derived adipocytes from superclavicular BAT in adult humans (Shinoda et al., 2015). However, the other study indicates that cold-induced BAT from adult human neck area consists of classical brown adipocytes (Cypess et al., 2013). In addition, it has also been shown that activated thermogenic fat in the supraclavicular region are composed of both classical brown and beige adipocytes (Jespersen et al., 2013).
WAT browning can exert a significant impact on whole-body metabolism in humans. Evidence shows that white adipocytes in patients with pheochromocytoma undergo direct transformation into brown adipocytes, which is associated with elevated thermogenesis and lower BMI in patients (Frontini et al., 2013). Body weight reduction has also been reported in patients with hibernoma due to ectopic de novo development of brown adipocytes (Allegra et al., 1983). However, the extent to which WAT browning contributes to weight loss is not clear until recently. According to one study on three young individuals, mathematical analysis with a modest assumption suggests a decrease in approximately 4.1 kg of adipose tissue over the course of one year, if human BAT were fully activated (Virtanen et al., 2009). Recent studies indicate that BAT activation in humans minimally contributes to the increase in energy expenditure which is only about 15–25 kcal/day, resulting in an estimated weight loss of 1 kg/year (Muzik et al., 2012; Porter et al., 2015). Furthermore, it is still unclear how much white fat can be converted into beige fat in adult humans. In addition to the effects on human body weight, increased activation of thermogenic fat induced by cold acclimation can potentially enhance energy metabolism and insulin sensitization (Chondronikola et al., 2014; Lee et al., 2014). It will be of clinical significance to induce white-to-brown transformation in the treatment of obesity and type 2 diabetes.
Cellular and physiological functions of brown and beige adipocytes have been extensively reviewed recently (Diaz et al., 2014; Kajimura et al., 2015; Pfeifer and Hoffmann, 2015). A growing number of studies indicate that the recruitment of beige adipocytes relies on various extrinsic factors such as hormones and secreted molecules derived from various tissues and organs. In this review, we will summarize recent advances in understanding how different organs and systems contribute to the development and function of beige adipocytes.
Central nervous system (CNS)
A recent emphasis has been placed on the role of the hypothalamus of the CNS in beige adipocyte development and function (Dodd et al., 2015; Ruan et al., 2014; Yang and Ruan, 2015). The hypothalamus not only senses body temperature fluctuation in cold, but also responds to peripheral signals including hormones and nutrients to modulate sympathetic outputs (Plum et al., 2007; Rezai-Zadeh and Munzberg, 2013). The arcuate nucleus (ARC) of the hypothalamus is one of the important nodes where these peripheral signals converge to regulate browning (Commins et al., 2000; Morrison et al., 2014). The AgRP/NPY neurons are hunger-promoting neurons expressing agouti-related protein and neuropeptide Y, while POMC/CART neurons are satiety neurons expressing proopiomelanocortin and cocaine-amphetamine-regulated transcript. These two major sets of neurons in the ARC respond, generally in opposite directions, to hormones such as leptin, insulin, and ghrelin, as well as nutrients such as glucose, amino acids, and fatty acids (Belgardt et al., 2009; Dietrich and Horvath, 2013; Stefanidis et al., 2014). Previous studies have implicated the role of central leptin in the regulation of WAT browning. Supported by direct genetic evidence leptin-stimulated phosphatidylinositol 3-kinase (PI3K) signaling in the CNS has been shown to modulate energy expenditure via activation of sympathetic nerve activity to perigonadal WAT resulting in BAT-like differentiation of WAT in mice (Plum et al., 2007). But more efforts will be required to identify the exact anatomical site and nature of the hypothalamic leptin-responsive neurons responsible for mediating sympathetic nerve activity in WAT. In addition to hormones, the effects of these two neuronal populations on WAT browning are closely correlated with the body’s energy state. In the fed state, insulin and leptin act synergistically on POMC neurons to stimulate beige adipocyte activation in inguinal WAT (Dodd et al., 2015). Genetic ablation of two phosphatases PTP1B and TCPTP that negatively regulate insulin and leptin pathways in POMC neurons leads to increased WAT browning (Dodd et al., 2015). In the fasting state, the hunger-promoting hormone ghrelin activates AgRP neurons, which inhibits browning in retroperitoneal WAT. O-GlcNAc transferase (OGT) is enriched in AgRP neurons, and its expression is increased in response to fasting and ghrelin (Ruan et al., 2014). OGT knockout in AgRP neurons inhibits neuronal excitability and abrogates the suppression of WAT browning by fasting and ghrelin. These studies demonstrate that AgRP and POMC neurons regulate WAT browning through directly modulating sympathetic nerve activity; however, neural circuits linking these neurons to sympathetic innervation onto different WAT depots need to be further explored (Harlan et al., 2011; Shi et al., 2013).
Enriched environment (EE) abundant with complex physical and social stimulations is important for increased neurogenesis, improved cognitive performance and resistance to cerebral insults (Cao et al., 2004; Monteiro et al., 2014). Recently EE has also been found to trigger beige adipocyte induction (Cao et al., 2011). EE induces hypothalamic expression of the brain-derived neurotrophic factor (BDNF), which subsequently increases β-adrenergic receptor (β-AR) and norepinephrine (NE) levels in WAT, and this SNS outflow in WAT ultimately results in browning of retroperitoneal and epididymal WAT and decreased adiposity in mice (Cao et al., 2011). It has also been reported that the ventromedial nucleus (VMH) and the paraventricular nucleus (PVN) are vital sites of BDNF action (Wang et al., 2007; Wang et al., 2010). Moreover, hypothalamic BDNF also regulates VEGF signaling in retroperitoneal WAT that is required for angiogenesis and browning induced by diverse physiological and pharmacological approaches (During et al., 2015). Although growing evidence demonstrates that acute exercise induces a significant increase in the BDNF level, which might benefit brain cognition, it has yet to be determined whether exercise-induced BDNF has effects on browning of WAT (Etnier et al., 2016; Ieraci et al., 2016; Tsai et al., 2016).
Recent studies show that central serotonin neurons are indispensable for sympathetic activation of brown and beige adipocytes in inguinal WAT in response to cold (McGlashon et al., 2015). In fact, our knowledge of neuronal circuits that control the browning process is still fragmentary. More studies are needed to expand our understanding of how the CNS modulates WAT browning.
Sympathetic nervous system (SNS)
The SNS is believed to be a master regulator of both the recruitment and activation of beige adipocytes (Diaz et al., 2014; Romanovsky, 2007; van Marken Lichtenbelt and Schrauwen, 2011). Either physiological stimuli such as chronic cold exposure or pharmacological agents such as β3-adrenergic receptor (β3-AR) agonists, thiazolidinedione (TZDs) and other PPARγ agonists can activate sympathetic nerve fibers in WATs (Harms and Seale, 2013). Since most sympathetic nerve fibers are actually noradrenergic, the propensity of WAT depots to undergo browning is accompanied by enhanced density of noradrenergic parenchymal nerve fibers (Murano et al., 2009). Of note, prolonged cold exposure induces WAT browning via eliciting a significant increase in the total number of sympathetic noradrenergic fibers as well as macrophage activation. This leads to the release of catecholamine, particularly norepinephrine (NE), to act on β3-AR and activate mitochondrial biogenesis in adipocytes (Bartness et al., 2014; Collins, 2011; Granneman et al., 2005). Moreover, chronic stimulation of WAT by β3-AR agonists results in white adipocytes transformation into a brown phenotype (Himms-Hagen et al., 2000; Murano et al., 2009). It has also been suggested that a major physiological action of PPARγ agonists is to induce UCP1 expression in both BAT and WAT (Sell et al., 2004). Interestingly, the use of TZDs and other PPARγ agonists may influence the central regulation so as to indirectly reduce sympathetic activity, but additional treatment of β3-AR agonists overcomes this situation and synergizes with PPARγ agonism to increase thermogenic energy expenditure in WAT (Sell et al., 2004; Wilson-Fritch et al., 2004). Overall, sympathetic activities vary between different WAT depots under the basal, cold-induced, and fasting-induced conditions (Brito et al., 2008; Ruan et al., 2014). However, the mechanisms by which the SNS differentially regulates browning in different WAT depots remain to be characterized.
Immune cells
Upon cold exposure, in addition to the sympathetic nerves, eosinophil-derived interleukin (IL)-4 has been found to induce catecholamine synthesis from alternatively activated macrophages (AAMs) that recruits and activates beige adipocyte development in inguinal WAT (Nguyen et al., 2011; Qiu et al., 2014). In response to epithelial cytokines or microbe infection, group 2 innate lymphoid cells (ILC2s) promote eosinophil and AAMs via releasing a large amount of type 2 cytokines (Halim et al., 2014; Molofsky et al., 2013; Moro et al., 2010; Neill et al., 2010). It has been recently identified that activated by IL-33, ILC2s induce beige adipocyte activation in abdominal subcutaneous WAT in humans and epididymal and inguinal WAT in mice (Brestoff et al., 2015; Molofsky et al., 2013). Mechanistically, ILC2s can produce IL-5 and IL-13 to activate eosinophils and AAMs to synthesize catecholamines. Independent of the adaptive immune system, IL-33-elicited ILC2s also drive the browning process by producing methionine-enkephalin (MetEnk) that directly acts on adipocytes to upregulate UCP1 expression (Brestoff et al., 2015). The same group further showed that activated ILC2 cells in thermoneutral mice stimulate the proliferation of PDGFRα+ adipocyte precursor cells, which then commit to the beige adipocyte lineage (Lee et al., 2015a).
Skeletal muscle
Acute and endurance exercise training brings about an increase in brown adipocyte-specific genes expression in WAT of mice (Xu et al., 2011). Skeletal muscle-derived signals such as myokines contribute to the conducive effects of exercise (Bassel-Duby and Olson, 2006). Irisin, a myokine stimulated in muscle upon exercise, has been shown to act on subcutaneous white adipocytes to induce browning in mice at least in part via PPARα (Bostrom et al., 2012; Jedrychowski et al., 2015). Similar to mice, plasma irisin in humans increases in response to acute exercise and decreases with weight loss after bariatric surgery (Huh et al., 2012; Jedrychowski et al., 2015). Although muscle mass is the strongest predictor of circulating irisin levels, current knowledge of the long-term effects of irisin on browning of WAT in humans are still absent. Meteorin-like (Metrnl), another myokine induced in muscle after exercise and in adipose tissue upon cold exposure, has been observed to induce the cytokines IL4/IL13 and AAM activation to promote production of catecholamine, ultimately leading to browning of both epididymal and subcutaneous WAT in mice (Rao et al., 2014). These observations suggest that muscle-derived myokines might engage type 2 innate immunity to control beige adipocyte activation. The cytokine IL-6, released from contracting skeletal muscle to the circulation (Steensberg et al., 2000), is also required for a full induction of beige cell biogenesis in murine inguinal WAT after exercise and cold exposure (Knudsen et al., 2014). IL-6 induces WAT browning at least partly through increasing PGC-1α activity (Knudsen et al., 2014).
Recent studies demonstrate that certain metabolites secreted from skeletal muscle after physical activity control WAT browning. Lactate induces thermogenic gene expression in murine and human adipocytes. In mice treated with PPARγ agonist, lactate triggers beige adipocyte activation in subcutaneous WAT by modifying intracellular redox (Carriere et al., 2014). The ketone body β-hydroxybutyrate (β-HB) that impacts cellular redox state is a robust inducer of browning in inguinal WAT (Carriere et al., 2014). β-aminoisobutyric acid (BAIBA) is a secreted metabolite from PGC-1α-expressing myocytes and its circulating levels in mice and humans positively correlates with exercise (Roberts et al., 2014). BAIBA is found to be regulated by PGC-1α and increase brown adipocyte-specific genes expression. Further mechanistic experiments in rodents demonstrate that BAIBA elicits browning of murine inguinal WAT in a specific PPARα-dependent manner (Roberts et al., 2014).
Heart
Atrial natriuretic peptides (ANP) and ventricular natriuretic peptides (BNP) are predominantly released from the atria and ventricles, respectively. These two cardiac natriuretic peptides act through the natriuretic peptide receptor A (NPRA), whose intracellular domain possesses a guanylyl cyclase activity to generate the second messenger cGMP. The natriuretic peptide receptor C (NPRC) is the clearance receptor that binds ANP and BNP and removes them from circulation. Cold exposure in mice is associated with an increased ratio of NPRA to NPRC. Infusion of BNP into mice dramatically increases Ucp1 and PGC-1α levels in WAT via the p38 MAPK pathway, indicating that natriuretic peptides promote WAT browning to boost energy expenditure in mice (Bordicchia et al., 2012). In addition, recent findings show that Roux-en-Y gastric bypass (RYGB) surgery leads to browning of gonadal WAT in female mice and this may be explained in part by the upregulation of ANP and BNP after RYGB (Neinast et al., 2015). Similar observations have been reported in humans undergoing RYGB surgery, although detailed mechanisms by which RYGB surgery promotes beige adipocyte biogenesis in supraclavicular adipose tissue are still unknown (Rachid et al., 2015).
Gut
The gastrointestinal tract is known as the largest endocrine organ that secrets a number of regulatory peptide hormones (Badman and Flier, 2005). The intestinal microbiota develops within the host, and its composition is continuously influenced by different physiological conditions (Koren et al., 2012; Liou et al., 2013; Ridaura et al., 2013). Cold exposure is known to alter microbiota composition, and transplantation of the microbiota from mice under prolonged cold exposure to germ-free mice is sufficient to promote browning of inguinal and perigonadal WAT (Chevalier et al., 2015). There is also the evidence that depletion of microbiota either by means of antibiotic treatment or in germ-free mice promotes beige fat development in inguinal subcutaneous and perigonadal visceral adipose tissues, which is mediated via enhanced type 2 cytokine signaling (Suarez-Zamorano et al., 2015). Re-colonization of antibiotic-treated or germ-free mice with microbiota reverses the browning phenotypes that are induced by microbiota depletion (Suarez-Zamorano et al., 2015). Collectively, these results hold promise for the induction of beige adipocyte in humans through the transplantation of functional microbiota.
Farnesoid X receptor (FXR) is a ligand-activated transcriptional factor expressed in diverse tissues including the intestine. Bile acids act as endogenous ligands for FXR and bile acids released during a meal can selectively activates intestinal FXR (Fang et al., 2015; Fang et al., 2008; Kemper et al., 2009; Lee et al., 2006). In mimicking this tissue-selective effect, gut-restricted FXR agonist fexaramine is able to reduce diet-induced weight gain and activate inguinal WAT browning in mice via enhanced β-adrenergic signaling (Fang et al., 2015). These results offer insight into intestinal FXR activation, instead of systemic FXR agonism, as a promising approach in the treatment of metabolic morbidities.
Liver
The autocrine/paracrine hormone fibroblast growth factor 21(FGF21) is a key member of FGF superfamily that is produced mainly from liver and could be induced after fasting (Badman et al., 2007; Inagaki et al., 2007). Pharmacological administration of FGF21 induces browning of inguinal and perirenal WAT, which is evidenced by increased levels of thermogenic genes and histological appearance of increased brown-like adipocytes (Fisher et al., 2012). One possible mechanism is that FGF21 post-transcriptionally regulates PGC-1α levels in WAT (Fisher et al., 2012). Later, it was reported that intraperitoneal injection of FGF21 normalizes hyperglycemia in diabetic mice independently of insulin action in the liver, but largely due to increased energy expenditure via activation of BAT and browning of subcutaneous WAT (Emanuelli et al., 2014). However, FGF21 has serious limitations in that both genetic and pharmacological gain-of-function of FGF21 are found to severely decrease bone mass in humans (Wei et al., 2012). It has been further demonstrated that FGF21 inhibits osteoblastogenesis but enhances bone marrow adipogenesis by potentiating the activity of peroxisome proliferator-activated receptor γ (PPAR-γ) (Wei et al., 2012). Recent human studies show that circulating FGF21 levels are elevated in lipodystrophy and metabolically unhealthy obesity (Berti et al., 2015; Miehle et al., 2016). Therefore, the clinical application of FGF21 as a potential drug for the treatment of obesity and type 2 diabetes appears less desirable.
Adipose Tissue
Apart from the tissues described above, adipose tissue itself can produce secreted factors that enhance the recruitment of beige adipocytes. A recent study reveals that adenosine released from brown adipocytes during the stimulation of sympathetic nerves plays a critical role in the induction of beige adipocytes (Gnad et al., 2014). The adenosine A2A receptor is the most abundant adenosine receptor in human and murine BAT. Although A2A levels are scarcely expressed in white adipocytes, either pharmacological stimulation of the A2A receptors or injection of lentiviral vectors expressing the A2A receptor into inguinal WAT induces beige adipocyte development (Gnad et al., 2014).
In addition, vascular endothelial growth factor (VEGF)-A secreted by adipocytes plays a pivotal role in adipose tissue angiogenesis (Cao, 2010; Hausman and Richardson, 2004). Up-regulation of VEGF-A in retroperitoneal WAT improves vascularization and leads to browning of WAT (During et al., 2015). Moreover, transgenic overexpression of VEGF in adipose tissue may also directly recruit brown and beige adipocytes and triggers browning of WAT; however, the molecular basis is unclear (Elias et al., 2012; Sun et al., 2012). It is still worth pointing out that cold exposure induces angiogenesis in both brown and white adipose tissues independently of hypoxia (Xue et al., 2009). In inguinal WAT, cold exposure not only results in the browning phenotype with multilocular UCP1-positive adipocytes, but also leads to an increased production of VEGF. However, there is no direct evidence that VEGF-mediated vascularization is sufficient or necessary to induce browning.
Disease-induced browning of WAT
Cancer
Cancer-associated cachexia (CAC) is characterized by systemic inflammation, body weight loss, atrophy of adipose tissue, and skeletal muscle wasting (Fearon et al., 2012). A systematic morphological analysis of WAT depots in the cachectic mouse models of several cancer types such as Kras-pancreatic and lung cancer identified a robust phenotypic switch from white to beige fat in subcutaneous WAT (Petruzzelli et al., 2014). WAT browning takes place in the early stages of CAC before skeletal muscle atrophy, whereas inhibiting inflammation or β-adrenergic signaling significantly reduces WAT browning and alleviates the severity of cachexia (Petruzzelli et al., 2014). WAT browning is also observed in cancer cachexia patients, characterized by increased UCP1 staining in intestine adipose tissue as well as fat surrounding the liver, kidney and pancreas (Petruzzelli et al., 2014). In pursuit of the mechanism underlying WAT browning in CAC mouse models, IL-6 signaling together with β-adrenergic activation was found to jointly trigger and sustain WAT browning in cachexia (Petruzzelli et al., 2014). Insight into how tumors induce the development of beige adipocytes is also enriched by a study on tumor-derived parathyroid-hormone-related protein (PTHrP) (Kir et al., 2014). Lewis lung carcinoma-derived PTHrP has been demonstrated to initiate WAT browning and muscle loss, and neutralization of PTHrP is able to prevent tumor-induced browning of WAT (Kir et al., 2014). Collectively, blockage of CAC-induced beige adipocyte biogenesis may underlie the translational value to ameliorate cachexia in cancer patients.
Benign tumors
Several case studies further demonstrate the impact of various benign human tumors on fat browning. Pheochromocytoma is a catecholamine-secreting tumor. In affected patients, omental white adipocytes can transdifferentiate into brown adipocytes due to ectopic adrenergic stimulation (Frontini et al., 2013). Another study in patients with benign adrenal tumors indicates a white-brown plasticity of the white fat in the periadrenal region (Lidell et al., 2013). Similarly, tissue sections from human hibernoma exhibit three different adipocyte morphologies: unilocular, multilocular, and paucilocular. The various intermediate forms of adipocytes suggest a reversible transition between white and brown adipocytes (Manieri et al., 2010).
HIV infection
In addition to cancer, the influence of other diseases on fat browning in adult humans has been documented. HIV-infected subjects with lipodystrophy are characterized by the presence of excessive dorsocervical fat. Dorsocervical fat accumulation is correlated with the down-regulation of brown and beige fat genes in nonlipomatous abdominal subcutaneous fat (Torriani et al., 2016). These observations indicate that WAT browning is impaired in lipodystrophic HIV patients. It is reasonable to speculate that stimulating browning of WAT might improve metabolic health in this population.
Tissue Non-specific Regulators
Beige adipocyte development is regulated by a large variety of intrinsic factors, some of which are not confined to a specific tissue. Such molecules and related signaling pathways important for beige adipocyte activation are highlighted below.
Cyclooxygenase (COX)-2/ and prostaglandin (PG)
COX-2 serves as a rate-limiting enzyme in the synthesis of PG. COX-2 is required for the induction of beige adipocytes in mice as a downstream effector of β-adrenergic signaling in visceral WAT depots under cold exposure (Madsen et al., 2010; Vegiopoulos et al., 2010). Notably, local COX-2 overexpression in intra-abdominal WAT is sufficient for WAT browning. In addition, microsomal prostaglandin E (PGE) synthase-1 (mPGES-1) has recently been shown to be necessary for murine beige adipocyte biogenesis from pre-adipocytes (Garcia-Alonso et al., 2013). Further mechanistic studies suggest that COX-2-PG pathway shifts the differentiation of defined mesenchymal progenitors toward a brown adipocyte phenotype by acting on both the cellular prostacyclin (PGI2) transmembrane receptor and the nuclear receptor PPARγ (Garcia-Alonso et al., 2013; Vegiopoulos et al., 2010).
Transforming growth factor (TGF)-β superfamily
Bone morphogenetic protein (BMPs) are the members of the TGF-β superfamily. BMPs have recently been implicated with the ability to stimulate beige adipocyte development. Genetic knockout of the type 1A BMP receptor (Bmpr1a) in brown adipogenic progenitor cells results in a severe paucity of classical brown adipocytes, which in turn increases sympathetic input to WAT with elevated circulating NE, thereby promoting compensatory browning in both inguinal and epididymal WAT (Schulz et al., 2013). Moreover, gain- and loss-of-function experiments show that bone morphogenetic protein 4 (BMP4) recruits beige adipocytes in inguinal WAT by targeting PGC-1α (Qian et al., 2013). It has also been reported that a subpopulation of adipogenic progenitors (Sca-1+/CD45−/Mac1−; referred to as Sca-1+ progenitor cells, ScaPCs) residing in skeletal muscle and inguinal WAT are highly inducible to differentiation into beige adipocytes upon stimulation with BMP7 (Schulz et al., 2011). In addition, BMP7 suppresses ROCK to facilitate beige adipocyte formation via mediating the G-actin-regulated transcriptional coactivator myocardin-related transcription factor A, MRTFA (McDonald et al., 2015). The mechanisms, however, by which TGFβ family members regulate ROCK are still unclear. The role of TGF-β signaling in regulating fat browning is further supported by the pharmacological studies of the activin receptor type IIB (ActRIIB), a type II receptor that binds to multiple ligands from the TGF-β superfamily such as the activin and BMP subgroups (Sako et al., 2010). Administration of a soluble ActRIIB protein comprised of a form of the extracellular domain of ActRIIB fused to a human Fc (ActRIIB-Fc) leads to an induction of beige adipocytes in epididymal WAT, yet the underlying mechanism is poorly defined (Koncarevic et al., 2012).
Concluding remarks
Systemic homeostasis is achieved through coordinated metabolic regulation among multiple tissues/organs. Being no exception, the development and activation of beige adipocytes are also controlled by signals derived from various tissues/organs. Despite the recent explosion in our understanding of such metabolic communication between beige adipocytes and other tissues/organs, it is still a long way to go to fully describe the contribution of each individual tissue or organ to the browning process and how beige adipocytes integrate these signals.
As we are gaining a better understanding of the induction of beige adipocytes, our knowledge concerning the contribution of beige fat cells to energy expenditure and whole-body metabolic homeostasis has also greatly improved.
There is evidence that the function of beige adipocytes in regulation of energy expenditure and thermogenesis may be not entirely mediated by UCP1 (Bal et al., 2012; Cannon and Nedergaard, 2010; Ribeiro et al., 2000). Recently, beige adipocytes are shown to be able to utilize creatine to stimulate mitochondrial respiration when ADP is limiting (Kazak et al., 2015). These data suggest that creatine-driven futile substrate cycle could be another important mechanism of thermoregulation in beige adipocytes independent of UCP1 (Kazak et al., 2015).
In addition to generating heat and mediating energy expenditure, mounting evidence suggests that beige adipocytes also contribute to whole-body glucose and lipid homeostasis. Adipocyte-specific expression of PRDM16 in obese mice not only leads to increased beige fat mass and significantly reduced adipose mass, but also greatly improves glucose tolerance (Seale et al., 2011). Apart from subcutaneous adiposity, hepatic steatosis turns out to be the major phenotype in mice with adipocyte specific deletion of PRDM16 (Cohen et al., 2014). Beige fat may also secrete molecules into the circulation to improve glucose homeostasis. Recent study reveals that mice transplanted with inguinal WAT from exercise-trained mice show increased glucose and fatty acid uptake than those receiving inguinal WAT from sedentary or sham-treated mice (Stanford et al., 2015). These findings indicate a potentially direct function for beige adipocytes in reducing circulating glucose and fatty acids, independently on its regulation of body weight. We need future studies to address new functions of beige adipocytes and their regulatory circuits apart from thermogenesis.
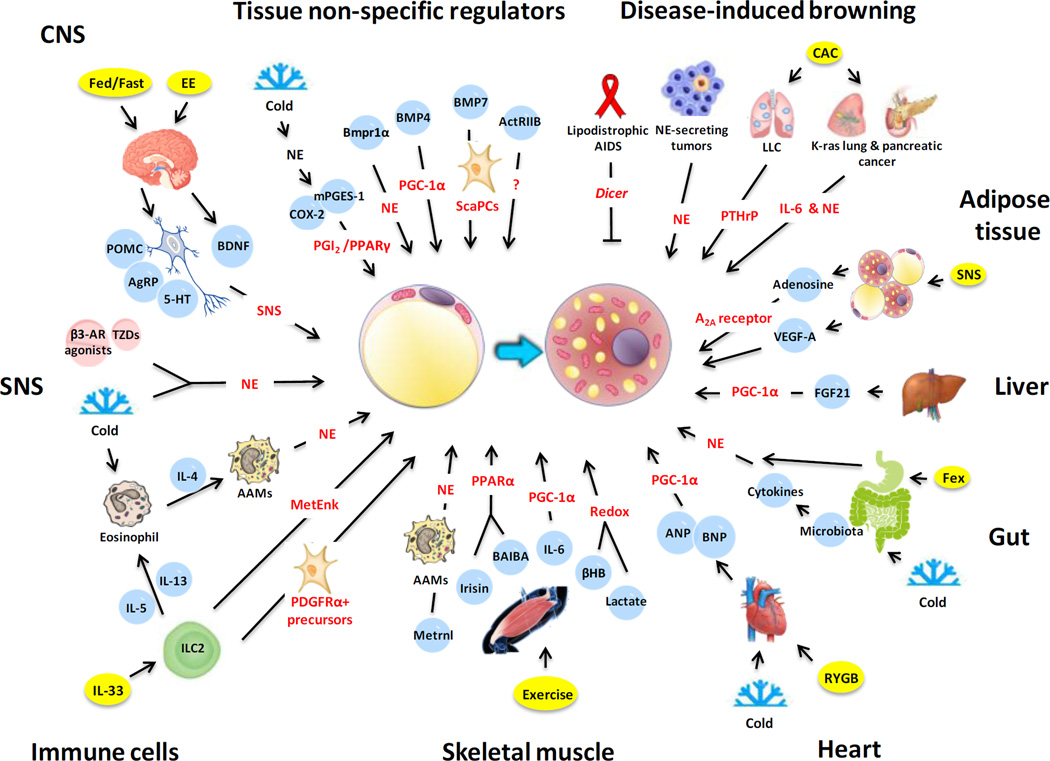
Figure 1. The inter-organ regulation of adipose tissue browning.
The schematic illustrates the cellular and molecular mechanisms of inter-organ regulation of adipose tissue browning in mice, human or both. A number of organs, tissues and cells have been found to either act alone or in concert to promote browning of white adipose tissue. This simplified overview delineates the regulation of beige adipocyte induction by central nervous system (CNS), sympathetic nervous system (SNS), immune cells, skeletal muscle, heart, gut, liver, adipose tissue, disease-associated, or tissue non-specific factors (for details, see the text).
Acknowledgments
We thank Hai-Bin Ruan for critical reading of the manuscript and all members of the Yang laboratory for stimulating discussions. This work was supported by National Institutes of Health (R01DK089098, R01DK102648, P01DK057751), American Cancer Society (RSG-14-244-01-TBE), State of Connecticut (DPH2014-0139), and Ellison Medical Foundation to XY, and China Scholarship Council Scholarship to SW.
References
- Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91:223–234. doi: 10.1002/path.1700910126. [DOI] [PubMed] [Google Scholar]
- Allegra SR, Gmuer C, O'Leary GP., Jr Endocrine activity in a large hibernoma. Hum Pathol. 1983;14:1044–1052. doi: 10.1016/s0046-8177(83)80260-3. [DOI] [PubMed] [Google Scholar]
- Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signalling in POMC and AgRP neurons. The Journal of physiology. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti L, Irmler M, Zdichavsky M, Meile T, Bohm A, Stefan N, Fritsche A, Beckers J, Konigsrainer A, Haring HU, et al. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Mol Metab. 2015;4:519–527. doi: 10.1016/j.molmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R1445–R1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34(Suppl 1):S7–S16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell metabolism. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanovic A, Hagemann S, et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Chi J, Cohen P. The Multifaceted Roles of PRDM16: Adipose Biology and Beyond. Trends Endocrinol Metab. 2016;27:11–23. doi: 10.1016/j.tem.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ: morphological perspectives of adipose tissues. The Proceedings of the Nutrition Society. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. beta-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Frontiers in endocrinology. 2011;2:102. doi: 10.3389/fendo.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. The Journal of biological chemistry. 2000;275:33059–33067. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF, Kwan HY, Kang C, Wong RH, Sul HS. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell. 2015;57:235–246. doi: 10.1016/j.molcel.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MB, Herzig S, Vegiopoulos A. Thermogenic adipocytes: from cells to physiology and medicine. Metabolism: clinical and experimental. 2014;63:1238–1249. doi: 10.1016/j.metabol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L. Adipose VEGF Links the White-to-Brown Fat Switch With Environmental, Genetic, and Pharmacological Stimuli in Male Mice. Endocrinology. 2015;156:2059–2073. doi: 10.1210/en.2014-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. The Journal of clinical investigation. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emont MP, Yu H, Wu J. Transcriptional control and hormonal response of thermogenic fat. The Journal of endocrinology. 2015;225:R35–R47. doi: 10.1530/JOE-15-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Wideman L, Labban JD, Piepmeier A, Pendleton DM, Dvorak K, Becofsky K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF) J Sport Exerc Psychol. 2016;29:1–33. doi: 10.1123/jsep.2015-0335. [DOI] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nature medicine. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Tsang S, Jones R, Ponugoti B, Yoon H, Wu SY, Chiang CM, Willson TM, Kemper JK. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. The Journal of biological chemistry. 2008;283:35086–35095. doi: 10.1074/jbc.M803531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell metabolism. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, Guerrieri M, Cinti S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochimica et biophysica acta. 2013;1831:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso V, Lopez-Vicario C, Titos E, Moran-Salvador E, Gonzalez-Periz A, Rius B, Parrizas M, Werz O, Arroyo V, Claria J. Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor gamma (PPARgamma) in the conversion of white-to-brown adipocytes. The Journal of biological chemistry. 2013;288:28230–28242. doi: 10.1074/jbc.M113.468603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. American journal of physiology. Endocrinology and metabolism. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism: clinical and experimental. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Madaio AI, Mallei A, Lee FS, Popoli M. Brain Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology. 2016;41:3070–3079. doi: 10.1038/npp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell metabolism. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell metabolism. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes & development. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell metabolism. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell metabolism. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PloS one. 2014;9:e84910. doi: 10.1371/journal.pone.0084910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA, et al. A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology. 2012;153:3133–3146. doi: 10.1210/en.2012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015a;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015b;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell metabolism. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell metabolism. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PloS one. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manieri M, Murano I, Fianchini A, Brunelli A, Cinti S. Morphological and immunohistochemical features of brown adipocytes and preadipocytes in a case of human hibernoma. Nutr Metab Cardiovasc Dis. 2010;20:567–574. doi: 10.1016/j.numecd.2009.04.020. [DOI] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashon JM, Gorecki MC, Kozlowski AE, Thirnbeck CK, Markan KR, Leslie KL, Kotas ME, Potthoff MJ, Richerson GB, Gillum MP. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell metabolism. 2015;21:692–705. doi: 10.1016/j.cmet.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehle K, Ebert T, Kralisch S, Hoffmann A, Kratzsch J, Schlogl H, Stumvoll M, Fasshauer M. Serum concentrations of fibroblast growth factor 21 are elevated in patients with congenital or acquired lipodystrophy. Cytokine. 2016;83:239–244. doi: 10.1016/j.cyto.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2014;24:239–248. doi: 10.1002/hipo.22218. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell metabolism. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat. 2009;214:171–178. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Frontiers in endocrinology. 2012;3:15. doi: 10.3389/fendo.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, Clegg DJ. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab. 2015;4:427–436. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504:163–167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell metabolism. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell metabolism. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol. 2015;55:207–227. doi: 10.1146/annurev-pharmtox-010814-124346. [DOI] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell metabolism. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Porter C, Chondronikola M, Sidossis LS. The Therapeutic Potential of Brown Adipocytes in Humans. Frontiers in endocrinology. 2015;6:156. doi: 10.3389/fendo.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid B, van de Sande-Lee S, Rodovalho S, Folli F, Beltramini GC, Morari J, Amorim BJ, Pedro T, Ramalho AF, Bombassaro B, et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int J Obes (Lond) 2015;39:1515–1522. doi: 10.1038/ijo.2015.94. [DOI] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Munzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiology & behavior. 2013;121:49–55. doi: 10.1016/j.physbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MO, Lebrun FL, Christoffolete MA, Branco M, Crescenzi A, Carvalho SD, Negrao N, Bianco AC. Evidence of UCP1-independent regulation of norepinephrine-induced thermogenesis in brown fat. Am J Physiol Endocrinol Metab. 2000;279:E314–E322. doi: 10.1152/ajpendo.2000.279.2.E314. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell metabolism. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nature cell biology. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D, Grinberg AV, Liu J, Davies MV, Castonguay R, Maniatis S, Andreucci AJ, Pobre EG, Tomkinson KN, Monnell TE, et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. The Journal of biological chemistry. 2010;285:21037–21048. doi: 10.1074/jbc.M110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Berger JP, Samson P, Castriota G, Lalonde J, Deshaies Y, Richard D. Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology. 2004;145:3925–3934. doi: 10.1210/en.2004-0321. [DOI] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, et al. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell metabolism. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature medicine. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. The Journal of physiology. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis A, Wiedmann NM, Adler ES, Oldfield BJ. Hypothalamic control of adipose tissue. Best Pract Res Clin Endocrinol Metab. 2014;28:685–701. doi: 10.1016/j.beem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nature medicine. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani M, Srinivasa S, Fitch KV, Thomou T, Wong K, Petrow E, Kahn CR, Cypess AM, Grinspoon SK. Dysfunctional Subcutaneous Fat With Reduced Dicer and Brown Adipose Tissue Gene Expression in HIV-Infected Patients. J Clin Endocrinol Metab. 2016;101:1225–1234. doi: 10.1210/jc.2015-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CL, Pan CY, Chen FC, Wang CH, Chou FY. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp Physiol. 2016;101:836–850. doi: 10.1113/EP085682. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301:R285–R296. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of lipid research. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R992–R1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. The Journal of clinical investigation. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Jun H, McDermott JR. Formation and activation of thermogenic fat. Trends in genetics. 2015;31:232–238. doi: 10.1016/j.tig.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R1115–R1125. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell metabolism. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Yang X, Ruan HB. Neuronal Control of Adaptive Thermogenesis. Frontiers in endocrinology. 2015;6:149. doi: 10.3389/fendo.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]